Drought Stress in Quinoa: Effects, Responsive Mechanisms, and Management through Biochar Amended Soil: A Review
Abstract
1. Introduction
2. Quinoa’s Botanical Characteristics and Germplasm Diversity
3. Quinoa Responses to Drought
3.1. Morphological Responses of Quinoa
3.2. Physiological and Biochemical Responses of Quinoa
3.3. Quinoa Anatomical Responses
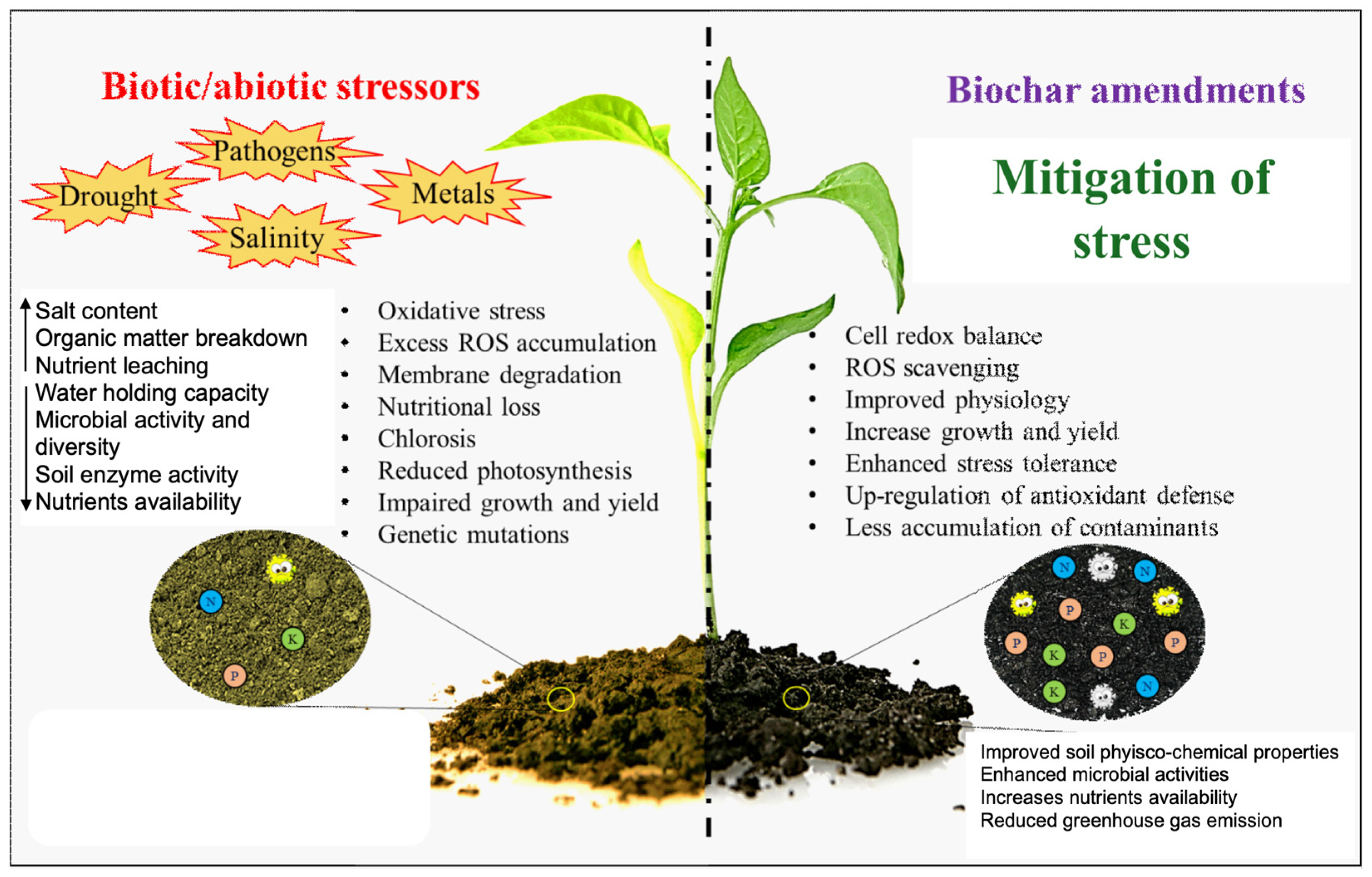
4. Biochar Production and Usage for Management of Drought Stress
5. Biochar-Amended Soils Under Drought Stress
5.1. Effect of Biochar on Soil Properties under Drought Stress
5.2. Effect of Biochar-Amended Soils on Plant Growth under Drought Stress
6. Role of Soils Amended with Biochar in Alleviating Drought Stress in Quinoa
7. Conclusions and Future Prospectives
- (i)
- Long-term field investigations are needed to elucidate the intricate interplay between biochar soil application and soil–plant systems under drought conditions.
- (ii)
- Exploring synergistic interactions between biochar application and established agricultural practices could unveil novel avenues for achieving sustainable agricultural production.
- (iii)
- Quinoa varietal response to biochar-amended soils under water stress conditions should be assessed through morphology, physiology and anatomical changes.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bisht, N.; Chauhan, P.S. Excessive and disproportionate use of chemicals cause soil contamination and nutritional stress. Soil Cont. Thr. Sust. Sol. 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Khan, Z.; Yang, X.-J.; Fu, Y.; Joseph, S.; Khan, M.N.; Khan, M.A.; Alam, I.; Shen, H. Engineered biochar improves nitrogen use efficiency via stabilizing soil water-stable macroaggregates and enhancing nitrogen transformation. Biochar 2023, 5, 52. [Google Scholar] [CrossRef]
- Kapazoglou, A.; Gerakari, M.; Lazaridi, E.; Kleftogianni, K.; Sarri, E.; Tani, E.; Bebeli, P.J. Crop wild relatives: A valuable source of tolerance to various abiotic stresses. Plants 2023, 12, 328. [Google Scholar] [CrossRef] [PubMed]
- Begna, T. Impact of drought stress on crop production and its management options. Int. J. Res. Agron. 2022, 8, 1–13. [Google Scholar] [CrossRef]
- Nazeer, S.; Akram, M.Z.; Ali, M. Amaranth as nutrition-rich and climatic resilient crop: A review. Agricult. Consp. Sci. 2022, 87, 281–293. Available online: https://xueshu.baidu.com/usercenter/paper/show?paperid=1v030cs02w3u0rx06u750v10ek381139&site=xueshu_se (accessed on 15 July 2024).
- Bazile, D. Global trends in the worldwide expansion of Quinoa cultivation. Biol. Life Sci. For. 2023, 25, 13. [Google Scholar] [CrossRef]
- Cui, H.; Yao, Q.; Xing, B.; Zhou, B.; Shah, S.S.; Qin, P. The performance of agronomic and quality traits of Quinoa under different altitudes in northwest of China. Agronomy 2024, 14, 1194. [Google Scholar] [CrossRef]
- Yadav, R.; Gore, P.G.; Gupta, V.; Siddique, K.H. Quinoa (Chenopodium quinoa Willd.)—A smart crop for food and nutritional security. In Neglected and Underutilized Crops; Academic Press: London, UK, 2023; pp. 23–43. [Google Scholar] [CrossRef]
- Liu, C.; Ma, R.; Tian, Y. An overview of the nutritional profile, processing technologies, and health benefits of Quinoa with an emphasis on impacts of processing. Crit. Rev. Food Sci. Nutr. 2022, 64, 5533–5550. [Google Scholar] [CrossRef]
- Agarwal, A.; Rizwana; Tripathi, A.D.; Kumar, T.; Sharma, K.P.; Patel, S.K.S. Nutritional and functional new perspectives and potential health benefits of Quinoa and Chia seeds. Antioxidants 2023, 12, 1413. [Google Scholar] [CrossRef]
- Patel, H.; Sharma, M.; Bains, A.; Chawla, P. Cultivation and production of pseudocereals. In Pseudocereals; CRC Press: Boca Raton, FL, USA, 2024; pp. 1–20. [Google Scholar] [CrossRef]
- Ain, Q.T.; Siddique, K.; Bawazeer, S.; Ali, I.; Mazhar, M.; Rasool, R.; Mubeen, B.; Ullah, F.; Unar, A.; Jafar, T.H. Adaptive mechanisms in Quinoa for coping in stressful environments: An update. PeerJ 2023, 11, e14832. [Google Scholar] [CrossRef]
- Hinojosa, L.; González, J.A.; Barrios-Masias, F.H.; Fuentes, F.; Murphy, K.M. Quinoa abiotic stress responses: A review. Plants 2018, 7, 106. [Google Scholar] [CrossRef]
- Alandia, G.; Rodríguez, J.P.; Palmgren, M.; Condori, B.; López-Marqués, R.L. Advances of biotechnology in quinoa production: A global perspective. In Biology and Biotechnology of Quinoa: Super Grain for Food Security; Varma, A., Ed.; Springer Nature: Singapore, 2021; pp. 79–111. [Google Scholar] [CrossRef]
- de Vries, F.; Lau, J.; Hawkes, C.; Semchenko, M. Plant–soil feedback under drought: Does history shape the future? Trends Ecol. Evol. 2023, 38, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Legg, S. IPCC, 2021: Climate Change 2021—The Physical Science basis. Interaction 2021, 49, 44–45. [Google Scholar] [CrossRef]
- Kim, J.-B.; Kim, S.-H.; Bae, D.-H. The impacts of global warming on arid climate and drought features. Theor. Appl. Climatol. 2023, 152, 693–708. [Google Scholar] [CrossRef]
- Shiade, S.R.G.; Fathi, A.; Taghavi Ghasemkheili, F.; Amiri, E.; Pessarakli, M. Plants’ responses under drought stress conditions: Effects of strategic management approaches—A review. J. Plant Nutr. 2022, 46, 2198–2230. [Google Scholar] [CrossRef]
- Babar, M.; Khalid, M.N.; Haq, M.W.U.; Hanif, M.; Ali, Z.; Awais, M.; Rasheed, Z.; Ali, M.F.; Iftikhar, I.; Saleem, S. A comprehensive review on drought stress response in Cotton at physiological, biochemical and molecular level. Pure Appl. Biol. 2023, 12, 610–622. [Google Scholar] [CrossRef]
- Ahmad, H.M.; Wang, X.; Fiaz, S.; Azeem, F.; Shaheen, T. Morphological and physiological response of Helianthus annuus L. to drought stress and correlation of wax contents for drought tolerance traits. Arab. J. Sci. Eng. 2022, 47, 6747–6761. [Google Scholar] [CrossRef]
- Munsif, F.; Shah, T.; Arif, M.; Jehangir, M.; Afridi, M.Z.; Ahmad, I.; Jan, B.L.; Alansi, S. Combined effect of salicylic acid and potassium mitigates drought stress through the modulation of physio-biochemical attributes and key antioxidants in Wheat. Saudi J. Biol. Sci. 2022, 29, 103294. [Google Scholar] [CrossRef]
- Wen, W.; Timmermans, J.; Chen, Q.; van Bodegom, P.M. A review of remote sensing challenges for food security with respect to salinity and drought threats. Remote Sen. 2021, 13, 6. [Google Scholar] [CrossRef]
- Mašek, O.; Brown, R.C.; Bakshi, S. Biochar production technology. In Biochar for Environmental Management; Routledge: New York, NY, USA, 2024; pp. 85–110. [Google Scholar] [CrossRef]
- Kamarudin, N.S.; Dahalan, F.A.; Hasan, M.; An, O.S.; Parmin, N.A.; Ibrahim, N.; Hamdzah, M.; Zain, N.A.M.; Muda, K.; Wikurendra, E.A. Biochar: A review of its history, characteristics, factors that influence its yield, methods of production, application in wastewater treatment and recent development. Biointerface Res. Appl. Chem. 2022, 12, 7914–7926. [Google Scholar] [CrossRef]
- Lehmann, J.; Abiven, S.; Azzi, E.; Fang, Y.; Singh, B.P.; Sohi, S.; Sundberg, C.; Woolf, D.; Zimmerman, A.R. Persistence of biochar: Mechanisms, measurements, predictions. In Biochar for Environmental Management; Routledge: New York, NY, USA, 2024; pp. 277–311. [Google Scholar]
- Whalen, J.K.; Ejack, L.; Gul, S.; Castro, L.L. Characteristics of biochar: Nutrient properties. In Biochar for Environmental Management; Routledge: London, UK, 2024; pp. 183–207. [Google Scholar] [CrossRef]
- Antonangelo, J.A.; Culman, S.; Zhang, H. Comparative analysis and prediction of cation exchange capacity via summation: Influence of biochar type and nutrient ratios. Front. Soil Sci. 2024, 4, 1371777. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, V.; Kumar, A. Soil and biochar: Attributes and actions of biochar for reclamation of soil and mitigation of soil borne plant pathogens. J. Soil Sci. Plant Nutr. 2024, 24, 1924–1939. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K. Soil hydro-physical properties affected by biomass-derived biochar and organic manure: A low-cost technology for managing acidic mountain sandy soils of north eastern region of India. Biomass Convers. Biorefinery 2024, 14, 6621–6635. [Google Scholar] [CrossRef]
- Libutti, A.; Francavilla, M.; Monteleone, M. Hydrological properties of a clay loam soil as affected by biochar application in a pot experiment. Agronomy 2021, 11, 489. [Google Scholar] [CrossRef]
- Kabir, E.; Kim, K.-H.; Kwon, E.E. Biochar as a tool for the improvement of soil and environment. Front. Environ. Sci. 2023, 11, 1324533. [Google Scholar] [CrossRef]
- Akram, M.Z.; Libutti, A.; Rivelli, A.R. Enhancing vegetative growth of Quinoa and soil properties under water shortage through targeted organic amendments. Biol. Life Sci. For. 2024, 30, 4. [Google Scholar] [CrossRef]
- Hailegnaw, N.S.; Mercl, F.; Pračke, K.; Száková, J.; Tlustoš, P. Mutual relationships of biochar and soil pH, CEC, and exchangeable base cations in a model laboratory experiment. J. Soils Sediments 2019, 19, 2405–2416. [Google Scholar] [CrossRef]
- Arshad, U.; Altaf, M.T.; Liaqat, W.; Ali, M.; Shah, M.N.; Jabran, M.; Ali, M.A. Biochar: Black gold for sustainable agriculture and fortification against plant pathogens—A Review. J. Crop Health 2024, 76, 385–396. [Google Scholar] [CrossRef]
- Long, X.-X.; Yu, Z.-N.; Liu, S.-w.; Qiu, R.-L. A systematic review of biochar aging and the potential eco-environmental risk in heavy metal contaminated soil. J. Haz. Mat. 2024, 472, 134345. [Google Scholar] [CrossRef]
- Kammann, C.I.; Linsel, S.; Gößling, J.W.; Koyro, H.-W. Influence of biochar on drought tolerance of Chenopodium quinoa Willd and on soil–plant relations. Plant Soil 2011, 345, 195–210. [Google Scholar] [CrossRef]
- Ramzani, P.M.A.; Shan, L.; Anjum, S.; Ronggui, H.; Iqbal, M.; Virk, Z.A.; Kausar, S. Improved Quinoa growth, physiological response, and seed nutritional quality in three soils having different stresses by the application of acidified biochar and compost. Plant Phys. Biochem. 2017, 116, 127–138. [Google Scholar] [CrossRef]
- Akram, M.Z.; Libutti, A.; Rivelli, A.R. Evaluation of vegetative development of Quinoa under water stress by applying different organic amendments. Agronomy 2023, 13, 1412. [Google Scholar] [CrossRef]
- Tabatabaei, I.; Alseekh, S.; Shahid, M.; Leniak, E.; Wagner, M.; Mahmoudi, H.; Thushar, S.; Fernie, A.R.; Murphy, K.M.; Schmöckel, S.M. The diversity of Quinoa morphological traits and seed metabolic composition. Sci. Data 2022, 9, 323. [Google Scholar] [CrossRef] [PubMed]
- El-Harty, E.H.; Ghazy, A.; Alateeq, T.K.; Al-Faifi, S.A.; Khan, M.A.; Afzal, M.; Alghamdi, S.S.; Migdadi, H.M. Morphological and molecular characterization of Quinoa genotypes. Agriculture 2021, 11, 286. [Google Scholar] [CrossRef]
- Apaza, V.; Cáceres, G.; Estrada, R.; Pinedo, R. Catalogue of Commercial Varieties of Quinoa in Perú; Food and Agriculture Organization: Rome, Italy, 2015; Available online: https://www.sidalc.net/search/Record/dig-fao-it-20.500.14283-I4596E/Description (accessed on 15 July 2024).
- Coronado, A.C.M.; Hernández, E.H.M.; Coronado, Y.M. Phenotypic diversity of agromorphological characteristics of quinoa (Chenopodium quinoa Willd.) germplasm in Colombia. Sci. Agric. 2022, 79, e20210017. [Google Scholar] [CrossRef]
- Voronov, S.; Pleskachiov, Y.; Shitikova, A.; Zargar, M.; Abdelkader, M. Diversity of the biological and proteinogenic characteristics of Quinoa genotypes as a multi-purpose crop. Agronomy 2023, 13, 279. [Google Scholar] [CrossRef]
- Habib, Z.; Ijaz, S.; Haq, I.U.; Hashem, A.; Avila-Quezada, G.D.; Abd_Allah, E.F.; Khan, N.A. Empirical phenotyping and genome-wide association study reveal the association of panicle architecture with yield in Chenopodium quinoa. Front. Microbiol. 2024, 15, 1349239. [Google Scholar] [CrossRef]
- Sarwar, A.G.; Khatun, M.M.; Fakir, M.S.A. Quinoa-a functional food crop: Morphological descriptors. J. Bangladesh Agric. Uni. 2023, 21, 12–22. [Google Scholar] [CrossRef]
- Maestro-Gaitán, I.; Granado-Rodríguez, S.; Poza-Viejo, L.; Matías, J.; Márquez-López, J.C.; Pedroche, J.J.; Cruz, V.; Bolaños, L.; Reguera, M. Quinoa plant architecture: A key factor determining plant productivity and seed quality under long-term drought. Environ. Exp. Bot. 2023, 211, 105350. [Google Scholar] [CrossRef]
- Casini, P. Varietà di quinoa adatta all’areale italiano. L’INFORMATORE AGRARIO 2017, 8, 51–55. Available online: https://flore.unifi.it/handle/2158/1100123 (accessed on 14 July 2024).
- Vásquez, S.C.; del Pozo, A.; Castillo, D.; Matus, I.; Gómez-Pando, L.; Zamudio-Ayala, D.; Mignone, C.M.; Bertero, H.D.; Calderini, D.F. The critical period for yield and grain protein determination in Quinoa (Chenopodium quinoa Willd.). Field Crops Res. 2024, 306, 109207. [Google Scholar] [CrossRef]
- Akram, M.Z.; Basra, S.M.A.; Hafeez, M.B.; Khan, S.; Nazeer, S.; Iqbal, S.; Saddiq, M.S.; Zahra, N. Adaptability and yield potential of new Quinoa lines under agro-ecological conditions of Faisalabad-Pakistan. Asian J. Agric. Biol. 2021, 2, 1–8. [Google Scholar] [CrossRef]
- Taaime, N.; El Mejahed, K.; Moussafir, M.; Bouabid, R.; Oukarroum, A.; Choukr-Allah, R.; El Gharous, M. Early sowing of quinoa cultivars, benefits from rainy season and enhances quinoa development, growth, and yield under arid condition in Morocco. Sustainability 2022, 14, 4010. [Google Scholar] [CrossRef]
- Hafeez, M.B.; Iqbal, S.; Li, Y.; Saddiq, M.S.; Basra, S.M.; Zhang, H.; Zahra, N.; Akram, M.Z.; Bertero, D.; Curti, R.N. Assessment of phenotypic diversity in the USDA collection of Quinoa links genotypic adaptation to germplasm origin. Plants 2022, 11, 738. [Google Scholar] [CrossRef]
- Manjarres-Hernández, E.H.; Arias-Moreno, D.M.; Morillo-Coronado, A.C.; Ojeda-Pérez, Z.Z.; Cárdenas-Chaparro, A. Phenotypic characterization of Quinoa (Chenopodium quinoa Willd.) for the selection of promising materials for breeding programs. Plants 2021, 10, 1339. [Google Scholar] [CrossRef]
- Romero, M.; Sanchez, A.M.M.; Pineda, E.; Ccamapaza, Y.; Zavalla, N. Genetic identity based on simple sequence repeat (SSR) markers for Quinoa (Chenopodium quinoa Willd.). Cienc. Investig. Agrar. 2019, 46, 166–178. [Google Scholar] [CrossRef]
- Gomez-Pando, L.R.; Aguilar-Castellanos, E.; Ibañez-Tremolada, M. Quinoa (Chenopodium quinoa Willd.) breeding. In Advances in Plant Breeding Strategies: Cereals; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer: Singapore, 2019; pp. 259–316. [Google Scholar] [CrossRef]
- Kilian, B.; Dempewolf, H.; Guarino, L.; Werner, P.; Coyne, C.; Warburton, M.L. Crop Science special issue: Adapting agriculture to climate change: A walk on the wild side. Crop Sci. 2021, 61, 32–36. [Google Scholar] [CrossRef]
- Bascuñán-Godoy, L.; Reguera, M.; Abdel-Tawab, Y.M.; Blumwald, E. Water deficit stress-induced changes in carbon and nitrogen partitioning in Chenopodium quinoa Willd. Planta 2016, 243, 591–603. [Google Scholar] [CrossRef]
- Von Baer, I.; Bazile, D.; Martinez, E.A. Cuarenta años de mejoramiento de Quinoa (Chenopodium quinoa Willd.) en la araucania: Origen de “la regalona-B”. Rev. Geográfica Valparaíso 2009, 42, 34–44. Available online: https://agritrop.cirad.fr/554885/ (accessed on 15 July 2024).
- Alandia, G.; Rodriguez, J.; Jacobsen, S.-E.; Bazile, D.; Condori, B. Global expansion of Quinoa and challenges for the Andean region. Glob. Food Secur. 2020, 26, 100429. [Google Scholar] [CrossRef]
- Angeli, V.; Miguel Silva, P.; Crispim Massuela, D.; Khan, M.W.; Hamar, A.; Khajehei, F.; Graeff-Hönninger, S.; Piatti, C. Quinoa (Chenopodium quinoa Willd.): An overview of the potentials of the “golden grain” and socio-economic and environmental aspects of its cultivation and marketization. Foods 2020, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Walia, S.S.; Kaur, K.; Kaur, T. Drought, Its different types and drought management strategies. In Rainfed Agriculture and Watershed Management; Springer: Singapore, 2024; pp. 17–26. [Google Scholar] [CrossRef]
- Cruz, P.; Joffre, R.; Saintenoy, T.; Vacher, J.J. Growing in scarcity: Pre-hispanic rain-fed agriculture in the semi-arid and frost-prone Andean Altiplano (Bolivia). Land 2024, 13, 619. [Google Scholar] [CrossRef]
- Abidi, I.; Daoui, K.; Abouabdillah, A.; Belqadi, L.; Mahyou, H.; Bazile, D.; Douaik, A.; Gaboun, F.; Hassane Sidikou, A.A.; Alaoui, S.B. Quinoa–Olive agroforestry system assessment in semi-arid environments: Performance of an innovative system. Agronomy 2024, 14, 495. [Google Scholar] [CrossRef]
- Matías, J.; Cruz, V.; Rodríguez, M.J.; Calvo, P.; Maestro-Gaitán, I.; Reguera, M. Evaluating yield, nutritional quality, and environmental impact of Quinoa straws across Mediterranean water environments. Plants 2024, 13, 751. [Google Scholar] [CrossRef]
- Grenfell-Shaw, L.; Tester, M. Abiotic stress tolerance in Quinoa. In The Quinoa Genome; Schmöckel, S.M., Ed.; Springer: Singapore, 2021; pp. 139–167. [Google Scholar] [CrossRef]
- Pathan, S.; Ndunguru, G.; Clark, K.; Ayele, A.G. Yield and nutritional responses of Quinoa (Chenopodium quinoa Willd.) genotypes to irrigated, rainfed, and drought-stress environments. Front. Sustain. Food Syst. 2023, 7, 1242187. [Google Scholar] [CrossRef]
- Harvey Infante, R.; Albesiano, S.; Leopoldo Arrieta, V.; Nubia Gómez, V. Morphological characterization of varieties of Chenopodium quinoa cultivated in the department of Boyacá, Colombia. Rev. UDCA Actual. Divulg. Científica 2018, 21, 329–339. [Google Scholar] [CrossRef]
- Otterbach, S.L.; Khoury, H.; Rupasinghe, T.; Mendis, H.; Kwan, K.H.; Lui, V.; Natera, S.H.; Klaiber, I.; Allen, N.M.; Jarvis, D.E. Characterization of epidermal bladder cells in Chenopodium quinoa. Plant Cell Environ. 2021, 29, 3836–3852. [Google Scholar] [CrossRef]
- Raney, J.A. Transcriptome Analysis of Drought induced Stress in Chenopodium quinoa. Master’s Thesis, Brigham Young University, Provo, UT, USA, 2012. Available online: https://scholarsarchive.byu.edu/etd/3915 (accessed on 14 July 2024).
- Zurita Silva, A.; Jacobsen, S.-E.; Razzaghi, F.; Álvarez Flores, R.; Ruiz, K.B.; Morales, A.; Silva Ascencio, H. Quinoa drought responses and adaptation. In State of the Art Report on Quinoa Around the World; Bazile, D., Bertero, D., Eds.; FAO and CIRAD Rome: Santiago, Chile, 2015; pp. 157–171. Available online: https://repositorio.uchile.cl/handle/2250/130689 (accessed on 15 July 2024).
- Tapia, M.E. The long journey of Quinoa: Who wrote its history. State Art Rep. Quinoa 2015, 3, 3–9. [Google Scholar]
- Iqbal, H.; Yaning, C.; Waqas, M.; Raza, S.T.; Shareef, M.; Ahmad, Z. Salinity and exogenous H2O2 improve gas exchange, osmoregulation, and antioxidant metabolism in Quinoa under drought stress. Physiol. Plant. 2023, 175, e14057. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I. Role of osmolytes in alleviation of oxidative stress. In Reactive Oxygen Species in Plants: The Right Balance; Springer: Singapore, 2023; pp. 173–202. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Pang, S.; Zheng, Q.; Quan, S.; Liu, Y.; Xu, T.; Liu, Y.; Qi, M. Function analysis of the ERF and DREB subfamilies in Tomato fruit development and ripening. Front. Plant Sci. 2022, 13, 849048. [Google Scholar] [CrossRef]
- Jacobsen, S.-E.; Liu, F.; Jensen, C.R. Does root-sourced ABA play a role for regulation of stomata under drought in Quinoa (Chenopodium quinoa Willd.). Sci. Hortic. 2009, 122, 281–287. [Google Scholar] [CrossRef]
- Kohan, K.; Kasraie, P.; Larijani, H.; Ghoshchi, F.; Oveysi, M. Response of Quinoa (Chenopodium quinoa) cultivars to organic acid under drought stress. Iran. J. Plant Physiol. 2023, 13, 4753–4763. [Google Scholar] [CrossRef]
- Nguyen, L.V.; Bertero, D.; Hoang, D.T.; Long, N.V. Variation in Quinoa roots growth responses to drought stresses. J. Agron. Crop Sci. 2022, 208, 830–840. [Google Scholar] [CrossRef]
- Fitter, A.; Stickland, T.; Harvey, M.; Wilson, G. Architectural analysis of plant root systems 1. Architectural correlates of exploitation efficiency. New Phytol. 1991, 118, 375–382. [Google Scholar] [CrossRef]
- Bouma, T.; Nielsen, K.L.; Van Hal, J.; Koutstaal, B. Root system topology and diameter distribution of species from habitats differing in inundation frequency. Funct. Ecol. 2001, 15, 360–369. [Google Scholar] [CrossRef]
- Singh, S.; Jain, A.; Varma, A. Root analysis of Quinoa plant. In Biology and Biotechnology of Quinoa: Super Grain for Food Security; Varma, A., Ed.; Springer: Singapore, 2022; pp. 153–165. [Google Scholar] [CrossRef]
- Gandarillas, A.; Rojas, W.; Bonifacio, A.; Ojeda, N. Quinoa in Bolivia: The Proinpa foundation’s perspective. In State of the Art Report of Quinoa in the World in 2013; Bazile, D., Bertero, D., Eds.; FAO and CIRAD Rome: Santiago, Chile, 2013; pp. 344–361. Available online: https://www.fao.org/3/a-i4042e.pdf (accessed on 15 July 2024).
- Alvarez-Flores, R.; Nguyen-Thi-Truc, A.; Peredo-Parada, S.; Joffre, R.; Winkel, T. Rooting plasticity in wild and cultivated Andean Chenopodium species under soil water deficit. Plant Soil 2018, 425, 479–492. [Google Scholar] [CrossRef]
- Flores, R.A.A. Réponses Morphologiques et Architecturales du Système Racinaire au Déficit Hydrique Chez des Chenopodium Cultivés et Sauvages d’Amérique Andine. Doctoral Dissertation, Centre d’Écologie Fonctionnelle et Évolutive, Montpellier, France, 2012. [Google Scholar]
- Issa Ali, O.; Fghire, R.; Anaya, F.; Benlhabib, O.; Wahbi, S. Physiological and morphological responses of two Quinoa cultivars (Chenopodium quinoa Willd.) to drought stress. Gesunde Pflanz. 2019, 71, 123–133. [Google Scholar] [CrossRef]
- Alvarez-Flores, R.; Winkel, T.; Nguyen-Thi-Truc, A.; Joffre, R. Root foraging capacity depends on root system architecture and ontogeny in seedlings of three Andean Chenopodium species. Plant Soil 2014, 380, 415–428. [Google Scholar] [CrossRef]
- Forouzandeh, M.; Parsa, S.; Mahmoodi, S.; Izanloo, A. Physiological, biochemical, and molecular responses of Quinoa (Chenopodium quinoa Willd.) to elicitors under drought stress. Plant Mol. Biol. Report. 2023, 1–17. [Google Scholar] [CrossRef]
- Gámez, A.L.; Soba, D.; Zamarreño, Á.M.; García-Mina, J.M.; Aranjuelo, I.; Morales, F. Effect of water stress during grain filling on yield, quality and physiological traits of Illpa and Rainbow quinoa (Chenopodium quinoa Willd.) cultivars. Plants 2019, 8, 173. [Google Scholar] [CrossRef]
- Sadak, M.S.; El-Bassiouny, H.M.S.; Dawood, M.G. Role of trehalose on antioxidant defense system and some osmolytes of Quinoa plants under water deficit. Bull. Natl. Res. Cent. 2019, 43, 5. [Google Scholar] [CrossRef]
- Killi, D.; Haworth, M. Diffusive and metabolic constraints to photosynthesis in Quinoa during drought and salt stress. Plants 2017, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Jacobsen, S.-E.; Andersen, M.N.; Nunez, N.; Andersen, S.; Rasmussen, L.; Mogensen, V. Leaf gas exchange and water relation characteristics of field Quinoa (Chenopodium quinoa Willd.) during soil drying. Eur. J. Agron. 2000, 13, 11–25. [Google Scholar] [CrossRef]
- Joshi, V.; Sahu, A.; Joshi, N.; Vyas, A.; Shah, K.; Chauhan, D.N.; Chauhan, N.S. Signaling crosstalk between cytokinins and abscisic acid in plant defense, growth, and development. In Hormonal Cross-Talk, Plant Defense and Development; Elsevier: Amsterdam, The Netherlands, 2023; pp. 149–170. [Google Scholar] [CrossRef]
- Sadak, M.S.; Bakhoum, G.S. Selenium-induced modulations in growth, productivity and physiochemical responses to water deficiency in Quinoa (Chenopodium quinoa) grown in sandy soil. Biocatal. Agric. Biotechnol. 2022, 44, 102449. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta 2020, 251, 3. [Google Scholar] [CrossRef]
- Al-Naggar, A.; Abd El-Salam, R.; Badran, A.; El-Moghazi, M. Genotype and drought effects on morphological, physiological and yield traits of Quinoa (Chenopodium quinoa Willd.). Asian J. Adv. Agric. Res. 2017, 3, 1–15. [Google Scholar] [CrossRef]
- Manaa, A.; Goussi, R.; Derbali, W.; Cantamessa, S.; Essemine, J.; Barbato, R. Photosynthetic performance of Quinoa (Chenopodium quinoa Willd.) after exposure to a gradual drought stress followed by a recovery period. Biochim. Biophy. Acta (BBA)-Bioenerg. 2021, 1862, 148383. [Google Scholar] [CrossRef]
- Prado, F.E.; Hilal, M.B.; Albornoz, P.L.; Gallardo, M.; Ruíz, V. Anatomical and physiological responses of four Quinoa cultivars to salinity at seedling stage. Ind. J. Sci. Technol. 2017, 10, 1–12. [Google Scholar] [CrossRef]
- Riccardi, M.; Pulvento, C.; Lavini, A.; d’Andria, R.; Jacobsen, S.E. Growth and ionic content of Quinoa under saline irrigation. J. Agron. Crop Sci. 2014, 200, 246–260. [Google Scholar] [CrossRef]
- Al-Naggar, A.; El-Salam, R.; Badran, A.; Boulos, S.; El-Moghazi, M. Heritability and genetic advance from selection for morphological, biochemical and anatomical traits of Chenopodium quinoa under water stress. Bionature 2018, 38, 66–85. Available online: https://www.researchgate.net/profile/A-M-Al-Naggar/publication/323879709_HERITABILITY_AND_GENETIC_ADVANCE_FROM_SELECTION_FOR_MORPHOLOGICAL_BIOCHEMICAL_AND_ANATOMICAL_TRAITS_OF_Chenopodium_quinoa_UNDER_WATER_STRESS/links/5ab10928458515ecebebe6c3/HERITABILITY-AND-GENETIC-ADVANCE-FROM-SELECTION-FOR-MORPHOLOGICAL-BIOCHEMICAL-AND-ANATOMICAL-TRAITS-OF-Chenopodium-quinoa-UNDER-WATER-STRESS.pdf (accessed on 14 July 2024).
- Milla-Moreno, E.A.; McKown, A.D.; Guy, R.D.; Soolanayakanahally, R.Y. Leaf mass per area predicts palisade structural properties linked to mesophyll conductance in Balsam Poplar (Populus balsamifera L.). Botany 2016, 94, 225–239. [Google Scholar] [CrossRef]
- Verheijen, F.; Graber, E.; Ameloot, N.; Bastos, A.C.; Sohi, S.; Knicker, H. Biochars in soils: New insights and emerging research needs. Eur. J. Soil Sci. 2014, 65, 22–27. [Google Scholar] [CrossRef]
- Aubertin, M.-L. Biochar-Compost Mixtures: Interactions and Impact on Carbon Sequestration and Soil Fertility. Ph.D. Thesis, Sorbonne Université, Paris, France, 2022. Available online: https://theses.hal.science/tel-03813566/ (accessed on 14 July 2024).
- Murtaza, G.; Ahmed, Z.; Usman, M.; Tariq, W.; Ullah, Z.; Shareef, M.; Iqbal, H.; Waqas, M.; Tariq, A.; Wu, Y. Biochar induced modifications in soil properties and its impacts on crop growth and production. J. Plant Nutr. 2021, 44, 1677–1691. [Google Scholar] [CrossRef]
- de Medeiros, E.V.; Lima, N.T.; de Sousa Lima, J.R.; Pinto, K.M.S.; da Costa, D.P.; Franco Junior, C.L.; Souza, R.M.S.; Hammecker, C. Biochar as a strategy to manage plant diseases caused by pathogens inhabiting the soil: A critical review. Phytoparasitica 2021, 49, 713–726. Available online: https://link.springer.com/article/10.1007/s12600-021-00887-y (accessed on 15 July 2024).
- Jaiswal, A.K.; Frenkel, O.; Debode, J.; Graber, E.R. How does biochar influence plant biotic stress? In Biochar for Environmental Management, 3rd ed.; Routledge: New York, NY, USA, 2024; pp. 589–612. [Google Scholar] [CrossRef]
- Imran, S.; Sarker, P.; Hoque, M.N.; Paul, N.C.; Mahamud, M.A.; Chakrobortty, J.; Tahjib-Ul-Arif, M.; Latef, A.A.H.A.; Hasanuzzaman, M.; Rhaman, M.S. Biochar actions for the mitigation of plant abiotic stress. Crop Pasture Sci. 2022, 74, 1–15. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Zhang, L.; Zheng, Y.; Liu, X.; Zhang, Y. The critical role of biochar to mitigate the adverse impacts of drought and salinity stress in plants. Front. Plant Sci. 2023, 14, 1163451. [Google Scholar] [CrossRef]
- Volpi, M.P.; Silva, J.C.; Hornung, A.; Ouadi, M. Review of the current state of pyrolysis and biochar utilization in europe: A scientific perspective. Clean Technol. 2024, 6, 152–175. [Google Scholar] [CrossRef]
- Hou, R.; Zhang, J.; Fu, Q.; Li, T.; Gao, S.; Wang, R.; Zhao, S.; Zhu, B. The boom era of emerging contaminants: A review of remediating agricultural soils by biochar. Sci. Total Environ. 2024, 931, 172899. [Google Scholar] [CrossRef]
- Shoudho, K.N.; Khan, T.H.; Ara, U.R.; Khan, M.R.; Shawon, Z.B.Z.; Hoque, M.E. Biochar in global carbon cycle: Towards sustainable development goals. Curr. Res. Green Sustain. Chem. 2024, 8, 100409. [Google Scholar] [CrossRef]
- Maisyarah, S.; Chen, J.-Y.; Hseu, Z.-Y.; Jien, S.-H. Retention and loss pathways of soluble nutrients in biochar-treated slope land soil based on a rainfall simulator. Soil Environ. Health 2023, 1, 100021. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Othman, M.H.D.; Liang, X.; Goh, H.H.; Gikas, P.; Chong, K.-K.; Chew, K.W. Challenges and opportunities for biochar to promote circular economy and carbon neutrality. J. Environ. Manag. 2023, 332, 117429. [Google Scholar] [CrossRef]
- Mishra, R.K.; Kumar, D.J.P.; Narula, A.; Chistie, S.M.; Naik, S.U. Production and beneficial impact of biochar for environmental application: A review on types of feedstocks, chemical compositions, operating parameters, techno-economic study, and life cycle assessment. Fuel 2023, 343, 127968. [Google Scholar] [CrossRef]
- Li, S.; Tasnady, D. Biochar for soil carbon sequestration: Current knowledge, mechanisms, and future perspectives. J. Carbon Res. 2023, 9, 67. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, M.N.; Zhang, K.; Luo, T.; Zhu, K.; Hu, L. The application of biochar alleviated the adverse effects of drought on the growth, physiology, yield and quality of Rapeseed through regulation of soil status and nutrients availability. Ind. Crops Prod. 2021, 171, 113878. [Google Scholar] [CrossRef]
- Amalina, F.; Abd Razak, A.S.; Krishnan, S.; Sulaiman, H.; Zularisam, A.; Nasrullah, M. Advanced techniques in the production of biochar from lignocellulosic biomass and environmental applications. Clean. Mater. 2022, 6, 100137. [Google Scholar] [CrossRef]
- Khater, E.-S.; Bahnasawy, A.; Hamouda, R.; Sabahy, A.; Abbas, W.; Morsy, O.M. Biochar production under different pyrolysis temperatures with different types of agricultural wastes. Sci. Rep. 2024, 14, 2625. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Cui, L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizabal, T.; Cayuela, M.L.; Sigua, G.; Novak, J.; Spokas, K. Feedstock choice, pyrolysis temperature and type influence biochar characteristics: A comprehensive meta-data analysis review. Biochar 2020, 2, 421–438. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Chen, W.; Yang, H.; Chen, H. The structure evolution of biochar from biomass pyrolysis and its correlation with gas pollutant adsorption performance. Bioresour. Technol. 2017, 246, 101–109. [Google Scholar] [CrossRef]
- Gale, N.V.; Halim, M.A.; Horsburgh, M.; Thomas, S.C. Comparative responses of early-successional plants to charcoal soil amendments. Ecosphere 2017, 8, e01933. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R. The biochar dilemma. Soil Res. 2014, 52, 217–230. [Google Scholar] [CrossRef]
- Haider, G.; Steffens, D.; Moser, G.; Müller, C.; Kammann, C.I. Biochar reduced nitrate leaching and improved soil moisture content without yield improvements in a four-year field study. Agric. Ecosyst. Environ. 2017, 237, 80–94. [Google Scholar] [CrossRef]
- Idbella, M.; Baronti, S.; Giagnoni, L.; Renella, G.; Becagli, M.; Cardelli, R.; Maienza, A.; Vaccari, F.P.; Bonanomi, G. Long-term effects of biochar on soil chemistry, biochemistry, and microbiota: Results from a 10-year field vineyard experiment. Appl. Soil Ecol. 2024, 195, 105217. [Google Scholar] [CrossRef]
- Pustahija, L.; Kern, W. Surface functionalization of (prolytic) Carbon—An overview. J. Crabon Res. 2023, 9, 38. [Google Scholar] [CrossRef]
- Amonette, J.E.; Joseph, S. Characteristics of biochar: Microchemical properties. In Biochar for Environmental Management; Routledge: New York, NY, USA, 2012; pp. 65–84. [Google Scholar]
- Pandian, K.; Vijayakumar, S.; Mustaffa, M.R.A.F.; Subramanian, P.; Chitraputhirapillai, S. Biochar—A sustainable soil conditioner for improving soil health, crop production and environment under changing climate: A review. Front. Soil Sci. 2024, 4, 1376159. [Google Scholar] [CrossRef]
- Akhil, D.; Lakshmi, D.; Kartik, A.; Vo, D.-V.N.; Arun, J.; Gopinath, K.P. Production, characterization, activation and environmental applications of engineered biochar: A review. Environ. Chem. Lett. 2021, 19, 2261–2297. [Google Scholar] [CrossRef]
- Faloye, O.T.; Ajayi, E.A.; Rostek, J.; Schroeren, V.; Babalola, T.; Fashina, A.; Horn, R. Hydraulic and pore functions of differently textured soils modified by biochar from different parts of the mango plant. Soil Tillage Res. 2024, 236, 105944. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.-J.; Novak, A.; Yang, Y.; Wang, J. Role of biochar in improving sandy soil water retention and resilience to drought. Water 2021, 13, 407. [Google Scholar] [CrossRef]
- Bolan, S.; Hou, D.; Wang, L.; Hale, L.; Egamberdieva, D.; Tammeorg, P.; Li, R.; Wang, B.; Xu, J.; Wang, T. The potential of biochar as a microbial carrier for agricultural and environmental applications. Sci. Total Environ. 2023, 886, 163968. [Google Scholar] [CrossRef] [PubMed]
- Boudjabi, S.; Ababsa, N.; Chenchouni, H. Enhancing soil resilience and crop physiology with biochar application for mitigating drought stress in Durum Wheat (Triticum durum). Heliyon 2023, 9, e22909. [Google Scholar] [CrossRef]
- Yavaş, İ.; Sürmen, M.; Kara, R.A.D.E.; Hussain, S. Biochar application and drought stress tolerance in plants: Overview and implications. In New Trends in Agriculture, Forestry and Aquaculture Sciences; Ersoy, N., Ed.; Duvar Publishing: Izmir, Turkey, 2022; pp. 209–224. [Google Scholar]
- Wei, B.; Peng, Y.; Lin, L.; Zhang, D.; Ma, L.; Jiang, L.; Li, Y.; He, T.; Wang, Z. Drivers of biochar-mediated improvement of soil water retention capacity based on soil texture: A meta-analysis. Geoderma 2023, 437, 116591. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Q.; Chen, Y.; Lu, H. Effects of biochar on plant growth and hydro-chemical properties of recycled concrete aggregate. Sci. Total Environ. 2023, 882, 163557. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, K.; Chen, B. Linking hydrophobicity of biochar to the water repellency and water holding capacity of biochar-amended soil. Environ. Pollut. 2019, 253, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Timms, W.; Mahmud, M.P. Optimising water holding capacity and hydrophobicity of biochar for soil amendment–A review. Sci. Total Environ. 2022, 851, 158043. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Mahmud, M.P.; Nguyen, M.D.; Timms, W. Evaluating fundamental biochar properties in relation to water holding capacity. Chemosphere 2023, 328, 138620. [Google Scholar] [CrossRef]
- Ruan, R.; Yuan, Y.; Wang, C.; Wang, Y. Biochar effects on drought tolerance in Maize roots are linked to K+ concentration, Ca2+ efflux, and apoplastic pH. Plant Growth Regul. 2024, 103, 307–320. [Google Scholar] [CrossRef]
- Gullap, M.K.; Severoglu, S.; Karabacak, T.; Yazici, A.; Ekinci, M.; Turan, M.; Yildirim, E. Biochar derived from hazelnut shells mitigates the impact of drought stress on Soybean seedlings. N. Z. J. Crop Hortic. Sci. 2024, 52, 19–37. [Google Scholar] [CrossRef]
- Albalasmeh, A.; Mohawesh, O.; Alqudah, A.; Unami, K.; Al-Ajlouni, Z.; Klaib, A. The potential of biochar application to enhance soil quality, yield, and growth of Wheat and Barley under rainfed conditions. Water Air Soil Pollut. 2023, 234, 463. [Google Scholar] [CrossRef]
- Amin, J.; Raza, M.A.S.; Iqbal, R.; Aslam, M.U.; Ibrahim, M.A.; Javed, M.A.; Ali, Q. Biochar enhances the sustainable growth and yield of drought-subjected Wheat. J. Tianjin Univ. Sci. Technol. 2023, 56, 123–135. [Google Scholar] [CrossRef]
- Baiamonte, G.; Crescimanno, G.; Parrino, F.; De Pasquale, C. Effect of biochar on the physical and structural properties of a sandy soil. Catena 2019, 175, 294–303. [Google Scholar] [CrossRef]
- Noureen, S.; Iqbal, A.; Muqeet, H.A. Potential of drought tolerant Rhizobacteria amended with biochar on growth promotion in Wheat. Plants 2024, 13, 1183. [Google Scholar] [CrossRef]
- Ullah, S.; Shah, S.; Jamal, A.; Saeed, M.F.; Mihoub, A.; Zia, A.; Ahmed, I.; Seleiman, M.F.; Mancinelli, R.; Radicetti, E. Combined Effect of Biochar and Plant Growth-Promoting Rhizbacteria on Physiological Responses of Canola (Brassica napus L.) Subjected to Drought Stress. J. Plant Growth Regul. 2024, 43, 1814–1832. [Google Scholar] [CrossRef]
- Ullah, N.; Ditta, A.; Imtiaz, M.; Li, X.; Jan, A.U.; Mehmood, S.; Rizwan, M.S.; Rizwan, M. Appraisal for organic amendments and plant growth-promoting rhizobacteria to enhance crop productivity under drought stress: A review. J. Agron. Crop Sci. 2021, 207, 783–802. [Google Scholar] [CrossRef]
- Tsolis, V.; Barouchas, P. Biochar as soil amendment: The effect of biochar on soil properties using VIS-NIR diffuse reflectance spectroscopy, biochar aging and soil microbiology—A review. Land 2023, 12, 1580. [Google Scholar] [CrossRef]
- Park, I.; Seo, Y.-S.; Mannaa, M. Recruitment of the rhizo-microbiome army: Assembly determinants and engineering of the rhizosphere microbiome as a key to unlocking plant potential. Front. Microbiol. 2023, 14, 1163832. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Ma, H.; Alimov, J.; Reckling, M.; Wirth, S.; Bellingrath-Kimura, S.D. Response of Soybean to hydrochar-based rhizobium inoculation in loamy sandy soil. Microorganisms 2020, 8, 1674. [Google Scholar] [CrossRef]
- Murtaza, G.; Usman, M.; Iqbal, J.; Tahir, M.N.; Elshikh, M.S.; Alkahtani, J.; Toleikienė, M.; Iqbal, R.; Akram, M.I.; Gruda, N.S. The impact of biochar addition on morpho-physiological characteristics, yield and water use efficiency of Tomato plants under drought and salinity stress. BMC Plant Biol. 2024, 24, 356. [Google Scholar] [CrossRef]
- Rivelli, A.R.; Akram, M.Z.; Libutti, A. Woody biochar rate and water shortage impact on early growth stages of Chenopodium quinoa Willd. Agronomy 2024, 14, 53. [Google Scholar] [CrossRef]
- Afshar, R.; Hashemi, M.; DaCosta, M.; Spargo, J.; Sadeghpour, A. Biochar application and drought stress effects on physiological characteristics of Silybum marianum. Commun. Soil Sci. Plant Anal. 2016, 47, 743–752. [Google Scholar] [CrossRef]
- Genesio, L.; Miglietta, F.; Baronti, S.; Vaccari, F.P. Biochar increases vineyard productivity without affecting Grape quality: Results from a four years field experiment in Tuscany. Agric. Ecosyst. Environ. 2015, 201, 20–25. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, J.; Wang, H.; Su, L.; Zhao, C. Biochar addition alleviate the negative effects of drought and salinity stress on Soybean productivity and water use efficiency. BMC Plant Biol. 2020, 20, 288. [Google Scholar] [CrossRef]
- Abideen, Z.; Koyro, H.-W.; Huchzermeyer, B.; Bilquees, G.; Khan, M.A. Impact of a biochar or a biochar-compost mixture on water relation, nutrient uptake and photosynthesis of Phragmites karka. Pedosphere 2020, 30, 466–477. [Google Scholar] [CrossRef]
- Abdou, N.M.; EL-Samnoudi, I.M.; Ibrahim, A.E.-A.M.; EL-Tawwab, A.R.A. Biochar amendment alleviates the combined effects of salinity and drought stress on water productivity, yield and quality traits of Sugar Beet (Beta vulgaris L.). J. Soil Sci. Plant Nutr. 2024, 24, 2091–2110. [Google Scholar] [CrossRef]
- Jabborova, D.; Abdrakhmanov, T.; Jabbarov, Z.; Abdullaev, S.; Azimov, A.; Mohamed, I.; AlHarbi, M.; Abu-Elsaoud, A.; Elkelish, A. Biochar improves the growth and physiological traits of Alfalfa, Amaranth and Maize grown under salt stress. PeerJ 2023, 11, e15684. [Google Scholar] [CrossRef] [PubMed]
- Babujia, L.C.; Silva, A.P.; Nakatani, A.S.; Cantão, M.E.; Vasconcelos, A.T.R.; Visentainer, J.V.; Hungria, M. Impact of long-term cropping of glyphosate-resistant transgenic Soybean [Glycine max (L.) Merr.] on soil microbiome. Trans. Res. 2016, 25, 425–440. [Google Scholar] [CrossRef]
- Hou, Q.; Zuo, T.; Wang, J.; Huang, S.; Wang, X.; Yao, L.; Ni, W. Responses of nitrification and bacterial community in three size aggregates of paddy soil to both of initial fertility and biochar addition. Appl. Soil Ecol. 2021, 166, 104004. [Google Scholar] [CrossRef]
- Han, S.; Li, H.; Rengel, Z.; Du, Z.; Hu, N.; Wang, Y.; Zhang, A. Biochar application promotes crops yield through regulating root development and the community structure of root endophytic fungi in Wheat-Maize rotation. Soil Tillage Res. 2023, 234, 105827. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, Q.; de Vries, W.; Ros, G.H.; Chen, X.; Muneer, M.A.; Zhang, F.; Wu, L. Effects of soil amendments on soil acidity and crop yields in acidic soils: A world-wide meta-analysis. J. Environ. Manag. 2023, 345, 118531. [Google Scholar] [CrossRef]
- Kartika, K.; Sakagami, J.-I.; Lakitan, B.; Yabuta, S.; Akagi, I.; Widuri, L.I.; Siaga, E.; Iwanaga, H.; Nurrahma, A.H.I. Rice husk biochar effects on improving soil properties and root development in Rice (Oryza glaberrima Steud.) exposed to drought stress during early reproductive stage. AIMS Agric. Food 2021, 6, 737–751. [Google Scholar] [CrossRef]
- Xuan, Y.; Yi, Y.; Liang, H.; Wei, S.; Chen, N.; Jiang, L.; Ali, I.; Ullah, S.; Wu, X.; Cao, T. Amylose content and RVA profile characteristics of noodle Rice under different conditions. Agron. J. 2020, 112, 117–129. [Google Scholar] [CrossRef]
- Sharma, S.; Rana, V.S.; Rana, N.; Prasad, H.; Sharma, U.; Patiyal, V. Biochar from fruit crops waste and its potential impact on fruit crops. Sci. Hortic. 2022, 299, 111052. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.; Zhang, L.; Li, Q.; Li, C.; Chen, G.; Zhang, S.; Liu, Q.; Hu, X. Evolution of the functionalities and structures of biochar in pyrolysis of Poplar in a wide temperature range. Bioresour. Technol. 2020, 304, 123002. [Google Scholar] [CrossRef]
- Almaroai, Y.A.; Eissa, M.A. Effect of biochar on yield and quality of Tomato grown on a metal-contaminated soil. Sci. Hortic. 2020, 265, 109210. [Google Scholar] [CrossRef]
- Jabborova, D.; Singh, P.K.; Saharan, B.S.; Ahmed, N.; Kumar, S.; Duhan, J.S. Biochar and AMF improve growth, physiological traits, nutrients of Turmeric and soil biochemical properties in drought stress. Agric. Res. 2024, 1–12. [Google Scholar] [CrossRef]
- Gonçalves, M.A.F.; da Silva, B.R.S.; Nobre, J.R.C.; Batista, B.L.; da Silva Lobato, A.K. Biochar mitigates the harmful effects of drought in Soybean through changes in leaf development, stomatal regulation, and gas exchange. J. Soil Sci. Plant Nutr. 2024, 24, 1940–1951. [Google Scholar] [CrossRef]
- Safari, S.; Nazari, F.; Vafaee, Y.; Teixeira da Silva, J.A. Impact of rice husk biochar on drought stress tolerance in perennial Ryegrass (Lolium perenne L.). J. Plant Growth Regul. 2023, 42, 810–826. [Google Scholar] [CrossRef]
- Afaf, A.; ALosaimi, J.S.; Alharby, H.F.; Alayafi, A.A. The importance of initial application of biochar on soil fertility to improve growth and productivity of Tomato plants (Solanum lycopersicum L.) under drought stress. Gesunde Pflanz. 2023, 75, 2515–2524. [Google Scholar] [CrossRef]
- Roy, R.; Núñez-Delgado, A.; Wang, J.; Kader, M.A.; Sarker, T.; Hasan, A.K.; Dindaroglu, T. Cattle manure compost and biochar supplementation improve growth of Onobrychis viciifolia in coal-mined spoils under water stress conditions. Environ. Res. 2022, 205, 112440. [Google Scholar] [CrossRef]
- Lalarukh, I.; Amjad, S.F.; Mansoora, N.; Al-Dhumri, S.A.; Alshahri, A.H.; Almutari, M.M.; Alhusayni, F.S.; Al-Shammari, W.B.; Poczai, P.; Abbas, M. Interactive effects of brassinosteroids and timber waste biochar enhances the drought tolerance capacity of Wheat plant. Sci. Rep. 2022, 12, 12842. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Souri, M.K.; Mousavi, A.; Sahebani, N. Biochar and vermicompost improve growth and physiological traits of Eggplant (Solanum melongena L.) under deficit irrigation. Chem. Biol. Technol. Agric. 2021, 8, 19. [Google Scholar] [CrossRef]
- Zaheer, M.S.; Ali, H.H.; Soufan, W.; Iqbal, R.; Habib-ur-Rahman, M.; Iqbal, J.; Israr, M.; El Sabagh, A. Potential effects of biochar application for improving Wheat (Triticum aestivum L.) growth and soil biochemical properties under drought stress conditions. Land 2021, 10, 1125. [Google Scholar] [CrossRef]
- Hoang, T.-L.-H.; Jang, D.-C.; Nguyen, Q.-T.; Na, W.-H.; Kim, I.-S.; Vu, N.-T. Biochar-improved growth and physiology of Ehretia asperula under water-deficit condition. Appl. Sci. 2021, 11, 10685. [Google Scholar] [CrossRef]
- Yang, A.; Akhtar, S.S.; Li, L.; Fu, Q.; Li, Q.; Naeem, M.A.; He, X.; Zhang, Z.; Jacobsen, S.-E. Biochar mitigates combined effects of drought and salinity stress in Quinoa. Agronomy 2020, 10, 912. [Google Scholar] [CrossRef]
- Gavili, E.; Moosavi, A.A.; Haghighi, A.A.K. Does biochar mitigate the adverse effects of drought on the agronomic traits and yield components of Soybean? Ind. Crops Prod. 2019, 128, 445–454. [Google Scholar] [CrossRef]
- Hashem, A.; Kumar, A.; Al-Dbass, A.M.; Alqarawi, A.A.; Al-Arjani, A.-B.F.; Singh, G.; Farooq, M.; Abd_Allah, E.F. Arbuscular mycorrhizal fungi and biochar improves drought tolerance in Chickpea. Saudi J. Biol. Sci. 2019, 26, 614–624. [Google Scholar] [CrossRef]
- Tanure, M.M.C.; da Costa, L.M.; Huiz, H.A.; Fernandes, R.B.A.; Cecon, P.R.; Junior, J.D.P.; da Luz, J.M.R. Soil water retention, physiological characteristics, and growth of Maize plants in response to biochar application to soil. Soil Tillage Res. 2019, 192, 164–173. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Abbas, T.; Adrees, M.; Zia-ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Qayyum, M.F.; Nawaz, R. Residual effects of biochar on growth, photosynthesis and cadmium uptake in Rice (Oryza sativa L.) under Cd stress with different water conditions. J. Environ. Manag. 2018, 206, 676–683. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Mahmood, A.; Zia-ur-Rehman, M.; Ibrahim, M.; Arshad, M.; Qayyum, M.F. Biochar application increased the growth and yield and reduced cadmium in drought stressed Wheat grown in an aged contaminated soil. Ecotoxicol. Environ. Saf. 2018, 148, 825–833. [Google Scholar] [CrossRef]
- Paneque, M.; José, M.; Franco-Navarro, J.D.; Colmenero-Flores, J.M.; Knicker, H. Effect of biochar amendment on morphology, productivity and water relations of Sunflower plants under non-irrigation conditions. Catena 2016, 147, 280–287. [Google Scholar] [CrossRef]
- Haider, G.; Koyro, H.-W.; Azam, F.; Steffens, D.; Müller, C.; Kammann, C. Biochar but not humic acid product amendment affected Maize yields via improving plant-soil moisture relations. Plant Soil 2015, 395, 141–157. [Google Scholar] [CrossRef]
- Batool, A.; Taj, S.; Rashid, A.; Khalid, A.; Qadeer, S.; Saleem, A.R.; Ghufran, M.A. Potential of soil amendments (biochar and gypsum) in increasing water use efficiency of Abelmoschus esculentus L. Moench. Fron. Plant Sci. 2015, 6, 733. [Google Scholar] [CrossRef]
- Danish, S.D.; Adeel Ameer, A.A.; Qureshi, T.; Uzma Younis, U.Y.; Hina Manzoor, H.M.; Amal Shakeel, A.S.; Muhammad Ehsanullah, M.E. Influence of biochar on growth and photosynthetic attributes of Triticum aestivum L. under half and full irrigation. Int. J. Biosci. 2014, 5, 101–108. [Google Scholar] [CrossRef]
- Ghorbani, M.; Konvalina, P.; Neugschwandtner, R.W.; Soja, G.; Bárta, J.; Chen, W.-H.; Amirahmadi, E. How do different feedstocks and pyrolysis conditions effectively change biochar modification scenarios? A critical analysis of engineered biochars under H2O2 oxidation. Energy Convers. Manag. 2024, 300, 117924. [Google Scholar] [CrossRef]
- Tourajzadeh, O.; Piri, H.; Naserin, A.; Chari, M. Investigation of the effect of biochar on the physical and chemical properties of soil under Quinoa cultivation under water and salinity stress conditions. Water Soil 2024, 38, 69–85. Available online: https://jsw.um.ac.ir/jufile?issue_pdf=5834&lang=en#page=70 (accessed on 15 July 2024).
- Tourajzadeh, O.; Piri, H.; Naserin, A.; mahdi Cahri, M. Effect of nano biochar addition and deficit irrigation on growth, physiology and water productivity of Quinoa plants under salinity conditions. Environ. Exp. Bot. 2024, 217, 105564. [Google Scholar] [CrossRef]
- Akram, M.Z.; Rivelli, A.R.; Libutti, A.; Liu, F.; Andreasen, C. Mitigation of drought stress for Quinoa (Chenopodium quinoa Willd.) varieties using woodchip biochar-amended soil. Plants 2024, 13, 2279. [Google Scholar] [CrossRef]
- Geerts, S.; Mamani, R.S.; Garcia, M.; Raes, D. Response of Quinoa (Chenopodium quinoa Willd.) to differential drought stress in the Bolivian Altiplano: Towards a deficit irrigation strategy within a water scarce region. In Proceedings of the 1st International Symposium on Land and Water Management for Sustainable Irrigated Agriculture, Adana, Turkey, 4–8 April 2006; pp. 1–9. [Google Scholar]
- Mohkami, A.; Yazdanpanah, N.; Saeidnejad, A. The Effect of vermicompost and biochar application on morphophysiological characteristics of Quinoa under drought stress conditions. Iran. J. Soil Water Res. 2022, 53, 129–140. [Google Scholar] [CrossRef]
- Alashti Rahimi, S.; Bahmanyar, M.; Ghajarsepanlu, M.; Sadegh Zade, F.; Mokhtassi, A. The effect of some organic and mineral amendments on soil macronutrient and micronutrient under Quinoa cultivation in stress status (water). J. Soil Manag. Sustain. Prod. 2021, 11, 25–48. [Google Scholar] [CrossRef]
| Feedstock | Pyrolysis T. (°C) | Plant Species | Application Rate | Water Stress Application Method | Effects on Plants | Ref. |
|---|---|---|---|---|---|---|
| Hazelnut shells (Corylus avellana L.) | 500 | Glycine max L. | 3 and 6% w/w | 75% and 50% of FC | Boosted growth and chlorophyll content | [137] |
| Maize (Zea mays L.) | 400 | Zea mays L. | 100 t/ha | 40% and 20% of FC | Increased K+ concentration, less Ca2+ efflux, and increased apoplastic pH in roots. | [136] |
| Acai seeds (Euterpe oleracea Mart.) | 700 | Glycine max L. | 2.5 to 10% w/w | With-held watering (8 days) | Increased biomass, including leaf and root DM, PN, WUE, PN/Ci, and gs. | [165] |
| Rice husk (Oryza sativa L.) | 500 | Lolium perenne L. | 5 and 10% w/w | 25% of FC | Enhanced root and shoot growth, leaf RWC and nutrient status, chlorophyll contents, and photosynthetic efficiency while reducing proline, H2O2, and MDA. | [166] |
| Natural wood | - | Solanum lycopersicum L. | 20 g/kg | 75% and 45% of FC | Enhanced morphological parameters, such as PH, LA, NB, FW, DW, and productivity. | [167] |
| Apple wood (Malus domestica (Suckow) Borkh.) | 400 | Onobrychis viciifolia L. | 0.8 to 4% w/w | 80 to 40% of FC | Enhanced leaf RWC and reduced MDA and H202 accumulation. | [168] |
| Timber waste | 390 | Triticum aestivum L. | 2% w/w | 75 and 35% of FC | Increased flavonoids, anthocyanin, phenolics, proteins, GB, APX, POD and SOD. | [169] |
| Date palm (Phoenix dactylifera L.) and pistachio (Pistacia vera L.) | 560 | Solanum melongena L. | 500 g/m2 | 100 to 50% of FC | Improved growth, yield, and WUE. | [170] |
| - | 700 | Triticum aestivum L. | 28 and 38 g/kg | Skip irrigation at tillering and grain formation | Higher mineral nutrient, Bray P, exchangeable K, soil C, N mineralization and respiration in the soil along with enhanced microbial activity. | [171] |
| Oak wood (Quercus robur L.) | 400 | Ehretia Asperula L. | 5 to 20 t/ha | With-held watering (10 days) | Increased PH, NL, FW and DW of roots, shoots, and leaves, chlorophyll content, and leaf RWC. Reduced relative ion leakage | [172] |
| Wheat straw (Triticum aestivum L.) | 550 | Glycine max L. | 5 and 10 g/kg | 80 to 25% of FC | Increased GY and improved WUE while reducing negative impact on productivity. | [151] |
| Corn straw (Zea mays L.) | 500 | Chenopodium quinoa Willd. | 5% w/w | DI and ARD by restoring water to 100% FC when consumed 90% of water | Improved water relations and growth and ameliorated plant water status. Creating a balance between chemical signal (leaf ABA) and hydraulic signal (Ψ). | [173] |
| Cattle manure | 600 | Glycine max L. | 1.25 to 5% w/w | 100 and 55% of FC | Improvement in plant growth and morphology increased gs. Higher rates (2.5, 5%) adversely affected the plant growth and production due to excessive salinity. | [174] |
| Woody branches of button mangrove (Conocarpus erectus L.) | 450 | Cicer arietinum L. | 3% w/w | 60% throughout the experiment and half exposed to 50% of FC for 6 weeks | Increased plant growth, PH, LA, enhanced chlorophyll content and carotenoids, increased stomatal pore aperture, gs, Pn, RWC, membrane stability index, and nutrient content. | [175] |
| Eucalyptus bark (Eucalyptus sp.) | 350 | Zea mays L. | 5 to 60 g/kg | With-held watering (4 days) | Raised water retention, and micro/macropore ratio while reducing BD, additionally, increased PH, plant nutritional status, and growth. However, raised water retention due to biochar could not overcome the drought problems in soil | [176] |
| Rice straw (Oryza sativa L.) | 450 | Oryza sativa L. | 3 and 5% w/w | 50% and 35% of FC | Increased DM production, chlorophyll contents, Pn, gs, WUE, E, reduction in H2O2 content, MDA and electrolyte leakage, enhanced enzymatic defense system. | [177] |
| Rice straw (Oryza sativa L.) | 450–550 | Triticum aestivum L. | 3 and 5% w/w | 70%, and 35% of FC | Increased plant growth and yield, chlorophyll contents, E, Pn, gs, WUE, increased antioxidant activity, decreased MDA, H2O2 and electrolyte leakage. | [178] |
| Pinewood (Pinus L.), sewage sludge, paper sludge, and grapevine wood (Vitis vinifera L.) | 550–620 | Helianthus annuus L. | Two experiments I: 15 t/ha II: 1.5 t/ha | - | Increased LA, PH, wider inflorescences, enhanced vegetative growth, seed production, and reduced gs with greater WUE. | [179] |
| Woodchip | 500–600 | Zea mays L. | 1.5 and 3% w/w | 60% and 25% of FC | Increased biomass, WUE, leaf RWC, and Ψπ, N use efficiency, photosynthesis because of stimulated electron transport rate of PSII. | [180] |
| Lantana stems (Lantana camara L.) | 450 | Abelmoschus esculentus L. | 1 and 3% w/w | 100 and 60% of FC | Improved WUE, gs, E, Pn, WUE, plant DM. | [181] |
| Cotton sticks (Gossypium arboretum L.) | 385 | Vitis vinifera L. | 1 and 2% w/w | 100% and 50% watering restoration | Increased PH, FW, DW, RL, chlorophyll, carotenoids and anthocyanin, and WHC. | [182] |
| Feedstock | Pyrolysis T. (°C) | Application Rate | Water Stress Application Stage | Water Stress Application Method | Effects on Plants | Ref. |
|---|---|---|---|---|---|---|
| Peanut hull residue (Arachis hypogaea L.) | 498 | 100 and 200 t/ha | After 27 days of sowing (flowering) | 60 and 20% of FC | Increased growth, LA, DT, leaf N, and WUE. | [36] |
| Maize cob (Zea mays L.) | 350 | 1% w/w | With-held watering every two weeks from the leaf development stage until maturity | 15–20% of FC | Improved plant growth, yield, physiological, chemical, and biochemical processes. Reduced anti-nutrients (phytate and polyphenols) and BD in soil, enhance bioavailability, and translocate essential nutrients from soil to plant. | [37] |
| Corn straw (Zea mays L.) | 500 | 5% w/w | Flowering | DI and ARD | ARD with biochar under salinity enhanced PH, SB, and grain. Balanced chemical signal (leaf ABA) and Ψ and increased iWUE. | [173] |
| Pistachio tree (Pistacia vera L.) | - | 20 t/ha | From emergence | 70, 100 and 130 mm of pan evaporation | Applying biochar + vermicompost enhanced chlorophyll, LAI, PH, PL, 1000 SW, and GY while decreasing proline content. | [188] |
| Rice straw (Oryza sativa L.) | 300 | 0.4, 0.8% w/w | 4 leaf stage and flowering | 30% of FC | Increased concentration of macro and micronutrients in soil. | [189] |
| Woodchip (Bw) and vineyard (Vitis vinifera L.) pruning (Bv) | - | 2% w/w | 12-leaf stage | 2 successive water stress cycles from FC to PWP | Bw increased plant growth, LA, FW and DW, main PL, NSP, and WUE. Reduced leaf Ψπ and TW:DW. Bv negatively affects the plant growth | [38] |
| Woodchip | - | 2 and 4% w/w | From E to FI | Restoration 100% and 50% ET | Enhanced NL, LA, biomass, DM, PL, and NSP. | [148] |
| Woodchip (Bw) and vineyard (Vitis vinifera L.) pruning (Bv) | - | I: 2% w/w Bw and Bv II: 2 and 4% Bw | I: at 12-leaf stage II: from E to FI | I: two successive water stress cycles from FC to PWP. II: restoring 100% and 50% ET | I: Bw enhanced biomass, DM, PL, NSP. II: 2% Bw modified soil pH, and EC and reduced BD. | [32] |
| Northern forest tree | 300 | 2, 4% w/w | From 8-leaf stage | 100, 80, 60% of FC | Increased P, K, N pH and EC of soil, while reducing the actual specific gravity and the apparent specific gravity of soil. | [184] |
| Dried forest leaves | - | 2, 4% w/w | After plant establishment | 100, 80, 60% of FC | Increased growth, PW, 1000 SW, and LAI. | [185] |
| Woodchip | - | 2% w/w | At 12 leaf stage | 2 successive water stress cycles from WHC to PWP | Increased root growth and development, plant growth and yield contributing traits | [186] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akram, M.Z.; Libutti, A.; Rivelli, A.R. Drought Stress in Quinoa: Effects, Responsive Mechanisms, and Management through Biochar Amended Soil: A Review. Agriculture 2024, 14, 1418. https://doi.org/10.3390/agriculture14081418
Akram MZ, Libutti A, Rivelli AR. Drought Stress in Quinoa: Effects, Responsive Mechanisms, and Management through Biochar Amended Soil: A Review. Agriculture. 2024; 14(8):1418. https://doi.org/10.3390/agriculture14081418
Chicago/Turabian StyleAkram, Muhammad Zubair, Angela Libutti, and Anna Rita Rivelli. 2024. "Drought Stress in Quinoa: Effects, Responsive Mechanisms, and Management through Biochar Amended Soil: A Review" Agriculture 14, no. 8: 1418. https://doi.org/10.3390/agriculture14081418
APA StyleAkram, M. Z., Libutti, A., & Rivelli, A. R. (2024). Drought Stress in Quinoa: Effects, Responsive Mechanisms, and Management through Biochar Amended Soil: A Review. Agriculture, 14(8), 1418. https://doi.org/10.3390/agriculture14081418








