Construction of Indicators of Low-Temperature Stress Levels at the Jointing Stage of Winter Wheat
Abstract
:1. Introduction
2. Materials and Methods
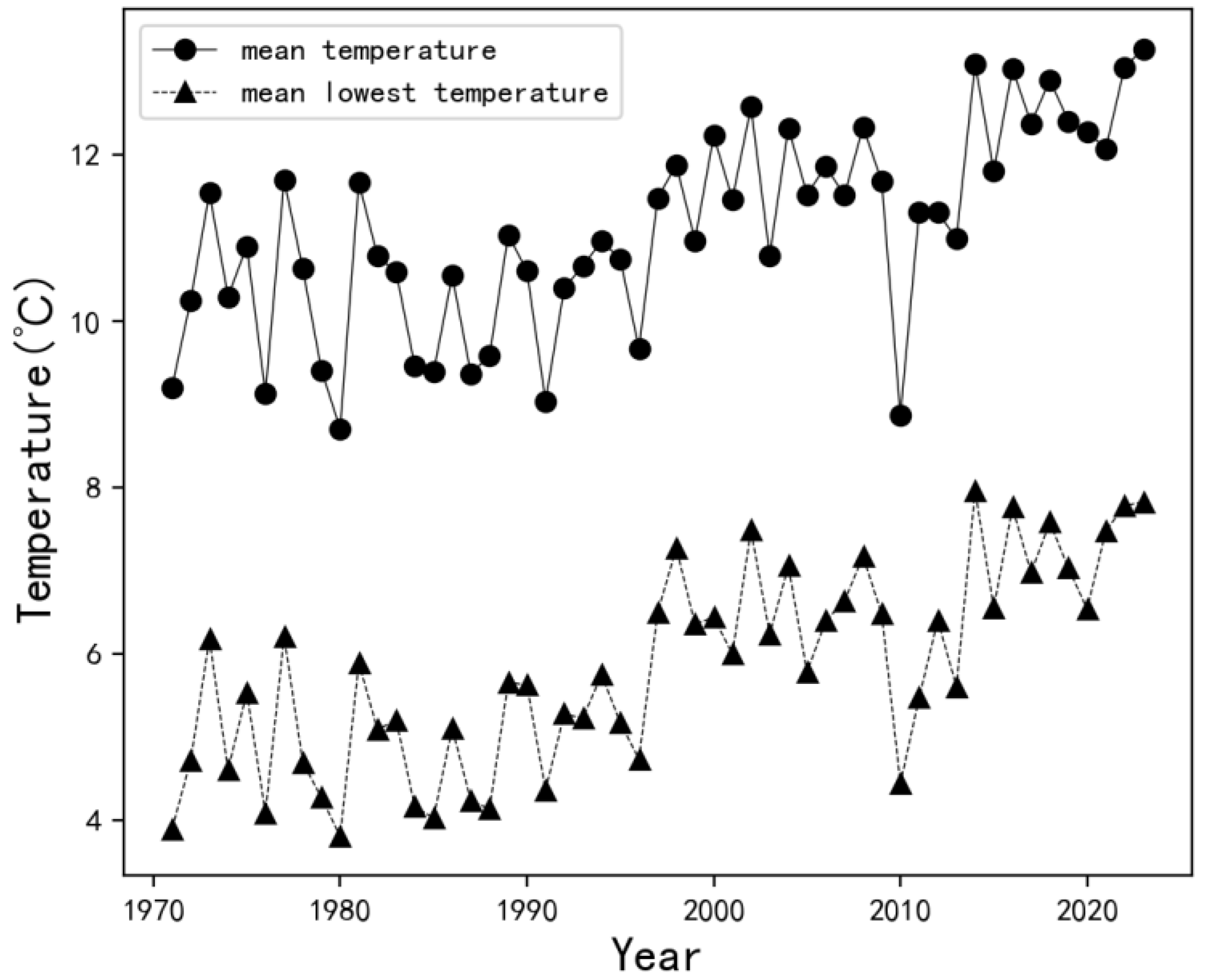
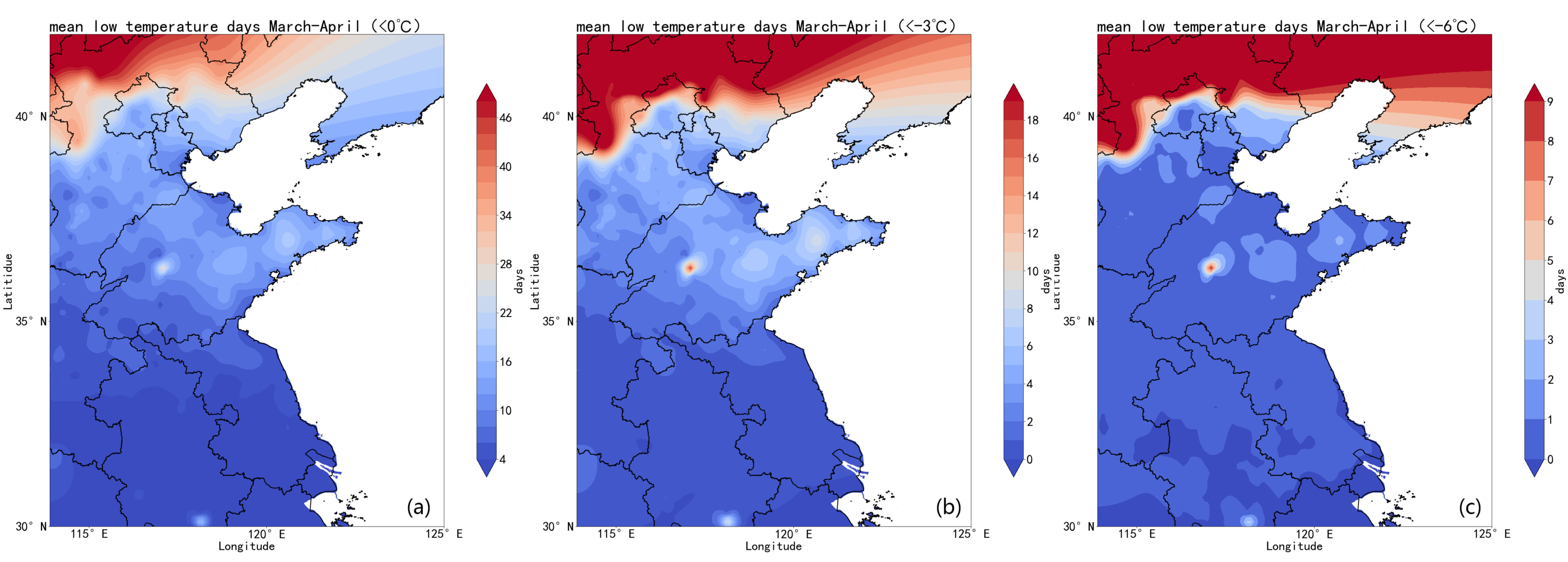
2.1. Meteorological Data Sources for the Study Area
2.2. Analysis of Low-Temperature Disasters in the Study Area
2.3. Experimental Materials
2.4. Experimental Design
2.5. Measurement Contents and Methods
2.5.1. The Measurement of Photosynthetic Parameters
2.5.2. Measurement of Photosynthetic Pigment Content
2.5.3. Measurement of Protective Enzyme Activity
2.5.4. Measurement of Endogenous Hormone Content
2.5.5. Measurement of Yield and Its Constituent Indicators
2.6. Statistical Analysis
3. Results
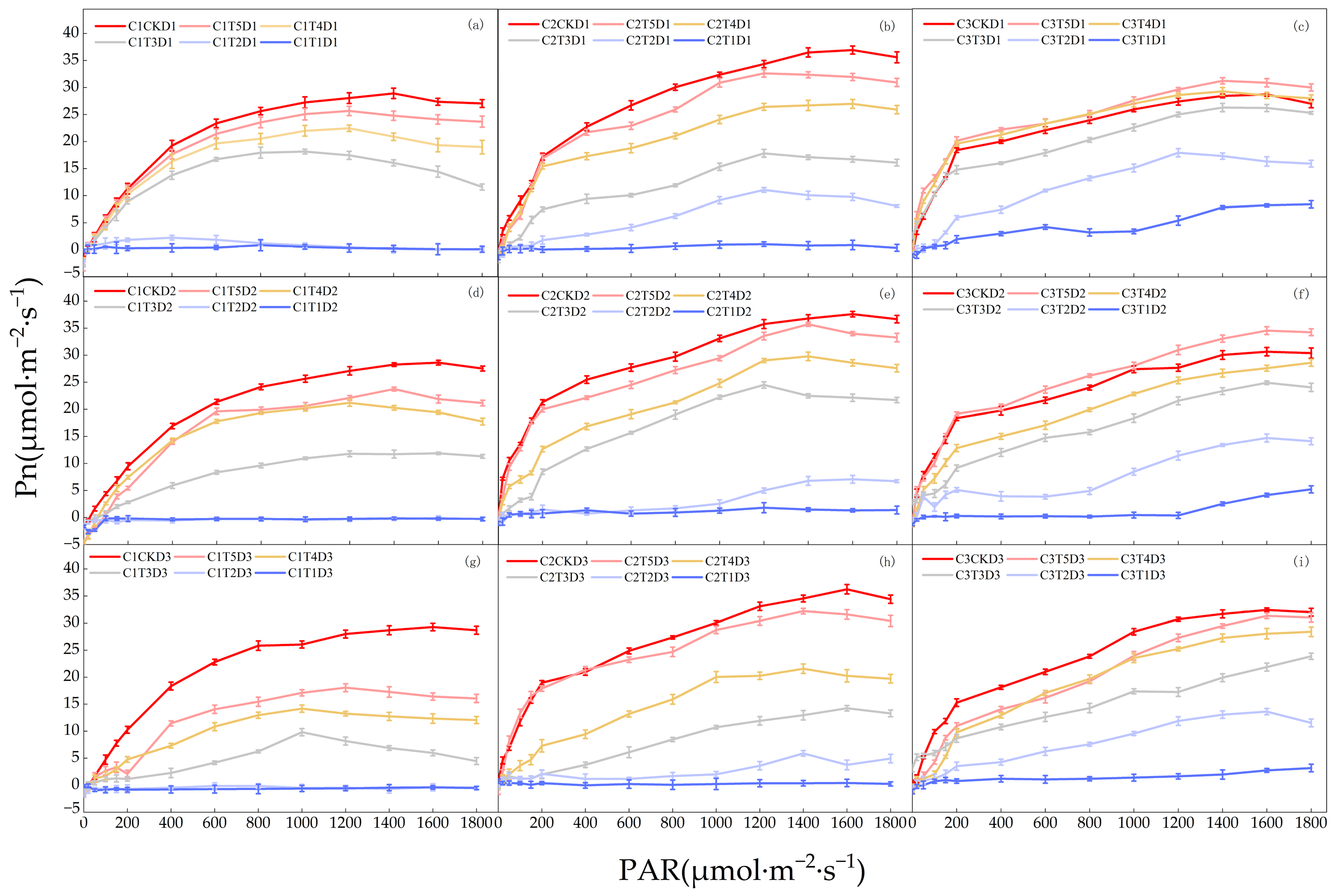
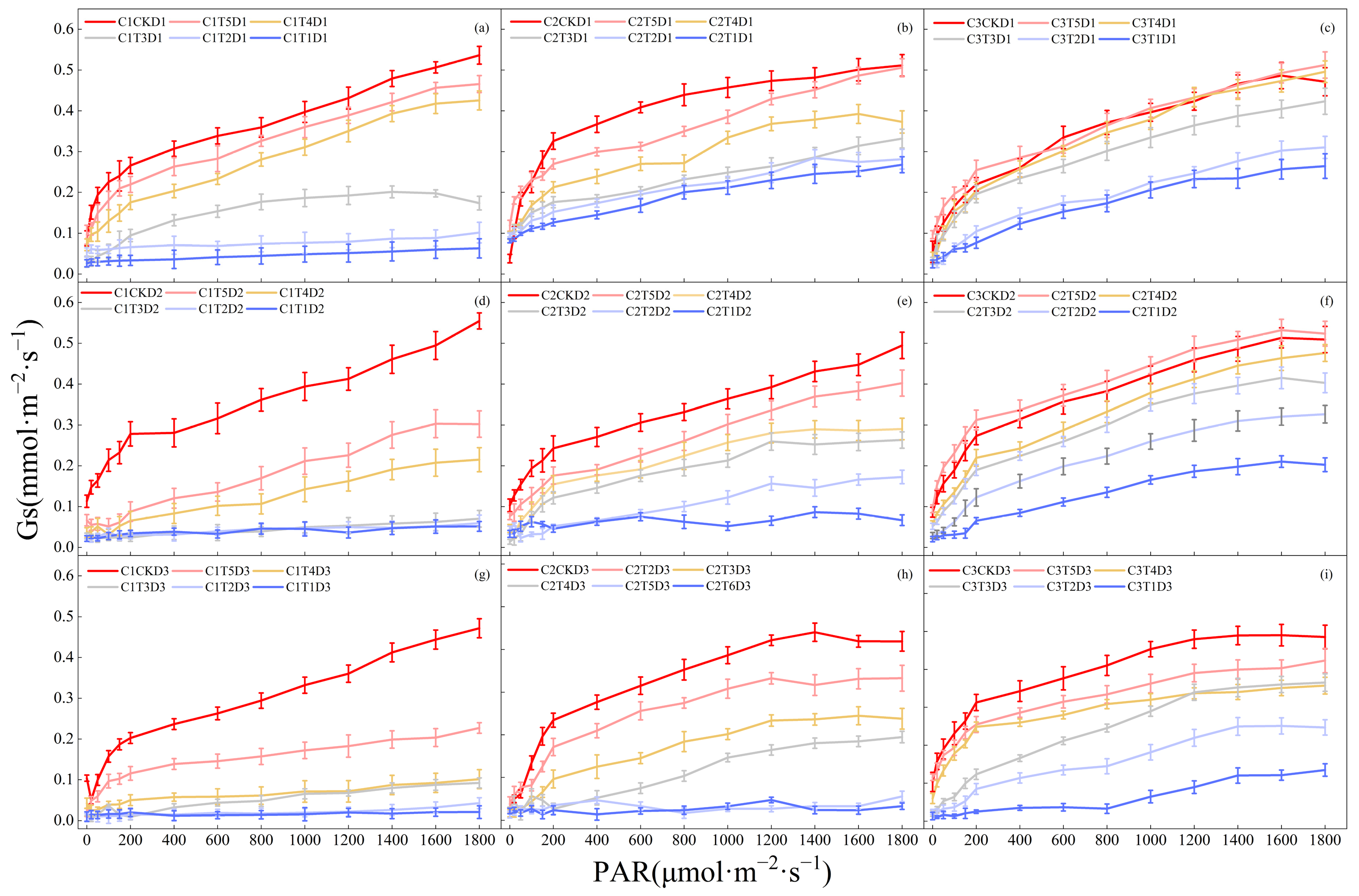
3.1. Effects of Low-Temperature Stress during the Jointing Stage on Photosynthetic Parameters of Leaves in Different Winter Wheat Varieties
3.2. Effects of Low-Temperature Stress during the Jointing Stage on Pigment Content in Leaves of Different Winter Wheat Varieties
3.3. Effects of Low-Temperature Stress during the Jointing Stage on the Activities of Protective Enzymes in Leaves of Different Winter Wheat Varieties
3.4. Effects of Low-Temperature Stress during the Jointing Stage on Endogenous Hormones in Leaves of Different Winter Wheat Varieties
3.5. Effects of Low-Temperature Stress during the Jointing Stage on the Yield of Different Winter Wheat Varieties
3.6. Constructing Indicators for Assessing Low-Temperature Disaster Levels in Different Winter Wheat Varieties under Low-Temperature Stress during the Jointing Stage
3.6.1. Correlation Analysis of Trait Indicators in Different Winter Wheat Varieties under Low-Temperature Stress during the Jointing Stage
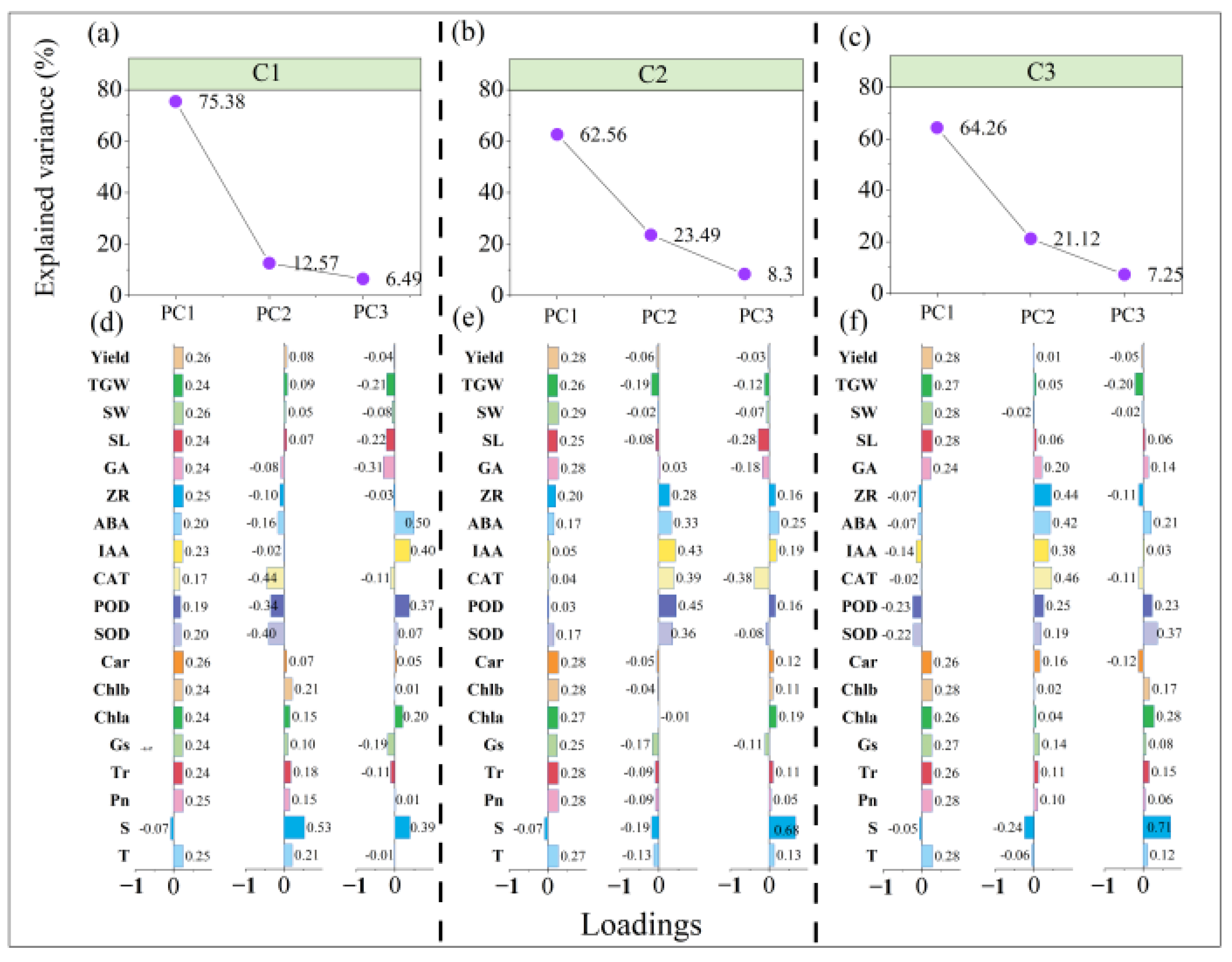
3.6.2. Principal Component Analysis of Trait Indicators in Different Winter Wheat Varieties under Low-Temperature Stress during the Jointing Stage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, J.; Han, Z.-Y.; Gao, X.-J.; Liu, Z.-J. Climatic impacts induced by winter wheat irrigation over North China simulated by the nonhydrostatic RegCM4.7. Adv. Clim. Change Res. 2024, 15, 197–210. [Google Scholar] [CrossRef]
- Tao, F.; Li, Y.; Chen, Y.; Yin, L.; Zhang, S. Daily, seasonal and inter-annual variations in CO2 fluxes and carbon budget in a winter-wheat and summer-maize rotation system in the North China Plain. Agric. For. Meteorol. 2022, 324, 109098. [Google Scholar] [CrossRef]
- Wang, D.; Rianti, W.; Gálvez, F.; van der Putten, P.E.L.; Struik, P.C.; Yin, X. Estimating photosynthetic parameter values of rice, wheat, maize and sorghum to enable smart crop cultivation. Crop Environ. 2022, 1, 119–132. [Google Scholar] [CrossRef]
- Karatayev, M.; Clarke, M.; Salnikov, V.; Bekseitova, R.; Nizamova, M. Monitoring climate change, drought conditions and wheat production in Eurasia: The case study of Kazakhstan. Heliyon 2022, 8, e08660. [Google Scholar] [CrossRef]
- Wu, X.; Liu, H.; Li, X.; Tian, Y.; Mahecha, M.D. Responses of Winter Wheat Yields to Warming-Mediated Vernalization Variations Across Temperate Europe. Front. Ecol. Evol. 2017, 5, 126. [Google Scholar] [CrossRef]
- Zhao, J.; Peng, H.; Yang, J.; Huang, R.; Huo, Z.; Ma, Y. Response of winter wheat to different drought levels based on Google Earth Engine in the Huang-Huai-Hai Region, China. Agric. Water Manag. 2024, 292, 108662. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, J.; Deng, X.-P. Winter wheat yield improvement by genetic gain across different provinces in China. J. Integr. Agric. 2023, 23, 468–483. [Google Scholar] [CrossRef]
- Albahri, A.S.; Khaleel, Y.L.; Habeeb, M.A.; Ismael, R.D.; Hameed, Q.A.; Deveci, M.; Homod, R.Z.; Albahri, O.S.; Alamoodi, A.H.; Alzubaidi, L. A systematic review of trustworthy artificial intelligence applications in natural disasters. Comput. Electr. Eng. 2024, 118, 109409. [Google Scholar] [CrossRef]
- Rastgoo, H.; Abbasi, E.; Bijani, M. Analysis of agricultural insurance vulnerability in the face of natural disasters: Insights from Iran. Environ. Sustain. Indic. 2024, 23, 100429. [Google Scholar] [CrossRef]
- Innes, P.J.; Tan, D.K.Y.; Van Ogtrop, F.; Amthor, J.S. Effects of high-temperature episodes on wheat yields in New South Wales, Australia. Agric. For. Meteorol. 2015, 208, 95–107. [Google Scholar] [CrossRef]
- Tian, Y.; Tian, X.; Yang, B.; Ma, J.; Shan, J.; Xing, F. Analysis of the impact of drying on common wheat quality and safety. Heliyon 2024, 10, e33163. [Google Scholar] [CrossRef] [PubMed]
- Abys, C.; Skakun, S.; Becker-Reshef, I. Two decades of winter wheat expansion and intensification in Russia. Remote Sens. Appl. Soc. Environ. 2024, 33, 101097. [Google Scholar] [CrossRef]
- Wang, B.; Li, L.; Feng, P.; Chen, C.; Luo, J.-J.; Taschetto, A.S.; Harrison, M.T.; Liu, K.; Liu, D.L.; Yu, Q.; et al. Probabilistic analysis of drought impact on wheat yield and climate change implications. Weather Clim. Extrem. 2024, 45, 100708. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J.; Huang, R.; Xie, H.; Qin, X.; Hu, Y. Estimating evapotranspiration and drought dynamics of winter wheat under climate change: A case study in Huang-Huai-Hai region, China. Sci. Total Environ. 2024, 949, 175114. [Google Scholar] [CrossRef] [PubMed]
- Shoukat, M.R.; Wang, J.; Habib-ur-Rahman, M.; Hui, X.; Hoogenboom, G.; Yan, H. Adaptation strategies for winter wheat production at farmer fields under a changing climate: Employing crop and multiple global climate models. Agric. Syst. 2024, 220, 104066. [Google Scholar] [CrossRef]
- Zhao, X.; Deng, G.; Xi, Y. Spatial–Temporal Characteristics and Driving Factors of Disaster-Induced Grain Yield Loss in China. Front. Environ. Sci. 2022, 10, 808565. [Google Scholar] [CrossRef]
- Donatti, C.I.; Nicholas, K.; Fedele, G.; Delforge, D.; Speybroeck, N.; Moraga, P.; Blatter, J.; Below, R.; Zvoleff, A. Global hotspots of climate-related disasters. Int. J. Disaster Risk Reduct. 2024, 108, 104488. [Google Scholar] [CrossRef]
- Cheng, W.; Li, Y.; Zuo, W.; Du, G.; Stanny, M. Spatio-temporal detection of agricultural disaster vulnerability in the world and implications for developing climate-resilient agriculture. Sci. Total Environ. 2024, 928, 172412. [Google Scholar] [CrossRef]
- Osawa, T. Evaluating the effectiveness of basin management using agricultural land for ecosystem-based disaster risk reduction. Int. J. Disaster Risk Reduct. 2022, 83, 103445. [Google Scholar] [CrossRef]
- Huang, C.; Li, N.; Zhang, Z.; Liu, Y. Examining the relationship between meteorological disaster economic impact and regional economic development in China. Int. J. Disaster Risk Reduct. 2024, 100, 104133. [Google Scholar] [CrossRef]
- Hu, J.; Ren, B.; Dong, S.; Liu, P.; Zhao, B.; Zhang, J. Poor development of spike differentiation triggered by lower photosynthesis and carbon partitioning reduces summer maize yield after waterlogging. Crop J. 2022, 10, 478–489. [Google Scholar] [CrossRef]
- Kang, M.; Wang, S.; Xu, Z.; Xu, C.; An, J.; Zhang, Y.; Zeng, Y.; Ali, I.; Tang, L.; Xiao, L.; et al. Simulating the effects of low-temperature stress during flowering stage on leaf-level photosynthesis with current rice models. Agric. For. Meteorol. 2024, 354, 110087. [Google Scholar] [CrossRef]
- Hayman, G.; Redhead, J.W.; Brown, M.; Pinnington, E.; Gerard, F.; Brown, M.; Fincham, W.; Robinson, E.L.; Huntingford, C.; Pywell, R.F. A framework for improved predictions of the climate impacts on potential yields of UK winter wheat and its applicability to other UK crops. Clim. Serv. 2024, 34, 100479. [Google Scholar] [CrossRef]
- Pang, H.; Lian, Y.; Zhao, Z.; Guo, H.; Li, Z.; Hu, J.; Ren, Y.; Lin, T.; Wang, Z. Compensatory effect of supplementary irrigation on winter wheat under warming conditions. Agric. Water Manag. 2024, 295, 108778. [Google Scholar] [CrossRef]
- Zeng, X.; Ma, L.; Yuan, J.; Xie, Y.; Guan, T.; Wang, X.; Ma, G.; Xu, Y.; Sun, W. Antioxidant metabolic system and comparative proteomics analysis in winter turnip rape (Brassica rapa L.) under cold stress. Oil Crop Sci. 2022, 7, 95–102. [Google Scholar] [CrossRef]
- Shah, T.; Latif, S.; Saeed, F.; Ali, I.; Ullah, S.; Abdullah Alsahli, A.; Jan, S.; Ahmad, P. Seed priming with titanium dioxide nanoparticles enhances seed vigor, leaf water status, and antioxidant enzyme activities in maize (Zea mays L.) under salinity stress. J. King Saud Univ. Sci. 2021, 33, 101207. [Google Scholar] [CrossRef]
- Ullah, S.; Afzal, I.; Shumaila, S.; Shah, W. Effect of naphthyl acetic acid foliar spray on the physiological mechanism of drought stress tolerance in maize (Zea mays L.). Plant Stress 2021, 2, 100035. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J.; Huang, Z.; Mi, L.; Xu, K.; Wu, J.; Fan, Y.; Ma, S.; Jiang, D. Effects of Low Temperature at Booting Stage on Sucrose Metabolism and Endogenous Hormone Contents in Winter Wheat Spikelet. Front. Plant Sci. 2019, 10, 498. [Google Scholar] [CrossRef]
- Li, Q.; Chang, X.-h.; Meng, X.-h.; Li, D.; Zhao, M.-h.; Sun, S.-l.; Li, H.-m.; Qiao, W.-c. Heat stability of winter wheat depends on cultivars, timing and protective methods. J. Integr. Agric. 2020, 19, 1984–1997. [Google Scholar] [CrossRef]
- Kosakivska, I.; Voytenko, L.; Vasyuk, V.; Shcherbatiuk, M. ABA-induced alterations in cytokinin homeostasis of Triticum aestivum and Triticum spelta under heat stress. Plant Stress 2024, 11, 100353. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Xie, J.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Abscisic acid and jasmonic acid are involved in drought priming-induced tolerance to drought in wheat. Crop J. 2021, 9, 120–132. [Google Scholar] [CrossRef]
- Love, B.; Molero, G.; Rivera-Amado, C.; Müller, M.; Munné-Bosch, S.; Reynolds, M.P.; Foulkes, M.J. Associations between endogenous spike cytokinins and grain-number traits in spring wheat genotypes. Eur. J. Agron. 2024, 152, 127011. [Google Scholar] [CrossRef]
- Malko, M.M.; Khanzada, A.; Wang, X.; Samo, A.; Li, Q.; Jiang, D.; Cai, J. Chemical treatment refines drought tolerance in wheat and its implications in changing climate: A review. Plant Stress 2022, 6, 100118. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Li, L.; Xu, X.; Yang, L.; Luo, Z.; Wang, B.; Ma, S.; Fan, Y.; Huang, Z. The Effects of Short-Term Exposure to Low Temperatures During the Booting Stage on Starch Synthesis and Yields in Wheat Grain. Front. Plant Sci. 2021, 12, 684784. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Song, Y.; Wang, J.; Zhang, Z.; Ma, S.; Jiang, K.; Pan, Z. Climatic threshold of crop production and climate change adaptation: A case of winter wheat production in China. Front. Ecol. Evol. 2022, 10, 1019436. [Google Scholar] [CrossRef]
- Hamani, A.K.M.; Abubakar, S.A.; Si, Z.; Kama, R.; Gao, Y.; Duan, A. Suitable split nitrogen application increases grain yield and photosynthetic capacity in drip-irrigated winter wheat (Triticum aestivum L.) under different water regimes in the North China Plain. Front. Plant Sci. 2022, 13, 1105006. [Google Scholar] [CrossRef]
- Yang, G.; Li, X.; Liu, P.; Yao, X.; Zhu, Y.; Cao, W.; Cheng, T. Automated in-season mapping of winter wheat in China with training data generation and model transfer. ISPRS J. Photogramm. Remote Sens. 2023, 202, 422–438. [Google Scholar] [CrossRef]
- Sani Shawai, R.; Liu, D.; Li, L.; Chen, T.; Li, M.; Cao, S.; Xia, X.; Liu, J.; He, Z.; Zhang, Y. QTL mapping for pre-harvest sprouting in a recombinant inbred line population of elite wheat varieties Zhongmai 578 and Jimai 22. Crop J. 2023, 11, 863–869. [Google Scholar] [CrossRef]
- Ma, Y.; Agathokleous, E.; Xu, Y.; Cao, R.; He, L.; Feng, Z. Cultivar-specific regulation of antioxidant enzyme activity and stomatal closure confer tolerance of wheat to elevated ozone: A two-year open-field study with five cultivars. Plant Stress 2024, 12, 100479. [Google Scholar] [CrossRef]
- Wu, J.; Gu, Y.; Sun, K.; Wang, N.; Shen, H.; Wang, Y.; Ma, X. Correlation of climate change and human activities with agricultural drought and its impact on the net primary production of winter wheat. J. Hydrol. 2023, 620, 129504. [Google Scholar] [CrossRef]
- Morozumi, T.; Kato, T.; Kobayashi, H.; Sakai, Y.; Tsujimoto, K.; Nakashima, N.; Buareal, K.; Lan, W.; Ninomiya, H. Row orientation influences the diurnal cycle of solar-induced chlorophyll fluorescence emission from wheat canopy, as demonstrated by radiative transfer modeling. Agric. For. Meteorol. 2023, 339, 109576. [Google Scholar] [CrossRef]
- Wu, L.; Quan, H.; Wu, L.; Zhang, X.; Feng, H.; Ding, D.; Siddique, K.H.M. Responses of winter wheat yield and water productivity to sowing time and plastic mulching in the Loess Plateau. Agric. Water Manag. 2023, 289, 108572. [Google Scholar] [CrossRef]
- Chao, E.; Wu, M.; Yue, D.; Yuan, Y.; Qiu, N.; Zhou, F. Promoting effect of low concentration strontium on photosynthetic performance of Chinese cabbage seedlings: Combined leaf characteristics, photosynthetic carbon assimilation and chlorophyll fluorescence. Ecotoxicol. Environ. Saf. 2024, 274, 116200. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, J.; Wang, J. Protective enzyme activity regulation in cotton (Gossypium hirsutum L.) in response to Scirpus planiculmis stress. Front. Plant Sci. 2022, 13, 1068419. [Google Scholar] [CrossRef]
- Ge, W.; Bu, H.; Wang, X.; Xia, Y.; Martinez, S.A.; Wang, X.; Qi, W.; Liu, K.; Du, G. Changes in endogenous hormone contents during seed germination of Anemone rivularis var. flore-minore. Glob. Ecol. Conserv. 2020, 24, e01200. [Google Scholar] [CrossRef]
- Zhuang, H.; Zhang, Z.; Cheng, F.; Han, J.; Luo, Y.; Zhang, L.; Cao, J.; Zhang, J.; He, B.; Xu, J.; et al. Integrating data assimilation, crop model, and machine learning for winter wheat yield forecasting in the North China Plain. Agric. For. Meteorol. 2024, 347, 109909. [Google Scholar] [CrossRef]
- Zhang, H.; Dean, L.; Wang, M.L.; Dang, P.; Lamb, M.; Chen, C. GWAS with principal component analysis identify QTLs associated with main peanut flavor-related traits. Front. Plant Sci. 2023, 14, 1204415. [Google Scholar] [CrossRef]
- Ahmed, N.; Zhang, Y.; Li, K.; Zhou, Y.; Zhang, M.; Li, Z. Exogenous application of glycine betaine improved water use efficiency in winter wheat (Triticum aestivum L.) via modulating photosynthetic efficiency and antioxidative capacity under conventional and limited irrigation conditions. Crop J. 2019, 7, 635–650. [Google Scholar] [CrossRef]
- Lin, F.; Li, C.; Xu, B.; Chen, J.; Chen, A.; Hassan, M.A.; Liu, B.; Xu, H.; Chen, X.; Sun, J.; et al. Late spring cold reduces grain number at various spike positions by regulating spike growth and assimilate distribution in winter wheat. Crop J. 2023, 11, 1272–1278. [Google Scholar] [CrossRef]
- Xiao, L.; Asseng, S.; Wang, X.; Xia, J.; Zhang, P.; Liu, L.; Tang, L.; Cao, W.; Zhu, Y.; Liu, B. Simulating the effects of low-temperature stress on wheat biomass growth and yield. Agric. For. Meteorol. 2022, 326, 109191. [Google Scholar] [CrossRef]
- Zhai, L.; Song, S.; Zhang, L.; Huang, J.; Lv, L.; Dong, Z.; Cui, Y.; Zheng, M.; Hou, W.; Zhang, J.; et al. Subsoiling before winter wheat alleviates the kernel position effect of densely grown summer maize by delaying post-silking root-shoot senescence. J. Integr. Agric. 2023; in press. [Google Scholar] [CrossRef]
- Liu, X.-J.; Yin, B.-Z.; Hu, Z.-H.; Bao, X.-Y.; Wang, Y.-D.; Zhen, W.-C. Physiological response of flag leaf and yield formation of winter wheat under different spring restrictive irrigation regimes in the Haihe Plain, China. J. Integr. Agric. 2021, 20, 2343–2359. [Google Scholar] [CrossRef]
- Gholizadeh, F.; Mirzaghaderi, G.; Marashi, S.H.; Janda, T. Polyamines-Mediated amelioration of cold treatment in wheat: Insights from morpho-physiological and biochemical features and PAO genes expression analyses. Plant Stress 2024, 11, 100402. [Google Scholar] [CrossRef]
- Onyemaobi, O.; Sangma, H.; Garg, G.; Wallace, X.; Kleven, S.; Dolferus, R. Transcriptome profiling of the chilling response in wheat spikes: I, acclimation response to long-term chilling treatment. Curr. Plant Biol. 2022, 31, 100255. [Google Scholar] [CrossRef]
- Cann, D.J.; Schillinger, W.F.; Hunt, J.R.; Porker, K.D.; Harris, F.A.J. Agroecological Advantages of Early-Sown Winter Wheat in Semi-Arid Environments: A Comparative Case Study From Southern Australia and Pacific Northwest United States. Front. Plant Sci. 2020, 11, 568. [Google Scholar] [CrossRef]
- Zhu, T.; Liu, B.; Liu, N.; Xu, J.; Song, X.; Li, S.; Sui, S. Gibberellin-related genes regulate dwarfing mechanism in wintersweet. Front. Plant Sci. 2022, 13, 1010896. [Google Scholar] [CrossRef]
- Shang, Q.; Wang, Y.; Tang, H.; Sui, N.; Zhang, X.; Wang, F. Genetic, hormonal, and environmental control of tillering in wheat. Crop J. 2021, 9, 986–991. [Google Scholar] [CrossRef]
- Xu, X.; He, W.; Zhang, H. A novel habitat adaptability evaluation indicator (HAEI) for predicting yield of county-level winter wheat in China base on multisource climate data from 2001 to 2020. Int. J. Appl. Earth Obs. Geoinf. 2023, 125, 103603. [Google Scholar] [CrossRef]
- Dong, Z.Q.; Jiang, M.Y.; Xue, X.P.; Pan, Z.H.; Li, N.; Zhao, H.; Hou, Y.Y. The applicability evaluation and drought validation of the WOFOST model for the simulation of winter wheat growth in Shandong Province, China. Heliyon 2022, 8, e12004. [Google Scholar] [CrossRef]

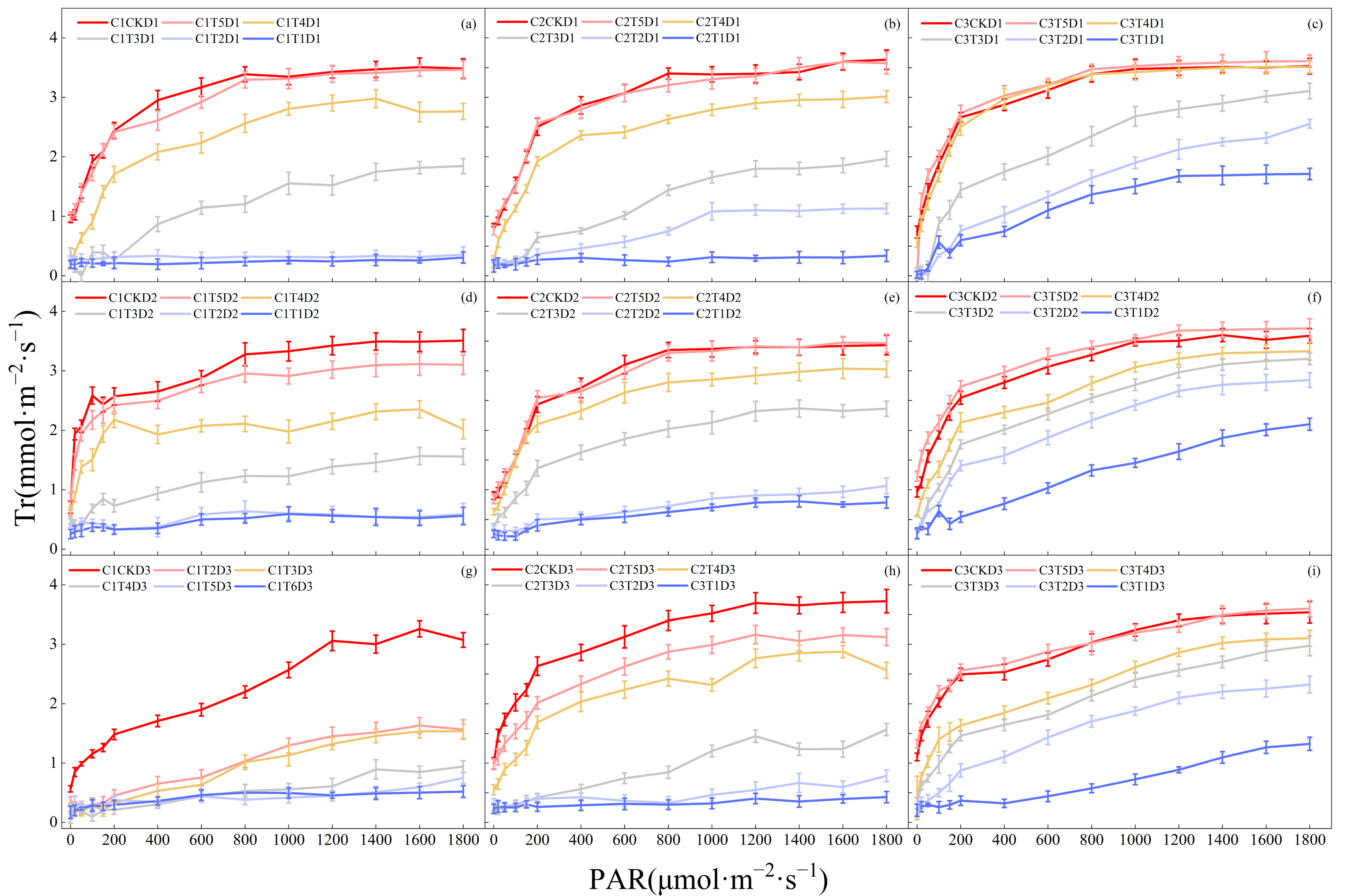

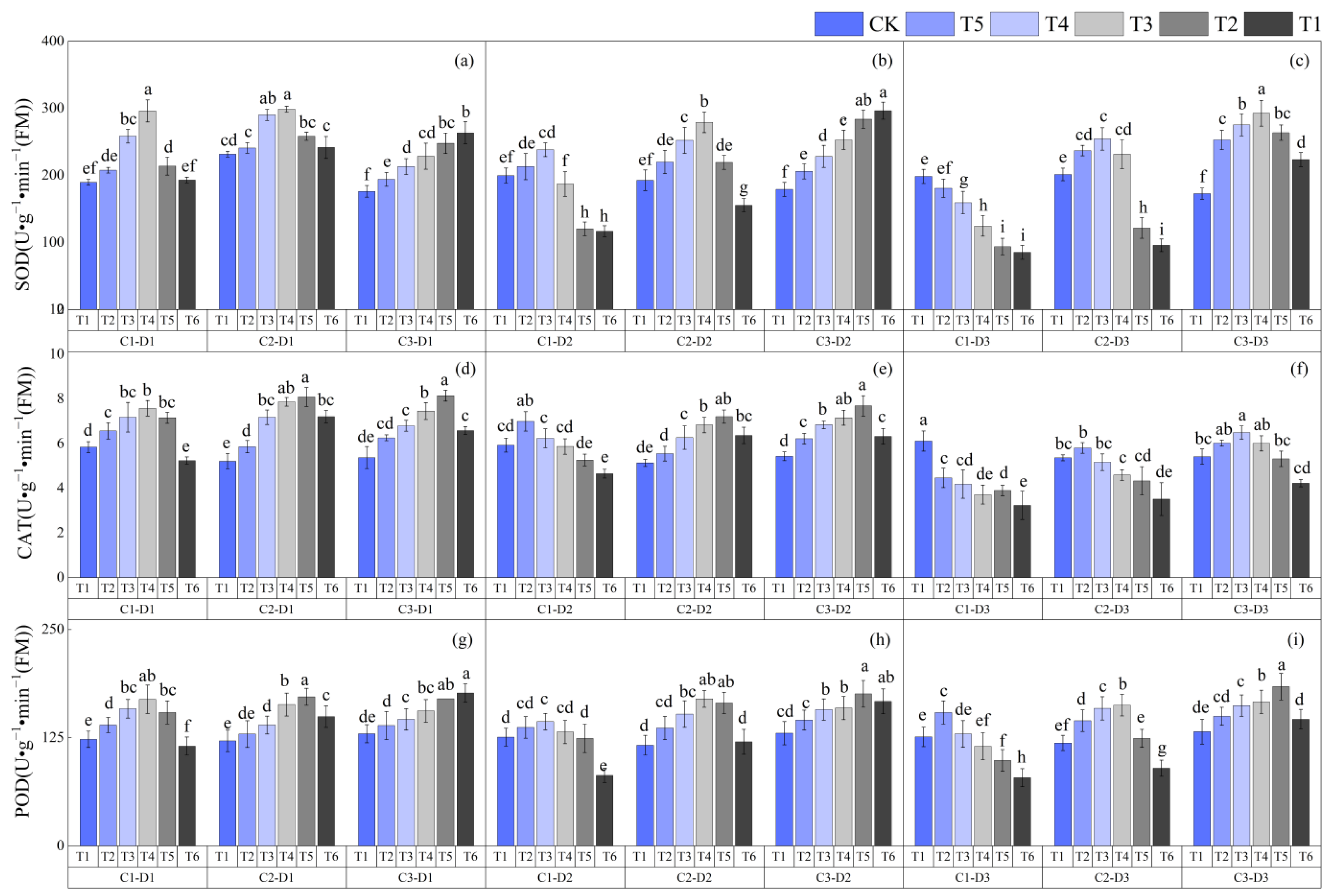
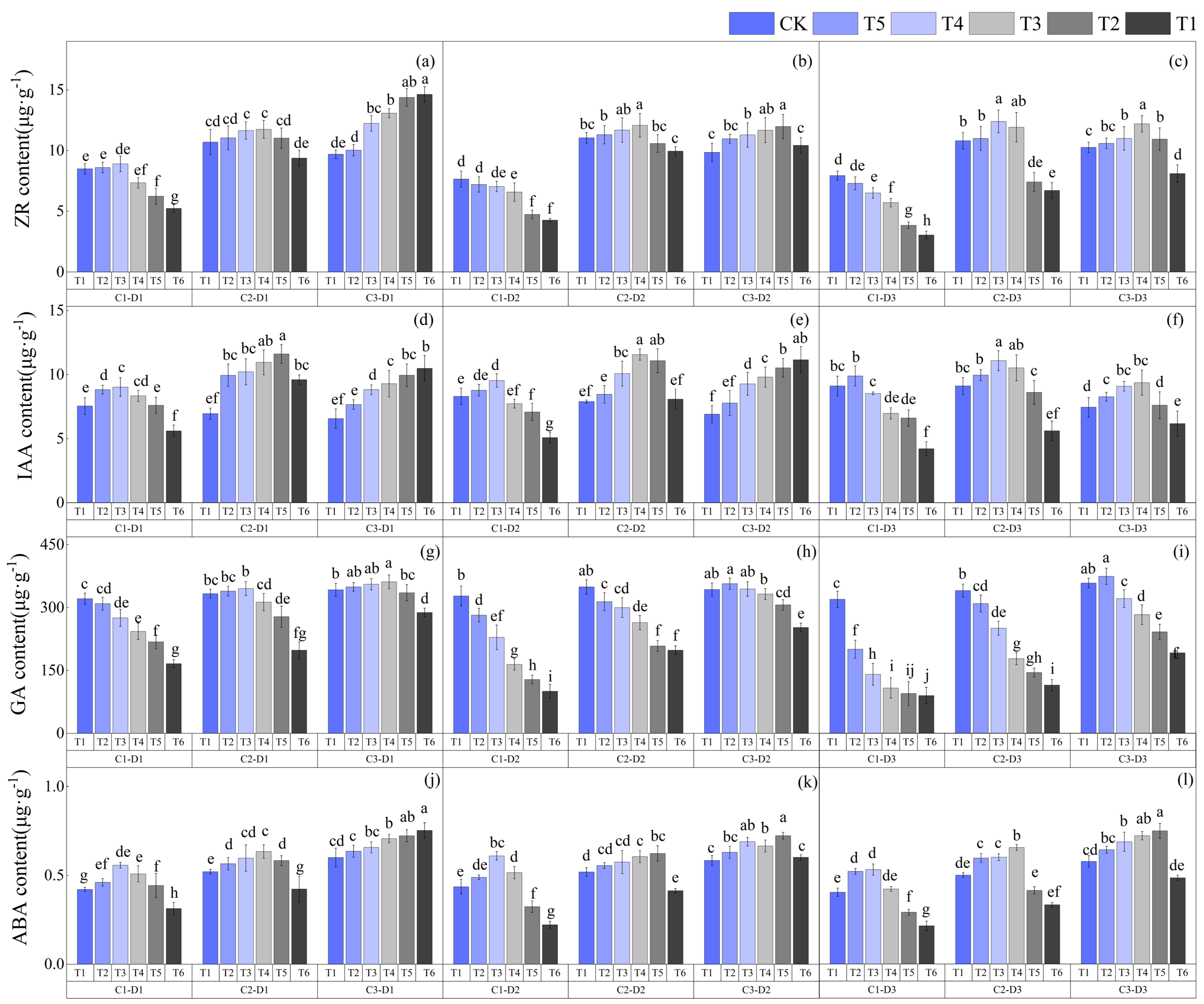
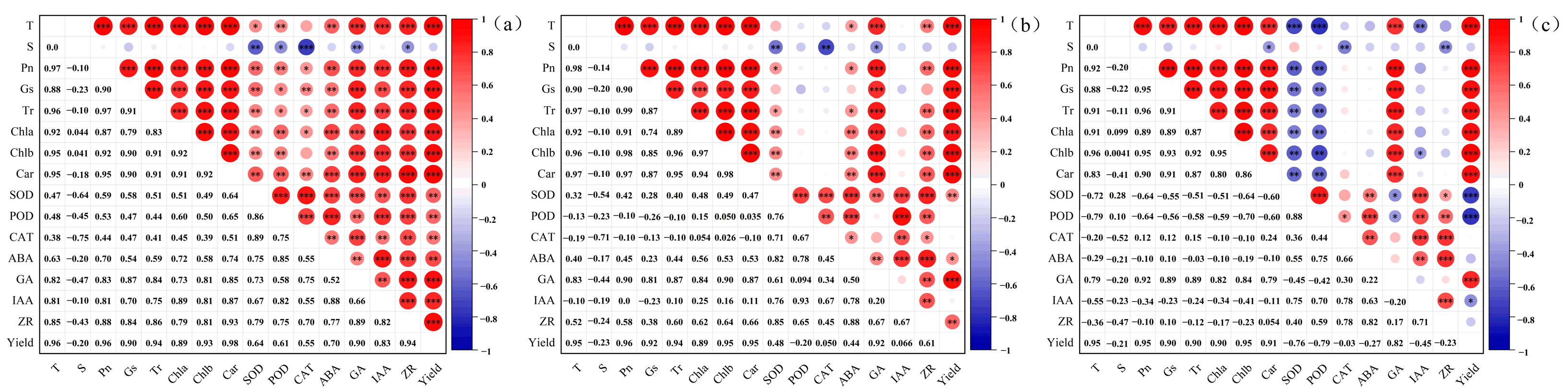
| Year | Cultivar | Stage | Temperature (Tmix/Tmax) (°C) | Duration(d) |
|---|---|---|---|---|
| 2021 | (C2)Jimai22 | Joint Stage | CK (8/18), T4(3/13), T3(0/10), | D1(2d), D2(4d), |
| T2(−3/7) | D3(6d) | |||
| 2022 | (C1)Zhenmai12, (C2)Jimai22, | CK (8/18), T5(6/16), T4(3/13), | D1(2d), D2(4d), | |
| (C3)Shannong38 | T3(0/10), T2(−3/7), T1(−6/4) | D3(6d) | ||
| 2023 | (C1)Zhenmai12, (C2)Jimai22, | CK (8/18), T5(6/16), T4(3/13), | D1(2d), D2(4d), | |
| (C3)Shannong38 | T3(0/10), T2(−3/7), T1(−6/4) | D3(6d) |
| Marker | Cultivated Species | Temperature [°C] | Duration [d] |
|---|---|---|---|
| C1CKD1/C2CKD1/C3CKD1 | Zhen Mai 12/Ji Mai 22/Shan Nong 28 | 18/8 | 2 |
| C1CKD2/C2CKD2/C3CKD2 | Zhen Mai 12/Ji Mai 22/Shan Nong 28 | 18/8 | 4 |
| C1CKD3/C2CKD3/C3CKD3 | Zhen Mai 12/Ji Mai 22/Shan Nong 28 | 18/8 | 6 |
| C1T5D1/C2T5D1/C3T5D1 | Zhen Mai 12/Ji Mai 22/Shan Nong 28 | 16/6 | 2 |
| C1T5D2/C2T5D2/C3T5D3 | Zhen Mai 12/Ji Mai 22/Shan Nong 28 | 16/6 | 4 |
| C1T5D3/C2T5D3/C3T5D3 | Zhen Mai 12/Ji Mai 22/Shan Nong 28 | 16/6 | 6 |
| C1T4D1/C2T4D1/C3T4D1 | Zhen Mai 12/Ji Mai 22/Shan Nong 28 | 13/3 | 2 |
| C1T4D2/C2T4D2/C3T4D2 | Zhen Mai 12/Ji Mai 22/Shan Nong 28 | 13/3 | 4 |
| C1T4D3/C2T4D3/C2T4D3 | Zhen Mai 12/Ji Mai 22/Shan Nong 28 | 13/3 | 6 |
| C1T3D1/C2T3D1/C3T3D1 | Zhen Mai 12/Ji Mai 22/Shan Nong 28 | 10/0 | 2 |
| C1T3D2/C2T3D2/C3T3D2 | Zhen Mai 12/Ji Mai 22/Shan Nong 28 | 10/0 | 4 |
| C1T3D3/C2T3D3/C3T3D3 | Zhen Mai 12/Ji Mai 22/Shan Nong 28 | 10/0 | 6 |
| C1T2D1/C2T2D1/C3T2D1 | Zhen Mai 12/Ji Mai 22/Shan Nong 28 | 7/−3 | 2 |
| C1T2D2/C2T2D2/C3T2D2 | Zhen Mai 12/Ji Mai 22/Shan Nong 28 | 7/−3 | 4 |
| C1T2D3/C2T2D3/C3T2D3 | Zhen Mai 12/Ji Mai 22/Shan Nong 28 | 7/−3 | 6 |
| C1T1D1/C2T1D1/C3T1D1 | Zhen Mai 12/Ji Mai 22/Shan Nong 28 | 4/−6 | 2 |
| C1T1D2/C2T1D2/C3T1D2 | Zhen Mai 12/Ji Mai 22/Shan Nong 28 | 4/−6 | 4 |
| C1T1D3/C2T1D2/C3T1D3 | Zhen Mai 12/Ji Mai 22/Shan Nong 28 | 4/−6 | 6 |
| Temperature (a) | Stress Days (d) | Spikelet Length (cm) | Spikelet Weight (g) | Thousand-Grain Weight (g) | Yield/Pot (g·pot−1) | Reduction Rate (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C1 | C2 | C3 | C1 | C2 | C3 | C1 | C2 | C3 | C1 | C2 | C3 | ||
| CK | D1-D2-D3 | 7.25 ± 0.13 Ba | 7.25 ± 0.13 Ba | 7.96 ± 0.23 Aa | 6.94 ± 0.27 Bab | 2.23 ± 0.12 Bab | 2.74 ± 0.2 Aa | 2.32 ± 0.21 Bab | 45.27 ± 4.95 ABab | 50.99 ± 2.63 Aa | 39.33 ± 3.39 Ba | 73.71 ± 3.83 Ba | 81.79 ± 2.78 Aa | 64.06 ± 3.14 Cab | — | — |
| T5 | D1 | 6.62 ± 0.28 Bb | 6.62 ± 0.28 Bb | 8.04 ± 0.3 Aa | 7.05 ± 0.31 Ba | 2.43 ± 0.22 Aa | 2.67 ± 0.18 Aab | 2.42 ± 0.18 Aa | 47.93 ± 2.28 Aa | 47.95 ± 2.28 Aab | 39.27 ± 2.28 Ba | 70.4 ± 2.28 Bab | 77.83 ± 2.28 Ab | 65.6 ± 2.28 Ca | −4.64 | 0.70 |
| D2 | 6.35 ± 0.29 Bbcd | 6.35 ± 0.29 Bbcd | 7.65 ± 0.29 Aabc | 6.94 ± 0.29 Bab | 2.34 ± 0.29 Aa | 2.6 ± 0.2 Aabc | 2.26 ± 0.2 Aab | 46.26 ± 2.29 Aab | 46.28 ± 2.29 Abc | 39.47 ± 2.29 Ba | 67.62 ± 2.29 Bbc | 76.64 ± 2.29 Abc | 65.08 ± 2.29 Bab | −6.04 | −0.06 | |
| D3 | 6.29 ± 0.29 Bbcd | 6.29 ± 0.29 Bbcd | 7.23 ± 0.31 Abcd | 7.08 ± 0.32 Aa | 2.34 ± 0.23 Aa | 2.58 ± 0.19 Aabc | 2.39 ± 0.19 Aa | 42.85 ± 2.29 Abc | 42.87 ± 2.29 Acd | 39.18 ± 2.29 Ba | 67.21 ± 2.29 Bbc | 74.3 ± 2.29 Abc | 65.22 ± 2.29 Bab | −9.00 | −0.06 | |
| T4 | D1 | 6.47 ± 0.3 Bbc | 6.47 ± 0.3 Bbc | 7.71 ± 0.31 Aab | 6.89 ± 0.33 Bab | 2.32 ± 0.23 Aa | 2.57 ± 0.2 Aabc | 2.24 ± 0.2 Aabc | 38.9 ± 2.3 Bcd | 40.53 ± 2.3 Ade | 38.94 ± 2.3 Ba | 68.37 ± 2.3 Bbc | 72.99 ± 2.3 Ac | 62.49 ± 2.3 Cabc | −10.56 | −4.09 |
| D2 | 6.3 ± 0.32 Bbcd | 6.3 ± 0.32 Bbcd | 7.4 ± 0.32 Abcd | 6.89 ± 0.32 ABab | 2.24 ± 0.32 Aab | 2.55 ± 0.22 Aabc | 2.19 ± 0.22 Aabc | 34.35 ± 2.32 Bdefg | 39.95 ± 2.32 Ade | 34.39 ± 2.32 Bbcd | 65.53 ± 2.32 Bc | 72.7 ± 2.32 Ac | 61.86 ± 2.32 Babc | −10.96 | −5.11 | |
| D3 | 5.99 ± 0.27 Bcde | 5.99 ± 0.27 Bcde | 6.61 ± 0.27 Aefg | 6.79 ± 0.27 Aabc | 2.1 ± 0.27 Aabc | 2.49 ± 0.17 Aabcd | 2.1 ± 0.17 Aabcd | 33.58 ± 2.27 Befg | 37.23 ± 2.27 Aefg | 33.62 ± 2.27 Bbcde | 64.35 ± 2.27 Bcd | 72.27 ± 2.27 Ac | 59.34 ± 2.27 Ccde | −11.46 | −8.97 | |
| T3 | D1 | 6.35 ± 0.29 Bbcd | 6.35 ± 0.29 Bbcd | 7.6 ± 0.29 Aabc | 6.79 ± 0.29 Babc | 2.08 ± 0.29 Aabc | 2.53 ± 0.19 Aabcd | 2.1 ± 0.19 Aabcd | 36.8 ± 2.29 Bde | 39.12 ± 2.29 Adef | 36.84 ± 2.29 Bab | 60.87 ± 2.29 Bde | 72.49 ± 2.29 Ac | 60.9 ± 2.29 Bbcd | −11.20 | −6.58 |
| D2 | 6.18 ± 0.29 Bbcd | 6.18 ± 0.29 Bbcd | 7.13 ± 0.29 Acde | 6.69 ± 0.29 ABabcd | 1.93 ± 0.29 Aabcd | 2.4 ± 0.19 Aabcde | 1.98 ± 0.19 Abcde | 35.56 ± 2.29 Bdef | 38.14 ± 2.29 Aef | 35.6 ± 2.29 ABab | 60.18 ± 2.29 Bdef | 72.4 ± 2.29 Ac | 58.31 ± 2.29 Bcde | −11.30 | −10.56 | |
| D3 | 5.93 ± 0.29 Bcde | 5.93 ± 0.29 Bcde | 7.22 ± 0.29 Abcd | 6.7 ± 0.29 Aabcd | 1.75 ± 0.29 Bbcde | 2.28 ± 0.19 Acdef | 1.95 ± 0.19 ABbcde | 30.32 ± 2.29 Bghi | 34.84 ± 2.29 Afgh | 30.36 ± 2.29 Bde | 56.44 ± 2.29 Bfg | 65.59 ± 2.29 Ad | 55.02 ± 2.29 Befg | −19.72 | −15.65 | |
| T2 | D1 | 5.87 ± 0.31 Bdef | 5.87 ± 0.31 Bdef | 7.03 ± 0.31 Adef | 6.41 ± 0.31 Bbcde | 1.8 ± 0.31 Bbcde | 2.33 ± 0.21 Abcdef | 1.88 ± 0.21 ABcde | 32.83 ± 2.29 Befgh | 35.41 ± 2.29 Afgh | 35.07 ± 2.29 Aabc | 58.22 ± 2.29 Bef | 67.77 ± 2.29 Ad | 57.24 ± 2.29 Bg | −16.90 | −12.07 |
| D2 | 5.54 ± 0.29 Befg | 5.54 ± 0.29 Befg | 6.65 ± 0.29 Aefg | 6.31 ± 0.29 Acde | 1.66 ± 0.29 Bcde | 2.28 ± 0.19 Acdef | 1.8 ± 0.19 Bde | 31.16 ± 2.29 Bfghi | 35.23 ± 2.29 Afgh | 33.4 ± 2.29 ABbcde | 52.56 ± 2.29 Bgh | 64.41 ± 2.29 Ad | 55.32 ± 2.29 Befg | −20.84 | −15.07 | |
| D3 | 5.3 ± 0.32 Bg | 5.3 ± 0.32 Bg | 6.53 ± 0.32 Afg | 6.17 ± 0.32 Ade | 1.52 ± 0.32 Ade | 2.05 ± 0.22 Aefg | 1.75 ± 0.22 Ade | 28.54 ± 2.32 Bgi | 33.49 ± 2.32 Agh | 30.78 ± 2.32 ABcde | 50.21 ± 2.32 Bhi | 59.89 ± 2.32 Ae | 52.54 ± 2.32 Bdef | −26.59 | −19.32 | |
| T1 | D1 | 5.55 ± 0.29 Cefg | 5.55 ± 0.29 Cefg | 7.04 ± 0.29 Adef | 6.36 ± 0.29 Bbcde | 1.66 ± 0.29 Bcde | 2.17 ± 0.19 Adefg | 1.79 ± 0.19 ABde | 30.84 ± 2.29 Bfghi | 35.07 ± 2.29 Afgh | 33.08 ± 2.29 ABbcde | 52.39 ± 2.29 Bfg | 63.78 ± 2.29 Ad | 53.43 ± 2.29 Bfg | −24.81 | −17.97 |
| D2 | 5.36 ± 0.29 Bfg | 5.36 ± 0.29 Bfg | 6.54 ± 0.29 Afg | 6.19 ± 0.29 Ade | 1.52 ± 0.29 Ade | 1.98 ± 0.19 Afg | 1.69 ± 0.19 Ae | 28.01 ± 2.29 Bi | 33.46 ± 2.29 Agh | 30.25 ± 2.29 ABde | 47.37 ± 2.29 Cij | 58.86 ± 2.29 Ae | 52.42 ± 2.29 Bg | −27.83 | −19.48 | |
| D3 | 5.1 ± 0.29 Bg | 5.1 ± 0.29 Bg | 6.44 ± 0.29 Ag | 5.98 ± 0.28 Ae | 1.37 ± 0.28 Be | 1.84 ± 0.17 Ag | 1.61 ± 0.17 ABe | 27.42 ± 2.26 Bi | 32.14 ± 2.26 Ah | 29.65 ± 2.25 ABe | 45.68 ± 2.25 Cj | 57.82 ± 2.24 Ae | 51.88 ± 2.24 Bg | −29.04 | −20.21 | |
| Cultivated Species | C1 | C2 | C3 |
|---|---|---|---|
| Stress Level | Comprehensive Score (Z) | Comprehensive Score (Z) | Comprehensive Score (Z) |
| No stress (0) | 2.23 < Z | 1.37 < Z | 1.16 < Z |
| Mild stress (1) | −0.02 < Z ≤ 2.23 | 0.33< Z ≤ 1.37 | 0.63 < Z ≤ 1.16 |
| Moderate stress (2) | −2.26 <Z ≤ −0.02 | −0.56 < Z ≤ 0.33 | 0.11 < Z ≤ 0.63 |
| Heavy stress (3) | Z ≤ −2.26 | Z ≤ −0.56 | Z ≤ 0.11 |
| Temperatures | CK | T5 | T4 | T3 | T2 | T1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cultivated Species | Stress Days | Z | Stress Level | Z | Stress Level | Z | Stress Level | Z | Stress Level | Z | Stress Level | Z | Stress Level |
| C1 | D1 | 2.28 | 0 | 1.71 | 1 | 1.52 | 1 | 1.01 | 1 | −0.15 | 2 | −2.29 | 3 |
| D2 | 2.31 | 0 | 1.37 | 1 | 0.96 | 1 | 0.67 | 1 | −0.96 | 2 | −3.47 | 3 | |
| D3 | 2.44 | 0 | 1.09 | 1 | 0.50 | 1 | −0.37 | 2 | −3.53 | 3 | −5.30 | 3 | |
| C2 | D1 | 1.77 | 0 | 1.46 | 0 | 1.03 | 1 | 0.65 | 1 | 0.17 | 2 | −0.40 | 3 |
| D2 | 1.63 | 0 | 1.15 | 0 | 0.86 | 1 | −0.04 | 2 | −0.25 | 2 | −0.61 | 3 | |
| D3 | 1.56 | 0 | 1.03 | 1 | 0.55 | 1 | −0.31 | 2 | −0.54 | 2 | −1.16 | 3 | |
| C3 | D1 | 1.23 | 0 | 1.20 | 0 | 1.16 | 0 | 0.72 | 1 | 0.53 | 2 | 0.15 | 2 |
| D2 | 1.24 | 0 | 1.19 | 0 | 0.82 | 1 | 0.55 | 2 | 0.26 | 2 | 0.12 | 2 | |
| D3 | 1.22 | 0 | 1.17 | 0 | 0.70 | 1 | 0.51 | 2 | 0.13 | 2 | −0.79 | 3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Huo, Z.; Yang, Z.; Zhang, L.; Wang, N.; Jiang, M.; Kong, R.; Mi, Q.; Li, M.; Wu, H. Construction of Indicators of Low-Temperature Stress Levels at the Jointing Stage of Winter Wheat. Agriculture 2024, 14, 1430. https://doi.org/10.3390/agriculture14081430
Zhang F, Huo Z, Yang Z, Zhang L, Wang N, Jiang M, Kong R, Mi Q, Li M, Wu H. Construction of Indicators of Low-Temperature Stress Levels at the Jointing Stage of Winter Wheat. Agriculture. 2024; 14(8):1430. https://doi.org/10.3390/agriculture14081430
Chicago/Turabian StyleZhang, Fengyin, Zhiguo Huo, Zaiqiang Yang, Lei Zhang, Na Wang, Mengyuan Jiang, Rui Kong, Qianchuan Mi, Meixuan Li, and Huizhen Wu. 2024. "Construction of Indicators of Low-Temperature Stress Levels at the Jointing Stage of Winter Wheat" Agriculture 14, no. 8: 1430. https://doi.org/10.3390/agriculture14081430






