Utilizing Principal Component Analysis to Assess the Effects of Complex Foliar Fertilizers Regarding Maize (Zea mays L.) Productivity
Abstract
1. Introduction
2. Materials and Methods
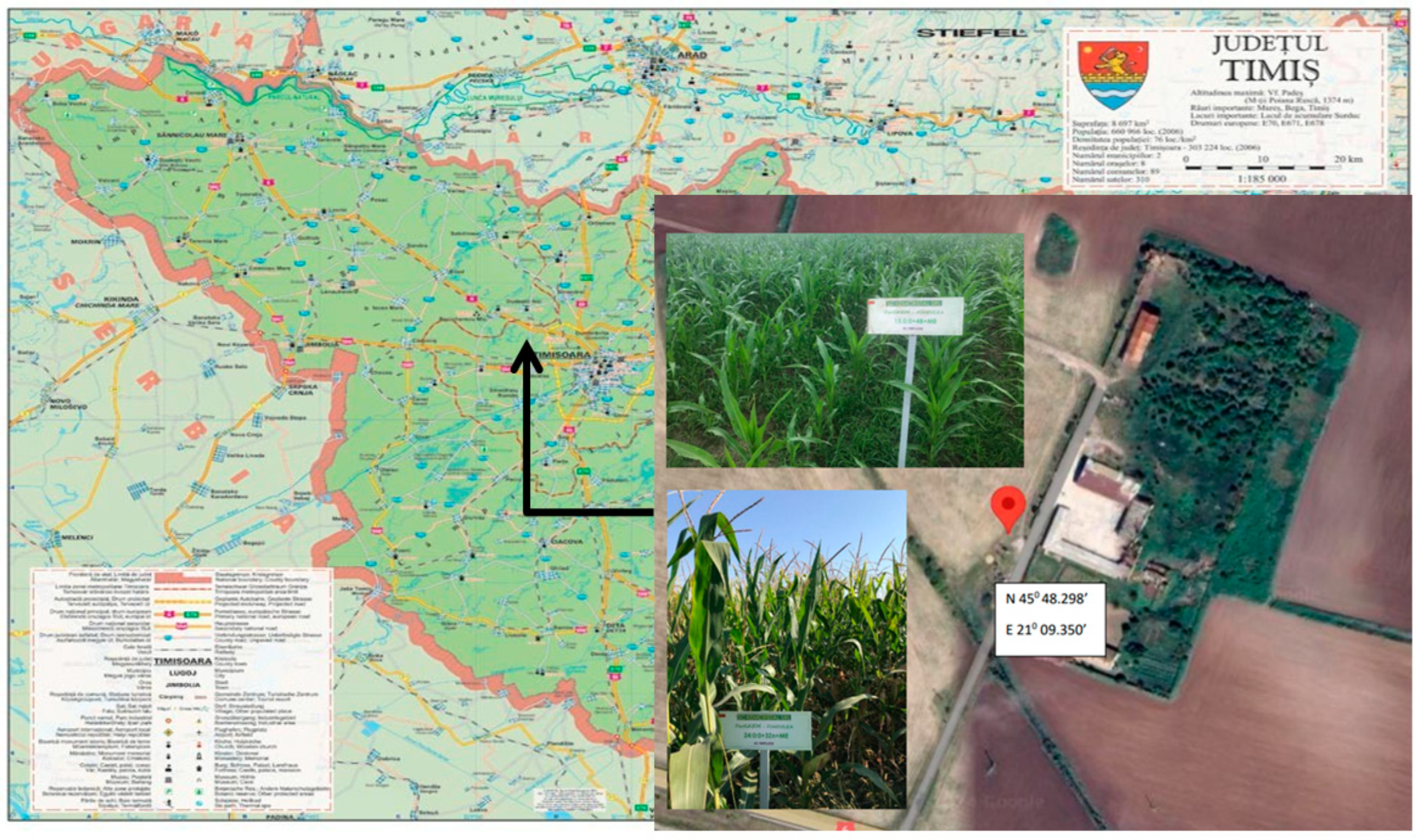
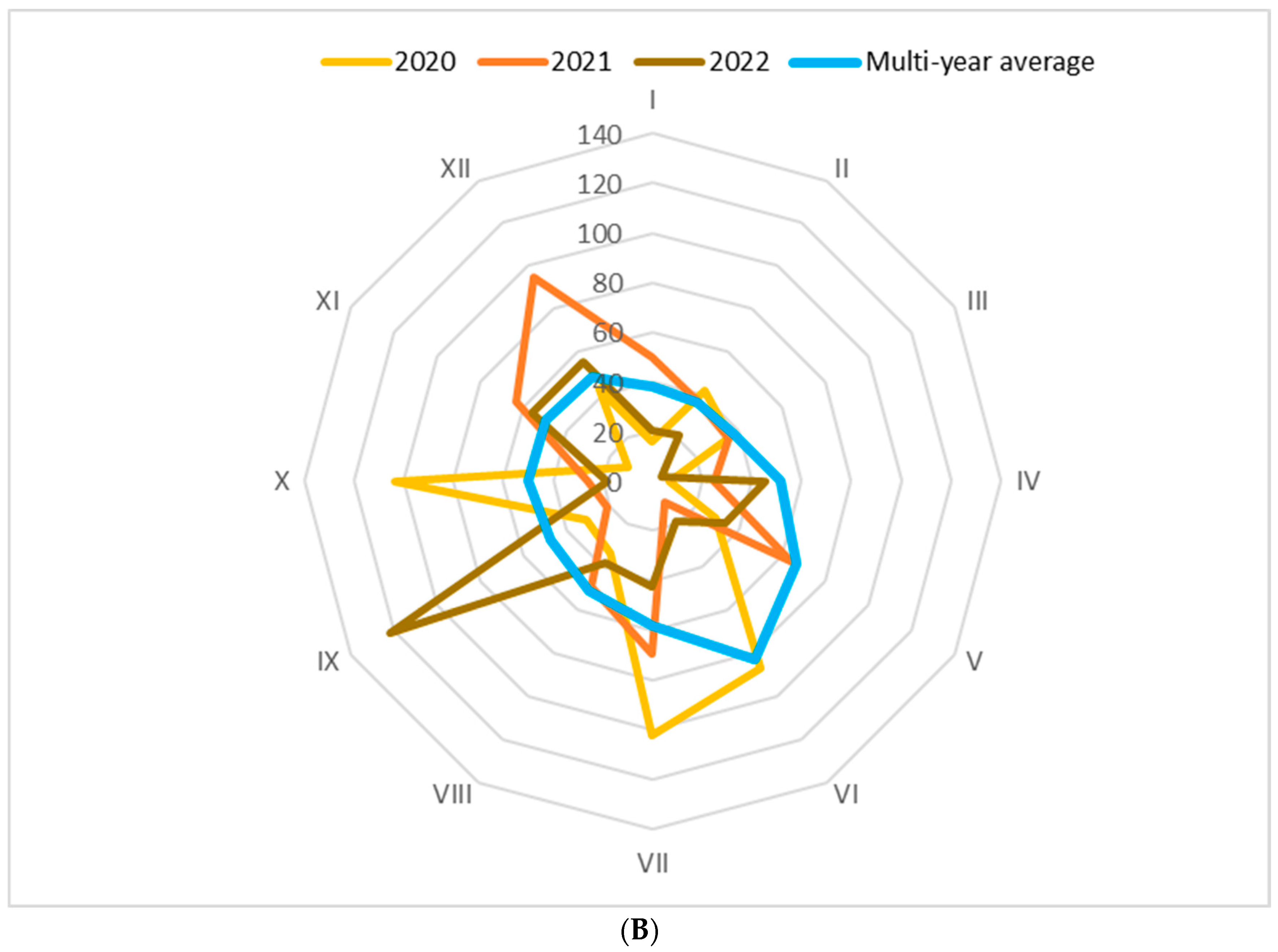
2.1. Natural Framework of Experimentation: Relief, Climate, and Soil
2.2. Sowing and Fertilization, Experimental Scheme
- Semi-late hybrid (FAO 450) with extraordinary production potential proven every year.
- It loses water very quickly at maturity.
- Very strong roots and stem.
- Intensive hybrid intended for farmers who apply cutting-edge technology.
- It is recommended for arid and semi-arid lowland areas in the south and west of the country.
- Non-irrigated: 60,000–65,000 harvestable plants/ha.
- Irrigated: 68,000–75,000 harvestable plants/ha
- number of rows per tin: 16–18
- number of seeds per row: 47–50
- MMB: 365–400 g.
2.3. Chemical Analysis and Statistical Data Processing Methods
- Humidity (%): this was performed by drying the samples at 110 °C in an electric oven, POL-EKO-equipment, Nitech-Romania, SR EN ISO 712:2010 [41].
- Ash (%): this was made by burning at 5500 °C using electric furnace equipment (Lenton Thermal Design, England), SR ISO 2171:2002 [42].
- Crude protein (%): this was classically determined by the Kjeldahl method, using a VELP kit (DK20 heating digestion and UDK 149 distillation unit) [43].
- Dietary fiber (%) extraction was carried out by the FOSS Fibertec device and method 2010&M6 [46].
- The mineralization of the samples from the maize grains was carried out by calcination followed by a wet mineralization of the ash using concentrated HNO3+HCl in the ratio 1:3 (aqua regia), followed by dilution. From the properly diluted solutions, total phosphorus content was determined colorimetrically using a CINTRA spectrophotometer (GBC Australia). Metal nutrients were determined by FAAS method (air-acetylene flame), using a fast sequential atomic absorption spectrometer VARIAN AA 240 FS (Australia) [47,48].
- The percentage content of carbohydrates was calculated by subtracting from 100 the sum of the other macronutrients.
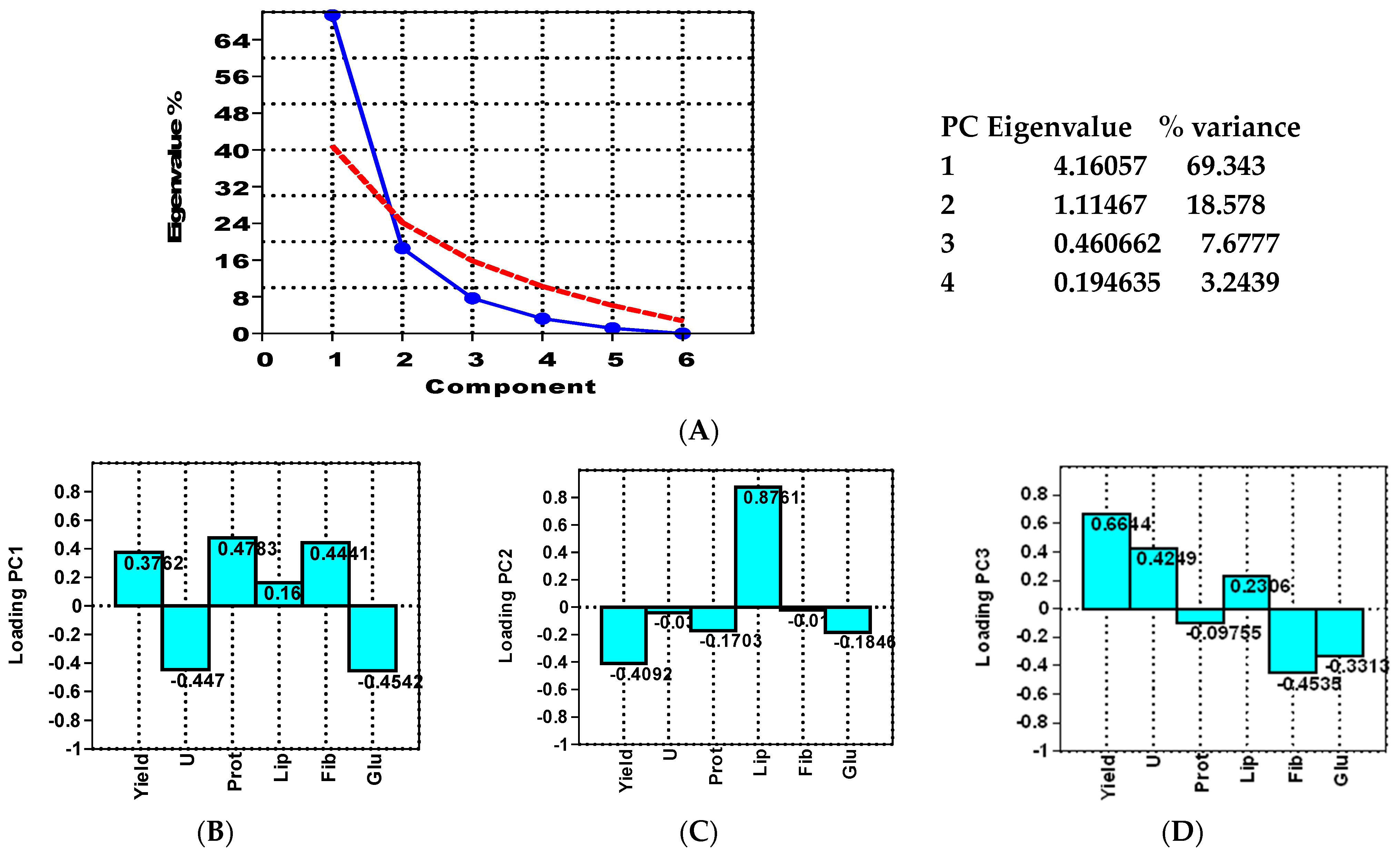
- Groups of variables: Based on the “loadings” (weights assigned to original variables in the principal components), PCA can group related quality parameters (e.g., protein and lipids).
- Groups of samples: Based on the “scores” (values of each sample on the principal components), PCA can group grain maize samples that respond similarly to the treatments (e.g., samples with high yield and similar protein content).
- PCA-nutrient: This model explores the interactions between treatments and key maize quality aspects (yield, moisture, protein, lipids, fibers, carbohydrates).
- PCA-mineral: This model focuses on the interactions between treatments and mineral content (yield, ash, macronutrients, and micronutrients).
3. Results and Discussions
3.1. Impact of FF on Production and Humidity of Maize Grain
3.2. The Influence of Foliar Fertilizers on the Protein, Lipid, Carbohydrate, and Fiber Content of Maize Grains
In summary, foliar fertilization with various mineral combinations, particularly those containing nitrogen and micronutrients such as zinc, can significantly influence the qualitative parameters of crop production. While it tends to increase protein content, it often leads to a decrease in lipid content, especially when nitrogen predominates. Carbohydrate content may also be affected, particularly by nitrogen fertilization. These findings underscore the importance of carefully balancing nutrient inputs to optimize both yield and quality in crop production.
- ✓
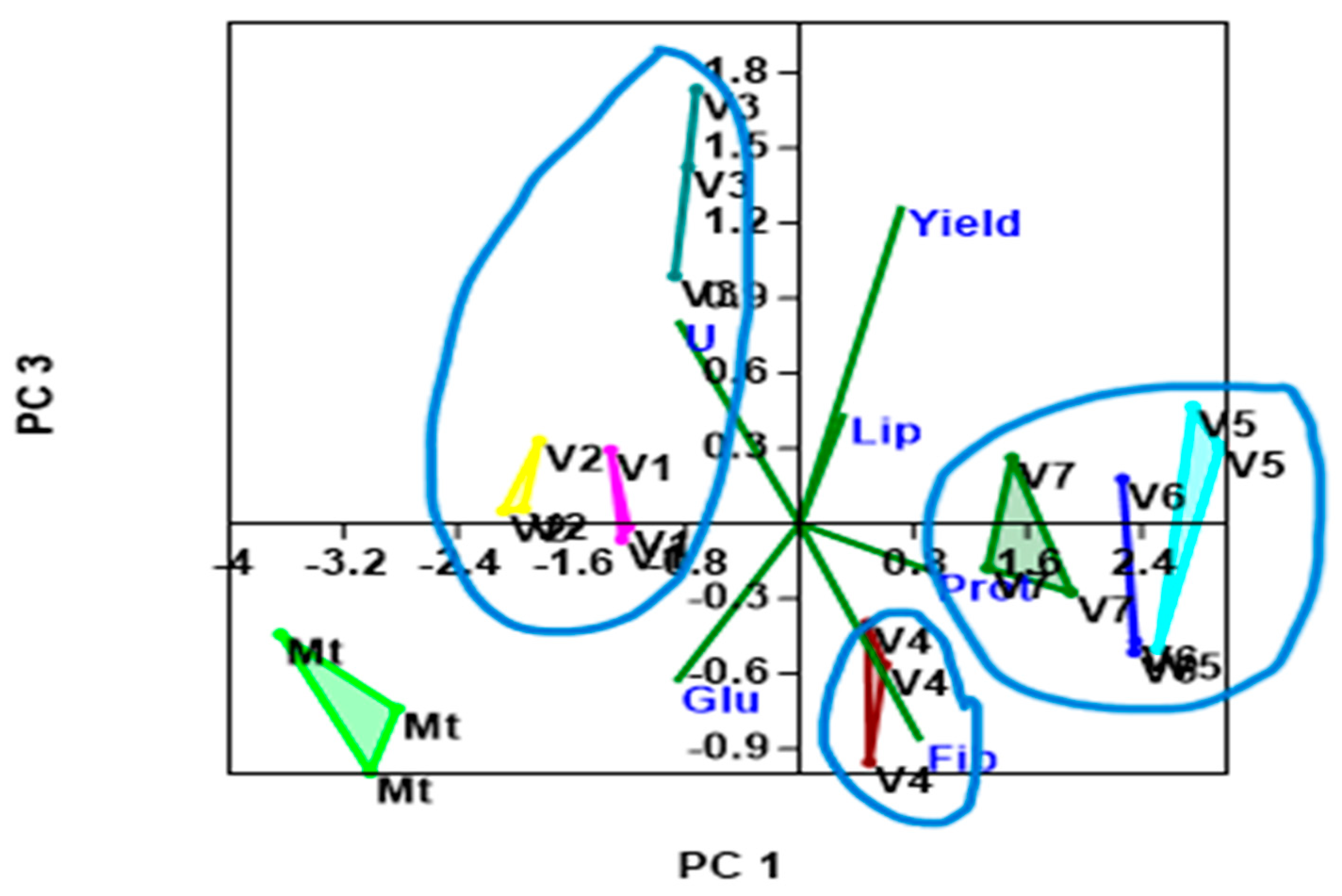
- Distinct Groupings:
- ✓
- Effects of foliar fertilization:
3.3. The Effect of Foliar Fertilization on Essential Macroelement Contents in Maize Seeds
3.3.1. The Impact on Macronutrient Content of Maize Grains
3.3.2. The Influence of FF on the Content of Some Micronutrients in Maize Grains
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://insse.ro/cms/sites/default/files/com_presa/com_pdf/prod_veg_r22.pdf (accessed on 12 April 2023).
- Available online: https://www.profit.ro/povesti-cu-profit/agribusiness/romania-locul-1-in-ue-la-porumb-boabe-si-floarea-soarelui-atat-la-suprafata-cultivata-cat-si-la-productia-realizata-20657884 (accessed on 12 April 2023).
- Available online: https://www.agrimedia.ro/articole/temeri-privind-viitorul-porumbului-in-europa (accessed on 2 May 2023).
- Available online: https://www.lumeasatului.ro/articole-revista/agrotehnica/7471-evolutia-suprafetelor-si-recoltelor-de-porumb-in-ultimii-30-de-ani.html (accessed on 2 May 2023).
- Penuelas, J.; Fernando Coello, F.; Sardans, J. A better use of fertilizers is needed for global food security and environmental sustainability. Agric. Food Secur. 2023, 12, 5. [Google Scholar] [CrossRef]
- Girma, K.; Martin, K.L.; Freeman, K.W.; Mosali, J.; Teal, R.K.; Raun, W.R.; Moges, S.M.; Arnall, D.B. Determination of Optimum Rate and Growth Stage for Foliar-Applied Phosphorus in Maize. Commun. Soil. Sci. Plant Anal. 2007, 38, 1137–1154. [Google Scholar] [CrossRef]
- Fernández, V.; Eichert, T. Uptake of Hydrophilic Solutes Through Plant Leaves: Current State of Knowledge and Perspectives of Foliar Fertilization. Crit. Rev. Plant Sci. 2009, 28, 36–68. [Google Scholar] [CrossRef]
- Fernández, V.; Brown, P.H. From plant surface to plant metabolism: The uncertain fate of foliar-applied nutrients. Front. Plant Sci. 2013, 4, 289. [Google Scholar] [CrossRef]
- Fernández, V.; Sotiropoulos, T.; Brown, P.H. Foliar Fertilization: Scientific Principles and Field Pratices; International Fertilizer Industry Association (IFA): Paris, France, 2013; ISBN 979-10-92366-00-6. [Google Scholar]
- White, P.J.; Brown, P.H. Plant nutrition for sustainable development and global health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef]
- Schreiber, L.; Schönherr, J. Water and Solute Permeability of Plant Cuticles. Measurement and Data Analysis; Springer-Verlag: Berlin/Heidelberg, Germany, 2009; pp. 1–29. [Google Scholar] [CrossRef]
- Yousaf, N.; Ishfaq, M.; Qureshi, H.A.; Saleem, A.; Yang, H.; Sardar, M.F.; Zou, C. Characterization of Root and Foliar-Applied Iron Oxide Nanoparticles (α-Fe2O3, γ-Fe2O3, Fe3O4, and Bulk Fe3O4) in Improving Maize (Zea mays L.) Performance. Nanomaterials 2023, 13, 3036. [Google Scholar] [CrossRef]
- Ishfaq, M.; Kiran, A.; Hafeezur, R.; Farooq, M.; Ijaz, N.H.; Nadeem, F.; Azeem, I.; Li, X.; Wakeel, A. Foliar nutrition: Potential and challenges under multifaceted agriculture. Environ. Exp. Bot. 2022, 200, 104909. [Google Scholar] [CrossRef]
- Otto, R.; Marques, J.P.R.; Pereira de Carvalho, H.W. Strategies for probing absorption and translocation of foliar applied nutrients. J. Exp. Bot. 2021, 72, 4600–4603. [Google Scholar] [CrossRef]
- Sager, M. Levels of Sulfur as an Essential Nutrient Element in the Soil-Crop-Food System in Austria. Agriculture 2012, 2, 1–11. [Google Scholar] [CrossRef]
- Carciochi, W.D.; Sadras, V.O.; Pagani, A.; Ciampitti, I.A. Co-limitation and stoichiometry capture the interacting effects of nitrogen and sulfur on maize yield and nutrient use efficiency. Eur. J. Agron. 2020, 113, 125973. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, H.; Xue, Y.; Gao, Y.; Qian, X.; Dai, H.; Liu, K.; Li, Q.; Li, Z. Effect of sulfur fertilizer on summer maize grain yield and soil water utilization under different irrigation patterns from anthesis to maturity. Agric. Water Manag. 2021, 250, 106828. [Google Scholar] [CrossRef]
- Wang, H.; Cui, S.; Fu, J.; Gong, H.; Liu, S. Sulfur Application Improves the Nutritional Quality of Maize by Regulating the Amino Acid Balance of Grains. Agronomy 2023, 13, 2912. [Google Scholar] [CrossRef]
- Matthes, M.S.; Robil, J.M.; McSteen, P. From element to development: The power of the essential micronutrient boron to shape morphological processes in plants. J. Exp. Bot. 2020, 71, 1681–1693. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, M.A.; Abreu, I.; Bell, R.W.; Bienert, M.D.; Brown, P.H.; Dell, B.; Fujiwara, T.; Goldbach, H.E.; Lehto, T.; Mock, H.P.; et al. Boron: An essential element for vascular plants: A comment on Lewis (2019)’ Boron: The essential element for vascular plants that never was. New Phytol. 2020, 226, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Fátima Gotz, L.; Silvestrin, F.; Motta, A.C.V.; Pauletti, V. Response to Application and Tissue Diagnosis of Boron Deficiency and Toxicity in Maize. Commun. Soil. Sci. Plant Anal. 2021, 52, 2898–2911. [Google Scholar] [CrossRef]
- Luz, P.H.C.; Marin, M.A.; Devechio, F.F.S.; Romualdo, L.M.; Zuñiga, A.M.G.; Oliveira, M.W.S.; Bruno, O.M. Boron Deficiency Precisely Identified on Growth Stage V4 of Maize Crop Using Texture Image Analysis. Commun. Soil. Sci. Plant Anal. 2018, 49, 159–169. [Google Scholar] [CrossRef]
- Lordkaew, S.; Dell, B.; Jamjod, S.; Rerkasem, B. Boron deficiency in maize. Plant Soil. 2011, 342, 207–220. [Google Scholar] [CrossRef]
- Kumar, S.; Mohapatra, T. Interaction Between Macro- and Micro-Nutrients in Plants. Front. Plant Sci. 2021, 12, 665. [Google Scholar] [CrossRef]
- Grabovskyi, M.; Kucheruk, P.; Pavlichenko, K.; Roubík, H. Influence of macronutrients and micronutrients on maize hybrids for biogas production. Environ. Sci. Pollut. Res. Int. 2023, 30, 70022–70038. [Google Scholar] [CrossRef]
- Sharma, R.K.; Agrawal, M. Biological Effects of Heavy Metals: An Overview. J. Environ. Biol. 2005, 26 (Suppl. S2), 301–313. [Google Scholar]
- Brankov, M.; Simíc, M.; Dolijanoviíc, Z.; Rajkovíc, M.; Mandíc, V.; Dragcevíc, V. The Response of Maize Lines to Foliar Fertilizing. Agriculture 2020, 10, 365. [Google Scholar] [CrossRef]
- Faraz, A.M.; Jamil, M.; Ahmad, K.; Bakar, A.M.; Abbas, M.S.; Nadeem, M.; Batool, T. Effect of Copper on Sunflower (Helianthus annus L.). Int. J. Sci. Res. Publ. 2020, 10, 205. [Google Scholar] [CrossRef]
- Available online: https://www.meteoromania.ro/servicii/date-meteorologice/ (accessed on 19 May 2023).
- Șmuleac, L.; Rujescu, C.; Șmuleac, A.; Imbrea, F.; Radulov, I.; Manea, D.N.; Ienciu, A.; Adamov, T.; Pașcalău, R. Impact of Climate Change in the Banat Plain, Western Romania, on the Accessibility of Water for Crop Production in Agriculture. Agriculture 2020, 10, 437. [Google Scholar] [CrossRef]
- Nortjé, G.P.; Laker, M.C. Soil Fertility Management in Conservation Agriculture. South Afr. J. Plant Soil. 2021, 38, 247–257. [Google Scholar] [CrossRef]
- Radulov, I.; Sala, F.; Berbecea, A.; Crista, F. Changes of soil microelements content after intesive mineral fertilization. Res. J. Agric. Sci. 2009, 41, 487–492. [Google Scholar]
- Barrow, N.J.; Hartemink, A.E. The effects of pH on nutrient availability depend on both soils and plants. Plant Soil. 2023, 487, 21–37. [Google Scholar] [CrossRef]
- Crista, F.; Radulov, I.; Crista, L.; Berbecea, A.; Lațo, A. Influence of mineral fertilization on the amino acid content and raw protein of wheat grain. J. Food Agric. Environ. (JFAE) 2012, 10, 47–50. [Google Scholar]
- Hybrid Description. Available online: https://search.app/QhEFA17P3J8xCtYB8 (accessed on 11 August 2024).
- BBCH. Available online: https://www.bionity.com/en/encyclopedia/BBCH-scale_%28maize%29.html (accessed on 28 March 2024).
- Adengo Herbicide. Available online: http://www.royalagroscience.com/en/bayer6/adengo-sc-4652.html (accessed on 19 March 2024).
- Available online: https://schachtner-sprayer.de/en (accessed on 19 March 2024).
- Available online: www.ospatimisoara.ro (accessed on 15 February 2023).
- Available online: www.renar.ro (accessed on 15 February 2023).
- SR EN ISO 712:2010; Determinarea Umidității. ISO: Geneva, Switzerland, 2010. Available online: https://magazin.asro.ro/ro/standard/181049 (accessed on 22 April 2023).
- SR ISO 2171:2002; Determinarea Cenușii la Cereale. ISO: Geneva, Switzerland, 2002. Available online: https://magazin.asro.ro/ro/standard/28087 (accessed on 22 April 2023).
- SR EN ISO 20483:2014; Metoda Kjeldahl, Cereale. ISO: Geneva, Switzerland, 2014. Available online: https://magazin.asro.ro/ro/standard/226094 (accessed on 22 April 2023).
- Norma Sanitară-Veterinară din 21 Februarie 2007, Metoda Soxhlet. Available online: https://legislatie.just.ro/Public/DetaliiDocument/80122 (accessed on 22 April 2023).
- Hărmănescu, M. Comparative researches on two direct transmethylation without prior extraction methods for fatty acids analysis in vegetal matrix with low fat content. Chem. Cent. J. 2012, 6, 8. [Google Scholar] [CrossRef]
- Available online: https://www.servitechexpres.ro/analizoare-furaje/sistem-determinare-fibra-foss-fibertec-8000/ (accessed on 22 April 2023).
- Bordean, D.M.; Borozan, A.B.; Cojocariu, L.; Moigradean, D.; Cojocariu, A.; Nica, D.; Pirvulescu, L.; Alda, S.; Horablaga, M. Seasonal variation in nutrient content of some leafy vegetables from Banat County, Romania. Rev. Agric. Rural Dev. 2013, 2, 170–174. [Google Scholar]
- Kiandokht Ghanati, K.; Farid Zayeri, F.; Hedayat Hosseinia, H. Potential Health Risk Assessment of Different Heavy Metals in Wheat Products. Iran J Pharm Res. 2019, 18, 2093–2100. [Google Scholar] [CrossRef]
- Boldea, M.; Sala, F.; Radulov, I.; Crista, F. A Mathematical Model on the Dependence between the Agricultural Production and Chemical Fertilizer. In International Conference on Numerical Analysis and Applied Mathematics; American Institute of Physics (AIP): College Park, MY, USA, 2010; pp. 1363–1366. [Google Scholar] [CrossRef]
- Olive, D.J. Principal Component Analysis. In Robust Multivariate Analysis; Springer: Cham, Switzerland, 2017; pp. 189–217. [Google Scholar]
- Gergen, I.; Hărmănescu, M. Application of principal component analysis in the pollution assessment with heavy metals of vegetable food chain in the old mining areas. Chem. Cent. J. 2012, 6, 156. [Google Scholar] [CrossRef] [PubMed]
- The PAST Software. Available online: https://www.nhm.uio.no/english/research/resources/past/ (accessed on 15 February 2023).
- Ter Braak, C.J.F.; Van Tongeren, O.F.R. Data Analysis in Community and Landscape Ecology; Jongman, R.H., Ed.; Pudoc Wageningen: Wageningen, The Netherlands, 1987; Available online: https://edepot.wur.nl/410376 (accessed on 22 April 2023).
- Cassim, B.M.A.R.; Martins Machado, A.P.; Fortune, D.; Moreira, F.R.; De Oliveira Zampar, E.J.; Batistand, M.A. Effects of Foliar Application of Urea and Urea-Formaldehyde/Triazone on Soybean and Maize Crops. Agronomy 2020, 10, 1549. [Google Scholar] [CrossRef]
- Yang, M.; Shi, L.; Xu, F.-S.; Lu, J.-W.; Wang, Y.H. Effects of Bo, Mo, Zn, and Their Interactions on Seed Yield of Rapeseed (Brassica napus L.). Pedosphere 2009, 19, 53–59. [Google Scholar] [CrossRef]
- Crista, F.; Radulov, I.; Imbrea, F.; Manea, D.N.; Boldea, M.; Gergen, I.; Ienciu, A.A.; Dunea-Banatean, I. The Study of the Impact of Complex Foliar Fertilization on the Yield and Quality of Sunflower Seeds (Helianhtus annuus L.) by Principal Component Analysis. Agronomy 2023, 13, 2074. [Google Scholar] [CrossRef]
- Ali, A.; Ali, Q.; Ahsan, M. Foliar application of micronutrients enhances growth, yield, and quality of maize (Zea mays L.) grown under two different irrigation regimes. Turk. J. Field Crops 2017, 22, 236–247. [Google Scholar]
- Sabourifard, H.; Estakhr, A.; Bagheri, M.; Hosseini, S.J.; Keshavarz, H. The quality and quantity response of maize (Zea mays L.) yield to planting date and fertilizers management. Food Chem. Adv. 2023, 2, 100196. [Google Scholar] [CrossRef]
- Crista, F.; Radulov, I.; Crista, L.; Lațo, A.; Stroia, C.; Băghină, N.; Gaica, I. Changing quality indicators of maize grain following mineral fertilizers application. Curr. Opin. Biotechnol. 2013, 24, S69. [Google Scholar] [CrossRef]
- Ferraz, G.A.S.; Ferraz, P.F.P.; Martins, F.B.; Silva, F.M.; Damasceno, F.A.; Barbari, M. Principal components in the study of soil and plant properties in precision coffee farming. Agron. Res. 2019, 17, 418–429. [Google Scholar] [CrossRef]
- Kabata, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd eds.; CRC Press: Boca Raton, FL, USA, 2001; p. 365. [Google Scholar]
- McDowell, L.R. Minerals in Animal and Human Nutrition; Academic Press: New York, NY, USA, 1992. [Google Scholar]
- Available online: https://www.vedantu.com/biology/role-of-macronutrients-and-micronutrients (accessed on 15 February 2023).


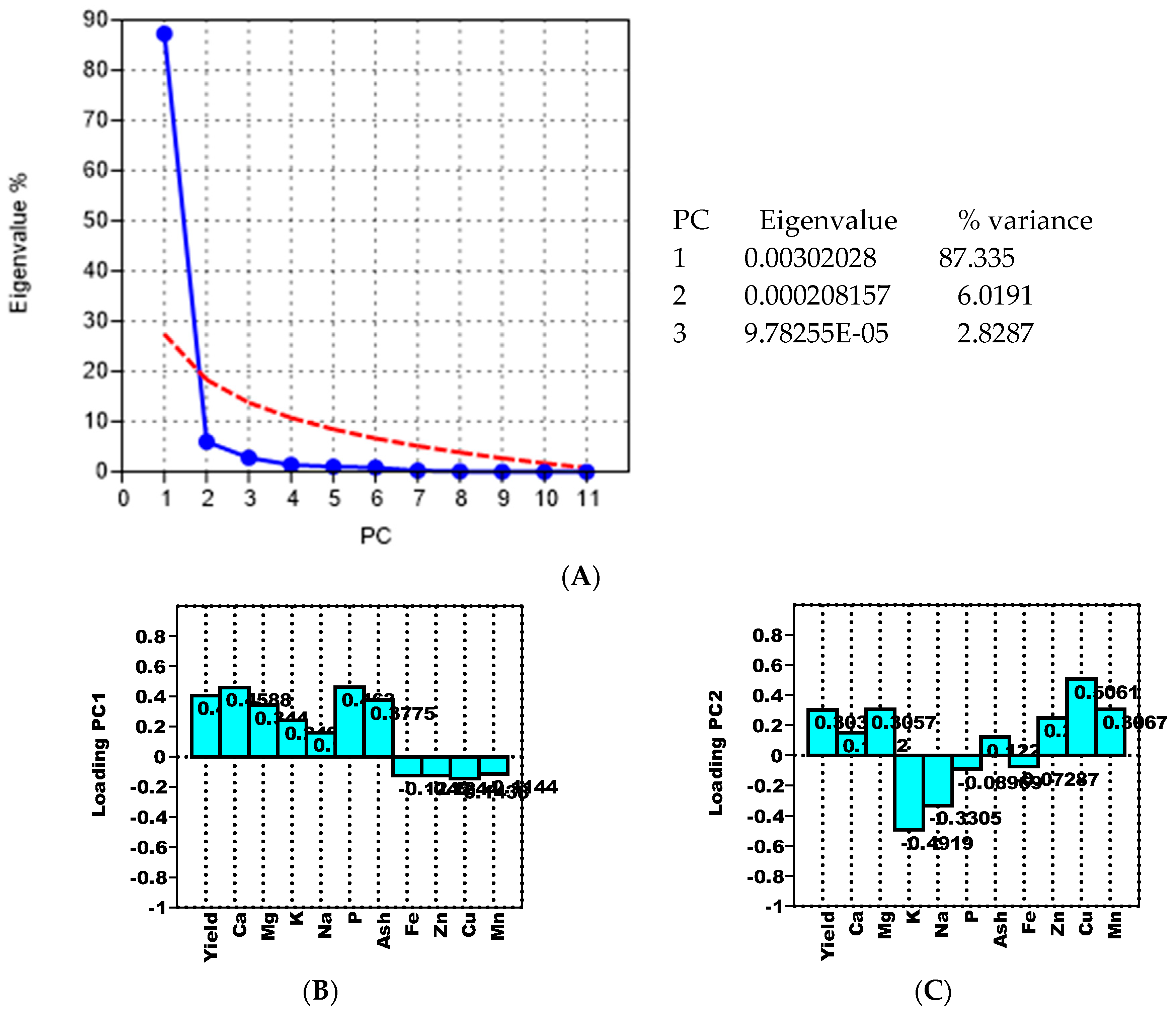
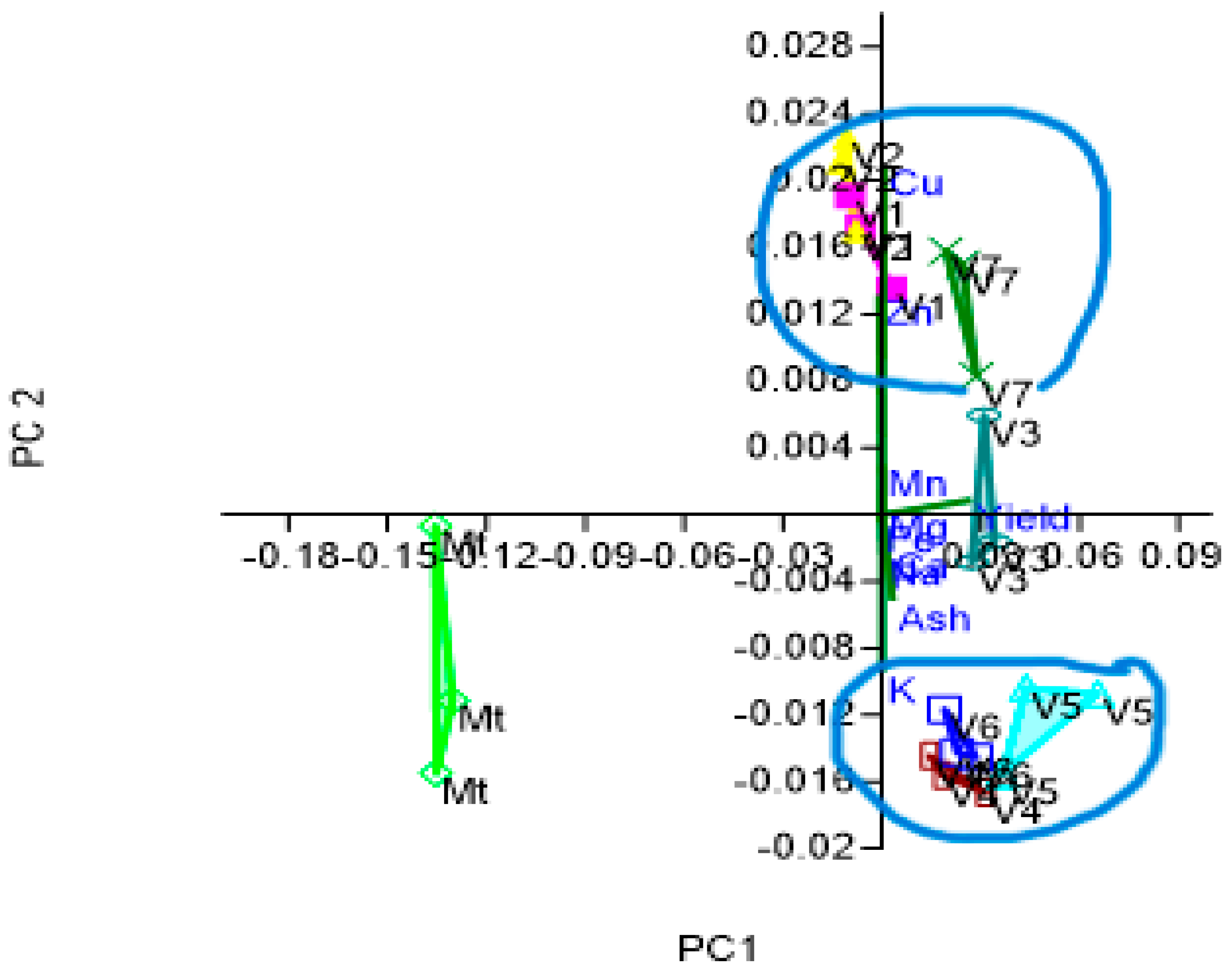
| Measured Parameters | Grain Yield | Humidity (Relative to the Fresh Mass) | |
|---|---|---|---|
| Units | kg ha−1 | % (Regarding Mt) | % |
| Variants | Mean/SD | Mean/SD | |
| V1 | 10,147 *** 146 | 134 | 13.10 NS 0.05 |
| V2 | 9997 *** 50 | 132 | 12.91 NS 0.06 |
| V3 | 10,987 *** 81 | 146 | 12.97 NS 0.14 |
| V4 | 10,723 *** 219 | 142 | 11.99 ** 0.17 |
| V5 | 11,433 *** 416 | 151 | 11.71 *** 0.20 |
| V6 | 10,777 *** 108 | 143 | 11.51 *** 0.23 |
| V7 | 10,813 *** 96 | 143 | 11.71 ** 0.27 |
| Mt | 7550 60 | 100 | 13.15 0.21 |
| Analyzed Nutrients | Proteins | Lipids | Carbohydrates | Fibers |
|---|---|---|---|---|
| Variants/Unit | %, Mean/SD | |||
| V1 | 9.40 *** 0.02 | 3.93 *** 0.03 | 72.36 ** 0.05 | 7.17 * 0.02 |
| V2 (S) | 9.18 *** 0.03 | 3.95 ** 0.10 | 72.76 NS 0.10 | 7.10 NS 0.01 |
| V3 (B) | 9.46 *** 0.11 | 4.45 NS 0.04 | 71.91 ** 0.28 | 7.10 NS 0.02 |
| V4 (S+B) | 10.97 *** 0.08 | 3.72 *** 0.12 | 72.09 ** 0.17 | 7.21 * 0.04 |
| V5 (Zn) | 11.62 *** 0.11 | 4.54 NS 0.10 | 70.91 ** 0.37 | 7.30 *** 0.02 |
| V6 (Zn) | 11.38 *** 0.02 | 4.65 NS 0.02 | 71.21 *** 0.23 | 7.27 ** 0.02 |
| V7 (B) | 10.81 *** 0.03 | 4.68 NS 0.07 | 71.58 ** 0.29 | 7.24 * 0.06 |
| Mt | 8.29 0.17 | 4.57 0.11 | 72.82 0.12 | 7.09 0.04 |
| Minerals | Ash | Ca | Mg | K | Na | P |
|---|---|---|---|---|---|---|
| Units | % | mg kg−1 | ||||
| Variants | Mean/SD | |||||
| V1 | 1.21 NS 0.01 | 7.10 * 0.03 | 128.07 *** 0.31 | 278.50 *** 0.10 | 34.67 * 0.15 | 211.07 NS 0.21 |
| V2 | 1.21 NS 0.00 | 7.08 * 0.03 | 127.97 ** 0.21 | 278.37 *** 0.06 | 34.73 * 0.21 | 211.70 * 0.40 |
| V3 | 1.22 NS 0.01 | 7.13 ** 0.02 | 127.37 * 0.12 | 278.83 *** 0.06 | 35.17 NS 0.15 | 212.53 *** 0.15 |
| V4 | 1.24 * 0.01 | 7.16 ** 0.01 | 127.70 ** 0.20 | 288.07 ** 0.06 | 34.63 ** 0.06 | 213.57 *** 0.32 |
| V5 | 1.22 NS 0.01 | 7.20 ** 0.02 | 128.40 *** 0.10 | 288.70 ** 0.20 | 35.20 NS 0.10 | 214.63 *** 0.21 |
| V6 | 1.24 NS 0.03 | 7.18 ** 0.01 | 128.23 *** 0.12 | 288.57 ** 0.21 | 35.40 * 0.10 | 214.57 *** 0.12 |
| V7 | 1.21 NS 0.02 | 7.16 ** 0.02 | 127.73 * 0.40 | 288.33 ** 0.15 | 35.23 NS 0.06 | 213.63 *** 0.25 |
| Mt | 1.17 0.04 | 6.96 0.05 | 126.77 0.25 | 286.8. 0.35 | 35.10 0.09 | 210.40 0.36 |
| Minerals | Fe | Zn | Cu | Mn |
|---|---|---|---|---|
| Unit | mg kg−1 | |||
| Variants | Mean/SD | |||
| V1 | 2.66 NS 0.06 | 2.34 * 0.01 | 0.33 * 0.00 | 0.48 NS 0.00 |
| V2 | 2.72 NS 0.02 | 2.29 NS 0.02 | 0.33 ** 0.00 | 0.49 * 0.00 |
| V3 | 2.76 NS 0.01 | 2.21 NS 0.06 | 0.32 NS 0.00 | 0.49 ** 0.00 |
| V4 | 2.71 NS 0.01 | 2.19 NS 0.02 | 0.32 NS 0.00 | 0.48 NS 0.00 |
| V5 | 2.66 NS 0.04 | 2.22 NS 0.02 | 0.31 NS 0.00 | 0.49 * 0.00 |
| V6 | 2.70 NS 0.02 | 2.28 NS 0.01 | 0.31 NS 0.00 | 0.48 * 0.00 |
| V7 | 2.70 NS 0.04 | 2.28 NS 0.03 | 0.33 ** 0.00 | 0.48 NS 0.00 |
| Mt | 2.72 0.03 | 2.25 0.04 | 0.31 0.00 | 0.48 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crista, L.; Radulov, I.; Crista, F.; Imbrea, F.; Manea, D.N.; Boldea, M.; Gergen, I.; Ienciu, A.A.; Lațo, A. Utilizing Principal Component Analysis to Assess the Effects of Complex Foliar Fertilizers Regarding Maize (Zea mays L.) Productivity. Agriculture 2024, 14, 1428. https://doi.org/10.3390/agriculture14081428
Crista L, Radulov I, Crista F, Imbrea F, Manea DN, Boldea M, Gergen I, Ienciu AA, Lațo A. Utilizing Principal Component Analysis to Assess the Effects of Complex Foliar Fertilizers Regarding Maize (Zea mays L.) Productivity. Agriculture. 2024; 14(8):1428. https://doi.org/10.3390/agriculture14081428
Chicago/Turabian StyleCrista, Laura, Isidora Radulov, Florin Crista, Florinel Imbrea, Dan Nicolae Manea, Marius Boldea, Iosif Gergen, Anișoara Aurelia Ienciu, and Alina Lațo. 2024. "Utilizing Principal Component Analysis to Assess the Effects of Complex Foliar Fertilizers Regarding Maize (Zea mays L.) Productivity" Agriculture 14, no. 8: 1428. https://doi.org/10.3390/agriculture14081428
APA StyleCrista, L., Radulov, I., Crista, F., Imbrea, F., Manea, D. N., Boldea, M., Gergen, I., Ienciu, A. A., & Lațo, A. (2024). Utilizing Principal Component Analysis to Assess the Effects of Complex Foliar Fertilizers Regarding Maize (Zea mays L.) Productivity. Agriculture, 14(8), 1428. https://doi.org/10.3390/agriculture14081428







