Influence of Biochar-Reinforced Hydrogel Composites on Growth and Biochemical Parameters of Bean Plants and Soil Microbial Activities under Different Moisture Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil and Biochar–Hydrogel Composites
2.2. Experimental Design
2.3. Biochemical Analysis of Plant Leaves
2.4. Soil Microorganisms
2.5. Statistical Analysis
3. Results
3.1. Soil pH and Electrical Conductivity
3.2. Effects of Biochar–Hydrogel Composite and Moisture Regime on Plants
3.2.1. Germination, Plant Growth, and Relative Water Content
3.2.2. Photosynthetic Pigments
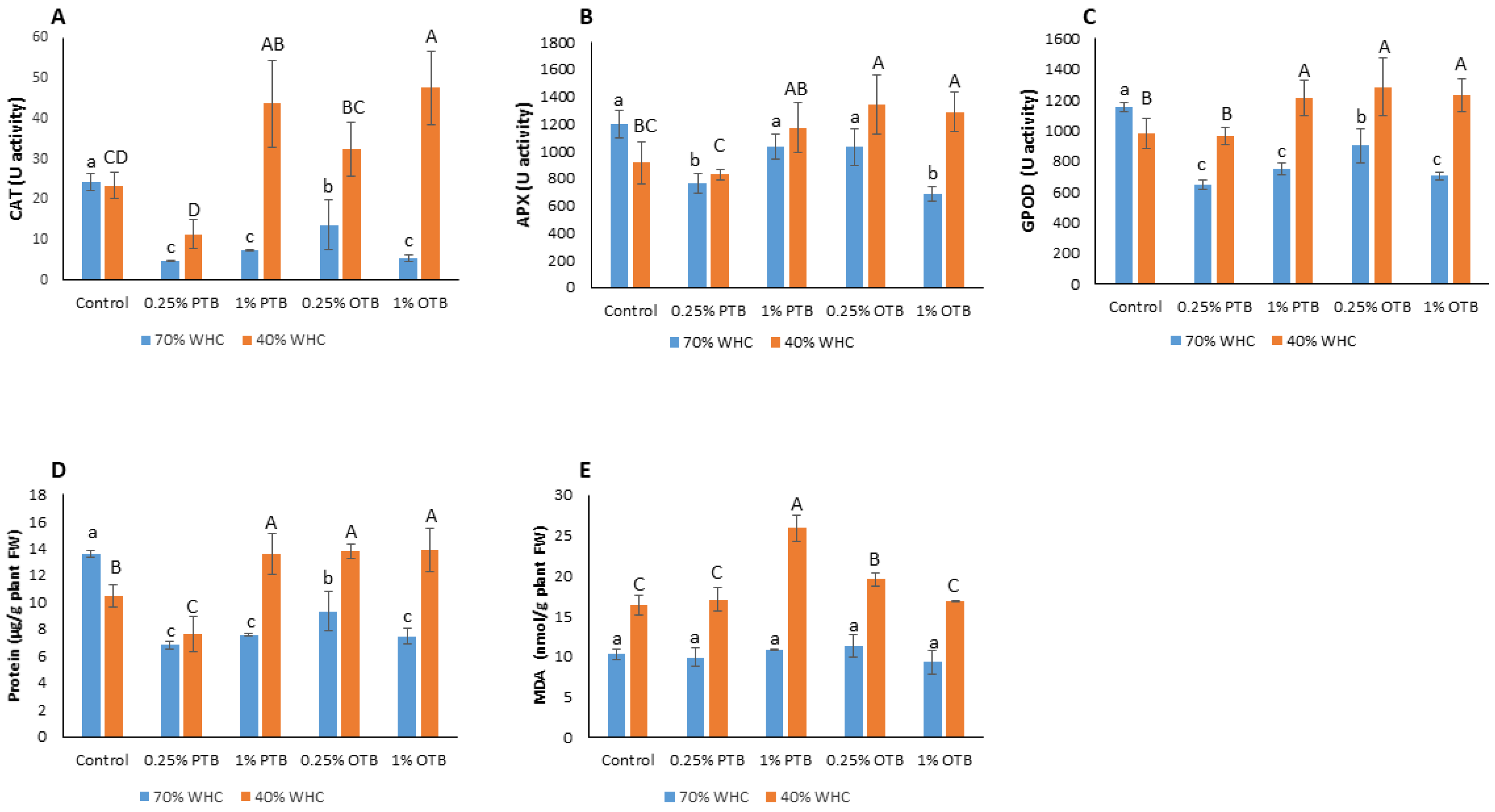
3.2.3. Biomarkers of Oxidative Stress
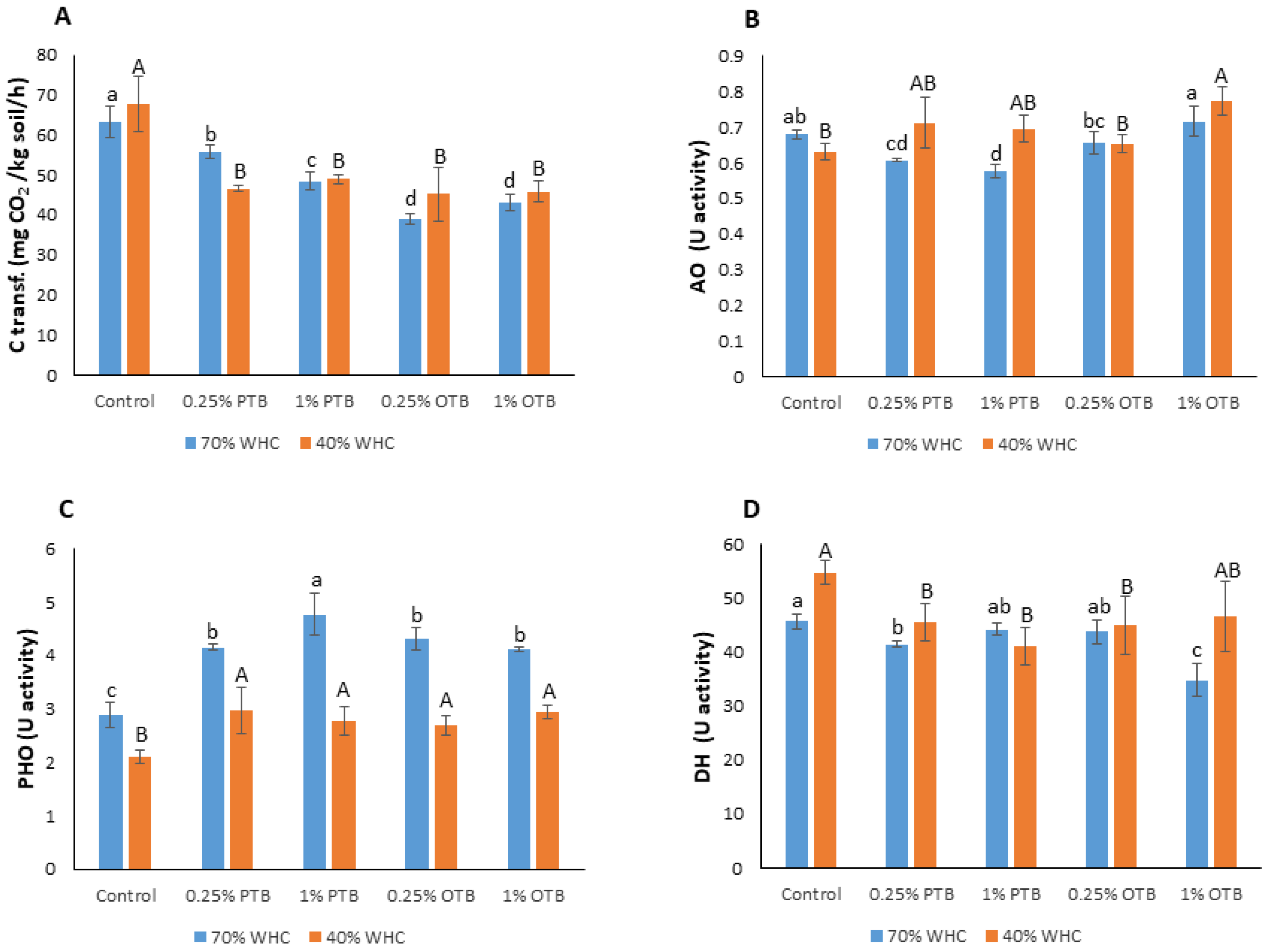
3.3. Effects of Biochar–Hydrogel Composite and Moisture Regime on Soil Microorganisms
3.4. Correlation Analysis
4. Discussion
4.1. Effects of Biochar–Hydrogel Composite and Moisture Regime on Plants
4.1.1. Germination, Plant Growth, and Relative Water Content
4.1.2. Photosynthetic Pigments
4.1.3. Biomarkers of Oxidative Stress
4.2. Effects of Biochar–Hydrogel Composite and Moisture Regime on Soil Microorganisms
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pereira, L.S. Water, Agriculture and Food: Challenges and Issues. Water Resour. Manag. 2017, 31, 2985–2999. [Google Scholar] [CrossRef]
- Saad, A.; Benyamina, A.E.H.; Gamatie, A. Water Management in Agriculture: A Survey on Current Challenges and Technological Solutions. IEEE Access 2020, 8, 38082–38097. [Google Scholar] [CrossRef]
- Ingrao, C.; Strippoli, R.; Lagioia, G.; Huisingh, D. Water scarcity in agriculture: An overview of causes, impacts and approaches for reducing the risks. Heliyon 2023, 9, e18507. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Majumder, S. Water management in agriculture: Innovations for efficient irrigation. In Modern Agronomy; Sil, P., Chhetri, P., Majumder, S., Santosh, D.T., Eds.; International Books & Periodical Supply Service: New Delhi, India, 2024; pp. 169–185. [Google Scholar]
- Bhardwaj, A.; Kumar, M.; Alshehri, M.; Keshta, I.; Abugabah, A.; Sharma, S.K. Smart water management framework for irrigation in agriculture. Environ. Technol. 2024, 45, 2320–2334. [Google Scholar] [CrossRef]
- Vedovello, P.; Sanches, L.V.; Teodoro, G.d.S.; Majaron, V.F.; Bortoletto-Santos, R.; Ribeiro, C.; Putti, F.F. An Overview of Polymeric Hydrogel Applications for Sustainable Agriculture. Agriculture 2024, 14, 840. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Bertaki, M. Sustainable water management in agriculture under climate change. Agric. Agric. Sci. Procedia 2015, 4, 88–98. [Google Scholar] [CrossRef]
- Saha, A.; Sekharan, S.; Manna, U. Superabsorbent hydrogel (SAH) as a soil amendment for drought management: A review. Soil Tillage Res. 2020, 204, 104736. [Google Scholar] [CrossRef]
- Abobatta, W. Impact of hydrogel polymer in agricultural sector. Adv. Agric. Environ. Sci. 2018, 1, 59–64. [Google Scholar] [CrossRef]
- Rizwan, M.; Rubina Gilani, S.; Iqbal Durani, A.; Naseem, S. Materials diversity of hydrogel: Synthesis, polymerization process and soil conditioning properties in agricultural field. J. Adv. Res. 2021, 33, 15–40. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Wilson, L.D. A Review on the design and hydration properties of natural polymer-based hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef]
- Mazloom, N.; Khorassani, R.; Zohuri, G.H.; Emami, H.; Whalen, J. Development and characterization of lignin-based hydrogel for use in agricultural soils: Preliminary evidence. Clean-Soil Air Water 2019, 47, 1900101. [Google Scholar] [CrossRef]
- Motamedi, E.; Motesharezedeh, B.; Shirinfekr, A.; Samar, S.M. Synthesis and swelling behavior of environmentally friendly starch-based superabsorbent hydrogels reinforced with natural char nano/micro particles. J. Environ. Chem. Eng. 2020, 8, 103583. [Google Scholar] [CrossRef]
- Wu, Y.; Brickler, C.; Li, S.; Chen, G. Synthesis of microwave-mediated biochar-hydrogel composites for enhanced water absorbency and nitrogen release. Polym. Test. 2021, 93, 106996. [Google Scholar] [CrossRef]
- Parvathy, P.C.; Jyothi, A.N.; John, K.S.; Sreekumar, J. Cassava starch based superabsorbent polymer as soil conditioner: Impact on soil physico-chemical and biological properties and plant growth. Clean-Soil Air Water 2014, 42, 1610–1617. [Google Scholar] [CrossRef]
- Abdallah, A.M. The effect of hydrogel particle size on water retention properties and availability under water stress. Int. Soil Water Conserv. Res. 2019, 7, 275–285. [Google Scholar] [CrossRef]
- Li, X.; He, J.-Z.; Liu, Y.-R.; Zheng, Y.-M. Effects of super absorbent polymers on soil microbial properties and Chinese cabbage (Brassica chinensis) growth. J. Soils Sediments 2013, 13, 711–719. [Google Scholar] [CrossRef]
- Solanki, R.; Bisen, B.; Pandey, S.K. Effect of super absorbent polymer and irrigation scheduling on growth attributes in acid lime. Int. J. Chem. Stud. 2021, 9, 360–363. [Google Scholar] [CrossRef]
- Tomadoni, B.; Salcedo, M.F.; Mansilla, A.Y.; Casalongué, C.A.; Alvarez, V.A. Macroporous alginate-based hydrogels to control soil substrate moisture: Effect on lettuce plants under drought stress. Eur. Polym. J. 2020, 137, 109953. [Google Scholar] [CrossRef]
- Mazloom, N.; Khorassani, R.; Zohury, G.H.; Emami, H.; Whalen, J. Lignin-based hydrogel alleviates drought stress in maize. Environ. Exp. Bot. 2020, 175, 104055. [Google Scholar] [CrossRef]
- Nassaj-Bokharaei, S.; Motesharezedeh, B.; Etesami, H.; Motamedi, E. Effect of hydrogel composite reinforced with natural char nanoparticles on improvement of soil biological properties and the growth of water deficit-stressed tomato plant. Ecotox. Environ. Saf. 2021, 223, 112576. [Google Scholar] [CrossRef]
- Chen, X.; Huang, L.; Mao, X.Y.; Liao, Z.W.; He, Z.L. A comparative study of the cellular microscopic characteristics and mechanisms of maize seedling damage from superabsorbent polymers. Pedosphere 2017, 27, 274–282. [Google Scholar] [CrossRef]
- Del Campo, A.D.; Hermoso, J.; Flors, J.; Lidón, A.; Navarro-Cerrillo, R.M. Nursery location and potassium enrichment in Aleppo pine stock 2. Performance under real and hydrogel-mediated drought conditions. Forestry 2011, 84, 235–245. [Google Scholar] [CrossRef]
- Rydlova, J.; Puschel, D. Arbuscular mycorrhiza, but not hydrogel, alleviates drought stress of ornamental plants in peat-based substrate. Appl. Soil Ecol. 2020, 146, 103394. [Google Scholar] [CrossRef]
- Shivakumara, L.R.; Demappa, T. Synthesis and swelling behavior of sodium alginate/poly(vinyl alcohol) hydrogels. Turk. J. Pharm. Sci. 2019, 16, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zeng, R.; Zhang, F.; Kan, J. Effects of sodium carboxymethyl cellulose on rheological properties and gelation behaviors of sodium alginate induced by calcium ions. LWT-Food Sci. Technol. 2019, 103, 131–138. [Google Scholar] [CrossRef]
- Campos, K.; Schwember, A.R.; Machado, D.; Ozores-Hampton, M.; Gil, P.M. Physiological and yield responses of green-shelled beans (Phaseolus vulgaris L.) grown under restricted irrigation. Agronomy 2021, 11, 562. [Google Scholar] [CrossRef]
- Nina, N.; Theoduloz, C.; Tapia, G.; Jimenez-Aspee, F.; Marquez, K.; Schmeda-Hirschmann, G. Changes in polyphenol composition, antioxidant capacity and enzyme inhibition in Phaseolus vulgaris L. submitted to hydric stress. Sci. Hortic. 2023, 317, 112070. [Google Scholar] [CrossRef]
- MAPA (Ministerio de Agricultura, Pesca y Alimentación). Métodos Oficiales de Análisis; Secretaría General Técnica: Madrid, Spain, 1994; Volume 3. [Google Scholar]
- Tsubota, T.; Tsuchiya, S.; Kusumoto, T.; Kalderis, D. Assessment of biochar produced by flame-curtain pyrolysis as a precursor for the development of an efficient electric double-layer capacitor. Energies 2021, 14, 7671. [Google Scholar] [CrossRef]
- Kalderis, D.; Tsuchiya, S.; Phillipou, K.; Paschalidou, P.; Pashalidis, I.; Tashima, D.; Tsubota, T. Utilization of pine tree biochar produced by flame-curtain pyrolysis in two non-agricultural applications. Bioresour. Technol. Rep. 2020, 9, 100384. [Google Scholar] [CrossRef]
- Doğaroğlu, Z.G. Two different biochar-doped hydrogels affect the growth of arugula (Eruca vesicaria) under different irrigation period. J. Soils Sediments 2023, 23, 232–245. [Google Scholar] [CrossRef]
- Zambrano, M.A.O.; Castillo, D.A.; Perez, L.R.; Teran, W. Cacao (Theobroma cacao L.) response to water stress: Physiological characterization and antioxidant gene expression profiling in commercial clones. Front. Plant Sci. 2021, 12, 700855. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gomez, C.; Obrador, A.; Gonzalez, D.; Babin, M.; Fernandez, M.D. Comparative study of the phytotoxicity of ZnO nanoparticles and Zn accumulation in nine crops grown in a calcareous soil and an acidic soil. Sci. Total Environ. 2018, 644, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids. Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Miguel, A.S.; Faure, M.; Ravanel, P.; Raveton, M. Biological responses of maize (Zea mays) plants exposed to chlorobenzenes. Case study of monochloro-, 1,4-dichloro- and 1,2,4-trichloro-benzenes. Ecotoxicology 2012, 21, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.; Cao, W.; Yun, L.; Hao, H.; Liang, C.; Liu, X.; Hong, F. Cerium under calcium deficiency-influence on the antioxidative defense system in spinach plants. Plant Soil 2009, 323, 285–294. [Google Scholar] [CrossRef]
- Aebi, H. Catalase invitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Gupta, A.K.; Ahmad, M. Study of the effect of refinery waste water exposure on Allium cepa’s enzymatic antioxidant defense: Probing potential biomarkers. Toxicol. Environ. Chem. 2011, 93, 1166–1179. [Google Scholar] [CrossRef]
- El-Shabrawi, H.; Kumar, B.; Kaul, T.; Reddy, M.K.; Singla-Pareek, S.L.; Sopory, S.K. Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma 2010, 245, 85–96. [Google Scholar] [CrossRef]
- Bradford, M.M. The rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Rogers, J.E.; Li, S.W. Effect of metals and other inorganic ions on soil microbial activity: Soil dehydrogenase assay as a simple toxicity test. Bull. Environ. Contam. Toxicol. 1985, 34, 858–865. [Google Scholar] [CrossRef]
- Freeman, C.; Liska, G.; Ostle, N.J.; Jones, S.E.; Lock, M.A. The use of fluorogenic substrates for measuring enzyme-activity in peatlands. Plant Soil 1995, 175, 147–152. [Google Scholar] [CrossRef]
- Kandeler, E. Potential nitrification. In Methods in Soil Biology; Spinger: Berlin/Heidelberg, Germany, 1996; pp. 146–149. [Google Scholar]
- Nahuelcura, J.; Ruiz, A.; Gomez, F.; Cornejo, P. The effect of arbuscular mycorrhizal fungi on the phenolic compounds profile, antioxidant activity and grain yields in wheat cultivars growing under hydric stress. J. Sci. Food Agric. 2022, 102, 407–416. [Google Scholar] [CrossRef]
- Hachemi, A.; Ali, O.S.; Belghazi, T.; Lahrouni, A.; El Mercht, S.; El Hassan, C.; El Messoussi, S. Effect of hydric and light stress on biomass, nutrient uptake and enzymatic antioxidants of Argania spinosa seedlings. Arch. Biol. Sci. 2021, 73, 145–153. [Google Scholar] [CrossRef]
- Urbanaviciute, I.; Bonfiglioli, L.; Pagnotta, M.A. One hundred candidate genes and their roles in drought and salt tolerance in wheat. Int. J. Mol. Sci. 2021, 22, 6378. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Morezuelas, A.; Barandalla, L.; Ritter, E.; Lacuesta, M.; de Galarreta, J.I.R. Physiological response and yield components under greenhouse drought stress conditions in potato. J. Plant Physiol. 2022, 278, 153790. [Google Scholar] [CrossRef]
- Galicia-Juarez, M.; Zavala-Garcia, F.; Sinagawa-Garcia, S.R.; Gutierrez-Diez, A.; Williams-Alanis, H.; Cisneros-Lopez, M.E.; Valle-Gough, R.E.; Flores-Garivay, R.; Santillano-Cazares, J. Identification of sorghum (Sorghum bicolor (L.) Moench) genotypes with potential for hydric and heat stress tolerance in Northeastern Mexico. Plants 2021, 10, 2265. [Google Scholar] [CrossRef]
- Satriani, A.; Catalano, M.; Scalcione, E. The role of superabsorbent hydrogel in bean crop cultivation under deficit irrigation conditions: A case-study in Southern Italy. Agric. Water Manag. 2018, 195, 114–119. [Google Scholar] [CrossRef]
- de Vasconcelos, M.C.; Gomes, R.F.; Sousa, A.A.L.; Moreira, F.J.C.; Rodrigues, F.H.A.; Fajardo, A.R.; Neto, L.G.P. Superabsorbent hydrogel composite based on starch/rice husk ash as a soil conditioner in melon (Cucumis melo L.) seedling culture. J. Polym. Environ. 2020, 28, 131–140. [Google Scholar] [CrossRef]
- Ali, O.S.; Hachemi, A.; Moumni, A.; Belghazi, T.; Lahrouni, A.; El Messoussi, S. Physiological and biochemical responses of argan (Argania spinosa (L.)) seedlings from containers of different depths under water stress. Not. Bot. Horti Agrobot. Cluj-Na. 2021, 49, 12482. [Google Scholar] [CrossRef]
- Waqar, A.; Bano, A.; Ajmal, M. Effects of PGPR bioinoculants, hydrogel and biochar on growth and physiology of soybean under drought stress. Commun. Soil Sci. Plant Anal. 2022, 53, 826–847. [Google Scholar] [CrossRef]
- M’Barki, N.; Chehab, H.; Aissaoui, F.; Dabbaghi, O.; Attia, F.; Mahjoub, Z.; Laamari, S.; Chihaoui, B.; del Giudice, T.; Jemai, A.; et al. Effects of mycorrhizal fungi inoculation and soil amendment with hydrogel on leaf anatomy, growth and physiology performance of olive plantlets under two contrasting water regimes. Acta Physiol. Plant. 2018, 40, 116. [Google Scholar] [CrossRef]
- Ayyaz, A.; Fang, R.; Ma, J.; Hannan, F.; Huang, Q.; Athar, H.-u.-R.; Sun, Y.; Javed, M.; Ali, S.; Zhou, W.; et al. Calcium nanoparticles (Ca-NPs) improve drought stress tolerance in Brassica napus by modulating the photosystem II, nutrient acquisition and antioxidant performance. NanoImpact 2022, 28, 100423. [Google Scholar] [CrossRef]
- Rane, J.; Singh, A.K.; Tiwari, M.; Prasad, P.V.V.; Jagadish, S.V.K. Effective use of water in crop plants in dryland agriculture: Implications of reactive oxygen species and antioxidative system. Front. Plant Sci. 2022, 12, 778270. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.A.; Silva, K.R.; Rossi, M.L.; Martinelli, A.P.; Gaspar, M.; Braga, M.R. Water retention and metabolic changes improve desiccation tolerance in Barbacenia graminifolia (Velloziaceae). Physiol. Plant. 2022, 174, e13783. [Google Scholar] [CrossRef] [PubMed]
- Priya, M.; Dhanker, O.P.; Siddique, K.H.M.; HanumanthaRao, B.; Nair, R.M.; Pandey, S.; Singh, S.; Varshney, R.K.; Prasad, P.V.V.; Nayyar, H. Drought and heat stress-related proteins: An update about their functional relevance in imparting stress tolerance in agricultural crops. Theor. Appl. Genet. 2019, 132, 1607–1638. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Campos, E.; Velasco, A.G.V.; Montero-Palmero, M.B.; Gutierrez-Manero, F.J.; Ramos-Solano, B. Modulation of photosynthesis and ROS scavenging response by beneficial bacteria in Olea europaea plantlets under salt stress conditions. Plants 2022, 11, 2748. [Google Scholar] [CrossRef]
- Zhang, L.; Guan, Y. Microbial investigations of new hydrogel-biochar composites as soil amendments for simultaneous nitrogen-use improvement and heavy metal immobilization. J. Hazard. Mater. 2022, 424, 127154. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Peñuelas, J. Drought decreases soil enzyme activity in a Mediterranean Quercus ilex L. forest. Soil Biol. Biochem. 2005, 37, 455–461. [Google Scholar] [CrossRef]
- Daou, L.; Perissol, C.; Luglia, M.; Calvert, V.; Criquet, S. Effects of drying-rewetting or freezing thawing cycles on enzymatic activities of different Mediterranean soils. Soil Biol. Biochem. 2016, 93, 142–149. [Google Scholar] [CrossRef]
- Kumar, S. Soil dehydrogenase enzyme activity in natural and mine soil—A review. Middle East J. Sci. Res. 2013, 13, 898–906. [Google Scholar]
- Preece, C.; Verbruggen, E.; Liu, L.; Weedon, J.T.; Peñuelas, J. Effects of past and current drought on the composition and diversity of soil microbial communities. Soil Biol. Biochem. 2019, 131, 28–39. [Google Scholar]


| Biochar-Doped Hydrogel Type | Maximum Swelling Degree in Free Water | Re-Swelling Capacity | ||
|---|---|---|---|---|
| 1st Cycle | 2nd Cycle | 3rd Cycle | ||
| 0.25% PTB-doped hydrogel | 77.52% | 51.5% | 57.1% | 55.5% |
| 1% PTB-doped hydrogel | 141.05% | 94.7% | 127.2% | 124.3% |
| 0.25% OTB-doped hydrogel | 87.50% | 96.8% | 84.9% | 82.6% |
| 1% OTB-doped hydrogel | 84.06% | 87.3% | 69.2% | 68.3% |
| Soil Moisture | pHw (1:2.5) | ECw (1:5) (µS/cm) | |
|---|---|---|---|
| 70% WHC | Control | 7.4 ± 0.1 a | 269 ± 19 ab |
| 0.25% PTB | 7.4 ± 0.1 a | 287 ± 23 a | |
| 1% PTB | 7.5 ± 0.1 a | 228 ± 30 bc | |
| 0.25% OTB | 7.44 ± 0.05 a | 208 ± 14 c | |
| 1% OTB | 7.37 ± 0.08 a | 227 ± 31 bc | |
| 40% WHC | Control | 7.30 ± 0.03 AB | 364 ± 28 BC |
| 0.25% PTB | 7.09 ± 0.03 C | 545 ± 37 A | |
| 1% PTB | 7.31 ± 0.05 A | 318 ± 15 C | |
| 0.25% OTB | 7.1 ± 0.1 C | 444 ± 75 B | |
| 1% OTB | 7.19 ± 0.06 BC | 337 ± 42 BC |
| Moisture Regime | Hydrogel Composite Type | Moisture Regime × Hydrogel Composite Type | ||
|---|---|---|---|---|
| Plant weight | 0.0072 | - | - | |
| Photosynthetic pigments | CHLa | 0.0000 | - | - |
| CHLb | 0.0000 | - | - | |
| Carotenoids | 0.0235 | - | - | |
| CHLa/b | - | - | 0.0147 | |
| Carot/CHLtotal | 0.0000 | - | - | |
| Stress oxidative biomarkers | ROS | - | - | - |
| CAT | 0.0000 | 0.0008 | 0.0012 | |
| APX | 0.0000 | 0.0025 | 0.0234 | |
| GPOD | 0.0000 | 0.0045 | - | |
| Protein | 0.0000 | 0.0008 | 0.0064 | |
| MDA | 0.0012 | 0.0252 | - | |
| Soil microorganisms | C transformation | - | 0.0102 | - |
| Ammonium oxidase | 0.0000 | 0.0013 | - | |
| Phosphatase | 0.0066 | - | - | |
| Dehydrogenase | 0.0142 | - | - |
| (A) Moisture Regime 70% WHC | |||||||
| CHLa | CHLb | Carotenoids | Protein | CAT | APX | GPOD | |
| CHLa | 1 | 0.99 *** | 1.00 *** | 0.98 *** | 0.98 *** | - | 0.94 *** |
| CHLb | 0.99 *** | 1 | 0.99 *** | 0.97 *** | 0.98 *** | - | 0.90 *** |
| Carotenoids | 1.00 *** | 0.99 *** | 1 | 0.99 *** | 0.98 *** | - | 0.95 *** |
| Protein | 0.98 *** | 0.97 *** | 0.99 *** | 1 | 0.98 *** | - | 0.98 *** |
| CAT | 0.98 *** | 0.98 *** | 0.98 *** | 0.98 *** | 1 | 0.72 *** | 0.95 *** |
| APX | - | - | - | - | 0.72 *** | 1 | - |
| GPOD | 0.94 *** | 0.90 *** | 0.95 *** | 0.98 *** | 0.95 *** | - | 1 |
| (B) Moisture Regime 40% WHC | |||||||
| CHLa | CHLb | Carotenoids | Protein | CAT | APX | GPOD | |
| CHLa | 1 | 0.97 *** | 0.96 *** | - | - | - | - |
| CHLb | 0.97 *** | 1 | 0.88 ** | - | - | - | - |
| Carotenoids | 0.96 *** | 0.88 ** | 1 | - | - | - | - |
| Protein | - | - | - | 1 | 0.94 *** | 0.81 *** | 0.81 *** |
| CAT | - | - | - | 0.94 *** | 1 | - | - |
| APX | - | - | - | 0.81 *** | - | 1 | 0.97 *** |
| GPOD | - | - | - | 0.81 *** | - | 0.97 *** | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Gómez, C.; Uysal, Y.; Doğaroğlu, Z.G.; Kalderis, D.; Gasparatos, D.; Fernández, M.D. Influence of Biochar-Reinforced Hydrogel Composites on Growth and Biochemical Parameters of Bean Plants and Soil Microbial Activities under Different Moisture Conditions. Agriculture 2024, 14, 1405. https://doi.org/10.3390/agriculture14081405
García-Gómez C, Uysal Y, Doğaroğlu ZG, Kalderis D, Gasparatos D, Fernández MD. Influence of Biochar-Reinforced Hydrogel Composites on Growth and Biochemical Parameters of Bean Plants and Soil Microbial Activities under Different Moisture Conditions. Agriculture. 2024; 14(8):1405. https://doi.org/10.3390/agriculture14081405
Chicago/Turabian StyleGarcía-Gómez, Concepción, Yağmur Uysal, Zeynep Görkem Doğaroğlu, Dimitrios Kalderis, Dionisios Gasparatos, and María Dolores Fernández. 2024. "Influence of Biochar-Reinforced Hydrogel Composites on Growth and Biochemical Parameters of Bean Plants and Soil Microbial Activities under Different Moisture Conditions" Agriculture 14, no. 8: 1405. https://doi.org/10.3390/agriculture14081405






