Impact of Fertilization and Seasonal Changes on Paddy Soil: Unveiling the Interplay between Agricultural Practices, Enzyme Activity, and Gene Diversity
Abstract
:1. Introduction
- (1)
- How do agricultural management and seasonal change influence soil enzyme activity, genetic richness, and diversity, and which factors exert a more significant impact?
- (2)
- Does the genetic richness and diversity within the soil lead to changes in enzyme activity?
2. Materials and Methods
2.1. Field Site and Soil Properties
2.2. Soil Enzyme Activity
2.3. Biolog EcoPlate™ Analysis
2.4. Soil Metagenomic Analysis
2.5. Statistical Analysis
2.6. Nucleotide Sequence Accession Numbers and Data Availability
3. Results
3.1. Soil Physicochemical Properties
3.2. Correlation among Soil Enzyme Activity, Soil Physicochemical Properties and Agricultural Management
3.3. Correlation among Soil Carbon Utilization, Soil Physicochemical Properties and Agricultural Management
3.4. Soil Metagenomic Analysis
3.5. Soil Gene Annotation According to CAZy, COG and GO Databases
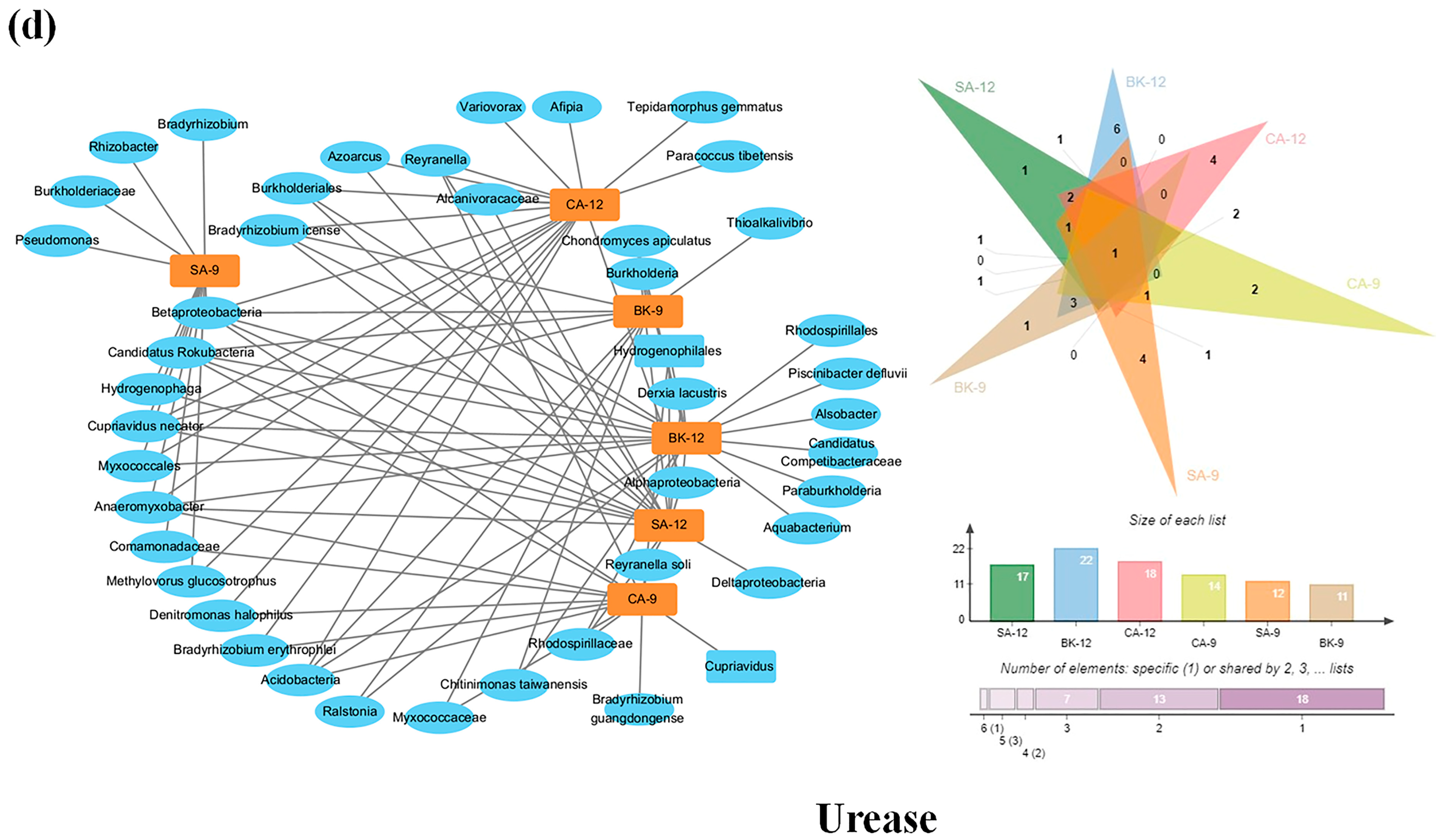
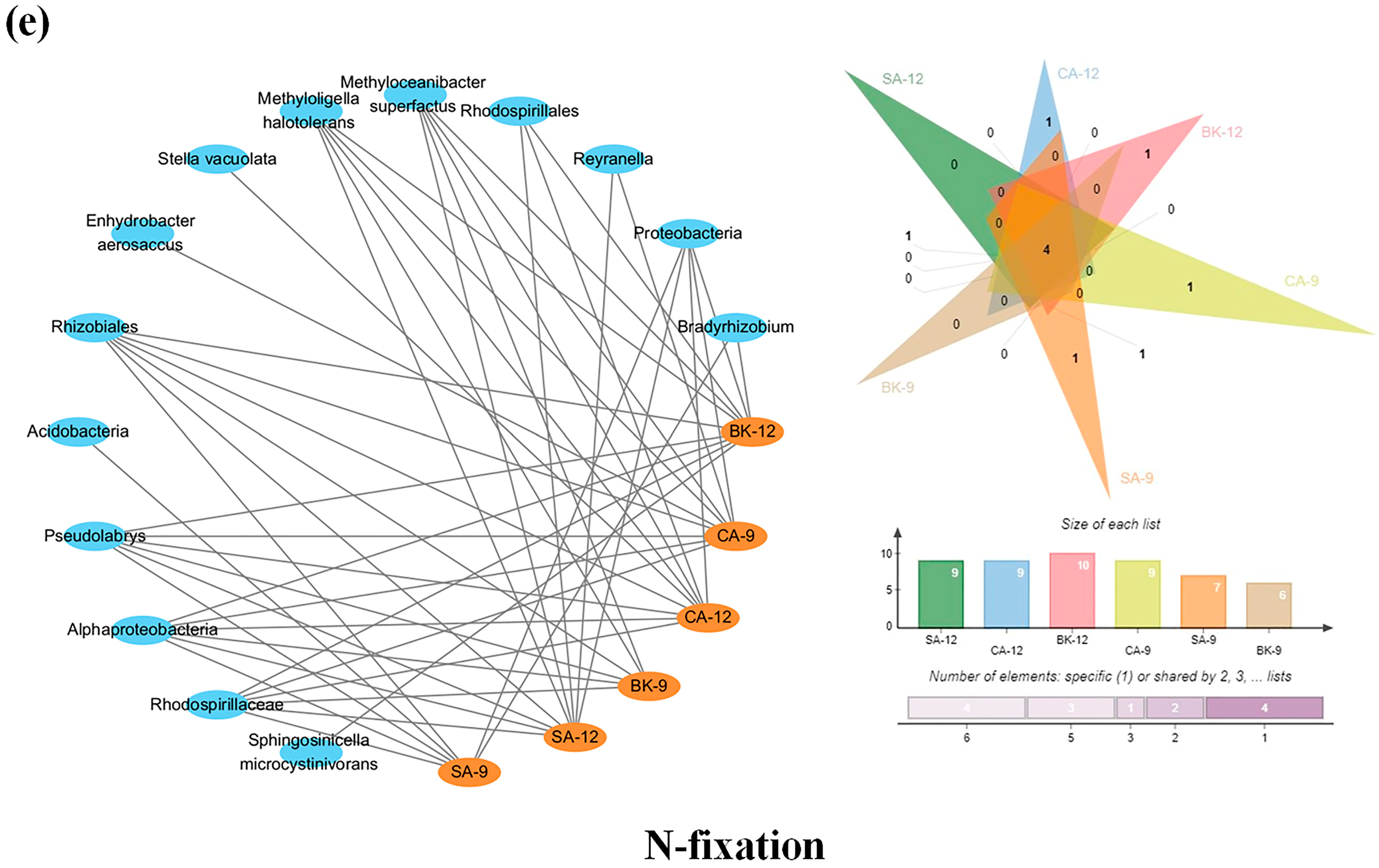
3.6. Soil Enzymes Sourced from Bacterial Diversity and Abundance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Porter, J.R.; Challinor, A.J.; Henriksen, C.B.; Howden, S.M.; Martre, P.; Smith, P. Invited review: Intergovernmental panel on climate change, agriculture, and food-A case of shifting cultivation and history. Glob. Change Biol. 2019, 25, 2518–2529. [Google Scholar] [CrossRef]
- Xu, D.; Ros, G.H.; Zhu, Q.; Xu, M.; Wen, S.; Cai, Z.; Zhang, F.; de Vries, W. Major drivers of soil acidification over 30 years differ in paddy and upland soils in China. Sci. Total Environ. 2024, 916, 170189. [Google Scholar] [CrossRef]
- Jimma, T.B.; Chemura, A.; Spillane, C.; Demissie, T.; Abera, W.; Ture, K.; Terefe, T.; Solomon, D.; Gleixner, S. Coupled impacts of soil acidification and climate change on future crop suitability in Ethiopia. Sustainability 2024, 16, 1468. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, U.B.; Sahu, P.K.; Paul, S.; Kumar, A.; Malviya, D.; Singh, S.; Kuppusamy, P.; Singh, P.; Paul, D.; et al. Linking soil microbial diversity to modern agriculture practices: A review. Int. J. Environ. Res. Public Health 2022, 19, 3141. [Google Scholar] [CrossRef]
- Nie, X.; Duan, X.; Zhang, M.; Zhang, Z.; Liu, D.; Zhang, F.; Wu, M.; Fan, X.; Yang, L.; Xia, X. Cadmium accumulation, availability, and rice uptake in soils receiving long-term applications of chemical fertilizers and crop straw return. Environ. Sci. Pollut. Res. 2019, 26, 31243–31253. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tang, H.; Liu, T.; Li, Y.; Huang, X.; Pi, J. Effects of long-term fertilization practices on heavy metal cadmium accumulation in the surface soil and rice plants of double-cropping rice system in Southern China. Environ. Sci. Pollut. Res. 2018, 25, 19836–19844. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, G.; Cui, N.; Liu, E.; Zhang, Y.; Liu, D.; Ren, X.; Jia, Z.; Zhang, P. Suitable fertilization can improve maize growth and nutrient utilization in ridge-furrow rainfall harvesting cropland in semiarid area. Front. Plant Sci. 2023, 14, 1198366. [Google Scholar] [CrossRef]
- Jin, X.; Cai, J.; Yang, S.; Li, S.; Shao, X.; Fu, C.; Li, C.; Deng, Y.; Huang, J.; Ruan, Y.; et al. Partial substitution of chemical fertilizer with organic fertilizer and slow-release fertilizer benefits soil microbial diversity and pineapple fruit yield in the tropics. Appl. Soil Ecol. 2023, 189, 104974. [Google Scholar] [CrossRef]
- Jiang, M.; Dong, C.; Bian, W.; Zhang, W.; Wang, Y. Effects of different fertilization practices on maize yield, soil nutrients, soil moisture, and water use efficiency in northern China based on a meta-analysis. Sci. Rep. 2024, 14, 6480. [Google Scholar] [CrossRef]
- Sun, L.; Wang, R.; Li, J.; Wang, Q.; Lyu, W.; Wang, X.; Cheng, K.; Mao, H.; Zhang, X. Reasonable fertilization improves the conservation tillage benefit for soil water use and yield of rain-fed winter wheat: A case study from the Loess Plateau, China. Field Crops Res. 2019, 242, 107589. [Google Scholar] [CrossRef]
- Hu, K.; Zhao, P.; Wu, K.; Yang, H.; Yang, Q.; Fan, M.; Long, G. Reduced and deep application of controlled-release urea maintained yield and improved nitrogen-use efficiency. Field Crops Res. 2023, 295, 108876. [Google Scholar] [CrossRef]
- Lian, Y.; Ali, S.; Zhang, X.; Wang, T.; Liu, Q.; Jia, Q.; Jia, Z.; Han, Q. Nutrient and tillage strategies to increase grain yield and water use efficiency in semi-arid areas. Agri. Water Manag. 2016, 178, 137–147. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, C.; Liu, M.; Yuan, L. Long-term application of manure reduced nutrient leaching under heavy N deposition. Nutr. Cycl. Agroecosyst. 2021, 119, 153–162. [Google Scholar] [CrossRef]
- Chen, J.; Sinsabaugh, R.L. Linking microbial functional gene abundance and soil extracellular enzyme activity: Implications for soil carbon dynamics. Glob. Change Biol. 2021, 27, 1322–1325. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, Y.; Chen, Y.; Li, Y.; Lin, Y.; Wu, J.; Cai, Y.; Chang, S.X. Enhanced soil potential N2O emissions by land-use change are linked to AOB-amoA and nirK gene abundances and denitrifying enzyme activity in subtropics. Sci. Total Environ. 2022, 850, 158032. [Google Scholar] [CrossRef]
- Lin, S.; Liu, Z.; Wang, Y.; Li, J.; Wang, G.; Ye, J.; Wang, H.; He, H. Soil metagenomic analysis on changes of functional genes and microorganisms involved in nitrogen-cycle processes of acidified tea soils. Front. Plant Sci. 2022, 13, 998178. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, K.; Wu, Y.; Wang, G. Abundance and structure composition of nirK and nosZ genes as well as denitrifying activity in heavy metal-polluted paddy soils. Geomicrobiol. J. 2018, 35, 100–107. [Google Scholar] [CrossRef]
- Wei, X.; Hu, Y.; Peng, P.; Zhu, Z.; Atere, C.T.; O’Donnell, A.G.; Wu, J.; Ge, T. Effect of P stoichiometry on the abundance of nitrogen-cycle genes in phosphorus-limited paddy soil. Biol. Fert. Soils 2017, 53, 767–776. [Google Scholar] [CrossRef]
- Ho, I. Acid phosphatase activity in forest soil. Forest Sci. 1979, 25, 567–568. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Arylsulfatase activity of soils. Soil Sci. Soc. Am. J. 1970, 34, 225–229. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Hardy, R.W.F.; Burns, R.C.; Holsten, R.D. Application of acetylene reduction assay for measurement of nitrogen fixation. Soil Biol. Biochem. 1973, 5, 47–81. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Wood, D.E.; Salzberg, S.L. Kraken: Ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014, 15, R46. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, C.; Xu, C.; Zhu, Q.; Huang, D. Effects of soil acidification and liming on the phytoavailability of cadmium in paddy soils of central subtropical China. Environ. Pollut. 2016, 219, 99–106. [Google Scholar] [CrossRef]
- He, X.; Lu, H.-l.; Wu, C.-m.; Xu, R.-k. Effects of inorganic alkalis and organic anions in biochars on acidic paddy soil resistance to acidification. J. Soils Sediments 2022, 22, 1201–1213. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The biological activities of β-glucosidase, phosphatase and urease as soil quality indicators: A review. J. Soil Sci. Plant Nut. 2017, 17, 794–807. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Ncube, B.; Meyer, A.H.; Olatunji, O.S.; Mulidzi, R.; Lewu, F.B. Soil pH, nitrogen, phosphatase and urease activities in response to cover crop species, termination stage and termination method. Heliyon 2021, 7, e05980. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-P.; Tsai, C.-F.; Rekha, P.D.; Ghate, S.D.; Huang, H.-Y.; Hsu, Y.-H.; Liaw, L.-L.; Young, C.-C. Agricultural management practices influence the soil enzyme activity and bacterial community structure in tea plantations. Bot. Stud. 2021, 62, 8. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Tsai, C.-F.; Hameed, A.; Chang, Y.-J.; Young, C.-C. Agricultural management and cultivation period alter soil enzymatic activity and bacterial diversity in litchi (Litchi chinensis Sonn.) orchards. Bot. Stud. 2021, 62, 13. [Google Scholar] [CrossRef]
- Zhu, J.; Qu, B.; Li, M. Phosphorus mobilization in the Yeyahu Wetland: Phosphatase enzyme activities and organic phosphorus fractions in the rhizosphere soils. Int. Biodeter. Biodegr. 2017, 124, 304–313. [Google Scholar] [CrossRef]
- Puissant, J.; Jassey, V.E.J.; Mills, R.T.E.; Robroek, B.J.M.; Gavazov, K.; De Danieli, S.; Spiegelberger, T.; Griffiths, R.; Buttler, A.; Brun, J.-J.; et al. Seasonality alters drivers of soil enzyme activity in subalpine grassland soil undergoing climate change. Soil Biol. Biochem. 2018, 124, 266–274. [Google Scholar] [CrossRef]
- Zhen, T.; Fan, W.; Wang, H.; Cao, X.; Xu, X. Monitoring soil microorganisms with community-level physiological profiles using biolog EcoPlates™ in Chaohu lakeside wetland, east China. Eurasian Soil Sci. 2020, 53, 1142–1153. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; He, J.S.; Feng, X. Iron-mediated soil carbon response to water-table decline in an alpine wetland. Nat. Commun. 2017, 8, 15972. [Google Scholar] [CrossRef]
- Li, R.; Luo, H.; Luo, L.; He, Y.; Deng, S.; Huang, C.; Deng, O.; Tang, X. Iron additions accelerate carbon loss from drained soil but promote carbon accumulation in waterlogged soil of Zoige plateau peatland. Int. Biodeter. Biodegr. 2024, 190, 105782. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Hu, X.; Hu, B.; Chu, G.; Tao, R. Three decades of organic manure and chemical fertilizers co-application enhanced rice productivity through increasing the diversity and key network module of soil bacterial community. J. Soils Sediments 2023, 23, 2175–2188. [Google Scholar] [CrossRef]
- Ding, L.-J.; Su, J.-Q.; Sun, G.-X.; Wu, J.-S.; Wei, W.-X. Increased microbial functional diversity under long-term organic and integrated fertilization in a paddy soil. Appl. Microbiol. Biot. 2018, 102, 1969–1982. [Google Scholar] [CrossRef]
- Daraz, U.; Erhunmwunse, A.S.; Dubeux, J.C.B.; Mackowiak, C.; Guerra, V.A.; Hsu, C.-M.; Ma, J.; Li, Y.; Yang, X.; Liao, H.-L.; et al. Soil bacterial communities across seven rhizoma peanut cultivars (Arachis glabrata Benth.) respond to seasonal variation. Microb. Ecol. 2023, 86, 2703–2715. [Google Scholar] [CrossRef]
- Wang, M.; Sun, M.; Zhao, Y.; Shi, Y.; Sun, S.; Wang, S.; Zhou, Y.; Chen, L. Seasonal changes of soil microbiota and its association with environmental factors in coal mining subsidence area. AMB Express 2023, 13, 147. [Google Scholar] [CrossRef]
- Jiang, D.M.; Zhao, L.; Zhang, C.Y.; Li, J.; Xia, Z.J.; Wang, J.; Wu, Z.H.; Li, Y.Z. Taxonomic analysis of Sorangium strains based on HSP60 and 16S rRNA gene sequences and morphology. Int. J. Syst. Evol. Microbiol. 2008, 58, 2654–2659. [Google Scholar] [CrossRef]
- Yuan, S.F.; Yue, X.J.; Hu, W.F.; Wang, Y.; Li, Y.Z. Genome-wide analysis of lipolytic enzymes and characterization of a high-tolerant carboxylesterase from Sorangium cellulosum. Front. Microbiol. 2023, 14, 1304233. [Google Scholar] [CrossRef]
- Wang, K.; Jia, R.; Li, L.; Jiang, R.; Qu, D. Community structure of Anaeromyxobacter in Fe(III) reducing enriched cultures of paddy soils. J. Soils Sediments 2020, 20, 1621–1631. [Google Scholar] [CrossRef]
- Itoh, H.; Xu, Z.; Mise, K.; Masuda, Y.; Ushijima, N.; Hayakawa, C.; Shiratori, Y.; Senoo, K. Anaeromyxobacter oryzae sp. nov., Anaeromyxobacter diazotrophicus sp. nov. and Anaeromyxobacter paludicola sp. nov., isolated from paddy soils. Int. J. Syst. Evol. Microbiol. 2022, 72, 005546. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Chen, H.; Yuan, R.; Wang, F.; Chen, Z.; Zhou, B. Metagenomic analysis of soil microbial community under PFOA and PFOS stress. Environ. Res. 2020, 188, 109838. [Google Scholar] [CrossRef] [PubMed]
- Frey, B.; Rast, B.M.; Qi, W.; Stierli, B.; Brunner, I. Long-term mercury contamination does not affect the microbial gene potential for C and N cycling in soils but enhances detoxification gene abundance. Front. Microbiol. 2022, 13, 1034138. [Google Scholar] [CrossRef]
- Bao, T.; Deng, S.; Yu, K.; Li, W.; Dong, A. Metagenomic insights into seasonal variations in the soil microbial community and function in a Larix gmelinii forest of Mohe, China. J. For. Res. 2021, 32, 371–383. [Google Scholar] [CrossRef]
- Xiao, H.; Yang, H.; Zhao, M.; Monaco, T.A.; Rong, Y.; Huang, D.; Song, Q.; Zhao, K.; Wang, D. Soil extracellular enzyme activities and the abundance of nitrogen-cycling functional genes responded more to N addition than P addition in an Inner Mongolian meadow steppe. Sci. Total Environ. 2021, 759, 143541. [Google Scholar] [CrossRef]

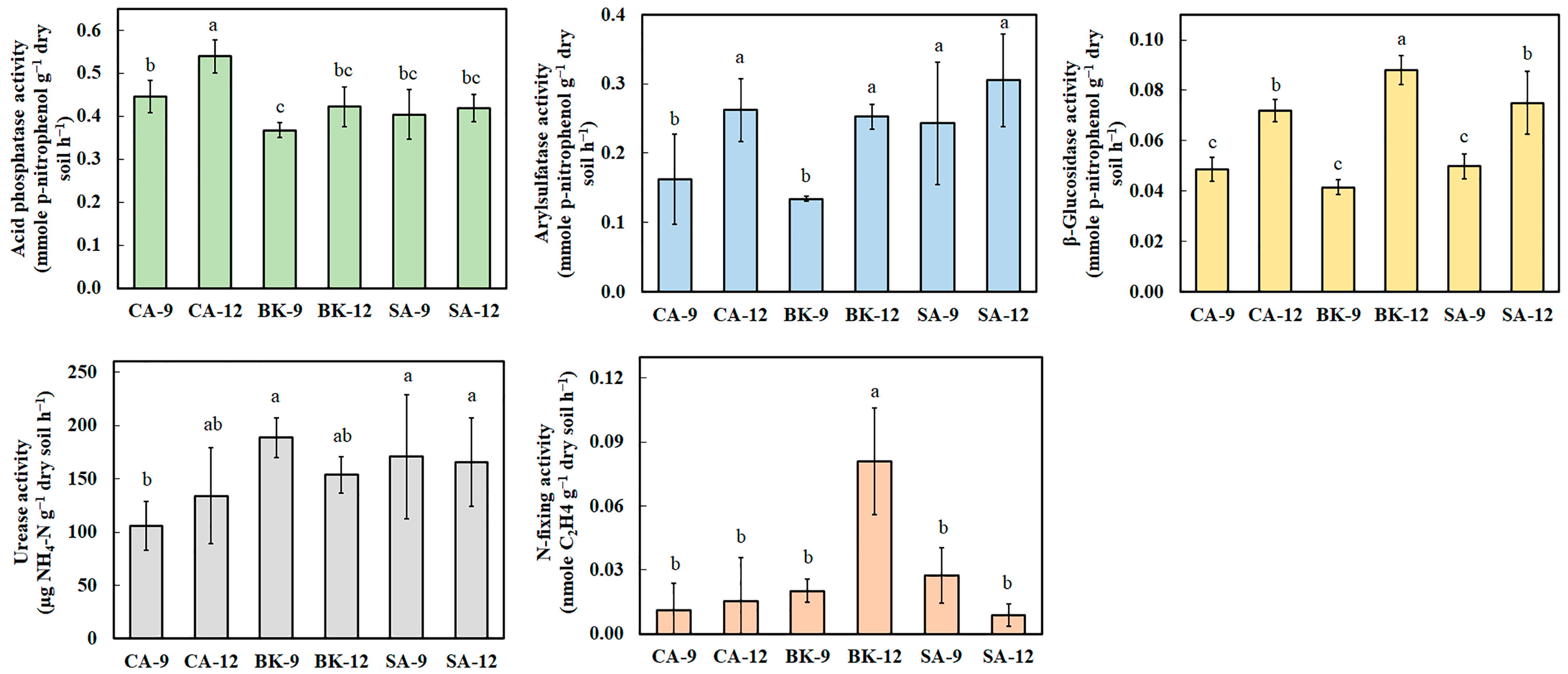

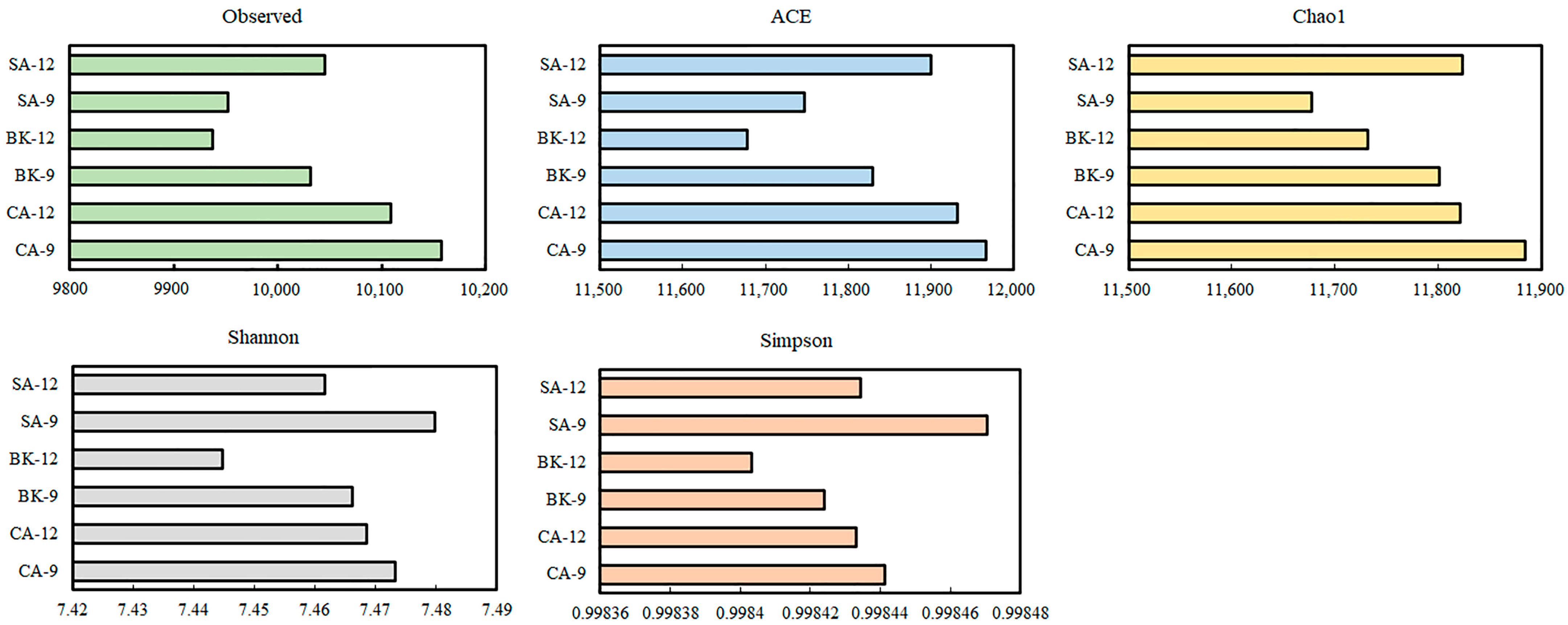
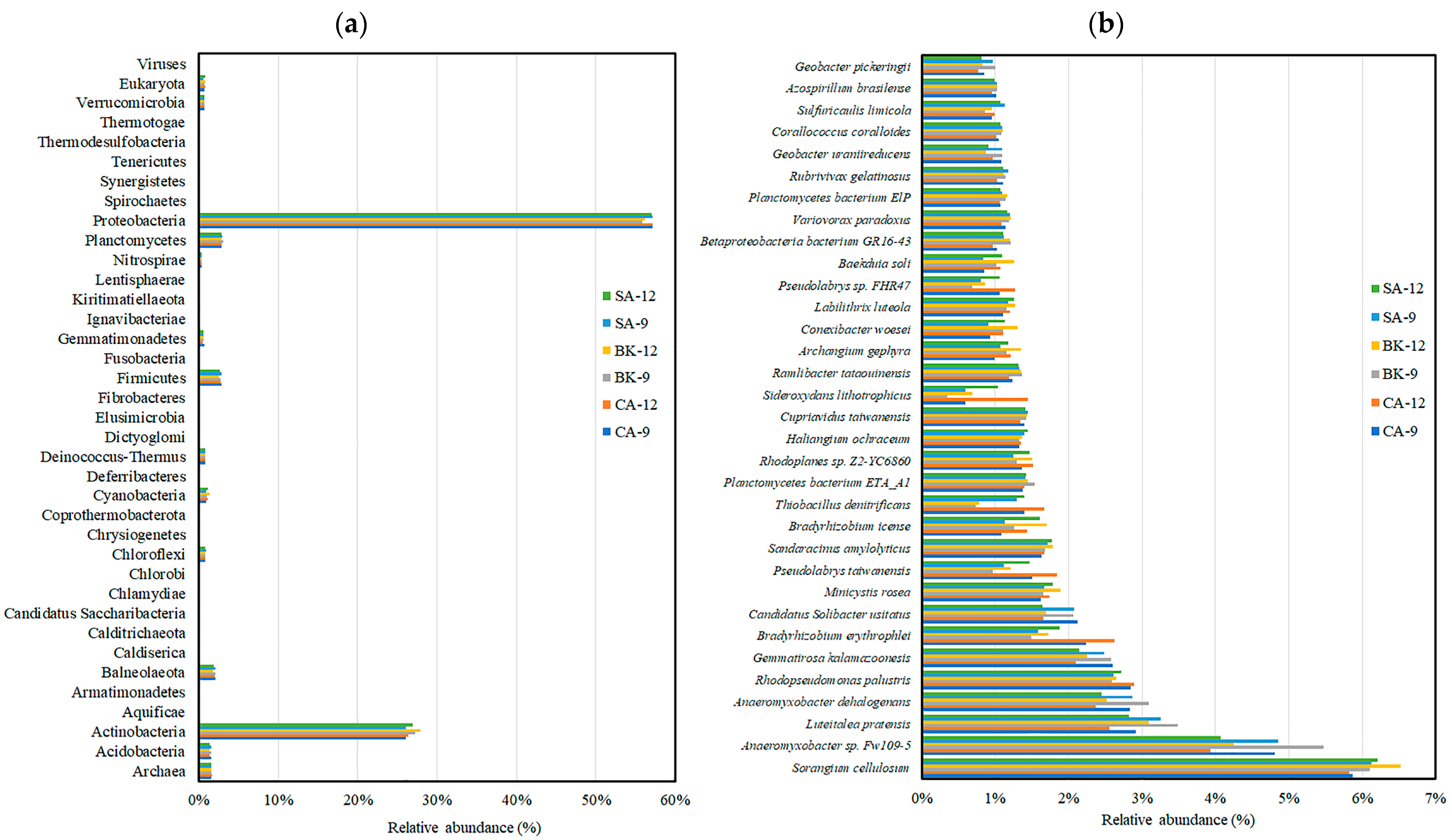
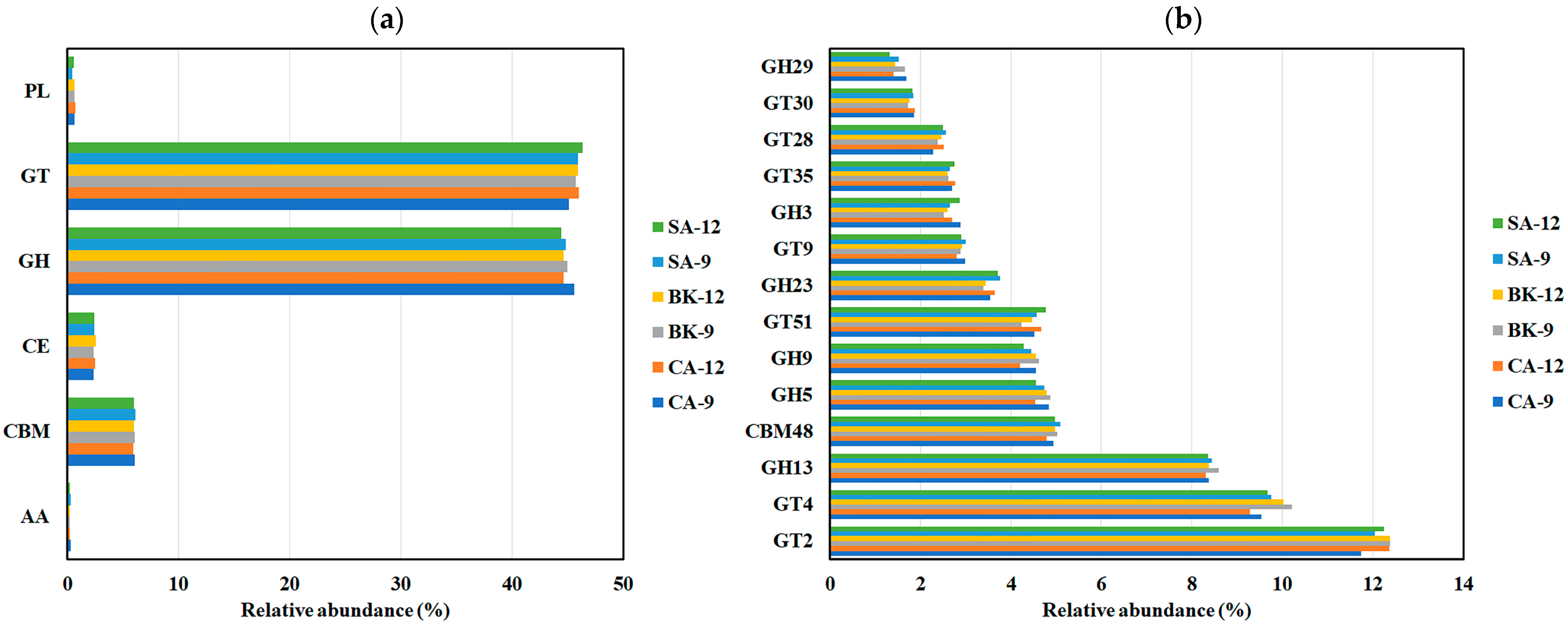
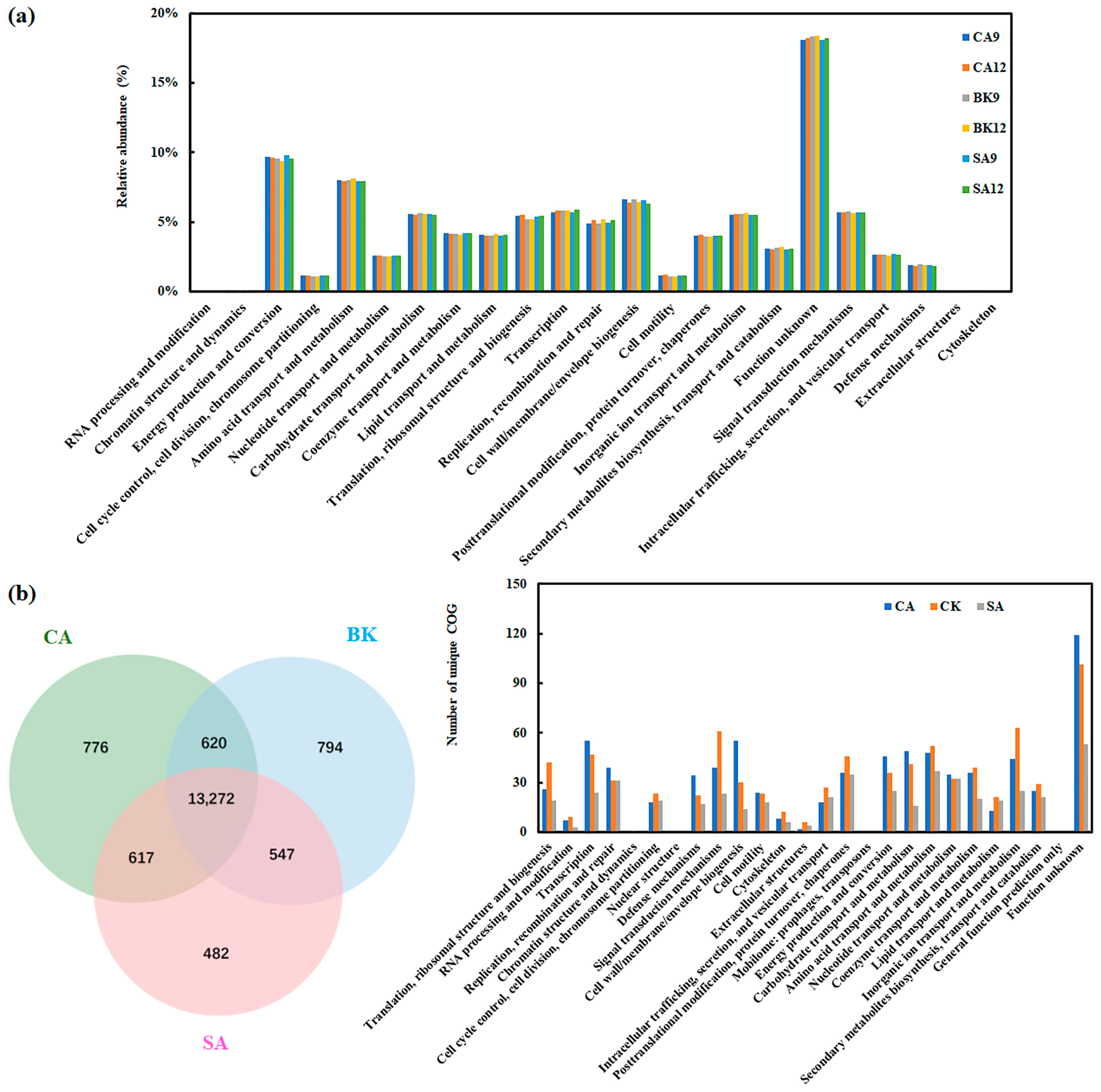
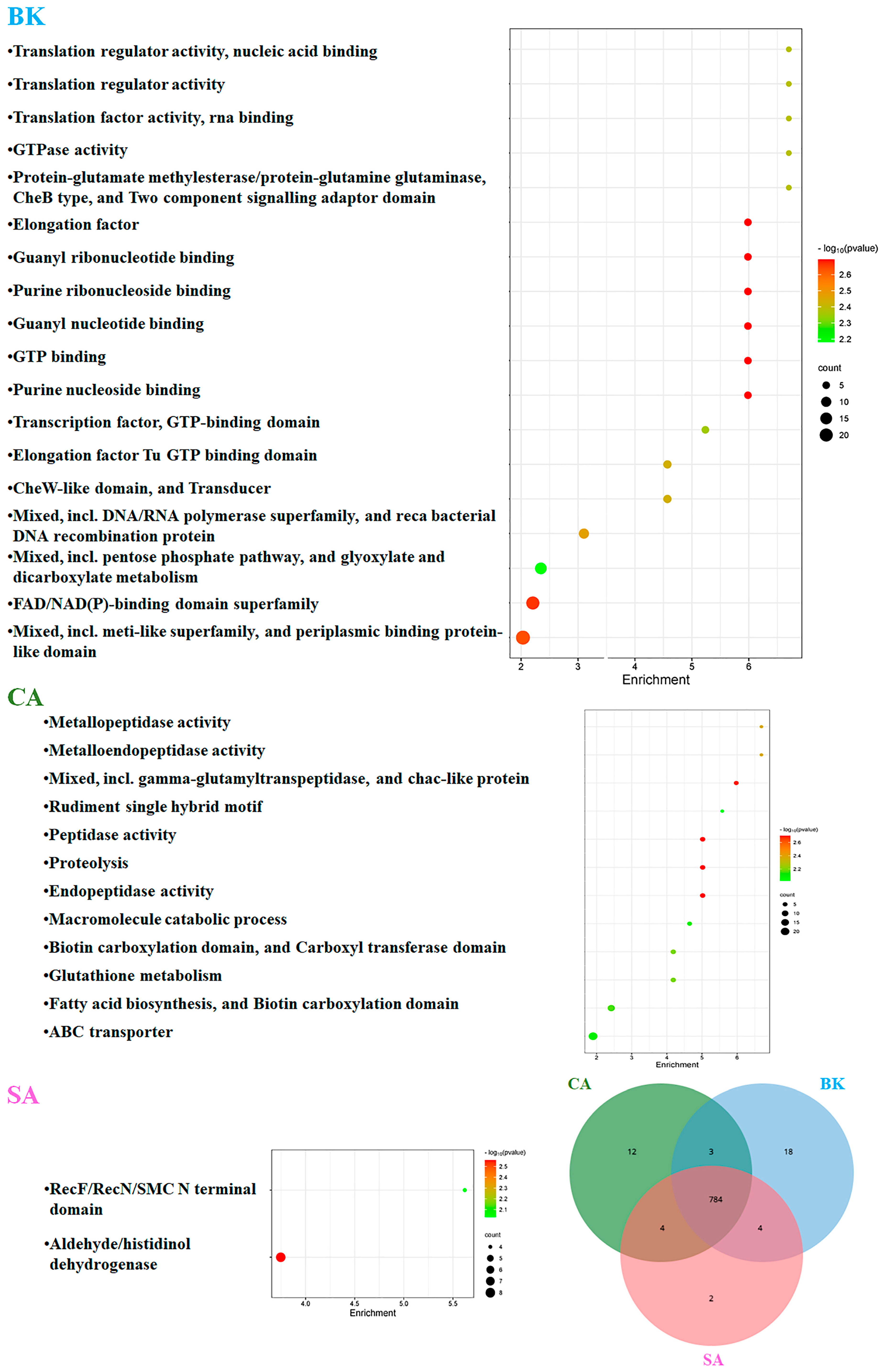
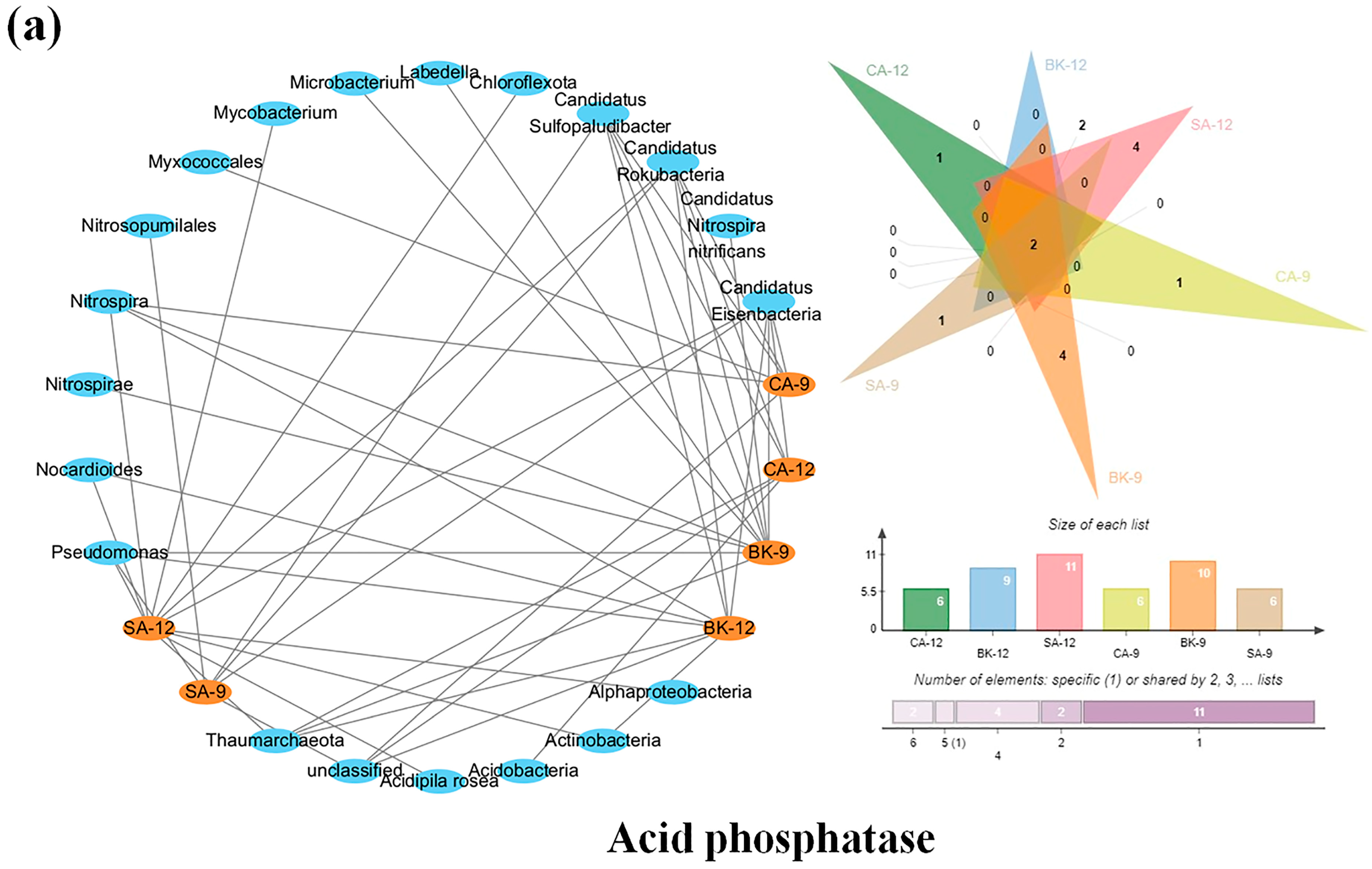

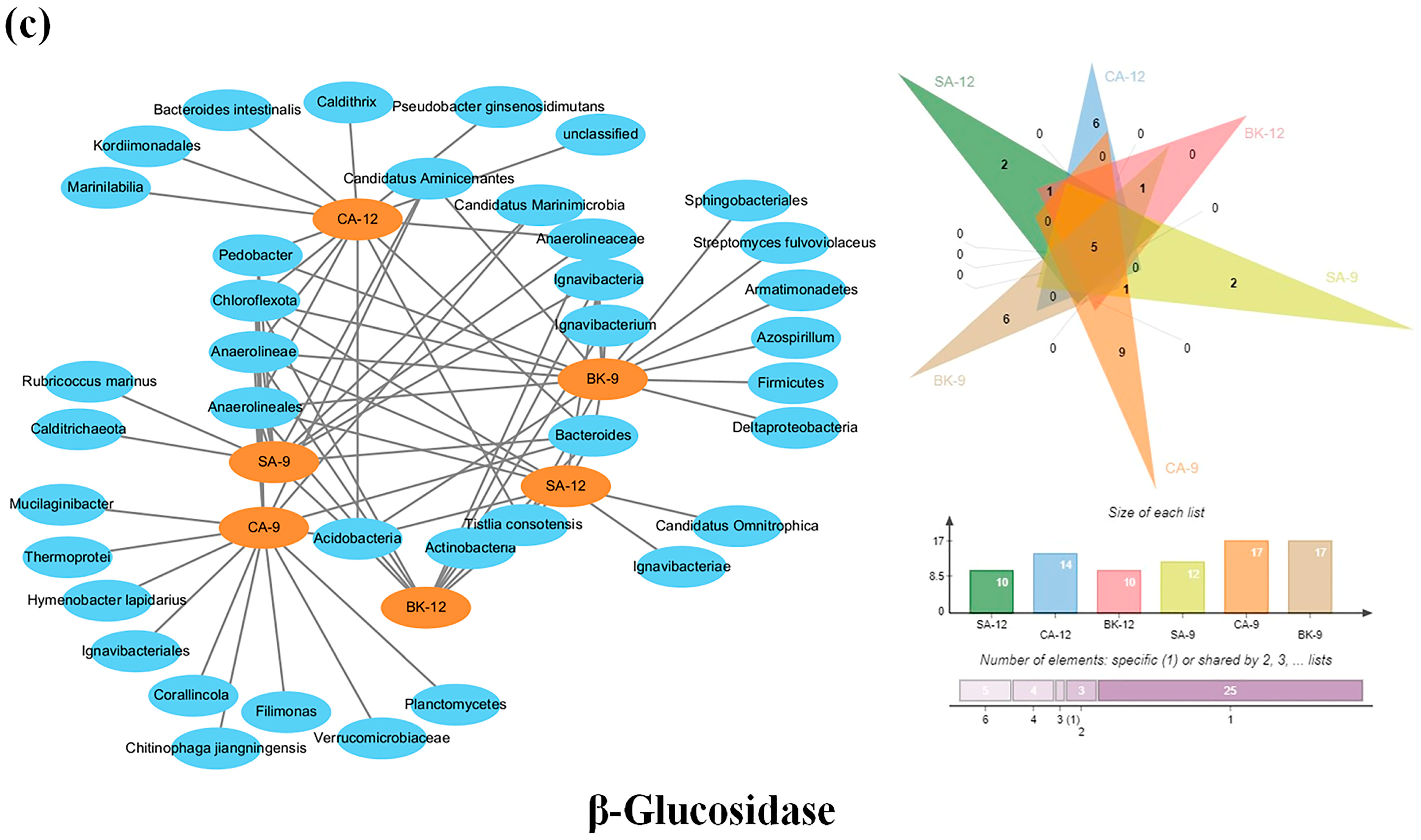

| Soils | P | K | Ca | Mg | Fe | Mn | Cu | Zn |
|---|---|---|---|---|---|---|---|---|
| mg/kg Soil | ||||||||
| CA-9 | 27.62 a | 86.35 a | 1626.32 a | 214.49 a | 1028.86 a | 131.85 a | 0.75 a | 1.14 c |
| CA-12 | 16.68 b | 59.25 b | 1601.28 a | 216.24 a | 852.72 b | 49.55 b | 0.65 a | 3.75 b |
| BK-9 | 14.34 b | 54.44 bc | 1492.46 a | 187.92 a | 1103.58 a | 137.40 a | 1.18 a | 1.31 c |
| BK-12 | 10.91 b | 45.60 c | 1560.92 a | 195.19 a | 909.85 b | 50.90 b | 1.05 a | 3.36 b |
| SA-9 | 12.16 b | 61.50 b | 1714.49 a | 262.53 a | 1074.20 a | 154.15 a | 0.93 a | 1.62 c |
| SA-12 | 6.75 b | 54.74 bc | 1784.36 a | 279.55 a | 865.28 b | 77.32 b | 0.94 a | 4.32 a |
| pH | EC | Total N | O.M. | P | K | Ca | Fe | |
|---|---|---|---|---|---|---|---|---|
| Acid phosphatase | −0.708 ** | 0.609 ** | 0.229 | 0.091 | 0.148 | 0.161 | 0.021 | −0.507 * |
| Arylsulfatase | −0.079 | 0.341 | 0.459 | 0.691 ** | −0.630 ** | −0.363 | 0.694 ** | −0.721 ** |
| β-Glucosidase | −0.004 | 0.060 | 0.079 | 0.273 | −0.453 | −0.484 * | 0.236 | −0.843 ** |
| Urease | 0.515 * | −0.390 | 0.249 | 0.105 | −0.640 ** | −0.534 * | 0.380 | 0.031 |
| N-fixation | 0.574 * | −0.362 | −0.179 | −0.029 | −0.179 | −0.488 * | −0.070 | −0.104 |
| Model Term | Enzymatic Activity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Acid Phosphatase | Arylsulfatase | β-Glucosidase | Urease | N-Fixation | ||||||
| F | p | F | p | F | p | F | p | F | p | |
| Test of within-subjects effects | ||||||||||
| Time | 8.145 | 0.011 * | 74.001 | <0.001 ** | 200.371 | <0.001 ** | 0.077 | 0.784 | 12.767 | 0.002 ** |
| Time × Management | 2.321 | 0.127 | 2.6 | 0.102 | 8.051 | 0.003 ** | 1.728 | 0.206 | 23.355 | <0.001 ** |
| Test of between-subjects effects | ||||||||||
| Intercept | 10,792.035 | <0.001 ** | 226.756 | <0.001 ** | 2014.698 | <0.001 ** | 387.223 | <0.001 ** | 92.288 | <0.001 ** |
| Management | 68.01 | <0.001 ** | 3.186 | 0.065 | 0.818 | 0.457 | 6.333 | 0.008 * | 12.326 | <0.001 ** |
| pH | EC | Total N | O.M. | P | K | Ca | Fe | |
|---|---|---|---|---|---|---|---|---|
| Richness | −0.206 | 0.044 | −0.097 | 0.115 | −0.443 | −0.548 * | 0.096 | −0.875 ** |
| AWCD | −0.352 | 0.191 | 0.008 | 0.140 | −0.399 | −0.438 | 0.163 | −0.910 ** |
| Shannon | −0.214 | 0.034 | −0.147 | 0.057 | −0.405 | −0.543 * | 0.047 | −0.863 ** |
| Model Term | Biolog | |||||
|---|---|---|---|---|---|---|
| Richness | AWCD | Shannon | ||||
| F | p | F | p | F | p | |
| Test of within-subjects effects | ||||||
| Time | 212.180 | <0.001 ** | 771.930 | <0.001 ** | 232.802 | <0.001 ** |
| Time × Management | 1.340 | 0.330 | 24.867 | 0.001 ** | 3.049 | 0.122 |
| Test of between-subjects effects | ||||||
| Intercept | 5163.559 | <0.001 ** | 2411.997 | <0.001 ** | 66,412.047 | <0.001 ** |
| Management | 0.206 | 0.819 | 1.883 | 0.232 | 0.632 | 0.564 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-P.; Huang, H.-Y.; Tsai, C.-F.; Young, C.-C. Impact of Fertilization and Seasonal Changes on Paddy Soil: Unveiling the Interplay between Agricultural Practices, Enzyme Activity, and Gene Diversity. Agriculture 2024, 14, 1424. https://doi.org/10.3390/agriculture14081424
Chen Y-P, Huang H-Y, Tsai C-F, Young C-C. Impact of Fertilization and Seasonal Changes on Paddy Soil: Unveiling the Interplay between Agricultural Practices, Enzyme Activity, and Gene Diversity. Agriculture. 2024; 14(8):1424. https://doi.org/10.3390/agriculture14081424
Chicago/Turabian StyleChen, Yu-Pei, Hsi-Yuan Huang, Chia-Fang Tsai, and Chiu-Chung Young. 2024. "Impact of Fertilization and Seasonal Changes on Paddy Soil: Unveiling the Interplay between Agricultural Practices, Enzyme Activity, and Gene Diversity" Agriculture 14, no. 8: 1424. https://doi.org/10.3390/agriculture14081424





