Soil Traits and Grapevine Rootstock Genotypes Modulate Arbuscular Mycorrhizal Rate and Species in a Mediterranean Environment
Abstract
:1. Introduction
2. Materials and Methods
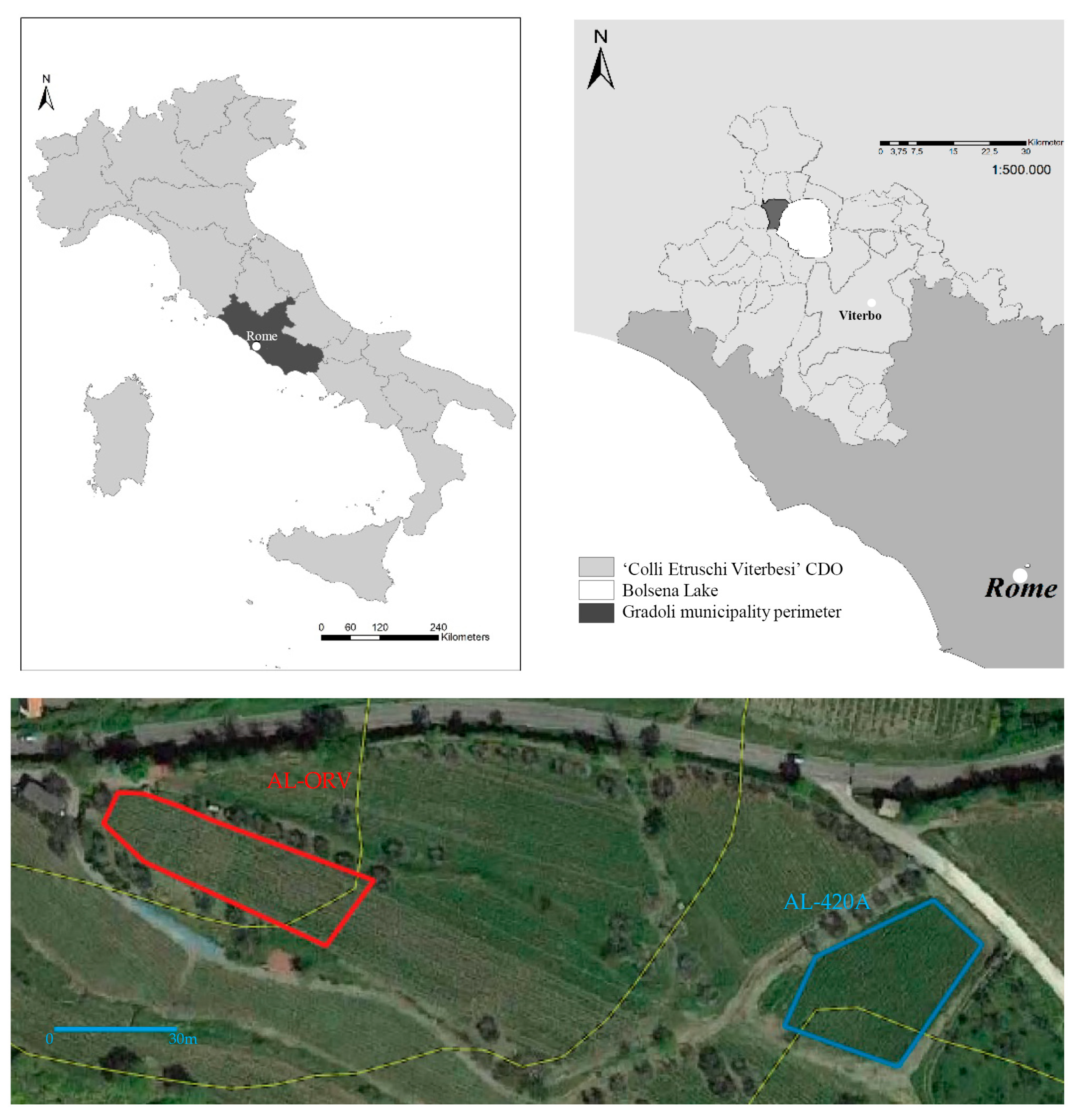
2.1. Study Site
2.2. Sampling Plan
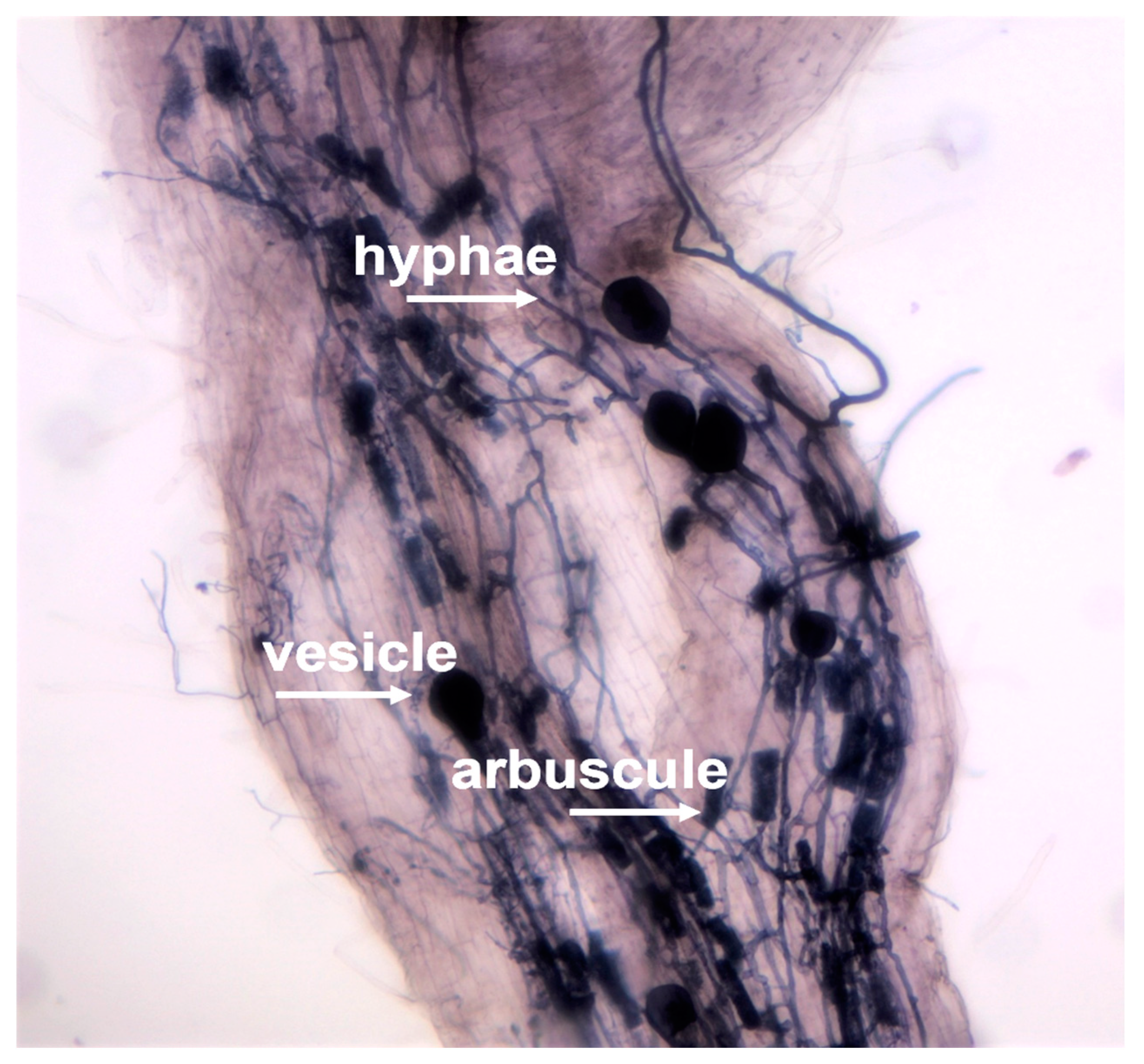
2.3. Isolation and Molecular Identification of AMFs from Grapevine Root System
2.4. Soil Physiochemical Characteristics and Quality Assessment
2.5. Data Statistical Analysis and Data Spatialization
3. Results
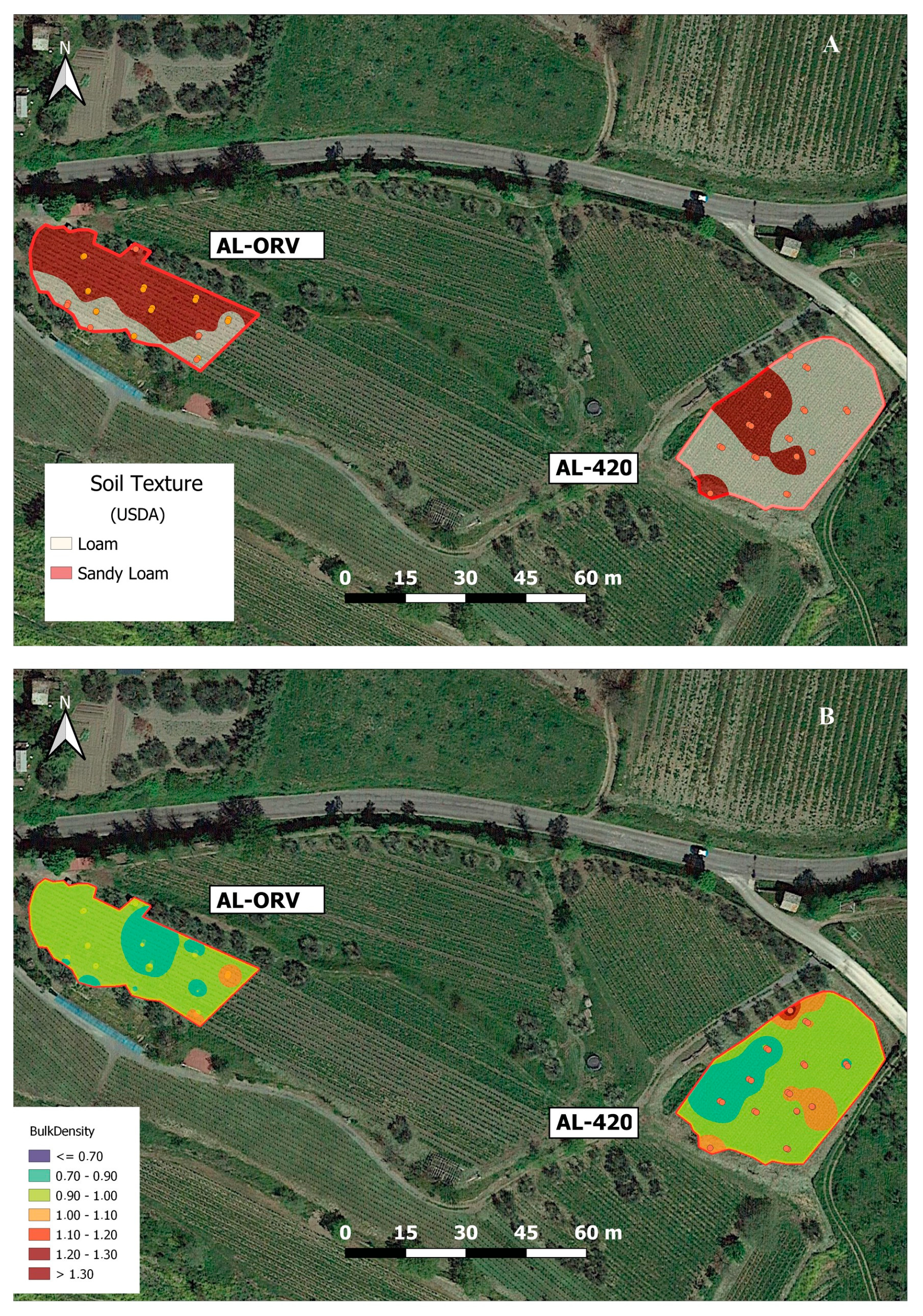
3.1. Vineyards’ Soil Traits and Data Spatialization
3.2. AMF Identification
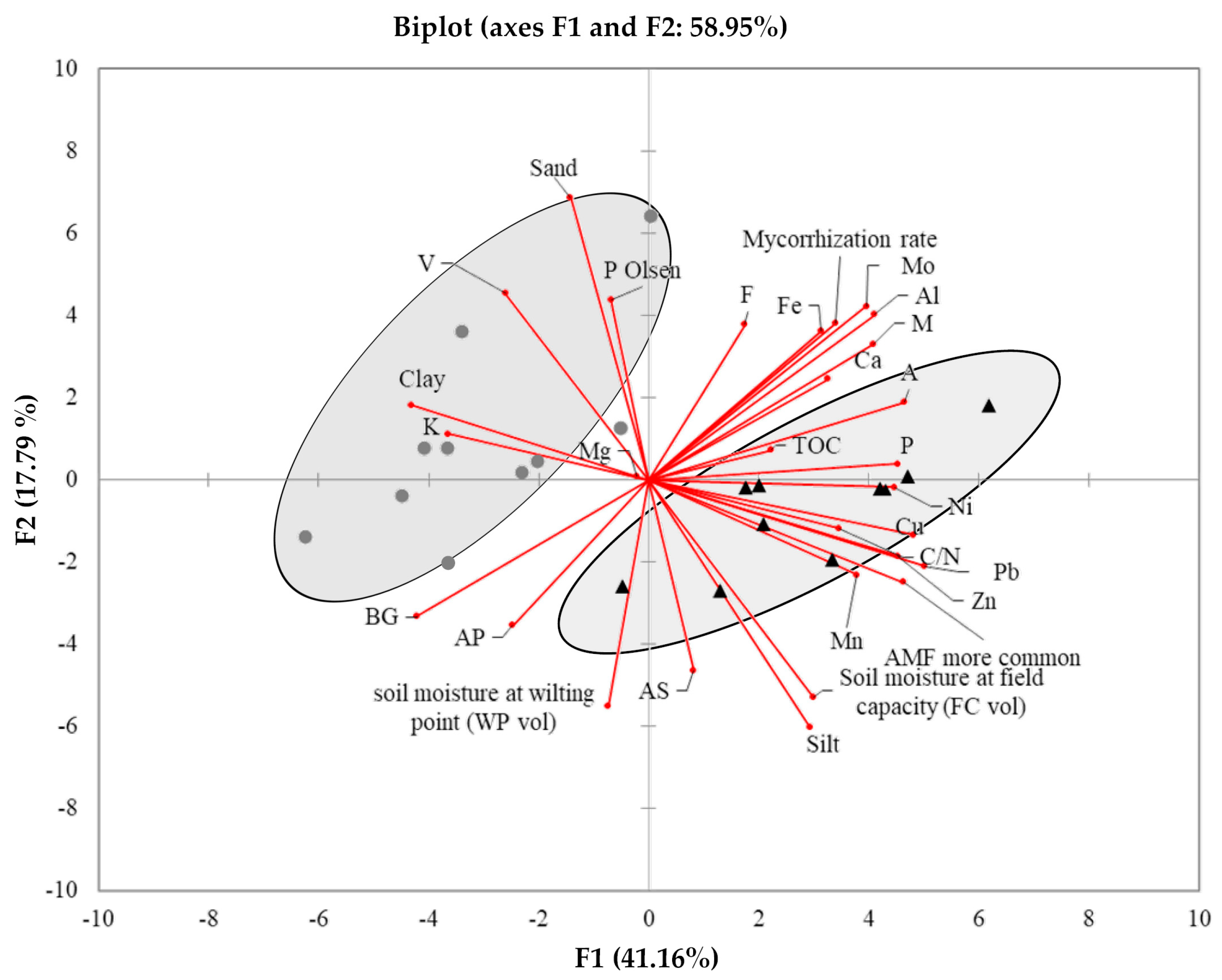
3.3. Correlation Among Soil Traits and AMF Specificities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO and ITPS. FAO (Food and Agriculture Organization of the UN) and ITPS (Intergovernmental Technical Panel on Soils) Status of the World’s Soil Resources (SWSR)—Technical Summary; FAO and ITPS: Rome, Italy, 2015; ISBN 978-92-5-109004-6. [Google Scholar]
- Seybold, C.A.; Herrick, J.E.; Brejda, J.J. Soil resilience: A fundamental component of soil quality. Soil Sci. 1999, 164, 224–234. [Google Scholar] [CrossRef]
- European Commission. Farm to Fork Strategy, for a Fair, Healthy and Environmentally-Friendly Food System. 2020. Available online: https://food.ec.europa.eu/horizontal-topics/farm-fork-strategy_en (accessed on 2 August 2024).
- European Commission. Biodiversity Strategy for 2030. 2020. Available online: https://environment.ec.europa.eu/strategy/biodiversity-strategy-2030_en (accessed on 2 August 2024).
- Panagos, P.; Montanarella, L.; Barbero, M.; Schneegans, A.; Aguglia, L.; Jones, A. Soil priorities in the European Union. Geoderma 2022, 29, e00510. [Google Scholar] [CrossRef]
- Belda, I.; Zarraonaindia, I.; Perisin, M.; Palacios, A.; Acedo, A. From vineyard soil to wine fermentation: Microbiome approximations to explain the “terroir” concept. Front. Microbiol. 2017, 8, 821. [Google Scholar] [CrossRef] [PubMed]
- Zarraonaindia, I.; Owens, S.M.; Weisenhorn, P.; West, K.; Hampton-Marcell, J.; Lax, S.; Bokulich, N.A.; Mills, D.A.; Martin, G.; Taghavi, S. The soil microbiome influences grapevine-associated microbiota. MBio 2015, 6, e02527-14. [Google Scholar] [CrossRef] [PubMed]
- Darriaut, R.; Lailheugue, V.; Masneuf-Pomarède, I.; Marguerit, E.; Martins, G.; Compant, S.; Ballestra, P.; Upton, S.; Ollat, N.; Lauvergeat, V. Grapevine rootstock and soil microbiome interactions: Keys for a resilient viticulture. Hortic. Res. 2022, 9, uhac019. [Google Scholar] [CrossRef]
- Giffard, B.; Winter, S.; Guidoni, S.; Nicolai, A.; Castaldini, M.; Cluzeau, D.; Coll, P.; Cortet, J.; Le Cadre, E.; d’Errico, G.; et al. Vineyard management and its impacts on soil biodiversity, functions, and ecosystem services. Front. Ecol. Evol. 2022, 10, 850272. [Google Scholar] [CrossRef]
- Biasi, R.; Brunori, E.; Vanino, S.; Bernardini, A.; Catalani, A.; Farina, R.; Bruno, R.; Chilosi, G. Soil-plant interaction mediated by indigenous AMF in grafted and own-rooted grapevines under field conditions. Agriculture 2023, 13, 1051. [Google Scholar] [CrossRef]
- EUROSTAT. Vineyards in the EU—Statistics. 2022. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Vineyards_in_the_EU_-_statistics#General_overview (accessed on 2 August 2024).
- ISTAT Agriculture Database. 2023. Available online: http://dati.istat.it/Index.aspx?QueryId=33706 (accessed on 2 August 2024).
- OIV. State of the World Vine and Wine Sector in 2022. 2022. Available online: https://www.oiv.int/sites/default/files/documents/OIV_2022_Activity_Report_2022.pdf (accessed on 1 June 2024).
- Bokulich, N.A.; Collins, T.S.; Masarweh, C.; Allen, G.; Heymann, H.; Ebeler, S.E.; Mills, D.A. Associations among wine grape microbiome, metabolome, and fermentation behavior suggest microbial contribution to regional wine characteristics. MBio 2016, 7, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Mezzasalma, V.; Sandionigi, A.; Bruni, I.; Bruno, A.; Lovicu, G.; Casiraghi, M.; Labra, M. Grape microbiome as a reliable and persistent signature of field origin and environmental conditions in Cannonau wine production. PLoS ONE 2017, 12, e0184615. [Google Scholar] [CrossRef]
- del Pilar Martínez-Diz, M.; Andrés-Sodupe, M.; Bujanda, R.; Díaz-Losada, E.; Eichmeier, A.; Gramaje, D. Soil-plant compartments affect fungal microbiome diversity and composition in grapevine. Fungal Ecol. 2019, 41, 234–244. [Google Scholar] [CrossRef]
- Trouvelot, S.; Bonneau, L.; Redecker, D.; van Tuinen, D.; Adrian, M.; Wipf, D. Arbuscular mycorrhiza symbiosis in viticulture: A review. Agron. Sustain. Dev. 2015, 35, 1449–1467. [Google Scholar] [CrossRef]
- Cameron, D.D.; Neal, A.L.; van Wees, S.C.; Ton, J. Mycorrhiza-induced resistance: More than the sum of its parts? Trends Plant Sci. 2013, 18, 539–545. [Google Scholar] [CrossRef]
- Aguilera, P.; Ortiz, N.; Becerra, N.; Turrini, A.; Gaínza-Cortés, F.; Silva-Flores, P.; Aguilar-Paredes, A.; Romero, J.K.; Jorquera-Fontena, E.; Mora, M.d.L. Application of arbuscular mycorrhizal fungi in vineyards: Water and biotic stress under a climate change scenario: New challenge for Chilean grapevine crop. Front. Microbiol. 2022, 13, 826571. [Google Scholar] [CrossRef]
- Hao, Z.; Fayolle, L.; van Tuinen, D.; Chatagnier, O.; Li, X.; Gianinazzi, S.; Gianinazzi-Pearson, V. Local and systemic mycorrhiza-induced protection against the ectoparasitic nematode Xiphinema index involves priming of defence gene responses in grapevine. J. Exp. Bot. 2012, 63, 3657–3672. [Google Scholar] [CrossRef] [PubMed]
- Bruisson, S.; Maillot, P.; Schellenbaum, P.; Walter, B.; Gindro, K.; Deglène-Benbrahim, L. Arbuscular mycorrhizal symbiosis stimulates key genes of the phenylpropanoid biosynthesis and stilbenoid production in grapevine leaves in response to downy mildew and grey mould infection. Phytochemistry 2016, 131, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Silva, A.; Figueiredo, A.; Sebastiana, M. First insights into the effect of mycorrhizae on the expression of pathogen effectors during the infection of grapevine with Plasmopara viticola. Sustainability 2021, 13, 1226. [Google Scholar] [CrossRef]
- Dey, M.; Ghosh, S. Arbuscular mycorrhizae in plant immunity and crop pathogen control. Rhizosphere 2022, 22, 100524. [Google Scholar] [CrossRef]
- Powell, K.S. A holistic approach to future management of grapevine Phylloxera. In Arthropod Management in Vineyards; Bostanian, N., Vincent, C., Isaacs, R., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 219–251. [Google Scholar] [CrossRef]
- Biasi, R.; Barbera, G.; Marino, E.; Brunori, E.; Nieddu, G. Viticulture as crucial cropping system for counteracting the desertification of coastal land. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on the 931, Lisbon, Portugal, 22–27 August 2010; pp. 71–77. [Google Scholar]
- Ollat, N.; Peccoux, A.; Papura, D.; Esmenjaud, D.; Marguerit, E.; Tandonnet, J.P.; Bordenave, L.; Cookson, S.J.; Barrieu, F.; Rossdeutsch, L.; et al. Rootstock as a component of adaptation to environment. In Grapevine in a Changing Environment: A Molecular and Ecophysiological Perspective; Geros, H., Chaves, M., Medrano, H., Delrot, S., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 68–108. [Google Scholar] [CrossRef]
- Moukarzel, R.; Ridgway, H.J.; Guerin-Laguette, A.; Jones, E.E. Grapevine rootstocks drive the community structure of arbuscular mycorrhizal fungi in New Zealand vineyards. J. Appl. Microbiol. 2021, 131, 2941–2956. [Google Scholar] [CrossRef]
- Moukarzel, R.; Ridgway, H.J.; Waller, L.; Guerin-Laguette, A.; Cripps-Guazzone, N.; Jones, E.E. Soil arbuscular mycorrhizal fungal communities differentially affect growth and nutrient uptake by grapevine rootstocks. Microb. Ecol. 2023, 86, 1035–1049. [Google Scholar] [CrossRef]
- De Santis, D.; Frangipane, M.T.; Brunori, E.; Cirigliano, P.; Biasi, R. Biochemical markers for enological potentiality in a grapevine aromatic variety under different soil types. Am. J. Enol. Vitic. 2017, 68, 100–111. [Google Scholar] [CrossRef]
- Biasi, R.; Farina, R.; Brunori, E. Family farming plays an essential role in preserving soil functionality: A study on active managed and abandoned traditional tree crop-based systems. Sustainability 2021, 13, 3967. [Google Scholar] [CrossRef]
- Biasi, R.; Cirigliano, P.; Di Francesco, G.; Brunori, E.; Muleo, R. Attuazione dei protocolli di 565 selezione clonale su biotipi di Aleatico individuate nell’area ‘tipica’ della Doc Aleatico di Gradoli e caratterizzazione-selezione del ‘Grechetto Rosso’ e di altri vitigni autoctoni dell’Alto Lazio: Risultati 567 della ricerca. Riv. Di Vitic. Ed. Enol. 2007, 4, 127–143. [Google Scholar]
- Lorenz, D.H.; Eichhorn, K.W.; Bleiholder, H.; Klose, R.; Meier, U.; Weber, E. Growth stages of the grapevine: Phenological growth stages of the grapevine (Vitis vinifera L. ssp. vinifera)—Codes and descriptions according to the extended BBCH scale. Aust. Grape Wine Res. 1995, 1, 100–103. [Google Scholar] [CrossRef]
- Vierheilig, H.; Coughlan, A.P.; Wyss, U.R.S.; Piché, Y. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl. Environ. Microbiol. 1998, 64, 5004–5007. [Google Scholar] [CrossRef]
- Redecker, D. Specific PCR primers to identify arbuscular mycorrhizal fungi within colonized roots. Mycorrhiza 2000, 10, 73–80. [Google Scholar] [CrossRef]
- Sadej, W.; Przekwas, K. Fluctuations of nitrogen levels in soil profile under conditions of a long-term fertilization experiment. Plant Soil. Environ. 2008, 54, 197. [Google Scholar] [CrossRef]
- Delle Politiche Agricole e Forestali (MiP.A.F.). Metodi Ufficiali di Analisi Biochimica del Suolo; D.M. 23/02/2004, Gazzetta Ufficiale n. 61, 13/03/2004; Ministero Delle Politiche Agricole e Forestali (MiP.A.F.): Genoa, Italy, 2004. [Google Scholar]
- Brdar-Jokanović, M. Boron toxicity and deficiency in agricultural plants. Int. J. Mol. Sci. 2020, 21, 1424. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Ra, FL, USA, 2001. [Google Scholar]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil. Sci. Plan. 1984, 15, 1409–14016. [Google Scholar] [CrossRef]
- Ziadi, N.; Simard, R.R.; Tran, T.S.; Allard, G. Soil-available phosphorus as evaluated by desorption techniques and chemical extractions. Can. J. Soil. Sci. 2001, 81, 167–174. [Google Scholar] [CrossRef]
- Olsen, S.R.; Dean, L.R. Phosphorus. In Methods of Soil Analysis; Olsen, S.R., Dean, L.A., Eds.; American Society of Agronomy: Madison, WI, USA, 1965; Volume 9, pp. 920–926. [Google Scholar]
- Van Genuchten, M.T. Closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Dotaniya, M.L.; Aparna, K.; Dotaniya, C.K.; Singh, M.; Regar, K.L. Role of soil enzymes in sustainable crop production. In Enzymes in Food Biotechnology; Kuddus, M., Ed.; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar] [CrossRef]
- Cenini, V.L.; Fornara, D.A.; McMullan, G.; Ternan, N.; Carolan, R.; Crawley, M.J.; Clément, J.-C.; Lavorel, S. Linkages between extracellular enzyme activities and the carbon and nitrogen content of soils. Soil Biol. Biochem. 2016, 96, 198–206. [Google Scholar] [CrossRef]
- Hu, Y.; Zheng, Q.; Noll, L.; Zhang, S.; Wanek, W. Direct measurement of the in situ decomposition of microbial-derived soil organic matter. Soil Biol. Biochem. 2020, 141, 107660. [Google Scholar] [CrossRef]
- Ndiaye, E.L.; Sandeno, J.M.; McGrath, D.; Dick, R.P. Integrative biological indicators for detecting change in soil quality. Am. J. Altern. Agric. 2000, 15, 26–36. [Google Scholar] [CrossRef]
- Pereira, G.W.; Valente, D.S.M.; de Queiroz, D.M.; Santos, N.T.; Fernandes-Filho, E.I. Soil mapping for precision agriculture using support vector machines combined with inverse distance weighting. Precis. Agric. 2022, 23, 1189–1204. [Google Scholar] [CrossRef]
- Possingham, J.V.; Obbink, J.G. Endotropic mycorrhiza and the nutrition of grape vines. Vitis 1971, 10, 120–130. [Google Scholar] [CrossRef]
- Valentine, A.J.; Mortimer, P.E.; Lintnaar, M.; Borgo, R. Drought responses of arbuscular mycorrhizal grapevines. Symbiosis 2006, 41, 127–133. [Google Scholar]
- Schreiner, R.P.; Mihara, K.L. The diversity of arbuscular mycorrhizal fungi amplified from grapevine roots (Vitis vinifera L.) in Oregon vineyards is seasonally stable and influenced by soil and vine age. Mycologia 2009, 101, 599–611. [Google Scholar] [CrossRef]
- López-García, Á.; Jurado-Rivera, J.A.; Bota, J.; Cifre, J.; Baraza, E. Space and vine cultivar interact to determine the arbuscular mycorrhizal fungal community composition. J. Fungi 2020, 6, 317. [Google Scholar] [CrossRef] [PubMed]
- Betancur-Agudelo, M.; Meyer, E.; Lovato, P.E. Arbuscular mycorrhizal fungus richness in the soil and root colonization in vineyards of different ages. Rhizosphere 2021, 17, 100307. [Google Scholar] [CrossRef]
- Pontes, J.S.D.; Sánchez-Castro, I.; Palenzuela, J.; Maia, L.C.; Silva, G.A.D.; Oehl, F. Scutellospora alterata, a new gigasporalean species from the semi-arid Caatinga biome in Northeastern Brazil. Mycotaxon 2021, 125, 169–181. [Google Scholar] [CrossRef]
- Blaszkowski, J.; Adamska, I.; Czerniawska, B. Arbuscular mycorrhizal fungi (Glomeromycota) of the Vistula Bar. Acta Mycol. 2002, 37, 39–62. [Google Scholar] [CrossRef]
- Muiruri, J.; Rimberia, F.K.; Mwashasha, M.R.; Kavoo, A. Abundance and diversity of arbuscular mycorrhizal fungal (AMF) spores isolated from the rhizosphere of papaya and other different cropping systems in Central Kenya. J. Agric. Sci. Technol. 2022, 21, 18–36. [Google Scholar] [CrossRef]
- Chagnon, P.L.; Bradley, R.L. Evidence that soil nutrient stoichiometry controls the competitive abilities of arbuscular mycorrhizal vs. root-borne non-mycorrhizal fungi. Fungal Ecol. 2013, 6, 557–560. [Google Scholar] [CrossRef]
- da Silva, K.J.; Fernandes, J.A.; Magurno, F.; Leandro, L.B.; Goto, B.T.; Theodoro, R.C. Phylogenetic review of Acaulospora (Diversisporales, Glomeromycota) and the homoplasic nature of its ornamentations. J. Fungi 2022, 8, 892. [Google Scholar] [CrossRef]
- Mukhongo, R.W.; Ebanyat, P.; Masso, C.; Tumuhairwe, J.B. Composition and spore abundance of arbuscular mycorrhizal fungi in sweet potato producing areas in Uganda. Front. Soil. Sci. 2023, 3, 1152524. [Google Scholar] [CrossRef]
- Juniper, S.; Abbott, L.K. Soil salinity delays germination and limits growth of hyphae from propagules of arbuscular mycorrhizal fungi. Mycorrhiza 2006, 16, 371–379. [Google Scholar] [CrossRef]
- Nopphakat, K.; Runsaeng, P.; Klinnawee, L. Acaulospora as the dominant arbuscular mycorrhizal fungi in organic lowland rice paddies improves phosphorus availability in soils. Sustainability 2021, 14, 31. [Google Scholar] [CrossRef]
- Palenzuela, J.; Azcón-Aguilar, C.; Brea, J.M.; Alves da Silva, G.; Oehl, F. Acaulospora baetica, a new arbuscular mycorrhizal fungal species from two mountain ranges in Andalucía (Spain). Nova Hedwig. 2015, 101, 463–474. [Google Scholar] [CrossRef]
- George, T.S.; Bulgarelli, D.; Carminati, A.; Chen, Y.; Jones, D.; Kuzyakov, Y.; Schnepf, A.; Wissuwa, M.; Roose, T. Bottom-up perspective—The role of roots and rhizosphere in climate change adaptation and mitigation in agroecosystems. Plant Soil 2024, 500, 1–27. [Google Scholar] [CrossRef]
- Borie, F.; Rubio, R.; Morales, A. Arbuscular Mycorrhizal Fungi and Soil Aggregation. In Proceedings of the Simposio Internacional Suelos, Ecología y Medioambiente, Temuco, Chile, 8–9 November 2007; Boletín N° 23. Sociedad Chilena de la Ciencia del Suelo Universidad de la Frontera: Temuco, Chile, 2008; p. 15. [Google Scholar]
- Hodge, A. Arbuscular mycorrhizal fungi influence decomposition of, but not plant nutrient capture from, glycine patches in soil. New Phytol. 2001, 151, 725–734. [Google Scholar] [CrossRef]
- Hoffland, E.; Kuyper, T.W.; Comans, R.N.; Creamer, R.E. Eco-functionality of organic matter in soils. Plant Soil 2020, 455, 1–22. [Google Scholar] [CrossRef]
- Mori, T.; Aoyagi, R.; Kitayama, K.; Mo, J. Does the ratio of β-1, 4-glucosidase to β-1, 4-N-acetylglucosaminidase indicate the relative resource allocation of soil microbes to C and N acquisition? Soil Biol. Biochem. 2021, 160, 108363. [Google Scholar] [CrossRef]
- Mori, T. Does ecoenzymatic stoichiometry really determine microbial nutrient limitations? Soil Biol. Biochem. 2020, 146, 107816. [Google Scholar] [CrossRef]
- Stege, P.W.; Messina, G.A.; Bianchi, G.; Olsina, R.A.; Raba, J. Determination of β-glucosidase activity in soils with a bioanalytical sensor modified with multiwalled carbon nanotubes. Anal. Bioanal. Chem. 2010, 397, 1347–1353. [Google Scholar] [CrossRef]
- Choi, J.; Summers, W.; Paszkowski, U. Mechanisms underlying establishment of arbuscular mycorrhizal symbioses. Annu. Rev. Phytopathol. 2018, 56, 135–160. [Google Scholar] [CrossRef]
- Burke, D.J.; Weintraub, M.N.; Hewins, C.R.; Kalisz, S. Relationship between soil enzyme activities, nutrient cycling and soil fungal communities in a northern hardwood forest. Soil Biol. Biochem. 2011, 43, 795–803. [Google Scholar] [CrossRef]
- Qin, M.; Zhang, Q.; Pan, J.; Jiang, S.; Liu, Y.; Bahadur, A.; Feng, H. Effect of arbuscular mycorrhizal fungi on soil enzyme activity is coupled with increased plant biomass. Eur. J. Soil. Sci. 2020, 71, 84–92. [Google Scholar] [CrossRef]
- Badiane, N.N.Y.; Chotte, J.L.; Pate, E.; Masse, D.; Rouland, C. Use of soil enzyme activities to monitor soil quality in natural and improved fallows in semi-arid tropical regions. Appl. Soil Ecol. 2001, 18, 229–238. [Google Scholar] [CrossRef]
- Deng, Y.; Feng, G.; Chen, X.; Zou, C. Arbuscular mycorrhizal fungal colonization is considerable at optimal Olsen-P levels for maximized yields in an intensive wheat-maize cropping system. Field Crop Res. 2017, 209, 1–9. [Google Scholar] [CrossRef]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef]
- Ambrosini, V.G.; Voges, J.G.; Canton, L.; Couto, R.D.R.; Ferreira, P.A.A.; Comin, J.J.; de Melo, G.W.B.; Brunetto, G.; Soares, C.R.F.S. Effect of arbuscular mycorrhizal fungi on young vines in copper-contaminated soil. Braz. J. Microbiol. 2015, 46, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Ballabio, C.; Panagos, P.; Lugato, E.; Huang, J.H.; Orgiazzi, A.; Jones, A.; Fernández-Ugalde, O.; Borrelli, P.; Montanarella, L. Copper distribution in European topsoils: An assessment based on LUCAS soil survey. Sci. Total Environ. 2018, 636, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, P.; Pérez-Tienda, J.; Meier, S.; Valderas, A.; Borie, F.; Azcón-Aguilar, C.; Ferrol, N. Copper compartmentalization in spores as a survival strategy of arbuscular mycorrhizal fungi in Cu-polluted environments. Soil Biol. Biochem. 2013, 57, 925–928. [Google Scholar] [CrossRef]
- Brunetto, G.; de Melo, G.W.B.; Terzano, R.; Del Buono, D.; Astolfi, S.; Tomasi, N.; Pii, R.; Cesco, S. Copper accumulation in vineyard soils: Rhizosphere processes and agronomic practices to limit its toxicity. Chemosphere 2016, 162, 293–307. [Google Scholar] [CrossRef]


| Mineral Elements | Range Values mg kg−1 | Reference | |
|---|---|---|---|
| Nitrogen (%) | N | 1–5 | [35] |
| Phosphorus Olsen (mg kg−1) | P | 20–60 | [36] |
| Available Potassium (mg kg−1) | K | 40–500 | [36] |
| Copper (mg kg−1) | Cu | 10–120 | [36] |
| Zinc (mg kg−1) | Zn | 10–150 | [36] |
| Iron(mg kg−1) | Fe | 50–150 | [36] |
| Manganese (mg kg−1) | Mn | 750–1000 | [36] |
| Molybdenous (mg kg−1) | Mo | 0.1–5 | [36] |
| Calcium (mg kg−1) | Ca | 10–250 (0.85–20 in non-calcareous soil) | [36] |
| Magnesium (mg kg−1) | Mg | Up to 400 (0.5–50 in non-calcareous soil) | [36] |
| Boron (mg kg−1) | B | 5–30 | [37] |
| HMs | |||
| Cadmium (mg kg−1) | Cd | 0.1–5 | [36] |
| Lead (mg kg−1) | Pb | 5–120 | [36] |
| Chromium (mg kg−1) | Cr | 10–150 | [36] |
| Nichel (mg kg−1) | Ni | 5–120 | [36] |
| Arsenic (mg kg−1) | As | 1–70 | [38] |
| Enzyme | Biogeochemical Cycle | Substrate | Soil Function | Reference |
|---|---|---|---|---|
| Arylsulfatase (AS) | S | Organic S compounds | S source for plants, nutrient for microbial biomass | [43] |
| Leucine-aminopeptidase (LA) | N | amino acid residues | N source for plants, nutrient for microbial biomass | [44] |
| N-acetylglusosaminidase (NAG) | N | chitin, peptidoglycan | [45] | |
| Beta-glucosidase (BG) | C | Cellobiose, cellotriose | C energy source for the growth and activity of soil microbes | [46] |
| Alkaline Phosphatase or Acid Phosphatase (AP) | P | Organic phosphorus | P source for plants, nutrient for microbial biomass | [43] |
| Mineral Elements | AL-ORV | AL-420A | |||
|---|---|---|---|---|---|
| Total Nitrogen (%) | N | 0.5 | ns | 0.34 | ns |
| Phosphorus Olsen (mg kg−1) | P | 30.16 | ns | 24.56 | ns |
| Available Phosphorus (mg kg−1) | P | 27.10 | b | 65.65 | a |
| Available Potassium (mg kg−1) | K | 1495.87 | a | 1185.46 | b |
| Copper (mg kg−1) | Cu | 39.70 | b | 65.17 | a |
| Zinc (mg kg−1) | Zn | 13.97 | b | 20.32 | a |
| Iron (mg kg−1) | Fe | 179.12 | ns | 195.12 | ns |
| Nickel (mg kg−1)) | Ni | 0.06 | b | 0.12 | a |
| Manganese (mg kg−1) | Mn | 61.60 | b | 84.07 | a |
| Molybdenous (mg kg−1) | Mo | 0.44 | ns | 0.65 | ns |
| Calcium (mg kg−1) | Ca | 1040.82 | ns | 1142.57 | ns |
| Magnesium (mg kg−1) | Mg | 392.05 | ns | 367.69 | ns |
| Boron (mg kg−1) | B | trace | - | trace | - |
| Heavy Metals | AL-ORV (mg kg−1) | AL-420A (mg kg−1) | |||
|---|---|---|---|---|---|
| Cadmium | Cd | 0.05 | ns | 0.06 | ns |
| Lead | Pb | 6.19 | b | 9.73 | a |
| Chromium | Cr | 0.07 | a | 0.0002 | b |
| Nickel | Ni | 0.06 | b | 0.12 | a |
| Arsenic | As | trace | - | trace | - |
| Enzymatic Activity | AL-ORV µmol g−1 h−1 | AL-420A µmol g−1 h−1 | |||
|---|---|---|---|---|---|
| Arylsulfatase | AS | 0.11 | ns | 0.13 | ns |
| Leucine-aminopeptidase | LA | 0.04 | ns | 0.04 | ns |
| N-acetylglusosaminidase | NAG | 0.35 | a | 0.27 | b |
| Beta-glucosidase | BG | 1.92 | a | 1.54 | b |
| Alkaline Phosphatase or Acid Phosphatase | AP | 1.08 | ns | 0.98 | ns |
| AMF Species | |||
|---|---|---|---|
| VARIABLES | F | ProbF | Sign. |
| Clay | 6.01 | 0.04 | * |
| AP | 8.44 | 0.02 | * |
| Mycorrhization rate | 7.90 | 0.02 | * |
| Cu | 7.50 | 0.02 | * |
| Ni | 23.66 | 0.00 | ** |
| Pb | 11.84 | 0.01 | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalani, A.; Brunori, E.; Chilosi, G.; Bernardini, A.; Vanino, S.; Migliore, M.; Farina, R.; Biasi, R. Soil Traits and Grapevine Rootstock Genotypes Modulate Arbuscular Mycorrhizal Rate and Species in a Mediterranean Environment. Agriculture 2024, 14, 1425. https://doi.org/10.3390/agriculture14081425
Catalani A, Brunori E, Chilosi G, Bernardini A, Vanino S, Migliore M, Farina R, Biasi R. Soil Traits and Grapevine Rootstock Genotypes Modulate Arbuscular Mycorrhizal Rate and Species in a Mediterranean Environment. Agriculture. 2024; 14(8):1425. https://doi.org/10.3390/agriculture14081425
Chicago/Turabian StyleCatalani, Alessia, Elena Brunori, Gabriele Chilosi, Alessandra Bernardini, Silvia Vanino, Melania Migliore, Roberta Farina, and Rita Biasi. 2024. "Soil Traits and Grapevine Rootstock Genotypes Modulate Arbuscular Mycorrhizal Rate and Species in a Mediterranean Environment" Agriculture 14, no. 8: 1425. https://doi.org/10.3390/agriculture14081425





