Epidemiology of Mycosphaerella Leaf Spot and Powdery Mildew and Agronomic Parameters of Strawberry Cultivars and Genotypes in the Highland Region of Southern Brazil
Abstract
1. Introduction
2. Materials and Methods
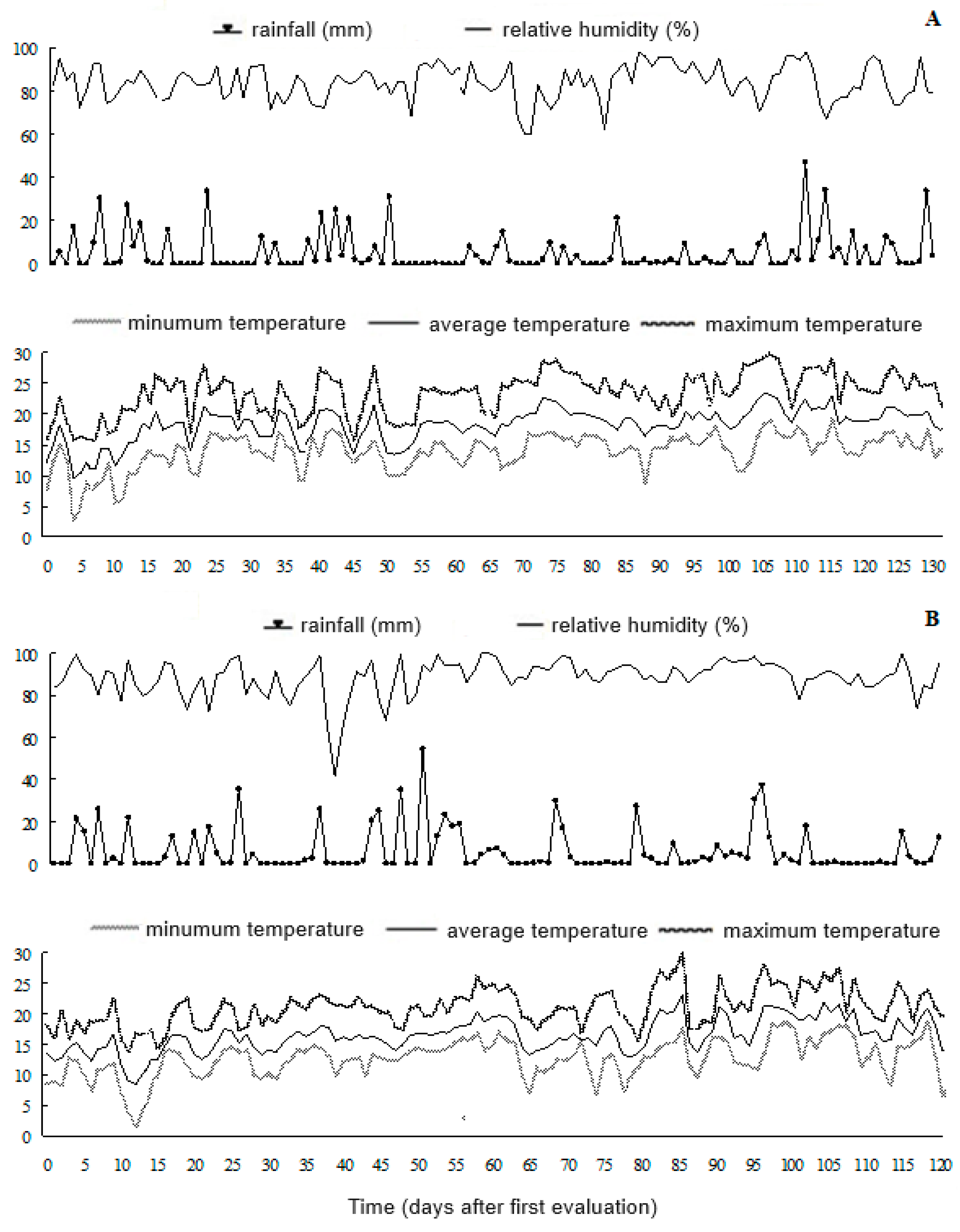
2.1. Meteorological Observations
2.2. Plant Materials
2.3. Disease’s Assessment
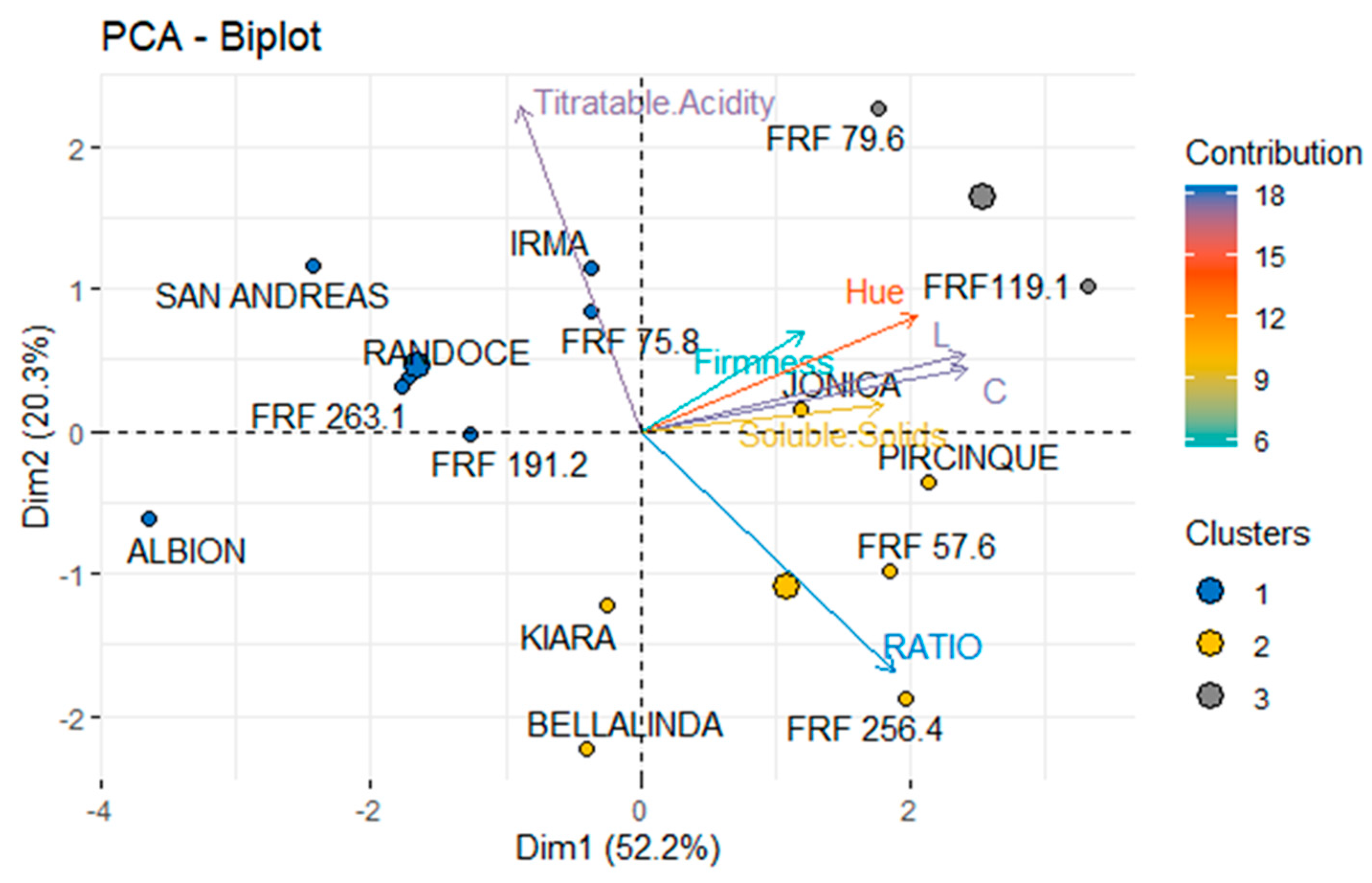
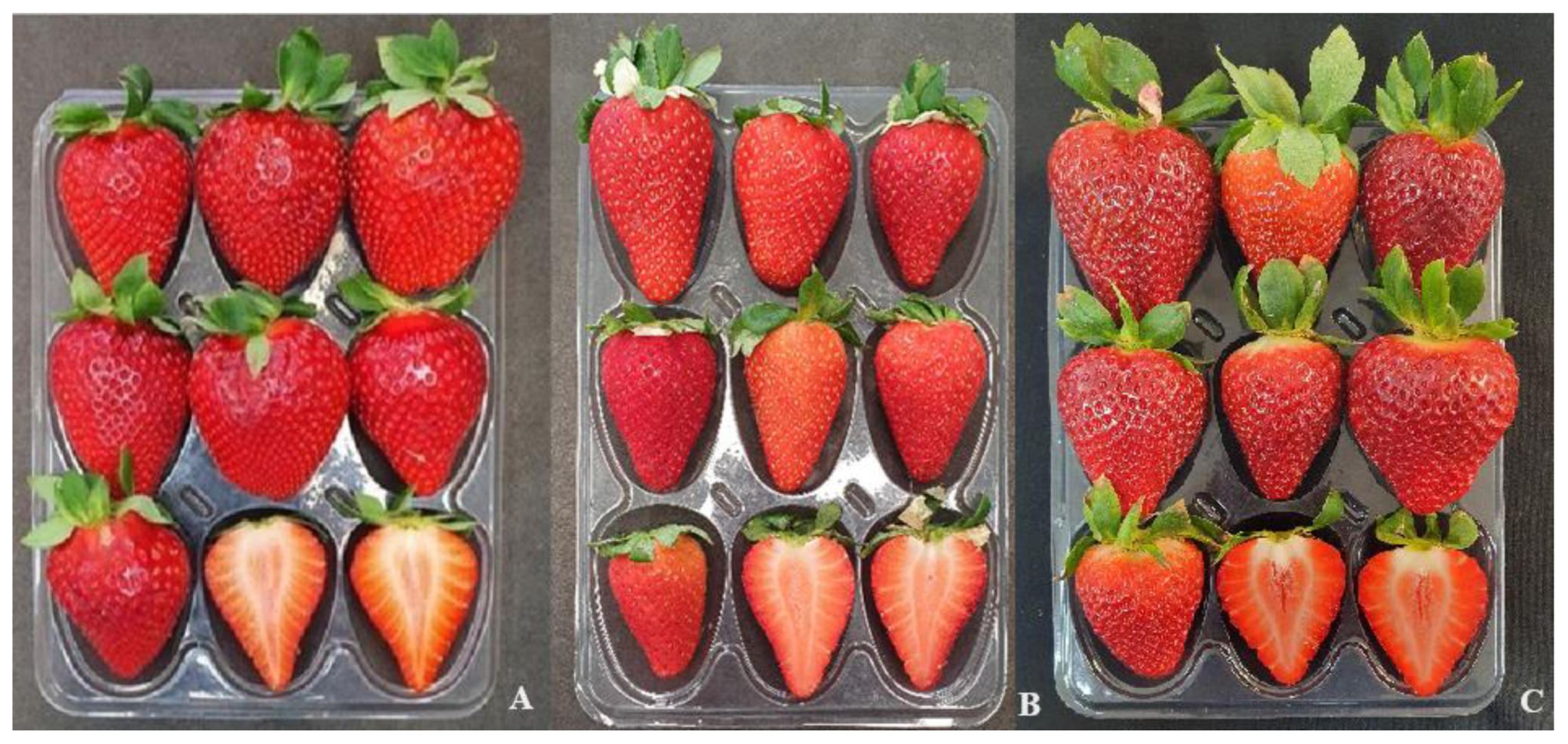
2.4. Fruit Agronomic Parameters
- (a)
- Texture Analyzer TA texturometer (Stable Micro Systems Ltd., Vienna Court, UK) was used to measure the L*, C*, °hue (color angle), and FPF parameters expressed in grams;
- (b)
- SS was measured using a digital refractometer and expressed as the sugar concentration in fruits (°Brix);
- (c)
- TA was measured using an automatic Titronic (titrator®) with 5 mL of juice in 45 mL of distilled water and titration in a sodium hydroxide solution (0.1 N) at pH 8.1;
- (d)
- SS/TA ratio was calculated as the ratio of SS content to that of TA.
2.5. Statistical Analysis
3. Results
3.1. Environment Conditions
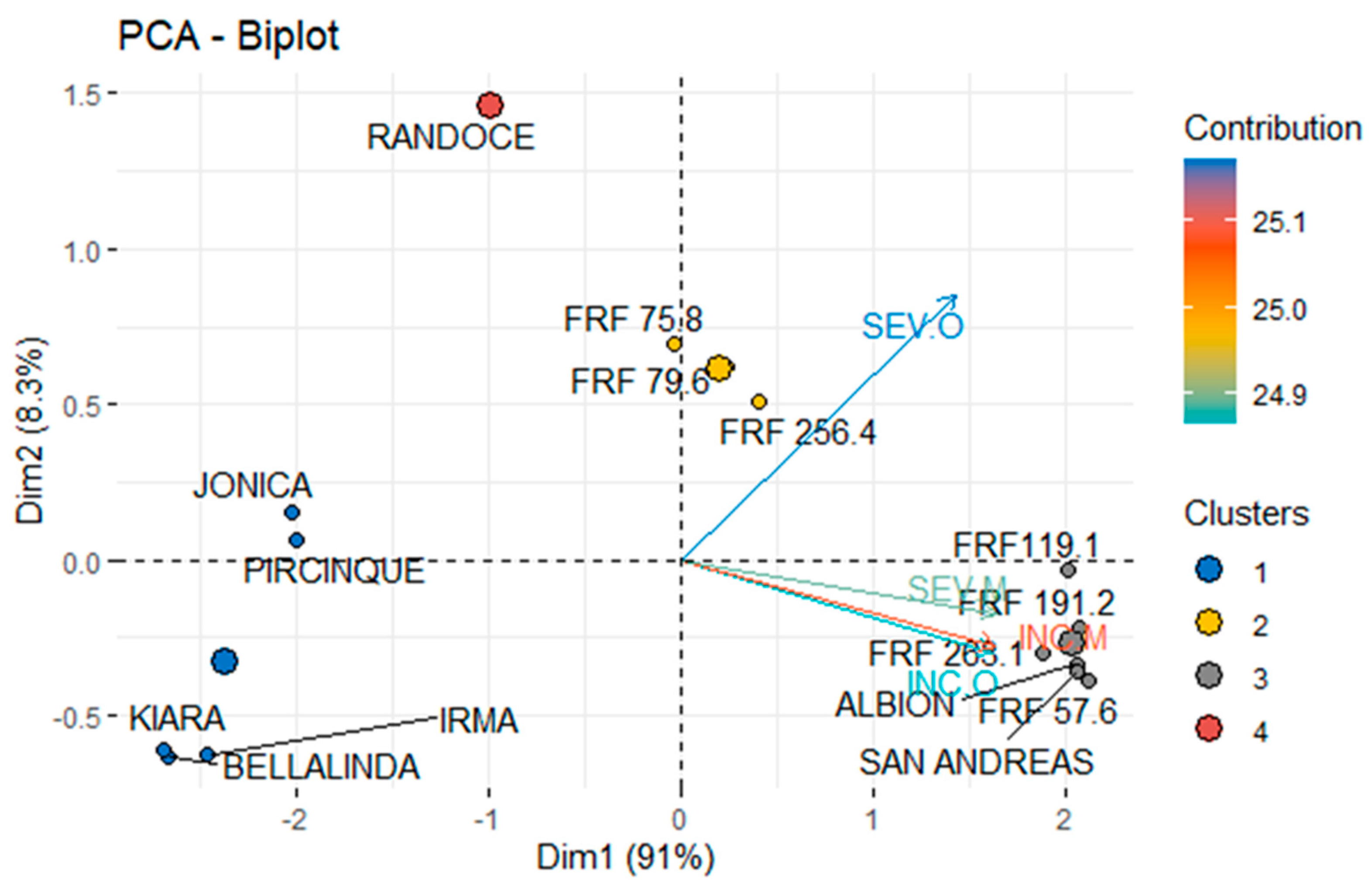
3.2. MLS and PM Intensity
3.3. Cultivars and Genotypes FAP
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fachinello, J.C.; Pasa, M.S.; Schmtiz, J.D.; Betemps, D.L. Situation and perspectives of temperate fruit crops in Brazil. Rev. Bras. Frut. 2011, 33, 109–120. [Google Scholar] [CrossRef]
- Faostat. Food and Agriculture Organization of United Nation. Statistical of Yearbook. Statistical of Strawberry Production in World. 2023. Available online: https://openknowledge.fao.org/ (accessed on 5 May 2024).
- Antunes, L.E.C.; Peres, N.A. Strawberry Production in Brazil and South America. Int. J. Fruit Sci. 2013, 13, 156–161. [Google Scholar] [CrossRef]
- Antunes, L.E.C.; Júnior, C.R.; Schwengber, J.E. Morangueiro, 1st ed.; Embrapa: Brasília, DF, Brazil, 2016; p. 146. [Google Scholar]
- Fagherazzi, A.F.; Bortolini, A.J.; Zanin, D.S.; Bisol, L.; Dos Santos, A.M.; Grimaldi, F.; Kretzschmar, A.A.; Baruzzi, G.; Faedi, W.; Lucchi, P.; et al. New strawberry cultivars and breeding activities in Brazil. Acta Hort. 2017, 1156, 167–170. [Google Scholar] [CrossRef]
- Jia, N.; Xie, Y.; Zhang, H.; Liu, H.; Feng, J.; Zhu, L.; Zhou, X. Effect of bacteriocin treatment on storage and quality of postharvest strawberry fruit. Adv. Mat. Res. 2012, 554, 1547–1552. [Google Scholar] [CrossRef]
- Heling, A.L.; Kuhn, O.J.; Stangarlin, J.R. Biological controlo f Mycosphaerella fragariae in strawberry. Sci. Agr. Paran. 2015, 14, 221–228. [Google Scholar]
- Fagherazzi, A.F.; Cocco, C.; Antunes, L.E.C.; De Souza, J.A.; Rufato, L. La fragolicoltura brasiliana guarda avanti. Riv. Fruttic. Ortofloric. 2014, 76, 20–25. [Google Scholar]
- da Silva, L.R.; Campos, A.A.A.; Moreira, L.C.; Barral, D.M.; de Andrade, G.F.P.; Guimarães, A.G.; da Silva, I.M.; Tannure, M.P.; Pinto, N.A.V.D.; da Costa, M.R.; et al. Agronomic characteristics and postharvest quality of strawberry in a semi-hydroponic cultivation system. Pesq. Agrop. Bras. 2024, 59, e03384. [Google Scholar] [CrossRef]
- Aldrighetti, A.; Pertot, I. Epidemiology and control of strawberry powdery mildew: A review. Phytop. Medit. 2023, 62, 427–453. [Google Scholar] [CrossRef]
- Carpenedo, S.; Antunes, L.E.C.; Treptow, R.O. Sensory characterization of strawberry grown in the region of Pelotas (Brazil). Hort. Bras. 2016, 34, 565–570. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Koppen-Geiger climate classification. Hydr. Earth Syst. Sci. 2007, 11, 633–1644. [Google Scholar] [CrossRef]
- dos Santos, H.G.; Jacomine, P.K.T.; dos Anjos, L.H.C.; de Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; de Almeida, J.A.; de Araujo Filho, J.C.; de Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Brasília, DF, Brazil, 2018; p. 306. [Google Scholar]
- SBCS. Sociedade Brasileira de Ciência do Solo. Manual de adubação e calagem para os estados de Rio Grande do Sul e Santa Catarina; Núcleo Regional Sul, Comissão de Química e Fertilidade do Solo—RS/SC: Xanxerê, SC, Brazil, 2016; p. 265. [Google Scholar]
- Fagherazzi, A.F.; Grimaldi, F.; Kretzschmar, A.A.; Molina, A.R.; Gonçalves, M.J.; Antunes, L.E.C.; Baruzzi, G.; Rufato, L. Strawberry production progress in Brazil. Acta Hort. 2017, 1156, 937–940. [Google Scholar] [CrossRef]
- Carisse, O.; Lefebvre, A.; Van der Heyden, H.; Roberge, L.; Brodeur, L. Analysis of incidence–severity relationships for strawberry powdery mildew as influenced by cultivar, cultivar type, and production systems. Plant Dis. 2013, 97, 354–362. [Google Scholar] [CrossRef]
- Mazaro, S.M.; Gouvea, A.; May De Mio, L.L.; Deschamps, C.; Biasi, L.A.; Citadin, I. Diagrammatic scale to evaluate the mycosphaerella blight severity in strawberry. Ciência Rural 2006, 36, 648–652. [Google Scholar] [CrossRef]
- Brahm, R.U.; Ueno, B.; De Oliveira, R.P. Reaction of strawberry cultivars to powdery mildew under greenhouse conditions. Rev. Bras. Frutic. 2005, 27, 219–221. [Google Scholar] [CrossRef][Green Version]
- de Jesus Junior, W.C. Análise Temporal de Epidemias. In Epidemiologia Aplicada ao Manejo de Doenças de Plantas; Vale, F.X.R., de Jesus Junior, W.C., Zambolim, L., Eds.; Perfil Editora: Belo Horizonte, MG, Brazil, 2004; pp. 125–192. [Google Scholar]
- Jeager, T.F. Categorical data analysis: Away from ANOVAs (transformation or not) and towards Logit Mixed Models. J. Mem. Lang. 2008, 59, 434–446. [Google Scholar] [CrossRef]
- Campbell, C.L.; Madden, L.V. Introduction to Plant Disease Epidemiology; Wiley: New York, NY, USA, 1990; p. 560. [Google Scholar]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Cienc. Agrotec. 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Carisse, O.; McNealis, V. Development of Action Threshold to Manage Common Leaf Spot and Black Seed Disease of Strawberry Caused by Mycosphaerella fragariae. Plant Dis. 2019, 103, 563–570. [Google Scholar] [CrossRef]
- Farooq, M.; Dodgson, J.; Hall, A. Examination of the morphology of Podosphaera aphanis cleistothecia and their role in over wintering of the fungus. Asp. App. Biol. 2007, 83, 55–58. [Google Scholar]
- Kader, A.A. Standardization and Inspection of Fresh Fruits and Vegetables. In Fresh Foods and Vegetables, 3rd ed.; Kader, A., Ed.; University of California: Oakland, CA, USA, 2002; pp. 287–299. [Google Scholar]
- Brugnara, E.C.; Colli, M.P. Leaf spot and leaflet removal in day-neutral strawberry cultivars under different cultivation conditions in organic management. Idesia 2014, 32, 89–92. [Google Scholar] [CrossRef]
- Carisse, O.; McNealis, V. Identification of weather conditions associated with the occurrence, severity, and incidence of black seed disease of strawberry caused by Mycosphaerella fragariae. Phytopathology 2018, 108, 83–93. [Google Scholar] [CrossRef]
- Gadoury, D.M.; Asalf, B.; Heidenreich, M.C.; Herrero, M.L.; Welser, M.J.; Seem, R.C.; Tronsmo, A.M.; Stensvand, A. Initiation, development, and survival of cleistothecia of Podosphaera aphanis and their role in the epidemiology of strawberry powdery mildew. Phytopathology 2010, 100, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Asano, S.; Yoshida, K.; Kitamura, S.; Taira, A.; Honda, Y.; Suzuki, T.; Takikawa, Y.; Kakutani, K.; Matsuda, Y.; et al. Analysis of conidiogenesis and lifelong conidial production from single conidiophores of Podosphaera aphanis on strawberry leaves using digital microscopic and electrostatic techniques. Aust. Plant Path. 2021, 50, 571–587. [Google Scholar] [CrossRef]
- Sombardier, A.; Dufour, M.C.; Blancard, D.; Corio-Costet, M.F. Sensitivity of Podosphaera aphanis isolates to DMI fungicides: Distribution and reduced cross-sensitivity. Pest Manag. Sci. 2010, 66, 35–43. [Google Scholar] [CrossRef]
- Sargent, D.J.; Buti, M.; Šurbanovski, N.; Brurberg, M.B.; Alsheikh, M.; Kent, M.P. Identification of QTLs for powdery mildew (Podosphaera aphanis; syn. Sphaerotheca macularis f. sp. fragariae) susceptibility in cultivated strawberry (Fragaria ×ananassa). PLoS ONE 2019, 14, e0222829. [Google Scholar] [CrossRef] [PubMed]
- Barankevicz, G.B.; Novello, D.; Resende, J.T.V.; Schwarz, K.; Santos, E.F. Physical and chemical characteristics of tomato hybrids pulp during frozen storage. Hort. Bras. 2015, 33, 7–11. [Google Scholar] [CrossRef][Green Version]
- Souza, D.C.; Ossani, P.C.; Resende, L.V.; Cirillo, M.A.; Silva, L.F.L.; Xavier, J.B. Genetic variability between commercial cultivars and experimental strawberry hybrids with emphasis on multi-factor analysis. Magistra 2019, 30, 48–59. [Google Scholar]
- Zanin, D.S.; Fagherazzi, A.F.; Santos, A.M.; Martins, R.; Kretzschmar, A.A.; Rufato, L. Agronomic performance of cultivars and advanced selections of strawberry in the South Plateau of Santa Catarina State. Rev. Ceres 2019, 66, 159–167. [Google Scholar] [CrossRef]
- Faedi, W.; Baruzzi, G.; Lucchi, P.; Magnani, S.; Carullo, A.; Maltoni, M.L.; Migani, M.; Sbrighi, P. The new ‘Pircinque’ strawberry cultivar released under Italy’s PIR Project. Acta Hort. 2014, 1049, 961–1966. [Google Scholar] [CrossRef]
| A | ||||||||||
| Cultivars/ Genotypes | Incidence | Average | Severity | Average | ||||||
| Days after First Appearance of Symptoms | Days after First Appearance of Symptoms | |||||||||
| 15° | 30° | 45° | 60° | 15° | 30° | 45° | 60° | |||
| PIRCINQUE | 6.21 Ad | 10.09 Gb | 11.94 Eb | 16.42 Da | 11.17 | 0.21 Db | 0.51 Ea | 0.57 Ea | 0.63 Fa | 0.48 |
| JONICA | 6.54 Dc | 10.33 Gb | 12.47 Ea | 14.34 Da | 10.92 | 0.33 Cb | 0.54 Ea | 0.57 Ea | 0.63 Fa | 0.51 |
| BELLALINDA | 4.91 Dc | 8.02 Hb | 9.59 Fb | 13.71 Da | 9.05 | 0.12 Da | 0.19 Fa | 0.21 Fa | 0.29 Ga | 0.20 |
| RANDOCE | 7.41 Dc | 11.71 Gb | 13.47 Eb | 15.94 Da | 12.13 | 0.40 Cc | 0.82 Db | 0.85 Db | 1.18 Ea | 0.81 |
| KIARA | 4.89 Db | 7.26 Ha | 8.68 Fa | 9.43 Ea | 7.56 | 0.11 Da | 0.18 Fa | 0.20 Fa | 0.30 Ga | 0.20 |
| IRMA | 4.95 Dc | 11.14 Gb | 11.94 Eb | 14.30 Da | 10.58 | 0.12 Da | 0.19 Fa | 0.22 Fa | 0.28 Ga | 0.20 |
| SAN ANDREAS | 20.65 Db | 43.57 Bc | 47.17 Bb | 53.48 Aa | 41.22 | 1.48 Ad | 2.70 Ac | 3.04 Ab | 4.08 Aa | 2.82 |
| ALBION | 20.65 Db | 40.90 Cc | 50.99 Ab | 54.01 Aa | 41.64 | 1.47 Ac | 2.76 Ab | 2.93 Ab | 3.88 Ba | 2.76 |
| LAM 119.1 | 24.00 Ad | 36.83 Dc | 47.85 Bb | 53.77 Aa | 40.61 | 1.55 Ac | 2.61 Ab | 2.79 Ab | 3.78 Ba | 2.68 |
| LAM 263.1 | 21.23 Bd | 42.20 Cc | 47.48 Bb | 53.33 Aa | 41.06 | 1.47 Ac | 2.75 Ab | 2.85 Ab | 3.76 Ba | 2.71 |
| PIR 256.4 | 13.14 Cc | 28.19 Eb | 32.85 Ca | 34.49 Ba | 27.17 | 0.77 Bb | 1.65 Ca | 1.74 Ca | 1.86 Da | 1.50 |
| PIR 75.8 | 12.85 Cd | 23.89 Fc | 27.14 Db | 30.86 Ca | 23.69 | 0.78 Bb | 2.03 Ba | 2.08 Ba | 2.13 Ca | 1.75 |
| PIR 79.6 | 12.45 Cc | 26.55 Eb | 31.61 Ca | 33.58 Ba | 26.05 | 0.79 Bb | 1.86 Ba | 1.95 Ba | 2.11 Ca | 1.68 |
| Average | 5.76 | 8.48 | 10.10 | 11.22 | 8.89 | 0.83 | 0.58 | 1.14 | 2.22 | 1.04 |
| CV (%) | 6.77 | 9.04 | ||||||||
| B | ||||||||||
| Cultivars/Genotypes | Incidence 1 | Average | Severity 2 | Average | ||||||
| Days after First Appearance of Symptoms | Days after First Appearance of Symptoms | |||||||||
| 15° | 30° | 45° | 60° | 15° | 30° | 45° | 60° | |||
| PIRCINQUE | 4.64 Cb 3 | 5.90 Gb | 6.89 Ga | 7.49 Ga | 6.23 | 0.38 Db | 0.43 Cb | 0.46 Ea | 0.48 Ca | 0.44 |
| JONICA | 4.85 Cb | 5.83 Gb | 6.63 Ga | 7.77 Ga | 6.27 | 0.36 Da | 0.44 Ca | 0.47 Ea | 0.51 Ca | 0.44 |
| BELLALINDA | 2.99 Db | 5.06 Ga | 5.48 Ga | 6.02 Aa | 4.89 | 0.10 Ea | 0.13 Da | 0.15 Fa | 0.17 Da | 0.14 |
| RANDOCE | 5.83 Cb | 7.81 Ga | 9.10 Fa | 9.74 Fa | 8.12 | 0.43 Cc | 0.85 Ab | 0.88 Cb | 1.06 Aa | 0.80 |
| KIARA | 3.02 Dc | 6.12 Gb | 6.26 Gb | 8.49 Fa | 5.97 | 0.09 Eb | 0.14 Da | 0.15 Fa | 0.18 Da | 0.14 |
| IRMA | 3.21 Dc | 6.58 Gb | 8.10 Fb | 9.71 Fa | 6.90 | 0.14 Ec | 0.17 Db | 0.19 Fb | 0.21 Da | 0.17 |
| SAN ANDREAS | 17.90 Ad | 30.66 Ac | 34.49 Ab | 37.61 Aa | 30.17 | 0.65 Bc | 0.80 Bb | 0.84 Db | 0.97 Ba | 0.81 |
| ALBION | 17.60 Ad | 28.11 Bc | 35.72 Ab | 38.47 Aa | 29.97 | 0.71 Ac | 0.86 Ab | 0.95 Ba | 0.98 Ba | 0.87 |
| FC 191.2 | 17.06 Ac | 29.68 Ab | 35.60 Aa | 37.47 Aa | 29.95 | 0.68 Aa | 0.82 Bc | 0.90 Cb | 1.02 Aa | 0.85 |
| FC 57.6 | 18.85 Ad | 27.68 Bc | 35.98 Ab | 38.28 Aa | 30.19 | 0.67 Bc | 0.81 Bb | 0.84 Db | 0.98 Ba | 0.82 |
| LAM 119.1 | 18.94 Ad | 25.61 Cc | 32.58 Bb | 35.73 Ba | 28.22 | 0.70 Ac | 0.85 Ab | 1.04 Ab | 1.07 Aa | 0.91 |
| LAM 263.1 | 18.91 Ac | 31.21 Ab | 34.87 Aa | 36.28 Ba | 30.32 | 0.65 Bc | 0.80 Bb | 0.84 Db | 0.97 Ba | 0.81 |
| PIR 256.4 | 13.80 Bd | 21.41 Dc | 25.57 Cb | 27.85 Ca | 22.16 | 0.65 Bc | 0.79 Bb | 0.83 Db | 1.00 Ba | 0.82 |
| PIR 75.8 | 11.93 Bc | 15.53 Fb | 17.33 Ea | 17.78 Ea | 15.64 | 0.66 Bc | 0.82 Bb | 0.86 Db | 0.97 Ba | 0.83 |
| PIR 79.6 | 12.59 Bc | 17.73 Eb | 20.58 Da | 22.13 Da | 18.26 | 0.64 Bc | 0.78 Bb | 0.82 Db | 1.00 Ba | 0.81 |
| Average | 11.48 | 17.66 | 21.01 | 22.72 | 18.22 | 0.50 | 0.63 | 0.68 | 0.77 | 0.64 |
| CV (%) 4 | 7.86 | 5.88 | ||||||||
| Cultivar/ Genotype | AUIDPC 1 | AUSDPC 2 | ||
|---|---|---|---|---|
| MLS | PM | MLS | PM | |
| Kiara | 326.38 g 3 | 271.86 g | 8.85 g | 6.15 g |
| Jonica | 498.53 f | 281.54 g | 23.65 f | 8.08 f |
| Irma | 490.60 f | 317.15 f | 9.19 g | 7.82 f |
| Bellalinda | 403.63 g | 225.64 g | 9.11 g | 6.11 g |
| Pircinque | 460.24 f | 282.70 g | 22.44 f | 9.80 f |
| Randoce | 552.81 e | 370.45 f | 36.71 e | 27.07 e |
| Albion | 1938.34 b | 1377.85 a | 125.47 a | 39.85 b |
| San Andreas | 1917.15 b | 1393,61 a | 127.75 a | 36.68 d |
| FRF PIR 256.4 | 1272.72 c | 1017.11 c | 70.59 d | 36.67 d |
| FRF PIR 79.6 | 1217.69 c | 834.98 d | 78.95 c | 36.22 d |
| FRF PIR 75.8 | 1093.26 d | 715.64 e | 83.38 c | 37.27 d |
| FRF 57.6 | 1923.75 b | 1383.17 a | 126.62 a | 38.07 c |
| FRF 191.2 | 2040.17 a | 1388.12 a | 127.60 a | 40.49 a |
| FRF LAM 119.1 | 1853.51 b | 1282.84 b | 121.02 b | 41.58 a |
| FRF LAM 263.1 | 1904.42 b | 1405.21 a | 123.26 b | 36.68 d |
| CV 4 | 12.77 | 15.05 | 18.93 | 11.17 |
| Cultivars/Genotypes | TFY | L | C | °Hue | F | SS | TA | SS/TA |
|---|---|---|---|---|---|---|---|---|
| PIRCINQUE | 667.79 a 1 | 37.83 a | 46.42 a | 33.13 a | 372.41 b | 8.36 a | 0.54 b | 15.99 b |
| JONICA | 596.63 a | 37.75 a | 45.23 b | 33.73 a | 375.05 b | 7.57 b | 0.54 b | 14.13 c |
| BELLALINDA | 470.05 b | 34.09 c | 42.42 c | 30.32 c | 343.94 c | 7.77 b | 0.48 b | 16.16 b |
| RANDOCE | 524.56 b | 34.07 c | 42.53 c | 29.68 d | 362.90 b | 7.68 b | 0.61 a | 12.72 c |
| KIARA | 643.12 a | 35.36 b | 43.31 c | 32.18 b | 379.73 b | 7.01 b | 0.49 b | 14.49 b |
| IRMA | 347.20 c | 35.74 b | 43.42 c | 33.46 a | 343.12 c | 7.43 b | 0.60 a | 12.40 c |
| SAN ANDREAS | 532.43 b | 33.13 d | 42.01 c | 30.47 c | 365.37 b | 7.21 b | 0.64 a | 11.45 c |
| ALBION | 499.09 b | 31.00 e | 37.60 d | 28.92 d | 325.14 c | 7.39 b | 0.59 a | 12.74 c |
| FC 191.2 | 410.02 c | 34.53 c | 42.56 c | 31.24 c | 366.71 b | 7.25 b | 0.57 b | 12.96 c |
| FC 57.6 | 535.66 b | 38.20 a | 47.93 a | 32.49 b | 375.35 b | 7.43 b | 0.48 b | 15.42 b |
| FRF LAM 119.1 | 680.32 a | 37.93 a | 47.18 a | 33.82 a | 448.60 a | 8.43 a | 0.55 b | 15.40 b |
| FRF LAM 263.1 | 474.05 b | 34.56 c | 41.93 c | 32.41 b | 358.11 b | 6.45 b | 0.56 b | 11.73 c |
| FRF PIR 256.4 | 484.15 b | 35.73 b | 45.61 b | 32.41 b | 342.53 c | 8.58 a | 0.49 b | 17.87 a |
| FRF PIR 75.8 | 483.25 b | 35.66 b | 45.09 b | 30.44 c | 421.81 a | 7.88 b | 0.61 a | 13.06 c |
| FRF PIR 79.6 | 558.59 b | 37.94 a | 47.16 a | 34.29 a | 370.99 b | 8.54 a | 0.66 a | 13.49 c |
| Means 2 | 527.13 | 35.57 | 44.03 | 31.93 | 370.12 | 7.66 | 0.56 | 14.00 |
| CV (%) 3 | 9.45 | 2.47 | 2.44 | 2.54 | 5.25 | 7.42 | 8.14 | 8.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, J.M.d.; Fagherazzi, A.F.; Nerbass, F.R.; Petry, D.; Kretzschmar, A.A.; Baruzzi, G.; Rufato, L.; Bogo, A. Epidemiology of Mycosphaerella Leaf Spot and Powdery Mildew and Agronomic Parameters of Strawberry Cultivars and Genotypes in the Highland Region of Southern Brazil. Agriculture 2024, 14, 1373. https://doi.org/10.3390/agriculture14081373
Lima JMd, Fagherazzi AF, Nerbass FR, Petry D, Kretzschmar AA, Baruzzi G, Rufato L, Bogo A. Epidemiology of Mycosphaerella Leaf Spot and Powdery Mildew and Agronomic Parameters of Strawberry Cultivars and Genotypes in the Highland Region of Southern Brazil. Agriculture. 2024; 14(8):1373. https://doi.org/10.3390/agriculture14081373
Chicago/Turabian StyleLima, Juliana Martins de, Antônio Felipe Fagherazzi, Francine Regianini Nerbass, Daiana Petry, Aike Anneliese Kretzschmar, Gianlucca Baruzzi, Leo Rufato, and Amauri Bogo. 2024. "Epidemiology of Mycosphaerella Leaf Spot and Powdery Mildew and Agronomic Parameters of Strawberry Cultivars and Genotypes in the Highland Region of Southern Brazil" Agriculture 14, no. 8: 1373. https://doi.org/10.3390/agriculture14081373
APA StyleLima, J. M. d., Fagherazzi, A. F., Nerbass, F. R., Petry, D., Kretzschmar, A. A., Baruzzi, G., Rufato, L., & Bogo, A. (2024). Epidemiology of Mycosphaerella Leaf Spot and Powdery Mildew and Agronomic Parameters of Strawberry Cultivars and Genotypes in the Highland Region of Southern Brazil. Agriculture, 14(8), 1373. https://doi.org/10.3390/agriculture14081373







