1. Introduction
Ginger (
Zingiber officinale Roscoe), a perennial herb of the ginger family, holds high medicinal and edible value and is an important cash crop globally [
1]. Many studies have reported that various compounds extracted from ginger have anti-cancer, antioxidant, anti-inflammatory, and other beneficial properties [
2,
3,
4,
5].
Ginger has been cultivated in China for over 2000 years. Today, China is a major player in the global ginger market, being one of the largest producers, consumers, and exporters of ginger [
6]. With more than 2700 years of cultivation history, China has a rich variety of ginger types in terms of form, color, flavor quality, and yield traits. Among the cultivars, little yellow ginger is particularly notable for its small tuber, high gingerol content, and greater medicinal value compared to ordinary ginger [
7]. Luoping County in Yunnan Province in China is the core producing area for this high-quality ginger. In 2016, Luoping small yellow ginger was registered under China’s National Geographical Indication Certification trademark. In 2020, it was included in the first batch of mutual recognition products listed in the China–EU Geographical Indication agreement and became a Sino–European geographical indication protected brand. However, with the increase in planting years and the low resistance of ginger to continuous cropping, Luoping is now facing the challenge of finding suitable land to grow small yellow ginger due to continuous cropping being an obstacle.
Ginger blast is one of the primary factors preventing the continuous cultivation of ginger and is the most severe disease affecting the ginger industry. In addition to the common characteristics of soil-borne diseases, ginger blast is highly infectious, aggressive, and devastating. Once ginger blast occurs in fields, the infection will spread rapidly, leading to a sharp reduction in ginger yield or to no yield at all [
8,
9]. Ginger blast is caused by
Ralstonia solanacearum, a bacterium that lives in the soil. Contact chemical agents and biological agents cannot completely eradicate it because they struggle to penetrate the deep soil and distribute evenly. When the climate becomes favorable, the surviving
R.
solanacearum will rapidly multiply and infect ginger plants, resulting in another outbreak of ginger blast. Current cultivation practices, disease-resistant varieties, and fungicides are not effective at controlling ginger blast [
10,
11].
Soil fumigation is one of the most effective techniques for controlling ginger blast. It involves using fumigants that release gases into the soil, which can be deeply and evenly distributed to kill soil pathogens before the planting of crops [
12]. Since 1950, methyl bromide, chloropicrin, metham-sodium, dazomet, and other fungicides have been used for soil fumigation around the world [
13]. Currently, the commercially available soil fumigants in China include chloropicrin, dazomet, metham-sodium, dimethyl disulfide (DMDS), and allyl isothiocyanate (AITC) [
14].
Dazomet and chloropicrin are both broad-spectrum soil fumigants widely used on fruit and vegetable crops, effective against nematodes, fungi, bacteria, and weeds [
15,
16,
17,
18]. Locascio et al. [
19] found that dazomet had a notable effect on controlling soil-borne diseases of tomatoes, effectively inhibiting fungal growth, killing nematodes, and significantly increasing tomato yield. A study by de Cal et al. [
20] showed that dazomet could effectively reduce the pathogenic fungus population in strawberry soil. Wang et al. [
21] reported that chloropicrin treatment could help overcome the continuous cropping obstacle and promote the growth of
Panax notoginseng. In another study, Wang et al. [
22] found that chloropicrin could significantly reduce the relative abundances of pathogens in soil and improve the survival rate of continuously cropped
P. notoginseng. Regarding the safety of fumigants, Cao et al. [
23] concluded that chloropicrin does not have significant long-term effects on soil microbial communities and stated that chloropicrin and its degradation products have no teratogenic effects.
At present, dazomet and chloropicrin have achieved good results in the prevention and control of soil-borne diseases in various crops. This study introduces the novel approach of using these two fumigants to control ginger blast. It also examines the changes in the soil bacterial community during the cultivation process and the flavor quality of Luoping small yellow ginger after harvest. The findings of this study can provide a theoretical and scientific basis for the efficient use of soil fumigants to control ginger blast in the field, thereby improving the yield and flavor quality of Luoping small yellow ginger.
2. Materials and Methods
2.1. Test Materials
The ginger variety used in this study was Chinese Luoping small yellow ginger. The soil fumigants were 99.5% chloropicrin, produced by Dalian Lufeng Chemical Co., Ltd., Dalian, China, and 98% dazomet particulate agent, produced by Nantong Shizhuang Chemical Co., Ltd., Nantong, China. The tarp covering the soil after fumigation was a 0.04 mm PVC tarp produced by Shandong Longxing Plastic Film Technology Co., Ltd., Weifang, Shandong, China.
2.2. Overview of the Test Site
The experimental site was located in a continuous ginger cropping field in Qingcaotang Village, Lushan Street, Luoping County, Qujing City, Yunnan Province, China (24.90472318° N, 104.33795941° E). The field test was conducted at two locations over two years. The physical and chemical properties of the test site are given in
Table 1.
2.3. Test Treatment
Over the two years of field trials, three treatments were established as the following: 75 g/m2 of dazomet, 52 g/m2 of chloropicrin, and a control group without any treatment. Three replicates were arranged for each treatment at each site. Each replicate area was 333 m2. All replicate fields were randomly arranged, and deep trenches (50 cm deep and 30 cm wide) were dug between different replicates to ensure isolation. In 2022, the fumigation operation was carried out on 29 March and the PVC tarp was removed on 13 April. In 2023, the fumigation operation was carried out on 13 March, and the tarp was removed on 28 March. The fumigation operation was carried out according to the “NY/T3129-2017 dazomet soil disinfection technical specification” and “NY/T2725-2015 technical specification for chloropicrin soil disinfection”.
Dazomet was mechanically mixed into the soil [
24,
25], and chloropicrin was administered using an injection method [
26,
27]. After fumigation, planting, fertilization, watering, and weeding were carried out according to conventional planting methods.
2.4. Sample Collection
In 2022, this study focused on the effect of the fumigants in controlling ginger blast and determining ginger yield. In 2023, experiments were conducted to verify the results, as well as to determine the effects of fumigation on the microflora in rhizospheric soil and the ginger quality (metabolome).
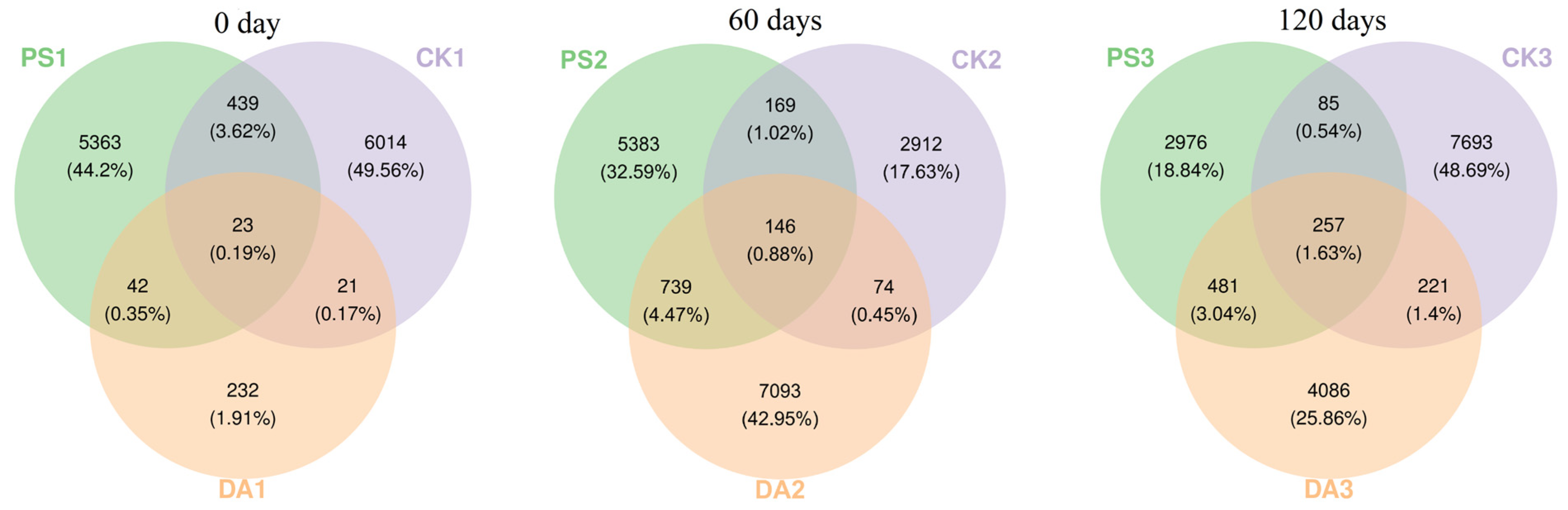
In 2023, rhizospheric soil was collected using a 5-point sampling method from the control site, the dazomet treatment site, and the chloropicrin treatment site, at 0, 60, and 120 days after tarp removal. The soil samples were transported in a dry ice box and then stored at −80 °C for 16S rRNA high-throughput sequencing. Information on the collected soil samples is given in
Table 2.
Regarding fresh ginger sample collection, on harvest day in 2023, 5 ginger rhizomes were randomly collected for each repeat for the control, dazomet treatment, and chloropicrin treatment, placed in a dry ice box, and taken back to the laboratory. The ginger rhizomes from each repeat were cut into small pieces and mixed evenly. A 0.5 kg sample from each mixture was taken and stored in the refrigerator at −80 °C for metabolomics analysis.
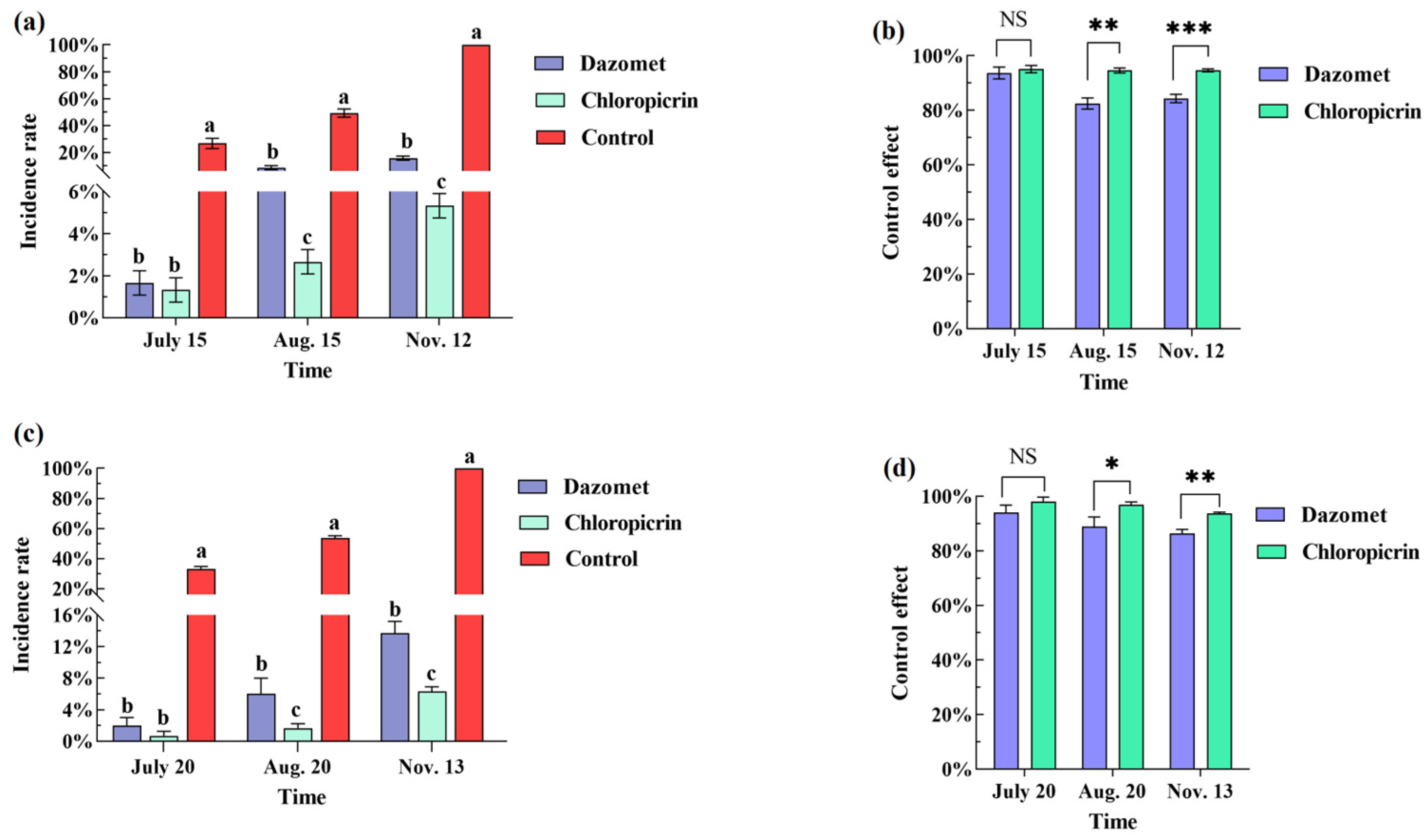
2.5. Investigation of Ginger Blast and Yield
In 2022 and 2023, the incidence of ginger blast was investigated at the early stage of ginger blast occurrence, at the outbreak, and at the harvest stage. A 5-point sampling method was adopted for each replicate, with 20 ginger plants investigated at each point. A total of 300 ginger plants were investigated for each treatment.
The investigation of small yellow ginger agronomic characteristics and yield was carried out on harvest day. The traits investigated included the plant height, stem diameter, branch number, and fresh weight of the ginger rhizome per plant. The investigation involved a 5-point sampling method for each repeat and taking 2 plants from each point for measurements.
For yield determination, the fresh weight of ginger rhizomes was determined by sampling all plants within 10 m
2 at each location. The final yield (kg/hm
2) was adjusted according to the incidence of ginger blast in the fields. The ginger rhizomes were graded according to the CODEX standard [
28].
We summarized and organized the collected data, and the significance of differences in the data for the ginger blast, agronomic trait, and yield were tested by one-way analysis of variance (ANOVA) with SPSS 26.0 software, Duncan’s new multiple range method, and Student’s t-test (p = 0.05). Plots were generated using GraphPadPrism 9 software.
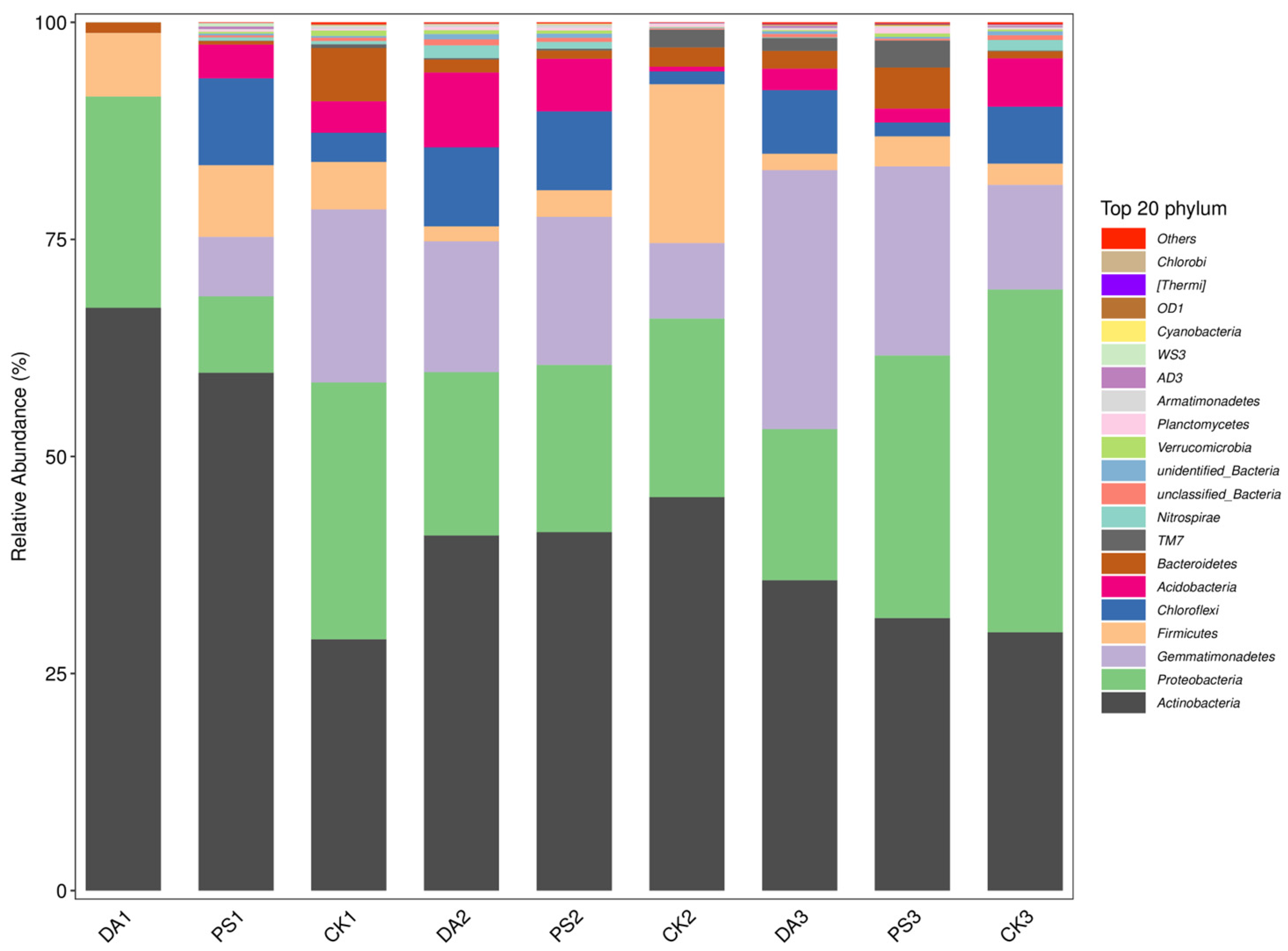
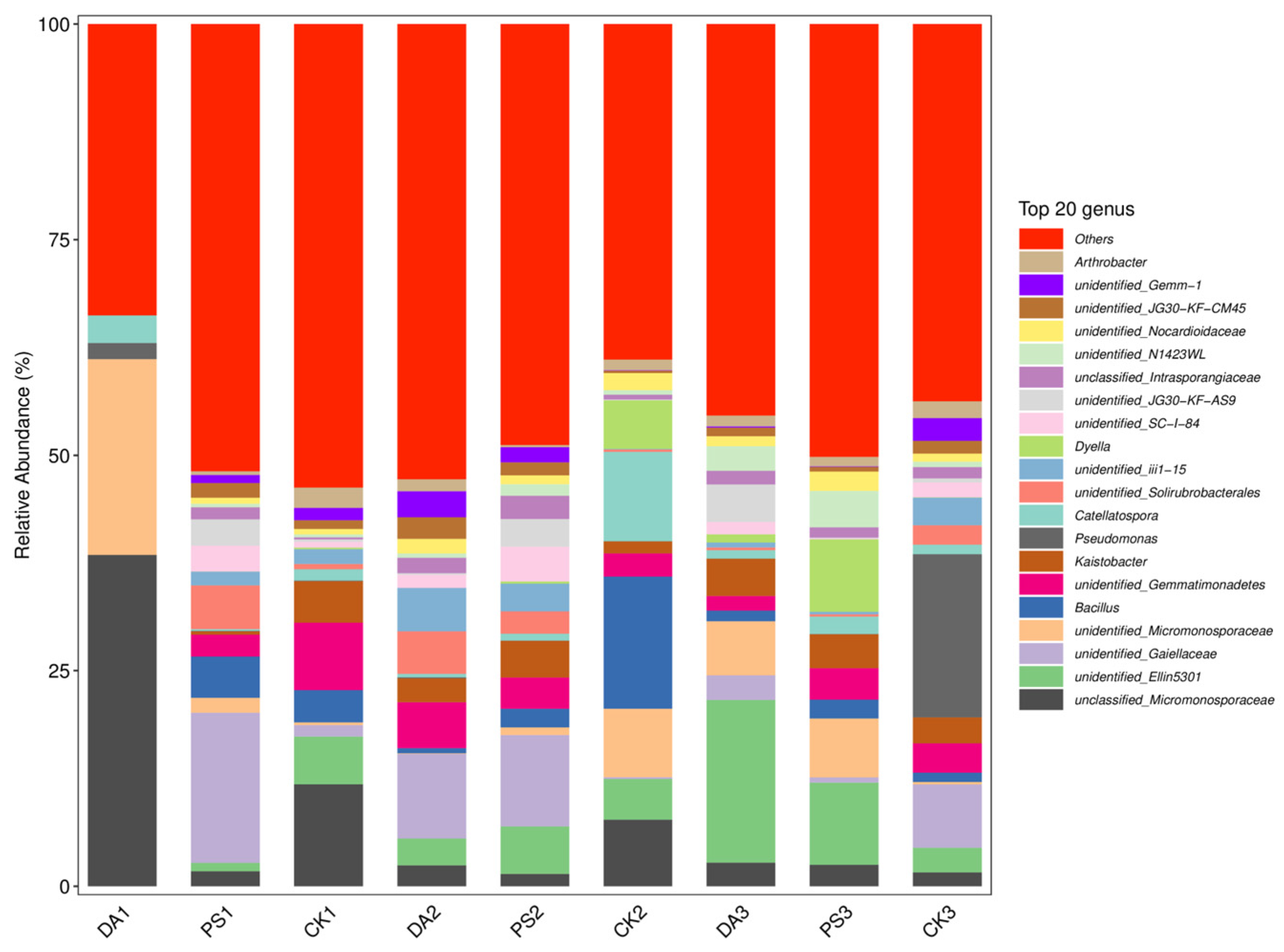
2.6. Sequencing and Data Analysis of the 16S rRNA of Soil Microorganisms
For 16S rRNA sequencing, the total DNA of soil microorganisms was extracted using an OMEGA Soil DNA Kit (M5635-02) (Omega Bio-Tek, Norcross, GA, USA). The 16S rRNA V3–V4 region was amplified using the bacterial universal primer sequences 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). After recovery and purification, the PCR products were sent to Shanghai Baipu Biotechnology Co., Ltd. (Shanghai, China), for high-throughput sequencing on the Illumina MiSeq platform.
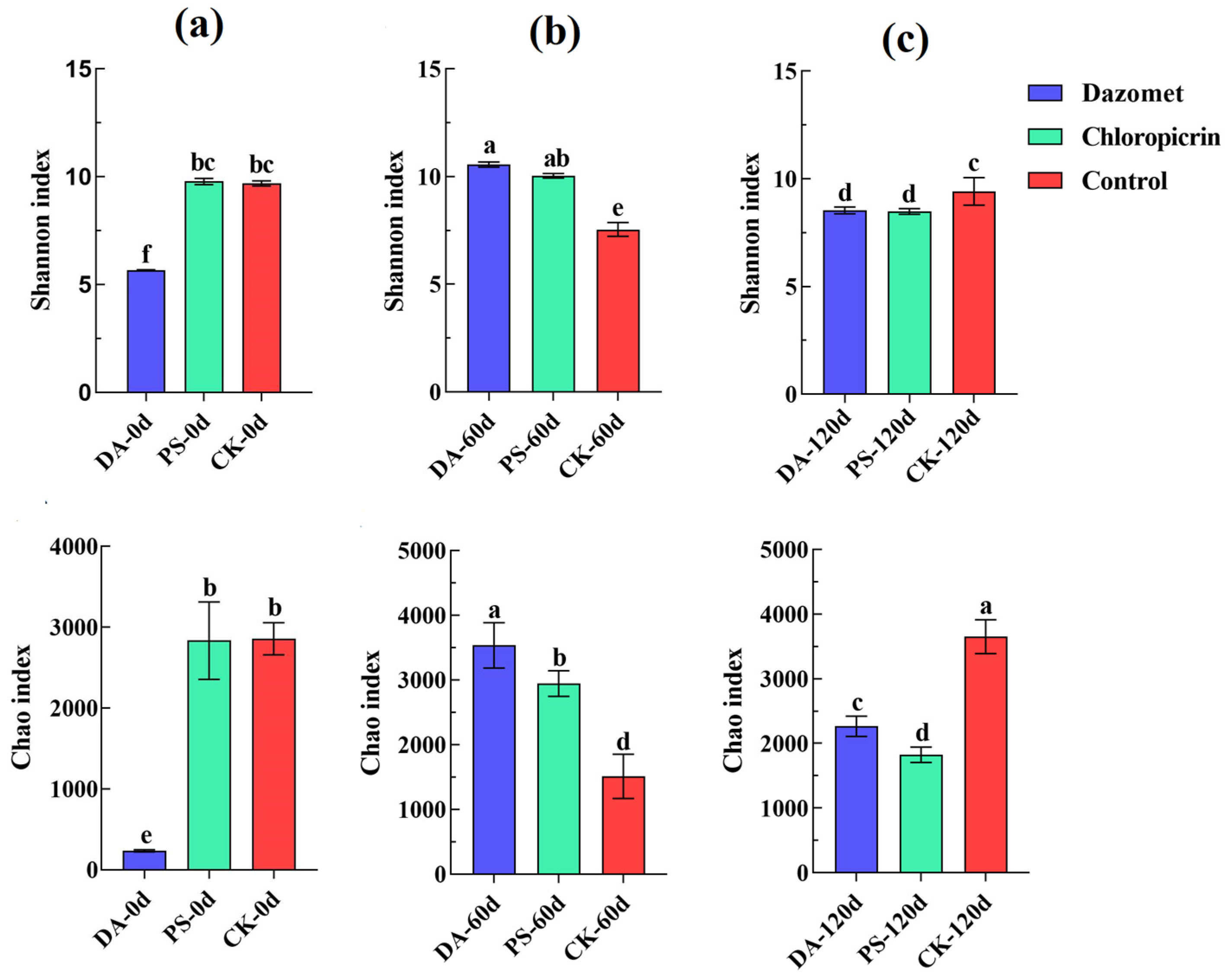
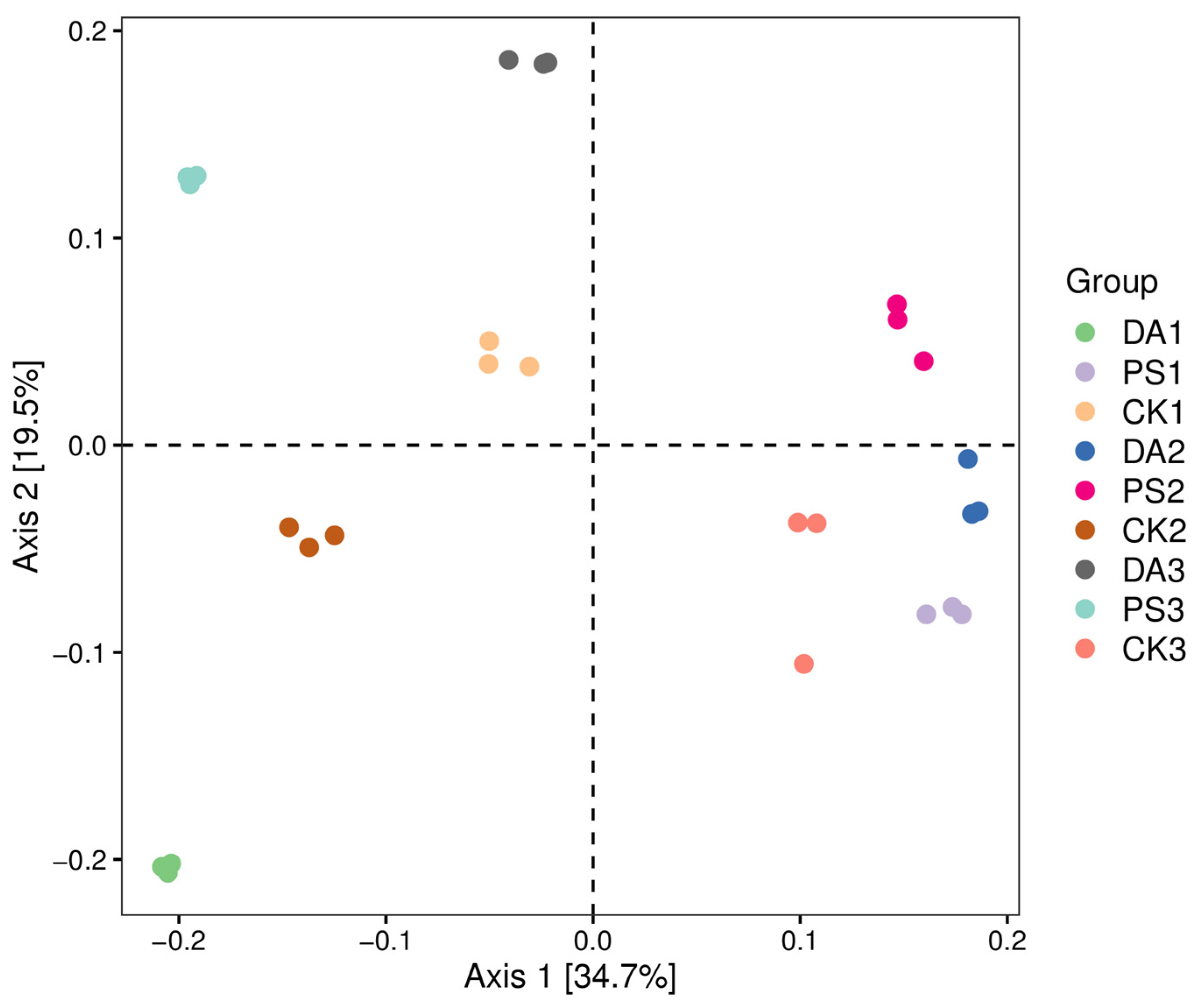
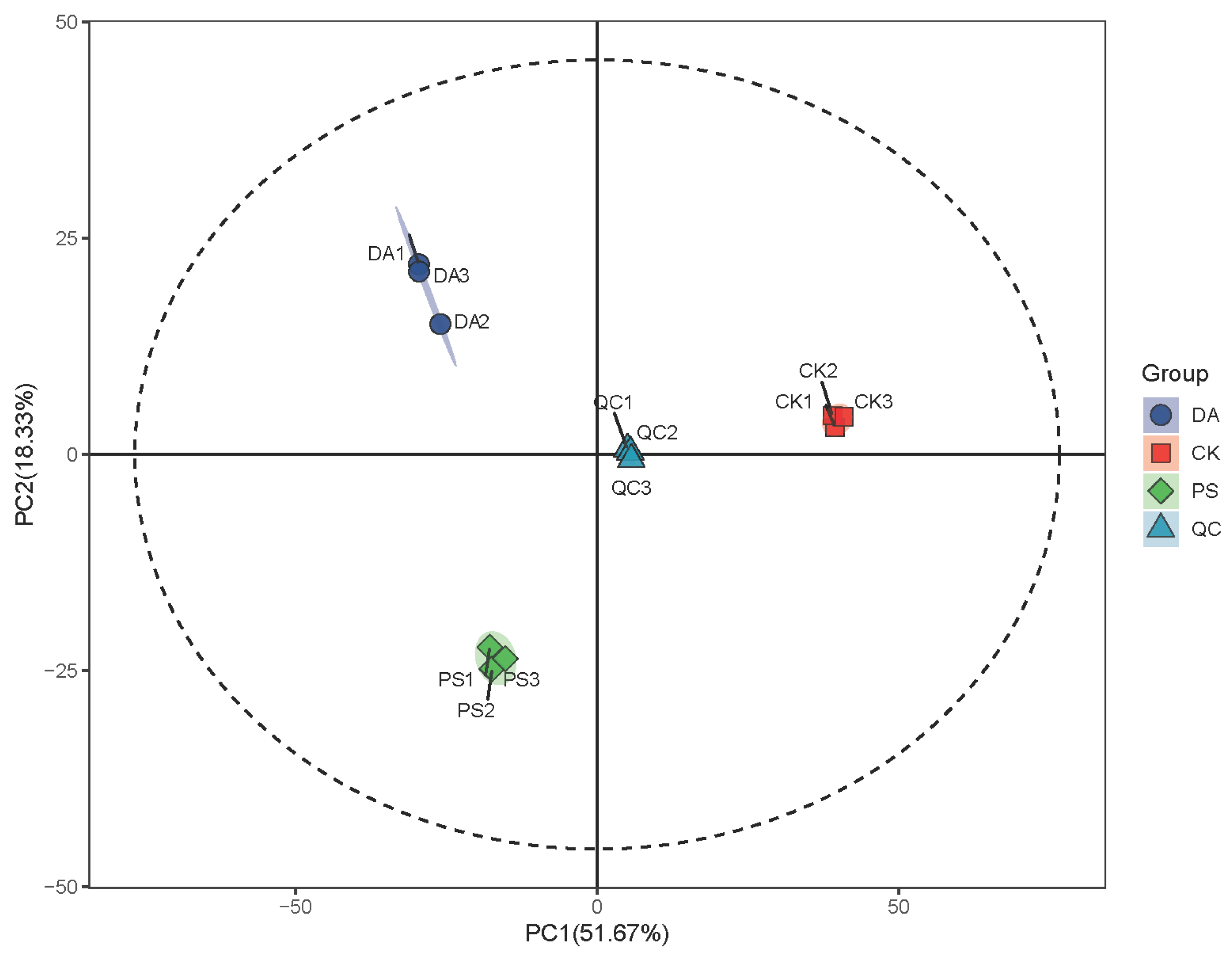
For data analysis, after sequencing, the DADA2 plugin was used for quality filtering, denoising, merging, and chimera removal. Then, 97% of non-chimera sequences were clustered, and OTU representative sequences and OTU tables were generated. The alpha diversity index was calculated using QIIME2 (2019.4) to compare the diversity and richness of different samples. Principal coordinate analysis (PCoA) was performed with R (4.0.3) and QIIME2 (2019.4) software to study the soil bacterial community compositions under different treatments according to the weighted UniFrac algorithm.
2.7. Determination and Data Analysis of the Metabolome of Small Yellow Ginger
For metabolome determination, the non-targeted metabolomic testing of small yellow ginger was conducted by Shanghai Baipu Biotechnology Co., Ltd.; a SHIMADZU-LC30 ultra-high performance liquid chromatography system (UHPLC) was used to isolate the metabolome. Chromatography was performed on an ACQUITY UPLC® HSS T3 (2.1 × 100 mm, 1.8 µm) column (Waters, Milford, MA, USA) with a QE Plus mass spectrometer (Thermo Scientific, MA, USA). Each sample was analyzed in both positive ion (+) and negative ion (−) modes using electrospray ionization (ESI), with ionization performed using a HESI source.
For data analysis, MSDIAL (4.9) software was used for peak alignment, retention time correction, and peak area extraction for the raw data. Metabolite structures were identified by precise mass number matching (mass tolerance < 10 ppm) and secondary spectrum matching (mass tolerance < 0.01 Da). Public databases such as HMDB, MassBank, GNPS, and self-established BP-DB were searched. The total peak area of the positive and negative ion data was normalized, and the peaks of positive and negative ions were integrated. Pattern recognition was carried out using the Python programming language. After processing the data by unit variance (UV) scaling, subsequent data analysis was conducted.
5. Conclusions
The results of this study showed that dazomet and chloropicrin could effectively control ginger blast, overcome the challenges of continuous cropping, and significantly improve the yield and quality of ginger. Treatment with both fumigants increased the relative abundance of some beneficial bacteria associated with plant resistance to soil-borne diseases. Additionally, both fumigants upregulated phenolic compound and terpenoid production, and chloropicrin treatment also increased the levels of amino acids and their derivatives. The upregulation of these substances can effectively improve flavor quality and enhance antioxidant, anti-inflammatory, and anti-tumoral properties in ginger.
For a long time, ginger blast has affected the healthy growth of small yellow ginger in Luoping County, Yunnan, China, causing the local ginger industry to shrink continuously. After two years of large-scale fumigation experiments, disinfecting soil via fumigation opened up a promising future, and the yield and quality of small yellow ginger improved. In the future, by combining soil fumigation with the addition of beneficial bacteria and bio-organic fertilizers, and integrating chemical fumigation into biological control technologies, the amount of chemical fumigants can be further reduced to achieve sustainable development in the Luoping small yellow ginger industry.