Effects of Plastic Mulch Residue on Soil Fungal Communities in Cotton
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area Overview
2.2. Experimental Design and Sample Collection
2.3. Sample Analysis and Data Processing
2.3.1. Sample Analysis
2.3.2. Data Processing
3. Results
3.1. Effects of Different Residue Levels on Soil Fungal Community Composition
3.2. Effects of Different Levels of Plastic Mulch Residue on Soil Fungal Alpha Diversity
3.3. Effects of Different Residual Mulch Levels on Soil Fungal Beta Diversity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Qi, H.; Zhao, G.Y.; Wang, Y.; Liu, J.G.; Geng, Z.; Dou, H.K.; Zhang, H.S.; Wang, Y.Q. Research progress on pollution hazards and prevention measures of residual film incotton field in China. Cotton Sci. 2021, 33, 169–179. [Google Scholar]
- Wang, J.; Luo, Y.; Teng, Y.; Ma, W.; Christie, P.; Li, Z. Soil contamination by phthalate esters in Chinese intensive vegetable production systems with different modes of use of plastic film. Environ. Pollut. 2013, 180, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.-Y.; Kong, S.-F.; Ni, H.-G. Contribution of mulch film to microplastics in agricultural soil and surface water in China. Environ. Pollut. 2021, 291, 118227. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Han, Y.; Wang, J.; Xu, J.; Li, Y.; Sun, M.; Zhao, F.; He, C.; Sun, Y.; Wang, Y.; et al. PBAT/PLA humic acid biodegradable film applied on solar greenhouse tomato plants increased lycopene and decreased total acid contents. Sci. Total Environ. 2023, 871, 162077. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Q.; Fan, B.; Zhang, J.; Li, W.; Zheng, X.; Lin, H.; Guo, L. Testing biodegradable films as alternatives to plastic films in enhancing cotton Gossypium hirsutum L. yield under mulched drip irrigation. Soil Tillage Res. 2019, 192, 196–205. [Google Scholar] [CrossRef]
- Yang, C.; Huang, Y.; Long, B.; Gao, X. Effects of biodegradable and polyethylene film mulches and their residues on soil bacterial communities. Environ. Sci. Pollut. Res. Int. 2022, 29, 89698–89711. [Google Scholar] [CrossRef] [PubMed]
- Barrios, E. Soil biota, ecosystem services and land productivity. Ecol. Econ. 2007, 64, 269–285. [Google Scholar] [CrossRef]
- Lavelle, P.; Decaëns, T.; Aubert, M.; Barot, S.; Blouin, M.; Bureau, F.; Margerie, P.; Mora, P.; Rossi, J.-P. Soil invertebrates and ecosystem services. Eur. J. Soil Biol. 2006, 42, 3–15. [Google Scholar] [CrossRef]
- Jin, Z.; Liu, G.; Zhou, M.; Xu, W. The Indicative Role of Soil Nutrients on Microbial Communities in Mountain Grassland Soils. Southwest China J. Agric. Sci. 2019, 32, 8. [Google Scholar]
- Wu, J.; Yang, R.; Wang, Y.; Qu, S.; Liu, Z.; Hou, C. Composition and Diversity of Rhizospheric Soil Fungal Communities Under Three Different Vegetation Types in Cao Hai Basin, Guizhou. Mycosystema 2020, 39, 13. [Google Scholar]
- Ai, C.; Zhang, S.; Zhang, X.; Guo, D.; Zhou, W.; Huang, S. Distinct responses of soil bacterial and fungal communities to changes in fertilization regime and crop rotation. Geoderma Int. J. Soil Sci. 2018, 319, 156–166. [Google Scholar] [CrossRef]
- Xing, J.F.; Wang, X.F.; Hu, C.; Wang, L.; Xu, Z.X.; He, X.W.; Wang, Z.B.; Zhao, P.F.; Liu, Q. Effects of residual mulching films with different mulching years on the diversity of soil microbial communities in typical regions. Heliyon 2022, 8, 12. [Google Scholar] [CrossRef]
- Liu, Y.; Mao, L.; He, X.; Cheng, G.; Ma, X.; An, L.; Feng, H. Rapid change of AM fungal community in a rain-fed wheat field with short-term plastic film mulching practice. Mycorrhiza 2012, 22, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.-C.; Sun, Y.; Tao, C.; Deng, X.; Xu, X.; Shen, Z.; Zhang, L.; Zheng, Z.; Huang, Y.; Hao, Y.; et al. Rhizosphere Microbiota Promotes the Growth of Soybeans in a Saline–Alkali Environment under Plastic Film Mulching. Plants 2013, 12, 1889. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, L.; Fu, B.; Huang, Z.; Gong, J. The wheat yields and water-use efficiency in the Loess Plateau: Straw mulch and irrigation effects. Agric. Water Manag. 2005, 72, 209–222. [Google Scholar] [CrossRef]
- Nomura, T.; Tani, S.; Yamamoto, M.; Nakagawa, T.; Toyoda, S.; Fujisawa, E.; Yasui, A.; Konishi, Y. Cytotoxicity and colloidal behavior of polystyrene latex nanoparticles toward filamentous fungi in isotonic solutions. Chemosphere 2016, 149, 84–90. [Google Scholar] [CrossRef]
- Collignon, A.; Hecq, J.-H.; Galgani, F.; Collard, F.; Goffart, A. Annual variation in neustonic micro- and meso-plastic particles and zooplankton in the Bay of Calvi (Mediterranean–Corsica). Mar. Pollut. Bull. 2014, 79, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, Y.; Wang, J.; Zhang, M.; Jia, W.; Qin, X. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ. Pollut. 2019, 254, 112983. [Google Scholar] [CrossRef]
- Qian, H.; Zhang, M.; Liu, G.; Lu, T.; Qu, Q.; Du, B.; Pan, X. Effects of soil residual plastic film on soil microbial community structure and fertility. Water Air Soil Pollut. 2018, 229, 261. [Google Scholar] [CrossRef]
- Wang, J.; Huang, M.; Wang, Q.; Sun, Y.; Zhao, Y.; Huang, Y. LDPE microplastics significantly alter the temporal turnover of soil microbial communities. Sci. Total Environ. 2020, 726, 138682. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, R.; Sun, J.; Sun, Y.; Xu, G.; Wang, F. Microplastics change soil properties, plant performance, and bacterial communities in salt-affected soils. J. Hazard. Mater. 2024, 471, 134333. [Google Scholar] [CrossRef]
- Huang, L. Effects of Polythylene Film Residues on Crop Fields, Soil Quality and Soil Microbial Diversity. Master’s Thesis, Lanzhou University, Lanzhou, China, 2017. [Google Scholar]
- Xu, C.; Wang, C. Current status and outlook of mulching cultivation at home and abroad. Chin. Hemp Ind. 2006, 28, 6–11. [Google Scholar]
- Zhang, Y.; Wang, Z.; Guo, H.; Meng, D.; Wang, Y.; Wong, P. Interaction between Microbes DNA and Atrazine inBlack Soil Analyzed by Spectroscopy. Clean Soil Air Water A J. Sustain. Environ. Saf. 2015, 43, 867–871. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; Chen, K.; Ma, L.; Ma, X. Effects of different mulching cultivation on soil enzyme activities and soil microorganisms of potato. Southwest J. Agric. 2015, 28, 2154–2157. [Google Scholar]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- National Bureau of Statistics. China Statistical Yearbook; China Statistics Press: Beijing, China, 2021. [Google Scholar]
- Wang, X.; Apaer, R.; Xu, Z.; Fan, R.; Wang, X.; Wang, S.; Zhang, S. Refined Climatic Zoning for Cotton in Hutubi County, Xinjiang Based on GIS. Desert Oasis Meteorol. 2023, 17, 139–145. [Google Scholar]
- Xinjiang Uygur Autonomous Region Bureau of Statistics. 2022 Xinjiang Statistical Yearbook; China Statistics Press: Beijing, China, 2022. [Google Scholar]
- Sun, C.; San, N.; Yang, Z.; Wu, Z.; Wang, Y. Analysis of factors influencing the amount of film residues in Xinjiang farmland. J. Environ. Eng. Technol. 2021, 1–12. [Google Scholar]
- Liu, C. Construction of Evaluation System and Grading of Residual Film Pollution in Cotton Fields in South Xinjiang. Master’s Thesis, Tarim University, Tarim, China, 2018. [Google Scholar]
- Li, H.; Liu, Q.; Zhou, M.; Wu, H.; Liu, X.; Xia, X.; He, W. Evaluation of Influencing Factors and Pollution Intensity of Mulch Residues in Agricultural Fields in Xinjiang Region. J. Agric. Resour. Environ. 2024, 41, 656–665. [Google Scholar]
- Corsaro, D. Exploring LSU and ITS rDNA Sequences for Acanthamoeba Identification and Phylogeny. Microorganisms 2022, 10, 1776. [Google Scholar] [CrossRef]
- Woese, C.R.; Fox, G.E. Phylogenetic Structure of the Prokaryotic Domain: The Primary Kingdoms. Proc. Natl. Acad. Sci. USA 1977, 74, 5088–5090. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Fungal Barcoding Consortium; Fungal Barcoding Consortium Author List; Bolchacova, E.; et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.S.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Anslan, S.; Bahram, M.; Wurzbacher, C.; Baldrian, P.; Tedersoo, L. Mycobiome diversity: High-throughput sequencing and identification of fungi. Nat. Rev. Microbiol. 2019, 17, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Cacho, A.; Smirnova, E.; Huzurbazar, S.; Cui, X. A Comparison of Base-calling Algorithms for Illumina Sequencing Technology. Brief. Bioinform. 2016, 17, 786–795. [Google Scholar] [CrossRef]
- Kuczynski, J.; Lauber, C.L.; Walters, W.A.; Parfrey, L.W.; Clemente, J.C.; Gevers, D.; Knight, R. Experimental and analytical tools for studying the human microbiome. Nat. Rev. Genet. 2012, 13, 47–58. [Google Scholar] [CrossRef]
- Amir, A.; McDonald, D.; Navas-Molina, J.A.; Kopylova, E.; Morton, J.T.; Zech Xu, Z.; Kightley, E.P.; Thompson, L.R.; Hyde, E.R.; Gonzalez, A. Deblur rapidly resolves single-nucleotide community sequence patterns. MSystems 2017, 2, e00191-16. [Google Scholar] [CrossRef] [PubMed]
- Hey, M.J.; Jackson, D.P.; Hong, Y. The salting-out effect and phase separation in aqueous solutions of electrolytes and poly(ethylene glycol). Polymer 2005, 46, 2567–2572. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, J.; Tan, G.; Men, F.; Zhu, J.; Li, R.; Jing, B. Research on rapid and efficient treatment method of fracturing drainback fluid. Ind. Water Treat. 2015, 12, 77–82. [Google Scholar]
- Chen, L.; Bao, W.; Guo, B.; Li, M.; Sun, H.; Zhao, J. Research on the performance of conjugated ultra-high temperature seawater-based fracturing fluid. J. Petrochem. High. Educ. 2020, 33, 57–61. [Google Scholar]
- Chen, L.; Bao, W.; Guo, B.; Wang, X.; Li, M.; Sun, H. Evaluation of thickening agent performance of seawater-based fracturing fluid with high temperature resistance. Oilfield Chem. 2020, 37, 17–28. [Google Scholar]
- Yuan, C.; Guo, R.; Chen, X.; Zhang, J. Performance optimization of seawater-based temperature-resistant polyacrylamide fracturing fluid. Chem. Ind. Eng. 2017, 34, 31–37. [Google Scholar]
- Zhang, C.; Jin, H.; Zhang, Y.; Zhang, R.; Zhang, S.; Zhang, Y.; Fan, J.; Song, F.; Zhang, L. Seawater-Based Fracturing Fluid and Its Preparation Method. CN102417814 B, 18 April 2012. [Google Scholar]
- Wang, H.B.; Li, Z.Y.; Si, Z.Y.; Hamani, A.K.M.; Huang, W.X.; Fan, K.; Wang, X.P.; Gao, Y. Alternative planting patterns of film-mulching cotton for alleviating plastic residue pollution in Aksu oasis, southern Xinjiang. Ind. Crops Prod. 2023, 203, 117205. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Zhou, X.; Wang, T.; Gao, Q.; Gao, Y.; Liu, S. Effects of Long-Term Different Fertilization Treatments on Black Soil Fungal Community Analyzed by Illumina MiSeq Sequencing Platform. Acta Microbiol. Sin. 2018, 58, 1658–1671. [Google Scholar]
- Bastida, F.; Torres, I.F.; Moreno, J.L.; Baldrian, P.; Ondoño, S.; Ruiz-Navarro, A.; Hernández, T.; Richnow, H.H.; Starke, R.; García, C.; et al. The active microbial diversity drives ecosystem multifunctionality and is physiologically related to carbon availability in Mediterranean semi-arid soils. Mol. Ecol. 2016, 25, 4660–4673. [Google Scholar] [CrossRef]
- Wang, J.; Lv, S.; Zhang, M.; Chen, G.; Zhu, T.; Zhang, S.; Teng, Y.; Christie, P.; Luo, Y. Effects of plastic film residues on occurrence of phthalates and microbial activity in soils. Chemosphere 2016, 151, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Long, B.; Huang, Q.; Li, J.; Zhou, W.; Yang, C. Integrated effects of residual plastic films on soil-rhizosphere microbeplant ecosystem. J. Hazard. Mater. 2023, 445, 130420. [Google Scholar] [CrossRef]
- Tian, X.; Yao, Y.; Chen, G.; Mao, Z.; Xie, B. Suppression of Meloidogyne incognita by the endophytic fungus Acremonium implicatum from tomato root galls. Int. J. Pest Manag. 2014, 60, 239–245. [Google Scholar] [CrossRef]
- Zhou, A.Y.; Ji, Q.S.; Kong, X.C.; Zhu, F.X.; Meng, H.; Li, S.Y.; He, H. Response of soil property and microbial community to biodegradable microplastics, conventional microplastics and straw residue. Appl. Soil Ecol. 2024, 196, 105302. [Google Scholar] [CrossRef]
- Kim, M.J.; Shim, C.K.; Kim, Y.K.; Hong, S.J.; Park, J.H.; Han, E.J.; Kim, S.C. Enhancement of Seed Dehiscence by Seed Treatment with Talaromyces flavus GG01 and GG04 in Ginseng Panax ginseng. Plant Pathol. J. 2017, 33, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.Y.; Wang, Y.E.; Xing, N.L.; Gu, B.Q.; Huang, Y.P.; Wang, Y.H. Identification of Pathogens Causing Root Rot in Cucurbits and Their Sensitivity to Fungicides in Zhejiang Province. J. Fruit Sci. 2023, 40, 1943–1951. [Google Scholar]
- Guo, D. Study on Wilt Disease of Simmondsia—II. Biological Characteristics of the Pathogen and the Development Pattern of the Disease. J. Southwest For. Coll. 1993, 3, 163–170. [Google Scholar]
- Ren, X.; Tang, J.; Liu, X.; Liu, Q. Effects of microplastics on greenhouse gas emissions and the microbial community in fertilized soil. Environ. Pollut. 2020, 256, 113347. [Google Scholar] [CrossRef] [PubMed]
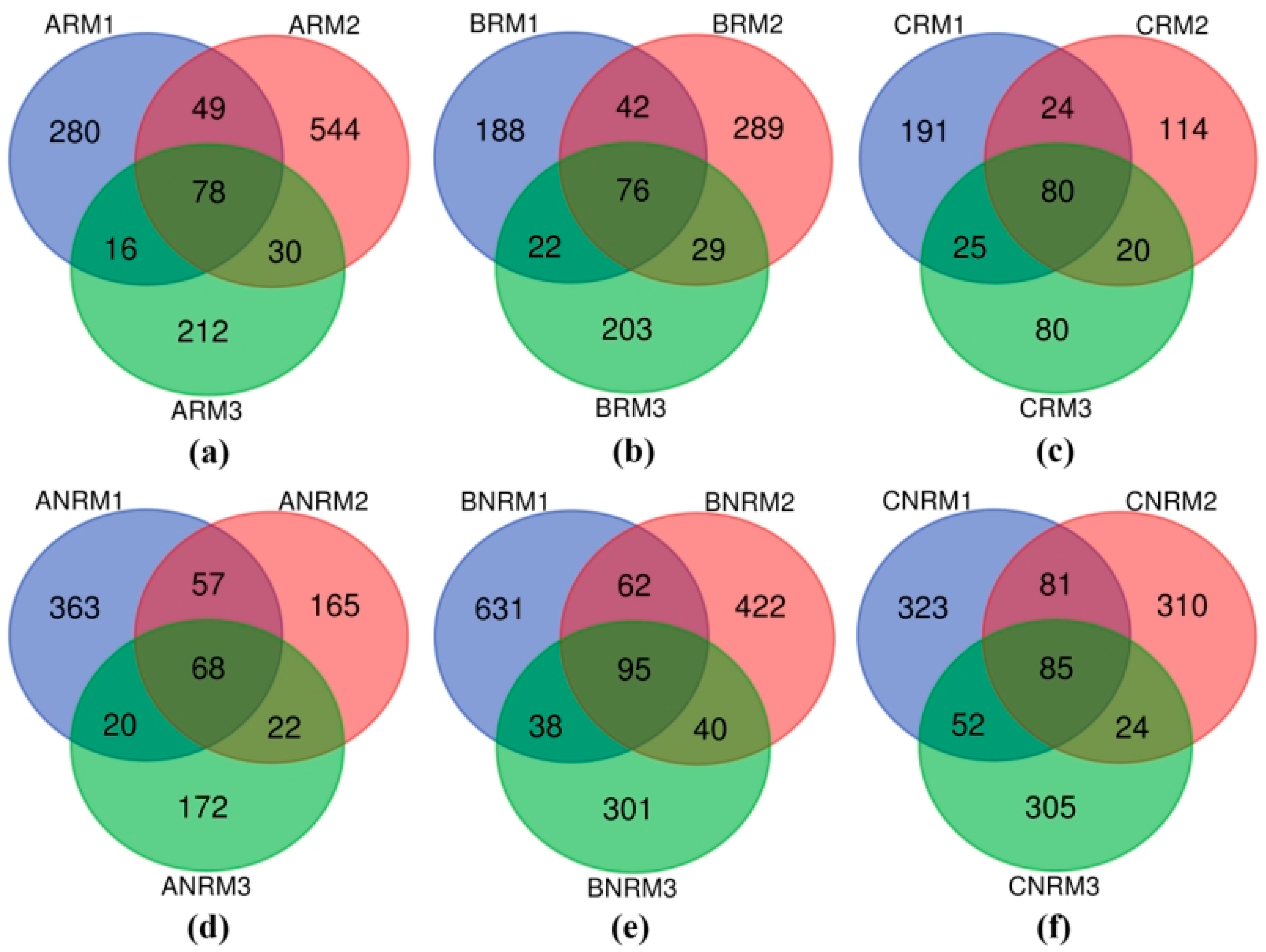
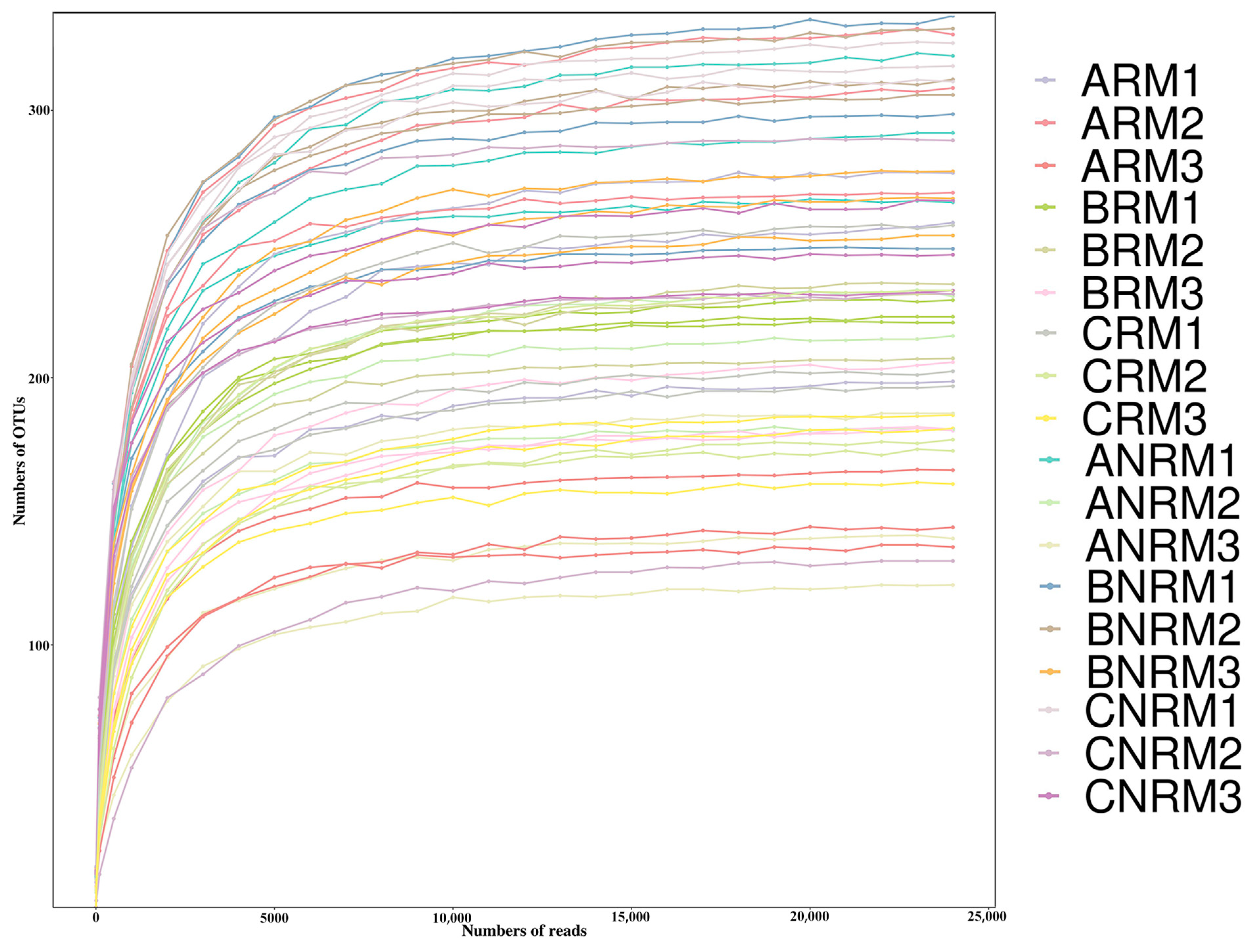


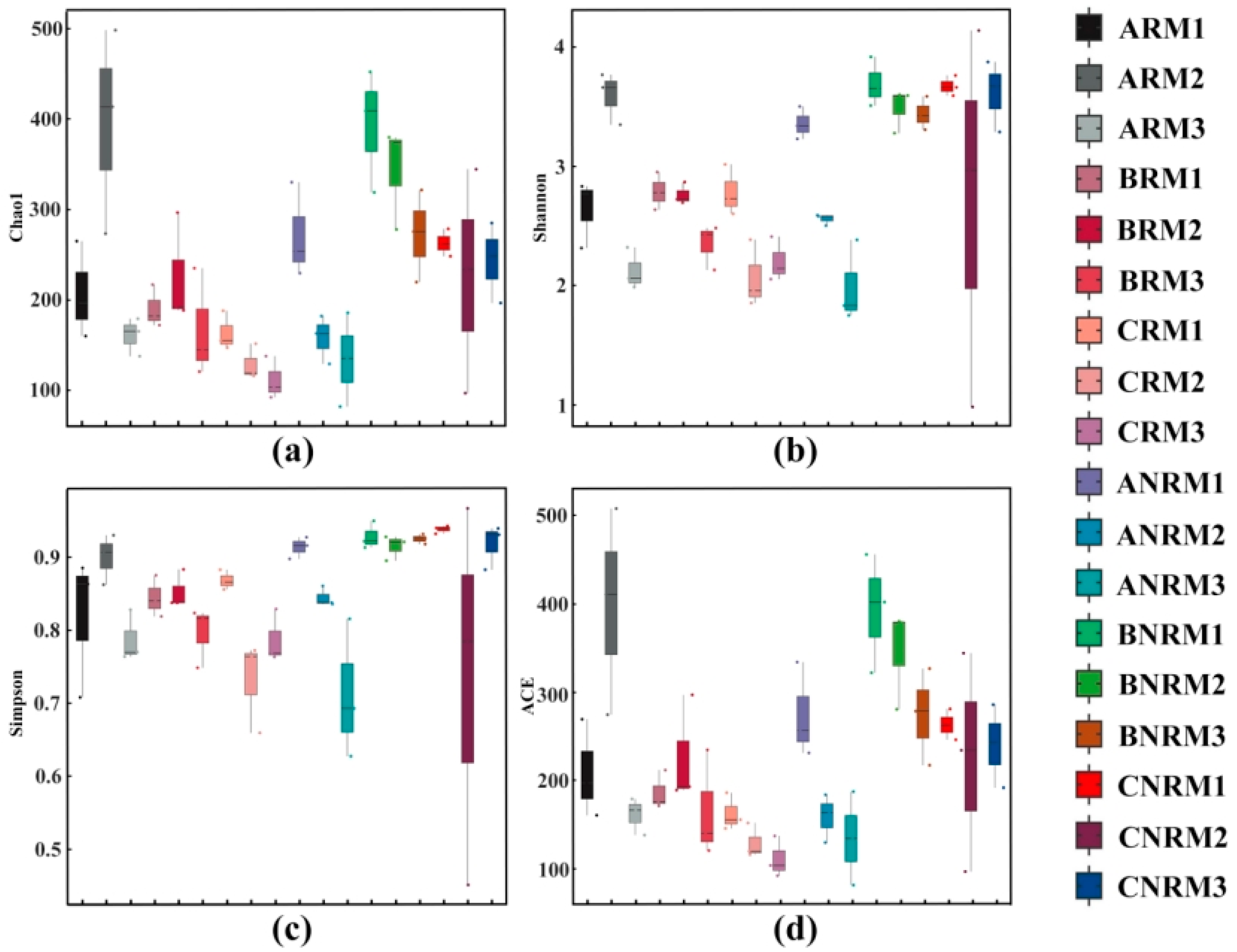


| Residual Mulch Amount | Aksu Region | Bayingolin Mongolian Autonomous Prefecture | Changji Hui Autonomous Prefecture | |||
|---|---|---|---|---|---|---|
| Rhizosphere Microorganisms | Non-Rhizosphere Microorganisms | Rhizosphere Microorganisms | Non-Rhizosphere Microorganisms | Rhizosphere Microorganisms | Non-Rhizosphere Microorganisms | |
| 0–75 kg/ha | ARM1 | ANRM1 | BRM1 | BNRM1 | CRM1 | CNRM1 |
| 75–150 kg/ha | ARM2 | ANRM2 | BRM2 | BNRM2 | CRM2 | CNRM2 |
| 150–225 kg/ha | ARM3 | ANRM3 | BRM3 | BNRM3 | CRM3 | CNRM3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, W.; Wu, H.; Xiang, Z.; Fan, Y.; Wang, S.; Guo, J. Effects of Plastic Mulch Residue on Soil Fungal Communities in Cotton. Agriculture 2024, 14, 1365. https://doi.org/10.3390/agriculture14081365
Song W, Wu H, Xiang Z, Fan Y, Wang S, Guo J. Effects of Plastic Mulch Residue on Soil Fungal Communities in Cotton. Agriculture. 2024; 14(8):1365. https://doi.org/10.3390/agriculture14081365
Chicago/Turabian StyleSong, Wenyue, Hongqi Wu, Zequn Xiang, Yanmin Fan, Shuaishuai Wang, and Jia Guo. 2024. "Effects of Plastic Mulch Residue on Soil Fungal Communities in Cotton" Agriculture 14, no. 8: 1365. https://doi.org/10.3390/agriculture14081365





