Analysis of Moisture Migration and Microstructural Characteristic of Green Sichuan Pepper (Zanthoxylum armatum) during the Hot-Air Drying Process Based on LF-NMR
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Materials
2.2. Experiment Instruments and Equipment
2.3. Experiment Methods
2.3.1. Experimental Operation
2.3.2. Low-Field Nuclear Magnetic Resonance Detection
2.3.3. Sample Microstructure Analysis
3. Analysis of Internal Moisture Distribution and Migration Patterns during Drying of Green Sichuan Pepper
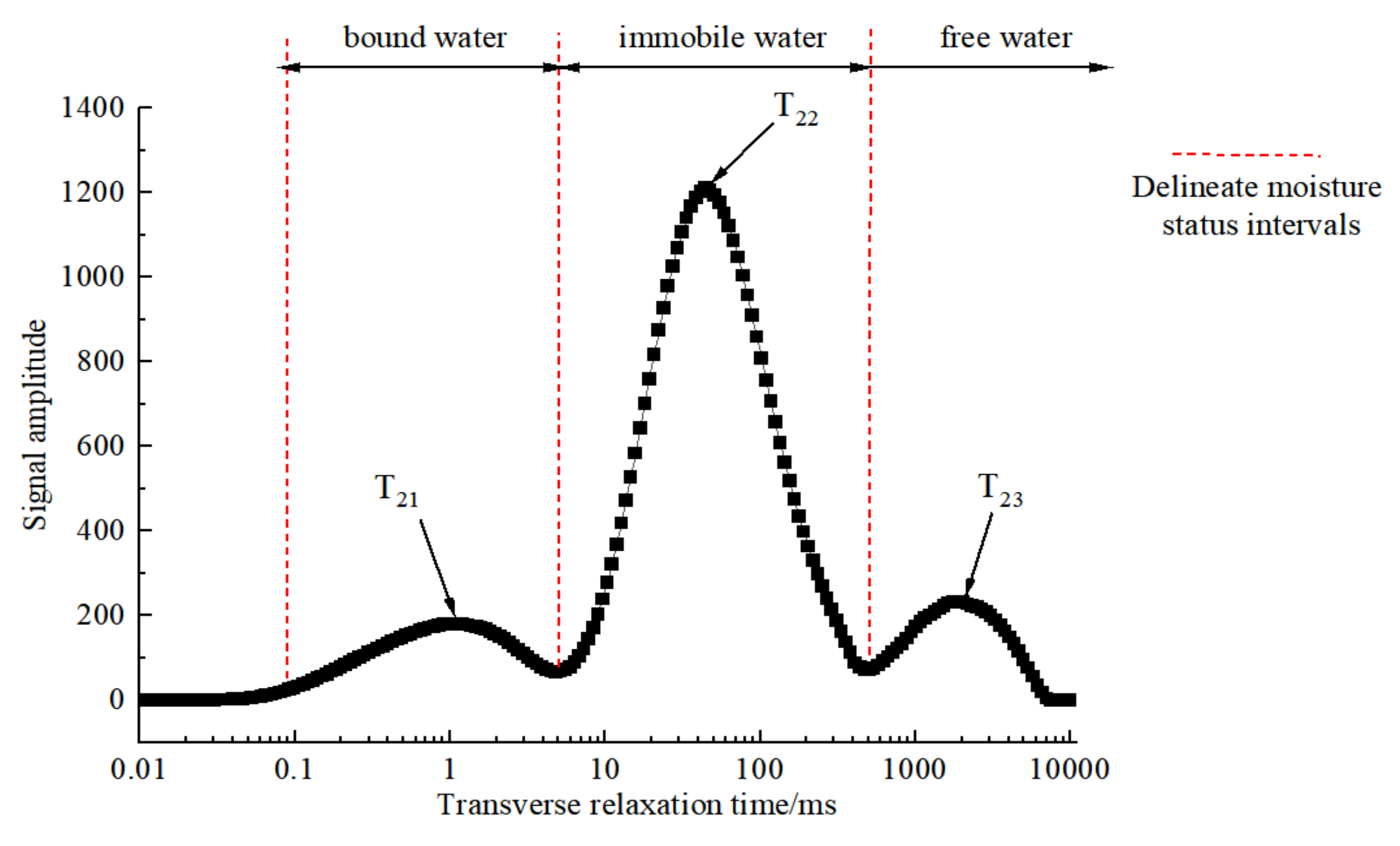
3.1. Moisture State and Distribution of Fresh Green Sichuan Pepper
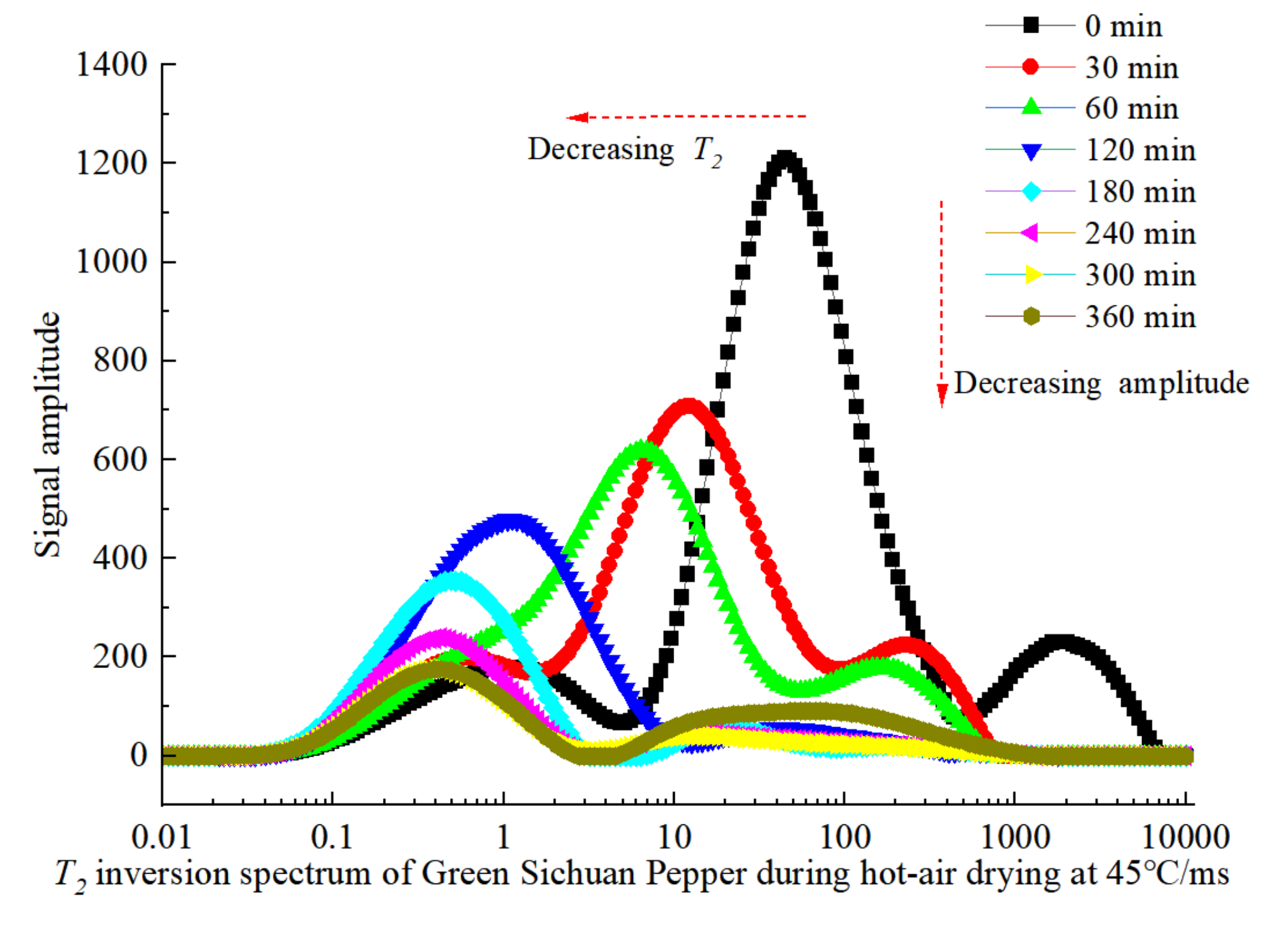
3.2. Variation of Moisture Migration during Hot-Air Drying
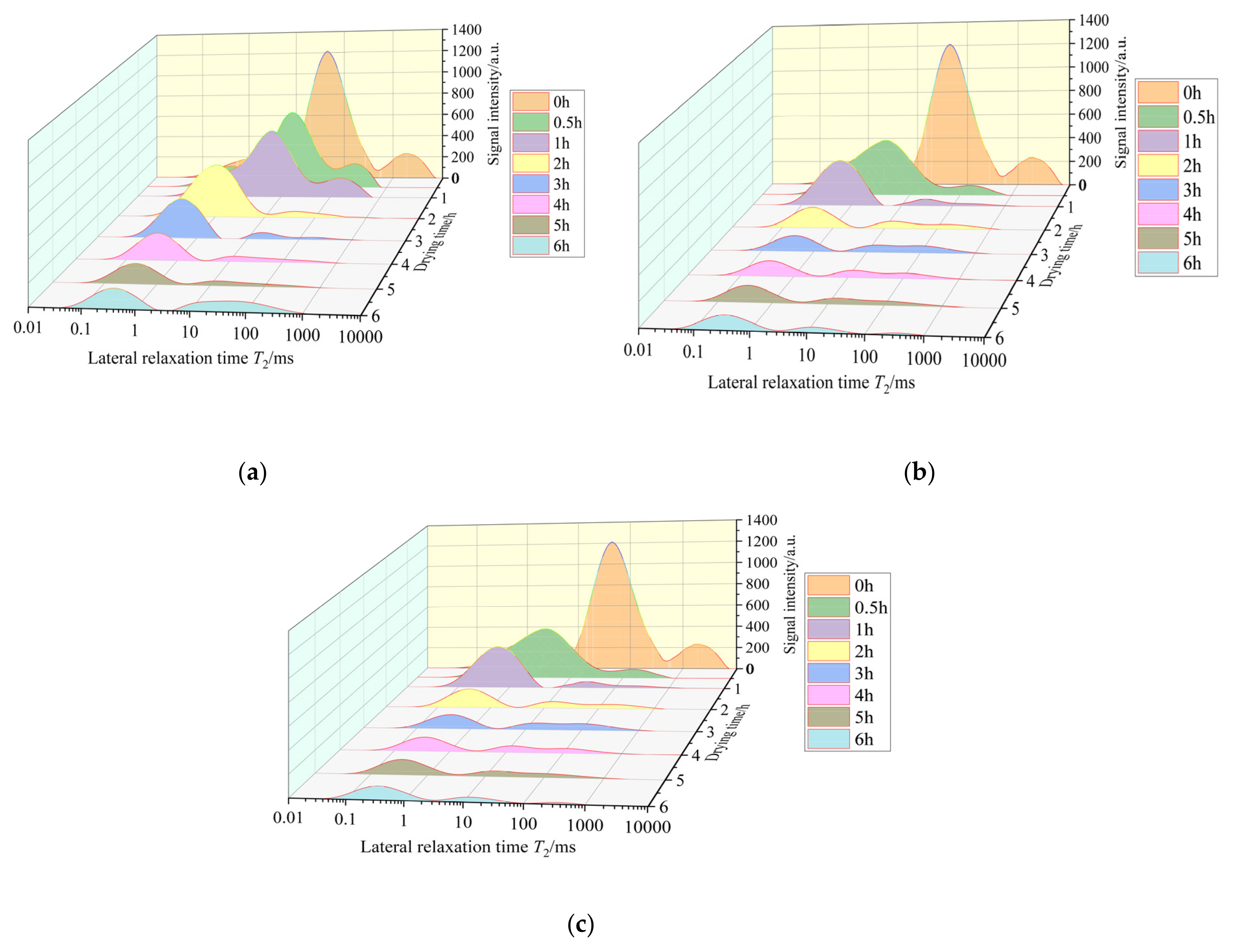
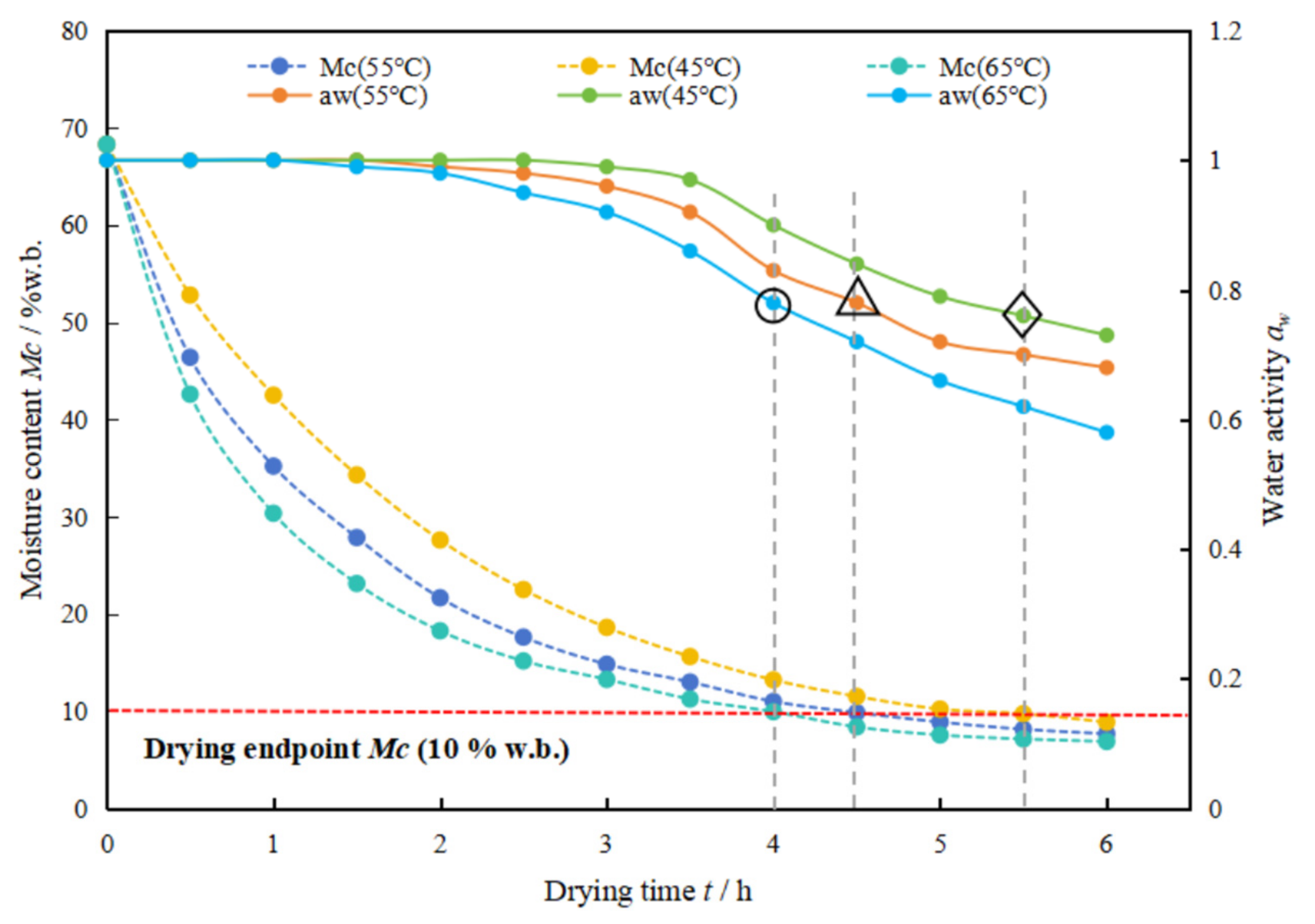
3.3. Effect of Hot Air Temperature on the Moisture Distribution and Migration Pattern of Green Sichuan Pepper
3.4. Moisture Content Prediction Model Based on Low-Field NMR Parameters
3.4.1. Correlation Analysis and Modelling of Parameters with Moisture Content
3.4.2. Model Validation
4. Microstructural Changes during Hot-Air Drying of Green Sichuan Pepper
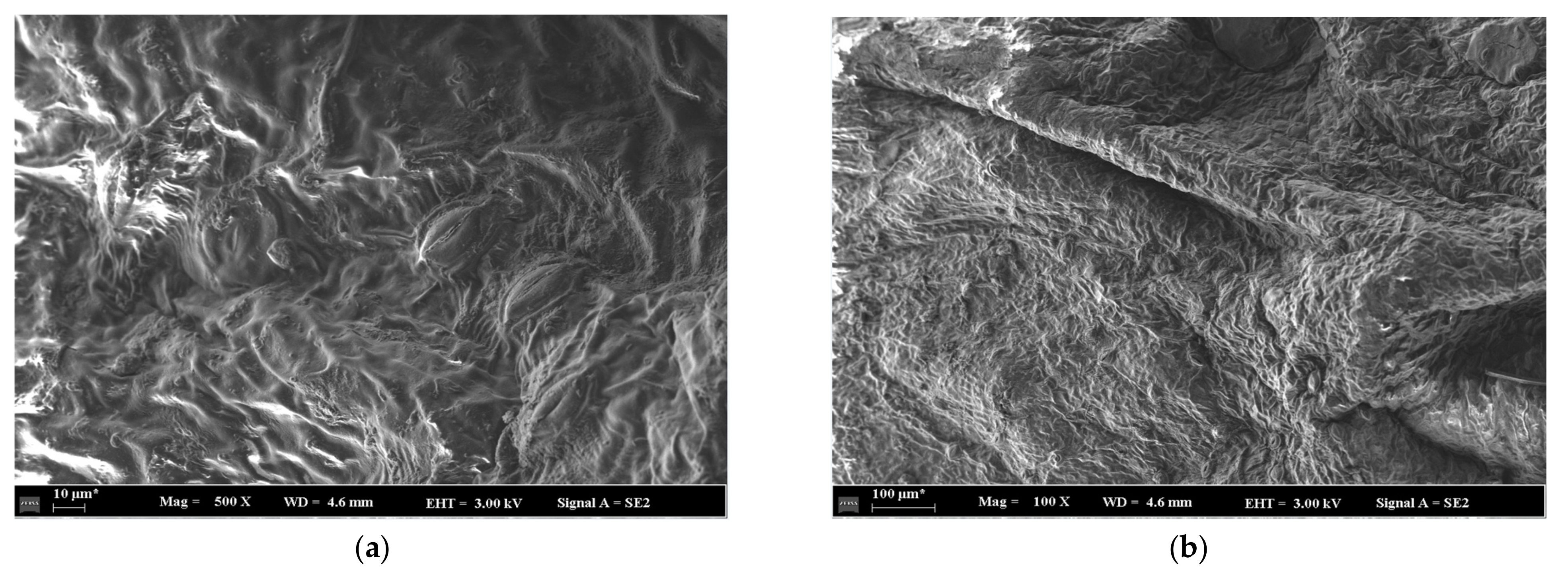
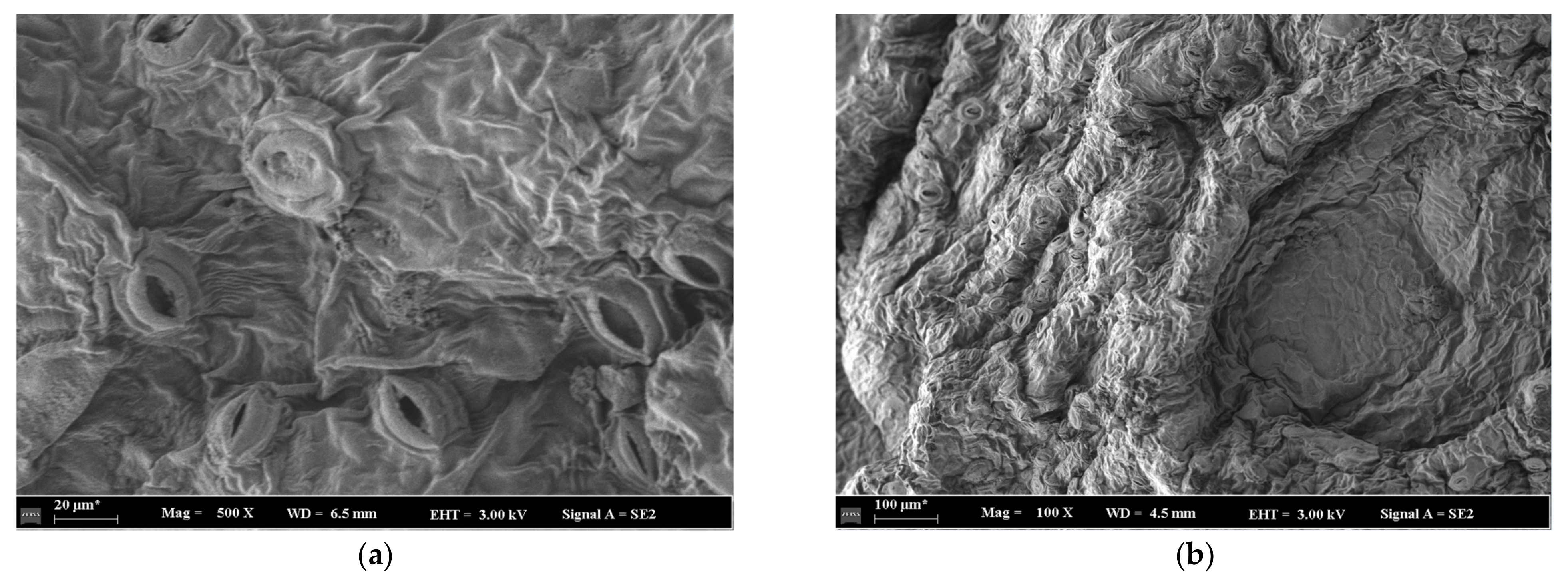
4.1. Analysis of Green Sichuan Pepper Microstructure
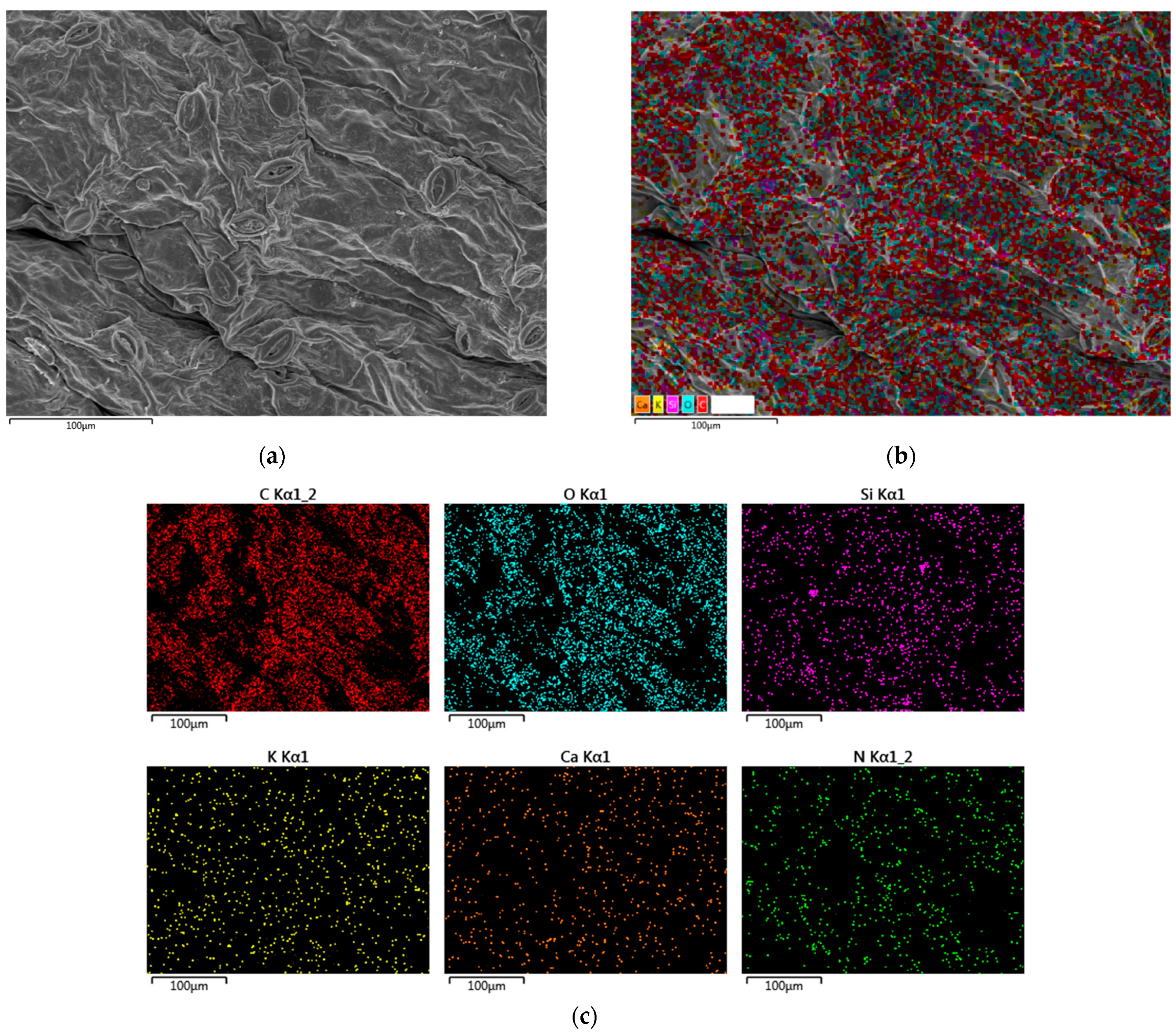
4.2. Analysis of Changes in Elemental Distribution
5. Conclusions
- (1)
- There are three states of water molecules present within the fresh Green Sichuan Pepper, including bound water, immobile water and free water, which can be symbolized by the relaxation time ranges of T21 (0.1–10 ms), T22 (10–500 ms) and T23 (500–10,000 ms), respectively. The majority of the water is immobile water (A22, accounting for 83.72%), which contributes to the considerable challenges associated with drying Green Sichuan Pepper.
- (2)
- Throughout the whole drying process, the immobile water and the free water consistently exhibit a declining trend, while the bound water initially increases and then decreases. The main dehydrated water during the drying process is ascertained to be the immobile water and free water. The higher the drying temperature is, the faster the immobile water and free water dehydrated rates are.
- (3)
- The LF-NMR parameter A2 shows a high correlation with the sample moisture content under different drying temperatures. The moisture content prediction models under 45, 55 and 65 °C were established and validated to be reliable by applying the index of relative error.
- (4)
- The pore opening degree increases with the increase of the drying temperature, which might reveal that the drying temperature can enhance the drying efficiency. The pore opening degree decreases with the increase of the drying time, which might reveal that drying efficiency decreases with the increase of drying time at the speed-down drying stage in Green Sichuan Pepper.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Zhang, Y.; Wei, X.; Wu, D.; Dai, J.; Liu, S.; Qin, W. Effect of radio frequency-assisted hot-air drying on drying kinetics and quality of Sichuan pepper (Zanthoxylum bungeanum maxim). LWT—Food Sci. Technol. 2021, 147, 111572. [Google Scholar] [CrossRef]
- Bhatt, V.; Sharma, S.; Kumar, N.; Sharma, U.; Singh, B. Simultaneous quantification and identification of flavonoids, lignans, coumarin and amides in leaves of Zanthoxylum armatum using UPLC-DAD-ESI-QTOF-MS/MS. J. Pharm. Biomed. 2017, 132, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Jing, N.; Wang, M.; Gao, M.; Zhong, Z.; Ma, Y.; Wei, A. Color sensory characteristics, nutritional components and antioxidant capacity of Zanthoxylum bungeanum Maxim. as affected by different drying methods. Ind. Crop. Prod. 2021, 160, 113167. [Google Scholar] [CrossRef]
- Yamazaki, E.; Inagaki, M.; Kurita, O.; Inoue, T. Antioxidant activity of Japanese pepper (Zanthoxylum piperitum DC.). Fruit. Food Chem. 2007, 100, 171–177. [Google Scholar] [CrossRef]
- Ma, Y.; Li, X.; Hou, L.; Wei, A. Extraction solvent affects the antioxidant, antimicrobial, cholinesterase and HepG2 human hepatocellular carcinoma cell inhibitory activities of Zanthoxylum bungeanum pericarps and the major chemical components. Ind. Crop. Prod. 2019, 142, 111872. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, X.; Battino, M.; Wei, X.; Shi, J.; Zhao, L.; Liu, S.; Xiao, J.; Shi, B.; Zou, X. A comparative overview on chili pepper (capsicum genus) and sichuan pepper (zanthoxylum genus): From pungent spices to pharma-foods. Trends Food Sci. Tech. 2021, 117, 148–162. [Google Scholar] [CrossRef]
- Lu, J.; Xiang, J.; Liu, T.; Gao, Z.; Liao, M. Sichuan Pepper Recognition in Complex Environments: A Comparison Study of Traditional Segmentation versus Deep Learning Methods. Agriculture 2022, 12, 1631. [Google Scholar] [CrossRef]
- Ojediran, J.O.; Okonkwo, C.E.; Adeyi, A.J.; Adeyi, O.; Olaniran, A.F.; George, N.E.; Olayanju, A.T. Drying characteristics of yam slices (Dioscorea rotundata) in a convective hot air dryer: Application of ANFIS in the prediction of drying kinetics. Heliyon 2020, 6, e03555. [Google Scholar] [CrossRef]
- Li, M.; Chen, Y.; Wang, X.; Cheng, S.; Liu, F.; Huang, L. Determination of drying kinetics and quality changes of Panax quinquefolium L. dried in hot-blast air. LWT—Food Sci. Technol. 2019, 116, 108563. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, B.; Han, C.; Wu, W.; Chen, T.; Dong, C.; Gao, C.; He, Z.; Zhang, F. Performance of Exergetic, Energetic and Techno-Economic Analyses on a Gas-Type Industrial Drying System of Black Tea. Foods 2022, 11, 3281. [Google Scholar] [CrossRef]
- Li, B.; Li, C.; Li, T.; Zeng, Z.; Ou, W.; Li, C. Exergetic, Energetic, and Quality Performance Evaluation of Paddy Drying in a Novel Industrial Multi-Field Synergistic Dryer. Energies 2019, 12, 4588. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Gong, C.; Jiao, S. Effect of radio frequency heating stress on sublethal injury of Salmonella Typhimurium in red pepper powder. LWT—Food Sci. Technol. 2020, 117, 108700. [Google Scholar] [CrossRef]
- Liu, S.; Wang, H.; Ma, S.; Dai, J.; Zhang, Q.; Qin, W. Radiofrequency-assisted hot-air drying of Sichuan pepper (Huajiao). LWT—Food Sci. Technol. 2021, 135, 110158. [Google Scholar] [CrossRef]
- Jin, H.; Wang, Y.; Lv, B.; Zhang, K.; Zhu, Z.; Zhao, D.; Li, C. Rapid Detection of Avocado Oil Adulteration Using Low-Field Nuclear Magnetic Resonance. Foods 2022, 11, 1134. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wang, X.; Chen, L. Rapid detection of peanut oil adulteration using low-field nuclear magnetic resonance and chemometrics. Food Chem. 2017, 216, 268–274. [Google Scholar] [CrossRef]
- Li, M.; Li, B.; Zhang, W. Rapid and non-invasive detection and imaging of the hydrocolloid-injected prawns with low-field NMR and MRI. Food Chem. 2018, 242, 16–21. [Google Scholar] [CrossRef]
- Kirtil, E.; Cikrikci, S.; McCarthy, M.J.; Oztop, M.H. Recent advances in time domain NMR & MRI sensors and their food applications. Curr. Opin. Food Sci. 2017, 17, 9–15. [Google Scholar]
- Cheng, S.; Wang, X.; Li, R.; Yang, H.; Wang, H.; Wang, H.; Tan, M. Influence of multiple freeze-thaw cycles on quality characteristics of beef semimembranous muscle: With emphasis on water status and distribution by LF-NMR and MRI. Meat Sci. 2019, 147, 44–52. [Google Scholar] [CrossRef]
- Tan, D.; Tang, X.; Zhang, Y.; Li, G.; Li, X.; Luo, Y.; Wu, S. Optimization of microwave vacuum drying process for horseshoe crisp slices and its moisture content variation. Food Res. Dev. 2024, 45, 107–115. [Google Scholar]
- Ren, A.; Cai, W.; Han, C.; Tang, X.; Duan, Z. LF-NMR combined with MRI analysis of moisture migration in black fungus during heat pump drying process. Food Res. Dev. 2023, 44, 10–16. [Google Scholar]
- Liu, J.; Li, L.; Xu, Q.; Wang, R.; Wu, L.; Li, Z. Research on the mechanism of low-pressure superheated steam drying of green radish chunks based on low field nuclear magnetic resonance. Packag. Food Mach. 2023, 41, 33–38. [Google Scholar]
- Ouyang, M.Y. Study on Color Change and Maillard Reaction Mechanism of Pumpkin Slices during Hot Air Drying; Hunan Agricultural University: Changsha, China, 2021. [Google Scholar]
- Chao, E.; Li, J.; Fan, L. Enhancing drying efficiency and quality of seed-used pumpkin using ultrasound, freeze-thawing and blanching pretreatments. Food Chem. 2022, 384, 132496. [Google Scholar] [CrossRef]
- Li, L.; Cheng, X.; Yang, S.; Luo, Z.; Liu, Z. A moisture content prediction model for hot air dried kiwifruit slices based on low field nuclear magnetic resonance. J. Agric. Eng. 2020, 36, 252–260. [Google Scholar]
- Wei, Z.; Zhu, W.; Bai, X.; Luo, L.; Ning, Y.; Si, M. Study on the moisture state of hot air dried peanut kernels based on low field nuclear magnetic resonance and electron microscopy. Chin. J. Cereals Oils 2022, 37, 213–220. [Google Scholar]
- Xu, X.; Ke, Y.; Tang, S.; Li, Y.; Sun, Y.; Wang, Q. Study on the Changes in Water Status of Yam During Hot Air Drying. Preserv. Process. 2020, 20, 177–180. [Google Scholar]
- Wang, J.; Geng, Y.; Liu, Y.; Hu, B.; Zhang, S.; Lu, Z.; Zeng, Q.; Chen, S. Exploring the Effect of Different Moisture Content on the Quality of Matured Chestnuts Based on Low Field Nuclear Magnetic Resonance Technology. J. Nucl. Agric. 2023, 37, 769–780. [Google Scholar]
- Chen, W.; Mu, H.; Wu, W.; Fang, X.; Han, Y.; Chen, H.; Gao, H.; Jin, L. Non destructive detection of moisture content in Australian nuts using low field nuclear magnetic resonance technology. J. Agric. Eng. 2020, 36, 303–309. [Google Scholar]
- Wen, Y. Dynamics and Quality Changes of Star Anise Dried by Far Infrared Irradiation and Hot Air; South China University of Technology: Guangzhou, China, 2019. [Google Scholar]
- Prothon, F.; Ahrne, L.; Sjoholm, I. Mechanismsand prevention of plant tissue collapse duringdehydration: A critical review. Crit. Rev. Infood Sci. Nutr. 2003, 43, 447–479. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Fu, Q.; Huang, H.; Li, M.; Li, L.; Xu, L. Drying characteristics and quality optimization of green prickly ashes during vacuum pulsed drying. Trans. Chin. Soc. Agric. Eng. Trans. CSAE 2021, 37, 279–287. [Google Scholar]
- Xu, F.; Jin, X.; Zhang, L.; Chen, X.D. Investigation on water status and distribution in broccoli and the effects of drying on water status using NMR and MRI methods. Food Res. Int. 2017, 96, 191–197. [Google Scholar] [CrossRef]
- Lv, W.; Li, S.; Han, Q.; Zhao, Y.; Wu, H. Study of the drying process of ginger (Zingiber officinale Roscoe) slices in microwave fluidized bed dryer. Dry. Technol. 2016, 34, 1690–1699. [Google Scholar] [CrossRef]
- Pan, Y.; Duan, Z.; Zhong, J. Analysis of internalmoisture changes of persimmon slices during intermittent microwavedrying using low-field NMR. Sci. Technol. Food-Dustry 2021, 42, 33–39. [Google Scholar]
- Li, B.; Peng, G.; Luo, C.; Meng, G.; Yang, L. Vacuum drying kinetics of Sichuan pepper based on Weibull distribution function. Food Ferment. Ind. 2017, 43, 58–64. [Google Scholar]
- Wang, X.; Jia, C.; Wang, X.; Li, M.; Liu, F.; Dong, H. Study on moisture changes and quality during hot air drying of figs. Food Res. Dev. 2022, 43, 71–78. [Google Scholar]
- Si, M.; Zhu, W.; Bai, X.; Luo, L.; Ning, Y. Research on the drying characteristics of oil sand bean heat pumps based on low field nuclear magnetic resonance technology. Chin. J. Cereals Oils 2023, 38, 19–26. [Google Scholar]
- Wang, Y. Scanning transmission electron microscopy technology and its application in traditional Chinese medicine research. Anal. Test. Tech. Instrum. 2022, 28, 280–288. [Google Scholar]





| Equipment | Type | Manufacturer |
|---|---|---|
| Electric constant temperature drier | 202-00 | Shanghai Guangdi Instrument Equipment Company, Shanghai, China |
| Thin-layer drying experiment bed | BC-2 | Changchun Jida Instrument Company, Changchun, China |
| Halogen water-determination meter | FBS-760A | Xiamen Forbes Testing Equipment Company, Xiamen, China |
| Low field magnetic resonance imaging analyzer | MesoMR12-060H-1 | Suzhou Newmai Analytical Instrument Company, Suzhou, China |
| Thermal field emission scanning electron microscope | Sigma300 | Carl Zeiss AG, Germany, Jena, Germany |
| Electronic scale | YP-6002B | Shanghai Lichen Instrument Technology Company, Shanghai, China |
| Sample sieve | 50 mesh; diameter: 20 cm | - |
| Drying Temperature/°C | Drying Time/h | T21/ms | T22/ms | T23/ms | A21 | A22 | A23 |
|---|---|---|---|---|---|---|---|
| 45 | 0 | 2.6 ± 0.3 | 47.7 ± 0.7 | 766.3 ± 1.1 | 6973.8 ± 1.4 | 37,433.8 ± 2.7 | 887.5 ± 1.3 |
| 0.5 | 1.8 ± 0.1 | 63.0 ± 0.6 | 486.3 ± 0.1 | 6099.1 ± 1.0 | 25,122.5 ± 1.3 | 4908.9 ± 3.1 | |
| 1 | 1.1 ± 0.0 | 166.4 ± 1.3 | - | 27,916.7 ± 2.1 | 4735.9 ± 0.5 | - | |
| 2 | 0.6 ± 0.1 | 41.5 ± 1.1 | 235.5 ± 0.1 | 18,921.1 ± 1.4 | 1911.8 ± 4.1 | 171.7 ± 1.5 | |
| 3 | 0.5 ± 0.2 | 19.3 ± 1.3 | 191.2 ± 0.1 | 14,230.3 ± 2.0 | 1577.8 ± 0.6 | 499.4 ± 0.6 | |
| 4 | 0.4 ± 0.0 | 13.7 ± 1.1 | - | 7574.3 ± 1.7 | 2201.5 ± 2.4 | - | |
| 5 | 0.4 ± 0.2 | 14.7 ± 0.3 | - | 5755.8 ± 0.6 | 1914.7 ± 1.7 | - | |
| 6 | 0.4 ± 0.3 | 13.2 ± 0.7 | - | 4849.3 ± 1.5 | 1475.1 ± 0.5 | ||
| 55 | 0 | 2.6 ± 0.3 | 47.7 ± 2.3 | 766.3 ± 1.3 | 6973.7 ± 1.5 | 37,433.8 ± 1.3 | 887.5 ± 0.8 |
| 0.5 | 1.7 ± 0.2 | 117.6 ± 1.5 | 622.3 ± 1.7 | 22,523.9 ± 1.6 | 3433.5 ± 1.5 | 87.7 ± 0.4 | |
| 1 | 0.9 ± 0.1 | 58.7 ± 1.7 | - | 21,129.8 ± 0.8 | 1962.2 ± 1.8 | - | |
| 2 | 0.5 ± 0.0 | 15.7 ± 0.5 | 109.7 ± 0.9 | 9936.8 ± 1.9 | 1385.5 ± 1.3 | 2233.3 ± 2.6 | |
| 3 | 0.4 ± 0.3 | 22.2 ± 0.5 | 6970.8 ± 0.3 | 1392.9 ± 0.9 | - | ||
| 4 | 0.4 ± 0.2 | 9.0 ± 0.3 | 51.1 ± 1.2 | 3898.9 ± 0.5 | 1044.1 ± 1.0 | 2037.0 ± 0.7 | |
| 5 | 0.4 ± 0.0 | 14.7 ± 0.3 | 252.3 ± 2.1 | 4349.6 ± 1.0 | 1395.3 ± 0.3 | 614.2 ± 1.3 | |
| 6 | 0.4 ± 0.1 | 12.7 ± 1.6 | 219.4 ± 0.6 | 3881.5 ± 0.4 | 1225.2 ± 0.3 | 335.1 ± 2.1 | |
| 65 | 0 | 2.6 ± 0.1 | 47.7 ± 1.5 | 766.3 ± 1.5 | 6973.7 ± 0.8 | 37,433.8 ± 2.3 | 887.5 ± 1.6 |
| 0.5 | 1.1 ± 0.0 | 155.2 ± 2.2 | - | 24,043.9 ± 0.3 | 2197.1 ± 1.7 | - | |
| 1 | 0.6 ± 0.0 | 29.3 ± 1.7 | - | 13,388.7 ± 1.1 | 2009.0 ± 2.0 | - | |
| 2 | 0.4 ± 0.1 | 14.7 ± 1.7 | 155.2 ± 1.9 | 5409.6 ± 0.3 | 1785.6 ± 0.1 | 1174.4 ± 2.1 | |
| 3 | 0.4 ± 0.4 | 16.8 ± 1.9 | 95.5 ± 1.7 | 3701.0 ± 1.6 | 1262.3 ± 0.7 | 1920.6 ± 3.7 | |
| 4 | 0.4 ± 0.1 | 15.7 ± 1.5 | 109.7 ± 2.8 | 3901.5 ± 1.4 | 1617.6 ± 3.3 | 1174.3 ± 2.9 | |
| 5 | 0.4 ± 0.0 | 13.7 ± 1.3 | - | 4342.3 ± 1.2 | 1959.1 ± 1.8 | - | |
| 6 | 0.2 ± 0.0 | 12.3 ± 0.5 | - | 3662.9 ± 0.3 | 1688.4 ± 1.4 | - |
| Index | T21/ms | T22/ms | T23/ms | A21 | A22 | A23 | A2 |
|---|---|---|---|---|---|---|---|
| 45 °C MC | 0.878 *** | 0.632 | 0.766 * | 0.344 | 0.771 * | 0.515 * | 0.986 * |
| 55 °C MC | 0.942 *** | 0.724 * | 0.719 * | 0.591 | 0.727 * | −0.151 | 0.977 *** |
| 65 °C MC | 0.413 | 0.617 | 0.729 | 0.537 ** | 0.811 * | −0.119 * | 0.993 ** |
| Drying Temperature/°C | Drying Time/h | A2 | Predicted Moisture Content/%w.b. | Measured Moisture Content/%w.b. | Relative Deviation/% |
|---|---|---|---|---|---|
| 45 °C | 0 | 43,295.02 | 72.31 | 69.84 | 3.54 |
| 0.5 | 37,130.57 | 62.14 | 62.21 | 0.11 | |
| 1 | 34,652.58 | 58.05 | 57.72 | 0.58 | |
| 2 | 23,004.5 | 38.83 | 43.95 | 11.65 | |
| 3 | 19,307.42 | 32.73 | 26.80 | 22.12 | |
| 4 | 9875.795 | 17.17 | 16.73 | 2.62 | |
| 5 | 7770.488 | 13.70 | 11.42 | 19.96 | |
| 6 | 6124.494 | 10.98 | 8.96 | 22.54 | |
| 55 °C | 0 | 43,399.5 | 74.23 | 69.91 | 6.18 |
| 0.5 | 34,730.34 | 59.15 | 53.18 | 11.23 | |
| 1 | 28,652.84 | 48.57 | 42.91 | 13.20 | |
| 2 | 17,004.35 | 28.31 | 25.47 | 11.13 | |
| 3 | 9607.7 | 15.43 | 12.69 | 21.63 | |
| 4 | 6975.21 | 10.85 | 9.74 | 11.49 | |
| 5 | 4510.169 | 6.57 | 5.97 | 9.90 | |
| 6 | 3924.869 | 5.55 | 5.73 | 3.20 | |
| 65 °C | 0 | 43,295.87 | 70.96 | 70.89 | 0.09 |
| 0.5 | 30,130.63 | 48.44 | 47.39 | 2.23 | |
| 1 | 20,495.58 | 31.97 | 26.73 | 19.59 | |
| 2 | 11,005.61 | 15.74 | 13.32 | 18.16 | |
| 3 | 8025.54 | 10.64 | 8.97 | 18.65 | |
| 4 | 5517.795 | 6.36 | 6.11 | 4.09 | |
| 5 | 5370.488 | 6.10 | 5.72 | 6.69 | |
| 6 | 4723.494 | 5.00 | 4.90 | 1.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Liu, C.; Luo, H.; Han, C.; Zhang, X.; Li, Q.; Gong, L.; Wang, P.; Zeng, Z. Analysis of Moisture Migration and Microstructural Characteristic of Green Sichuan Pepper (Zanthoxylum armatum) during the Hot-Air Drying Process Based on LF-NMR. Agriculture 2024, 14, 1361. https://doi.org/10.3390/agriculture14081361
Li B, Liu C, Luo H, Han C, Zhang X, Li Q, Gong L, Wang P, Zeng Z. Analysis of Moisture Migration and Microstructural Characteristic of Green Sichuan Pepper (Zanthoxylum armatum) during the Hot-Air Drying Process Based on LF-NMR. Agriculture. 2024; 14(8):1361. https://doi.org/10.3390/agriculture14081361
Chicago/Turabian StyleLi, Bin, Chuandong Liu, Hang Luo, Chongyang Han, Xuefeng Zhang, Qiaofei Li, Lian Gong, Pan Wang, and Zhiheng Zeng. 2024. "Analysis of Moisture Migration and Microstructural Characteristic of Green Sichuan Pepper (Zanthoxylum armatum) during the Hot-Air Drying Process Based on LF-NMR" Agriculture 14, no. 8: 1361. https://doi.org/10.3390/agriculture14081361
APA StyleLi, B., Liu, C., Luo, H., Han, C., Zhang, X., Li, Q., Gong, L., Wang, P., & Zeng, Z. (2024). Analysis of Moisture Migration and Microstructural Characteristic of Green Sichuan Pepper (Zanthoxylum armatum) during the Hot-Air Drying Process Based on LF-NMR. Agriculture, 14(8), 1361. https://doi.org/10.3390/agriculture14081361





