Migration of Microplastics in the Rice–Duckweed System under Different Irrigation Modes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. MPs
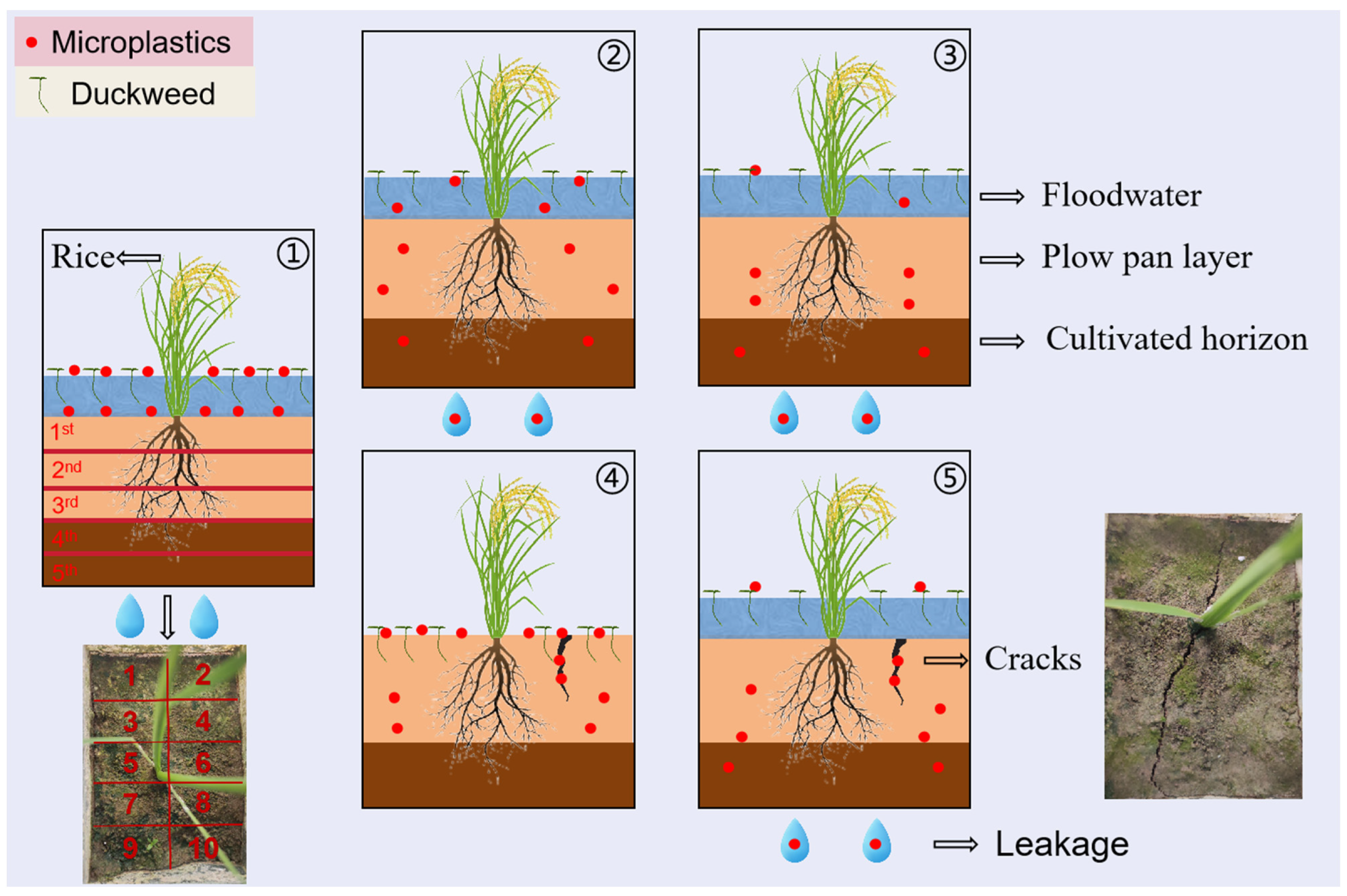
2.3. Experiment Setup
2.4. Measurement and Calculations
2.4.1. Water Depth
2.4.2. Rice Growth Parameters
2.4.3. DNA Extraction, Amplicon Sequencing, and Data Analysis in Microorganisms
2.4.4. Quantity and Characterization of MPs
2.4.5. Soil Cracks
2.5. Statistical Analysis
3. Results
3.1. The Change in Water Depth
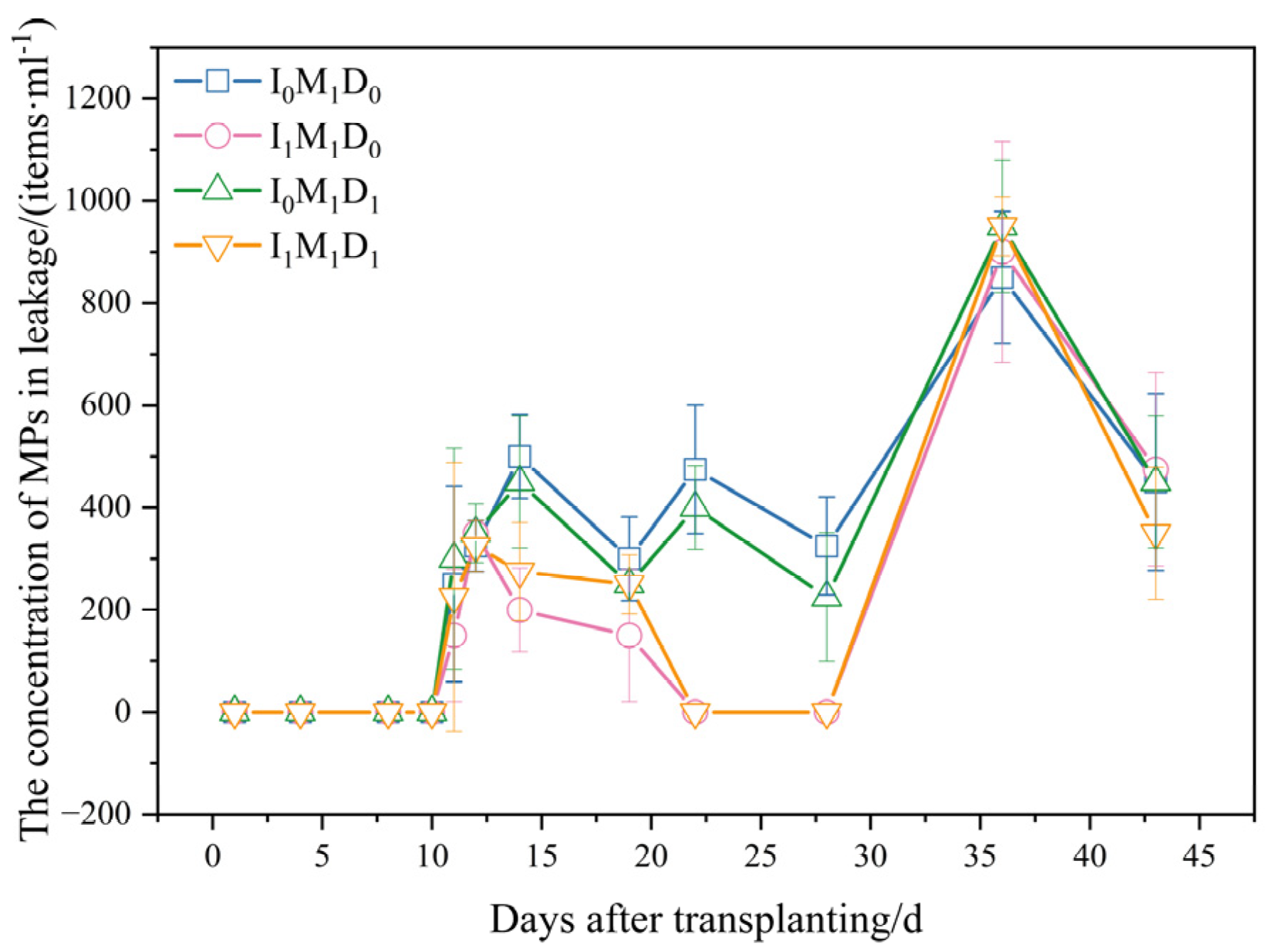
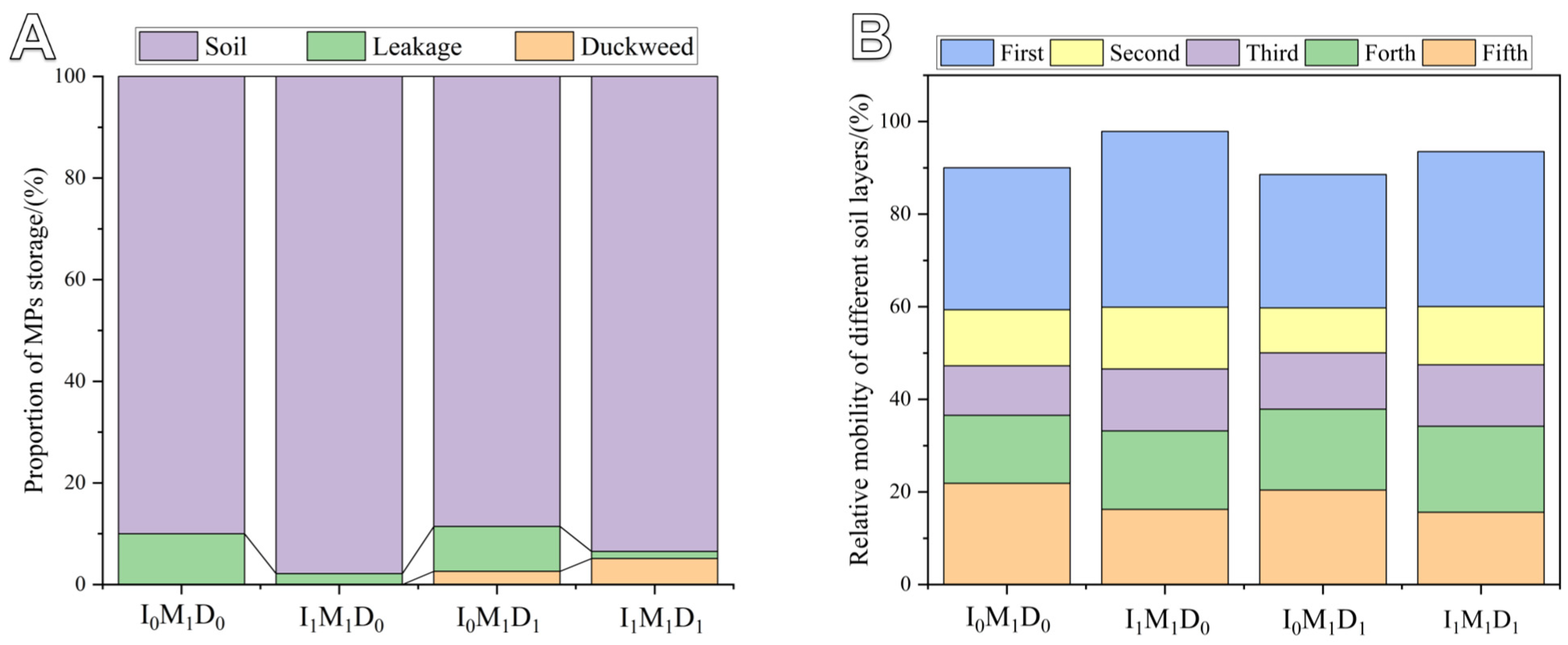
3.2. Numbers of MPs in Leakage
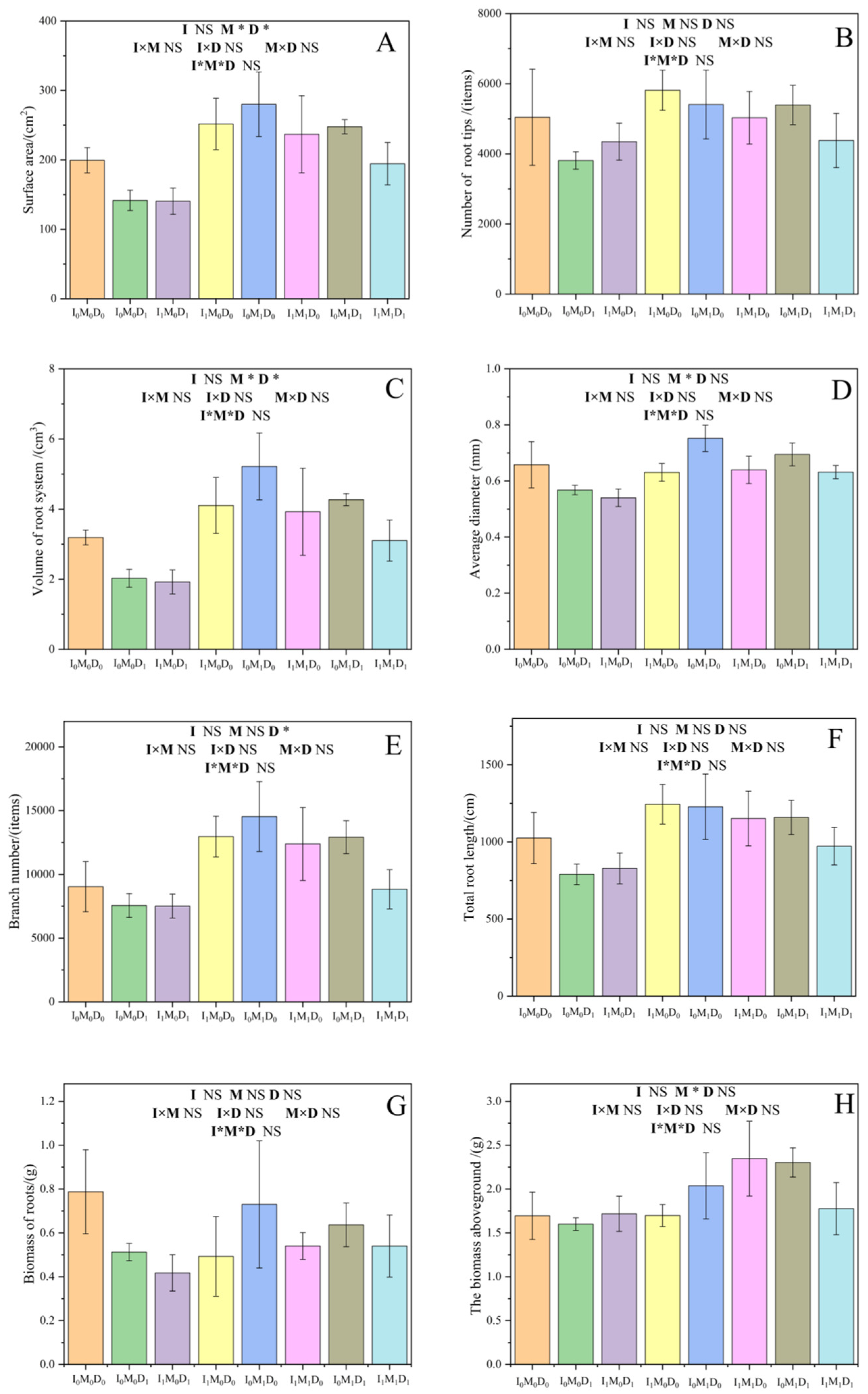
3.3. Rice Growth Indicators
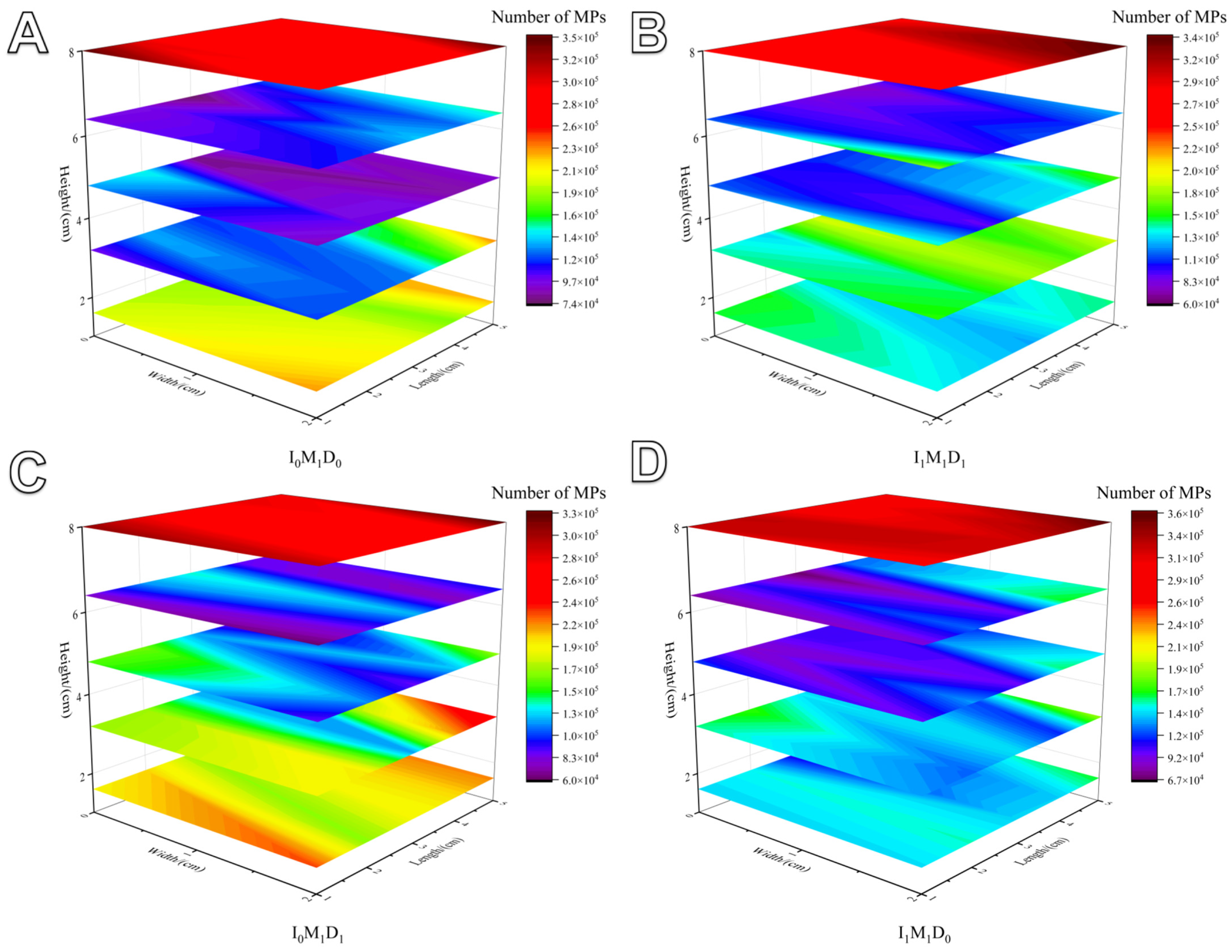
3.4. MP Distribution and Relative Mobility in Paddy Field Systems
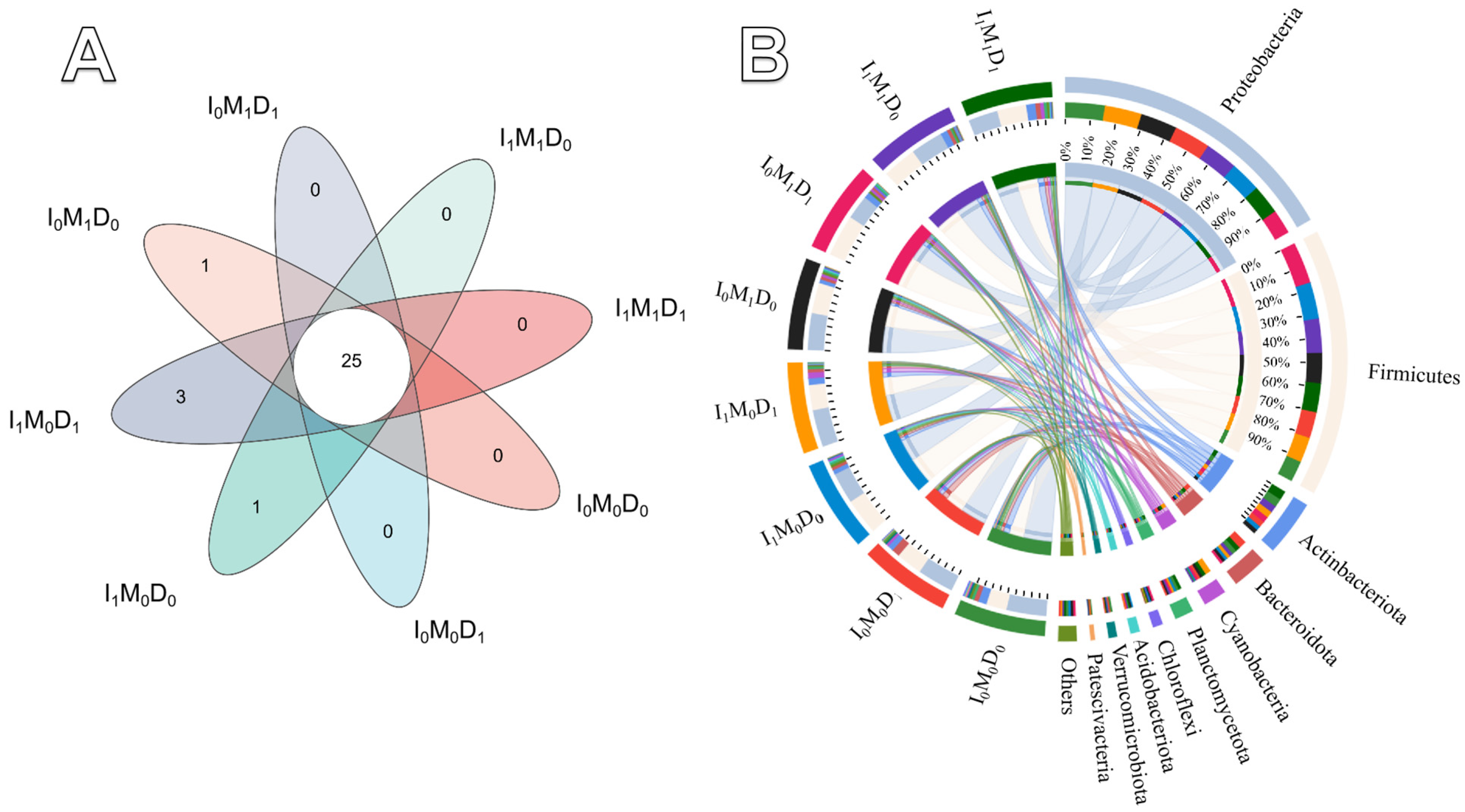
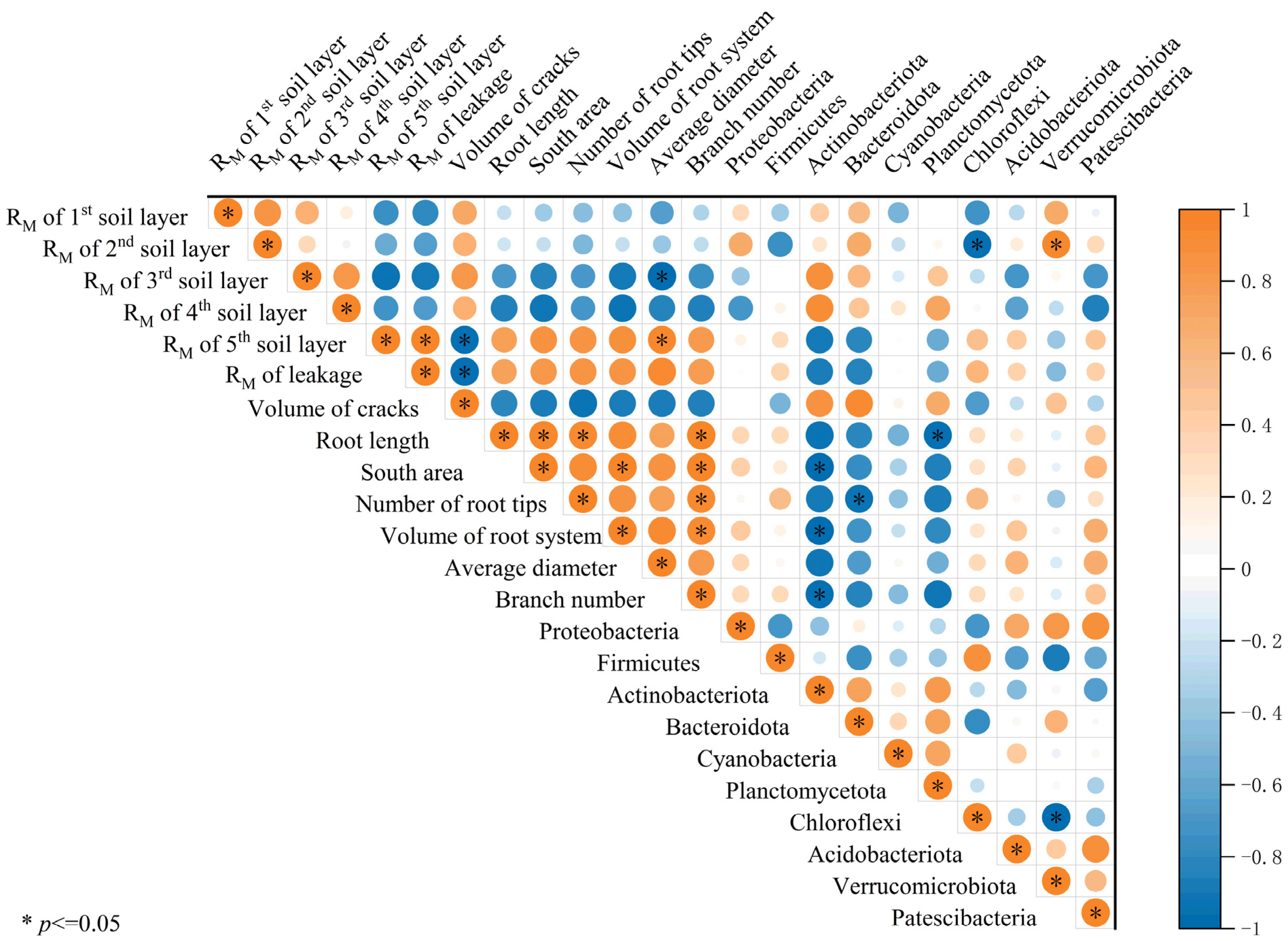
3.5. Microbial Community Diversity and Abundance and Pearson Correlation Analysis
4. Discussion
4.1. Influence of MPs on Microorganism and Rice Growth Parameters
4.2. Migration of MPs in Paddy Field Systems
4.3. Joint Regulation of MPs Migration by Irrigating-Duckweed
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, J.K.H.; Lee, K.K.; Tang, K.H.D.; Yap, P.-S. Microplastics in the Freshwater and Terrestrial Environments: Prevalence, Fates, Impacts and Sustainable Solutions. Sci. Total Environ. 2020, 719, 137512. [Google Scholar] [CrossRef]
- Ray, S.S.; Lee, H.K.; Huyen, D.T.T.; Chen, S.-S.; Kwon, Y.-N. Microplastics Waste in Environment: A Perspective on Recycling Issues from PPE Kits and Face Masks during the COVID-19 Pandemic. Environ. Technol. Innov. 2022, 26, 102290. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Feng, X.; Liu, Y.; Adams, C.A.; Sun, Y.; Zhang, S. Micro(Nano)Plastics and Terrestrial Plants: Up-to-Date Knowledge on Uptake, Translocation, and Phytotoxicity. Resour. Conserv. Recycl. 2022, 185, 106503. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, X.; Zhang, J.; Huang, X. Distribution and Migration Characteristics of Microplastics in Farmland Soils, Surface Water and Sediments in Caohai Lake, Southwestern Plateau of China. J. Clean. Prod. 2022, 366, 132912. [Google Scholar] [CrossRef]
- Yuan, Y.; Qin, Y.; Wang, M.; Xu, W.; Chen, Y.; Zheng, L.; Chen, W.; Luo, T. Microplastics from Agricultural Plastic Mulch Films: A Mini-Review of Their Impacts on the Animal Reproductive System. Ecotoxicol. Environ. Saf. 2022, 244, 114030. [Google Scholar] [CrossRef]
- Tian, L.; Jinjin, C.; Ji, R.; Ma, Y.; Yu, X. Microplastics in Agricultural Soils: Sources, Effects, and Their Fate. Curr. Opin. Environ. Sci. Health 2022, 25, 100311. [Google Scholar] [CrossRef]
- Lian, J.; Liu, W.; Meng, L.; Wu, J.; Zeb, A.; Cheng, L.; Lian, Y.; Sun, H. Effects of Microplastics Derived from Polymer-Coated Fertilizer on Maize Growth, Rhizosphere, and Soil Properties. J. Clean. Prod. 2021, 318, 128571. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, T.; van Groenigen, K.J.; Yang, Y.; Wang, P.; Cheng, K.; Zhu, Z.; Wang, J.; Li, Y.; Guggenberger, G.; et al. Rice Paddy Soils Are a Quantitatively Important Carbon Store According to a Global Synthesis. Commun. Earth Environ. 2021, 2, 154. [Google Scholar] [CrossRef]
- Yang, Y.; Jin, Z.; Mueller, N.D.; Driscoll, A.W.; Hernandez, R.R.; Grodsky, S.M.; Sloat, L.L.; Chester, M.V.; Zhu, Y.G.; Lobell, D.B. Sustainable Irrigation and Climate Feedbacks. Nat. Food 2023, 4, 654–663. [Google Scholar] [CrossRef]
- Katsumi, N.; Kusube, T.; Nagao, S.; Okochi, H. Accumulation of Microcapsules Derived from Coated Fertilizer in Paddy Fields. Chemosphere 2021, 267, 129185. [Google Scholar] [CrossRef]
- Lv, W.; Zhou, W.; Lu, S.; Huang, W.; Yuan, Q.; Tian, M.; Lv, W.; He, D. Microplastic Pollution in Rice-Fish Co-Culture System: A Report of Three Farmland Stations in Shanghai, China. Sci. Total Environ. 2019, 652, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, L.; Gong, L.; Li, G.; Xiu, W.; Yang, X.; Tan, B.; Zhao, J.; Zhang, G. Differences, Links, and Roles of Microbial and Stoichiometric Factors in Microplastic Distribution: A Case Study of Five Typical Rice Cropping Regions in China. Front. Microbiol. 2022, 13, 985239. [Google Scholar] [CrossRef]
- Zhou, C.Q.; Lu, C.H.; Mai, L.; Bao, L.J.; Liu, L.Y.; Zeng, E.Y. Response of Rice (Oryza sativa L.) Roots to Nanoplastic Treatment at Seedling Stage. J. Hazard. Mater. 2021, 401, 123412. [Google Scholar] [CrossRef]
- Ma, J.; Aqeel, M.; Khalid, N.; Nazir, A.; Alzuaibr, F.M.; Al-Mushhin, A.A.M.; Hakami, O.; Iqbal, M.F.; Chen, F.; Alamri, S.; et al. Effects of Microplastics on Growth and Metabolism of Rice (Oryza sativa L.). Chemosphere 2022, 307, 135749. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, R.; Zhang, S.; Sun, Y.; Wang, F. Uptake and Translocation of Nano/Microplastics by Rice Seedlings: Evidence from a Hydroponic Experiment. J. Hazard. Mater. 2022, 421, 126700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, Y.B.; He, H.R.; Zhang, J.F.; Ma, G.S. You Are What You Eat: Microplastics in the Feces of Young Men Living in Beijing. Sci. Total Environ. 2021, 767, 144345. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, H.; Liao, Y.; Ye, Z.; Li, M.; Klobučar, G. Ecotoxicity and Genotoxicity of Polystyrene Microplastics on Higher Plant Vicia Faba. Environ. Pollut. 2019, 250, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Bosker, T.; Bouwman, L.J.; Brun, N.R.; Behrens, P.; Vijver, M.G. Microplastics Accumulate on Pores in Seed Capsule and Delay Germination and Root Growth of the Terrestrial Vascular Plant Lepidium Sativum. Chemosphere 2019, 226, 774–781. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, M.; Song, Z.; Qiu, W. Microplastic Particles Increase Arsenic Toxicity to Rice Seedlings. Environ. Pollut. 2020, 259, 113892. [Google Scholar] [CrossRef]
- Sun, M.; Wang, Y.; Wang, Y.; Chen, G.; Zhao, L.; Zhang, Y. Photosynthetic Characteristics of Japonica Rice Cultivar Nanjing 5055 and Its Parents. Jiangsu J. Agric. Sci. 2020, 36, 1–9. [Google Scholar] [CrossRef]
- Mateos-Cárdenas, A.; Scott, D.T.; Seitmaganbetova, G.; van, P.; John, O.H.; Marcel, A.K.J. Polyethylene Microplastics Adhere to Lemna minor (L.), yet Have No Effects on Plant Growth or Feeding by Gammarus duebeni (Lillj.). Sci. Total Environ. 2019, 689, 413–421. [Google Scholar] [CrossRef]
- Taylor, S.E.; Pearce, C.I.; Sanguinet, K.A.; Hu, D.; Chrisler, W.B.; Kim, Y.-M.; Wang, Z.; Flury, M. Polystyrene Nano- and Microplastic Accumulation at Arabidopsis and Wheat Root Cap Cells, but No Evidence for Uptake into Roots. Environ. Sci. Nano 2020, 7, 1942–1953. [Google Scholar] [CrossRef]
- Verma, P.K.; Verma, S.; Pandey, N. Root System Architecture in Rice: Impacts of Genes, Phytohormones and Root Microbiota. 3 Biotech 2022, 12, 239. [Google Scholar] [CrossRef]
- Yu, Y.; Li, X.; Feng, Z.; Xiao, M.; Ge, T.; Li, Y.; Yao, H. Polyethylene Microplastics Alter the Microbial Functional Gene Abundances and Increase Nitrous Oxide Emissions from Paddy Soils. J. Hazard. Mater. 2022, 432, 128721. [Google Scholar] [CrossRef] [PubMed]
- Lampayan, R.M.; Rejesus, R.M.; Singleton, G.R.; Bouman, B.A.M. Adoption and Economics of Alternate Wetting and Drying Water Management for Irrigated Lowland Rice. Field Crops Res. 2015, 170, 95–108. [Google Scholar] [CrossRef]
- Bo, Y.; Jägermeyr, J.; Yin, Z.; Jiang, Y.; Xu, J.; Liang, H.; Zhou, F. Global Benefits of Non-Continuous Flooding to Reduce Greenhouse Gases and Irrigation Water Use without Rice Yield Penalty. Glob. Change Biol. 2022, 28, 3636–3650. [Google Scholar] [CrossRef]
- Hong, C.; Wang, Z.; Wang, Y.; Zong, X.; Qiang, X.; Li, Q.; Shaghaleh, H.; Alhaj Hamoud, Y.; Guo, X. Response of Duckweed to Different Irrigation Modes under Different Fertilizer Types and Rice Varieties: Unlocking the Potential of Duckweed (Lemna minor L.) in Rice Cultivation as “Fertilizer Capacitors”. Agric. Water Manag. 2024, 292, 108681. [Google Scholar] [CrossRef]
- Peng, S.; Buresh, R.J.; Huang, J.; Yang, J.; Zou, Y.; Zhong, X.; Wang, G.; Zhang, F. Strategies for Overcoming Low Agronomic Nitrogen Use Efficiency in Irrigated Rice Systems in China. Field Crops Res. 2006, 96, 37–47. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, X.; Liu, J.; Zhang, Y.; Chau, S.; Bhattarai, N.; Wang, Y.; Li, Y.; Connor, T.; Li, Y. Impacts of Irrigated Agriculture on Food–Energy–Water–CO2 Nexus across Metacoupled Systems. Nat. Commun. 2020, 11, 5837. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Li, X.; Zhang, Y.; Gao, W.; Jiang, J.; Mo, A.; He, D. Size/Shape-Dependent Migration of Microplastics in Agricultural Soil under Simulative and Natural Rainfall. Sci. Total Environ. 2022, 815, 152507. [Google Scholar] [CrossRef] [PubMed]
- Bigalke, M.; Fieber, M.; Foetisch, A.; Reynes, J.; Tollan, P. Microplastics in Agricultural Drainage Water: A Link between Terrestrial and Aquatic Microplastic Pollution. Sci. Total Environ. 2022, 806, 150709. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Li, Y.; Bian, P.; Hu, Y.; Zhang, J.; Shen, W. Effects of Irrigation on the Fate of Microplastics in Typical Agricultural Soil and Freshwater Environments in the Upper Irrigation Area of the Yellow River. J. Hazard. Mater. 2023, 447, 130766. [Google Scholar] [CrossRef]
- Alhaj Hamoud, Y.; Guo, X.; Wang, Z.; Shaghaleh, H.; Chen, S.; Hassan, A.; Bakour, A. Effects of Irrigation Regime and Soil Clay Content and Their Interaction on the Biological Yield, Nitrogen Uptake and Nitrogen-Use Efficiency of Rice Grown in Southern China. Agric. Water Manag. 2019, 213, 934–946. [Google Scholar] [CrossRef]
- Greve, A.; Andersen, M.S.; Acworth, R.I. Investigations of Soil Cracking and Preferential Flow in a Weighing Lysimeter Filled with Cracking Clay Soil. J. Hydrol. 2010, 393, 105–113. [Google Scholar] [CrossRef]
- Stewart, R.D.; Abou Najm, M.R.; Rupp, D.E.; Lane, J.W.; Uribe, H.C.; Arumí, J.L.; Selker, J.S. Hillslope Run-off Thresholds with Shrink-Swell Clay Soils. Hydrol. Process. 2015, 29, 557–571. [Google Scholar] [CrossRef]
- Huang, L.; Lu, Y.; Gao, X.; Du, G.; Ma, X.; Liu, M.; Guo, J.; Chen, Y. Ammonium-Induced Oxidative Stress on Plant Growth and Antioxidative Response of Duckweed (Lemna minor L.). Ecol. Eng. 2013, 58, 355–362. [Google Scholar] [CrossRef]
- Chen, S.; Xu, J.; Peng, L.; Cheng, Z.; Kuang, X.; Li, D.; Peng, C.; Song, H. Cadmium Accumulation in Rice Grains Is Mitigated by Duckweed-like Hydrophyte through Adsorption and Increased Ammonia Nitrogen. Sci. Total Environ. 2023, 890, 164510. [Google Scholar] [CrossRef]
- Chen, G.; Yu, Y.; Li, W.; Yan, B.; Zhao, K.; Dong, X.; Cheng, Z.; Lin, F.; Li, L.; Zhao, H.; et al. Effects of Reaction Conditions on Products and Elements Distribution via Hydrothermal Liquefaction of Duckweed for Wastewater Treatment. Bioresour. Technol. 2020, 317, 124033. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Bao, S.; Liu, H.; Yu, J.; Wang, Y.; Shao, H.; Ouyang, Y.; An, S. Effects of Different Vegetation Zones on CH4 and N2O Emissions in Coastal Wetlands: A Model Case Study. Sci. World J. 2014, 2014, 412183. [Google Scholar] [CrossRef]
- Sun, H.; A, D.; Feng, Y.; Vithanage, M.; Mandal, S.; Shaheen, S.M.; Rinklebe, J.; Shi, W.; Wang, H. Floating Duckweed Mitigated Ammonia Volatilization and Increased Grain Yield and Nitrogen Use Efficiency of Rice in Biochar Amended Paddy Soils. Chemosphere 2019, 237, 124532. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhang, L.; Li, S.; Liu, H.; Zhai, L.; Zhou, F.; Ye, Y.; Ruan, S.; Wen, W. Effects and Potential of Water-Saving Irrigation for Rice Production in China. Agric. Water Manag. 2019, 217, 374–382. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, Z.; Guo, X.; Lakthan, S.; Chen, S.; Xiao, Z.; Alhaj Hamoud, Y. Effects of Different Irrigation Treatments on Aquaculture Purification and Soil Desalination of Paddy Fields. Water 2019, 11, 1424. [Google Scholar] [CrossRef]
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every Base Matters: Assessing Small Subunit rRNA Primers for Marine Microbiomes with Mock Communities, Time Series and Global Field Samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Mohedano, R.A.; Tonon, G.; Costa, R.H.R.; Pelissari, C.; Belli Filho, P. Does Duckweed Ponds Used for Wastewater Treatment Emit or Sequester Greenhouse Gases? Sci. Total Environ. 2019, 691, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Escobar, A.; Tellez, J.; Gonzalez-Sosa, E. Use of Digital Photography for Analysis of Canopy Closure. Agrofor. Syst. 2005, 65, 175–185. [Google Scholar] [CrossRef]
- Schroeder, A.B.; Dobson, E.T.A.; Rueden, C.T.; Tomancak, P.; Jug, F.; Eliceiri, K.W. The ImageJ Ecosystem: Open-source Software for Image Visualization, Processing, and Analysis. Protein Sci. 2021, 30, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Constant, M.; Billon, G.; Breton, N.; Alary, C. Extraction of Microplastics from Sediment Matrices: Experimental Comparative Analysis. J. Hazard. Mater. 2021, 420, 126571. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Phuong, N.N.; Poirier, L.; Lagarde, F.; Kamari, A.; Zalouk-Vergnoux, A. Microplastic Abundance and Characteristics in French Atlantic Coastal Sediments Using a New Extraction Method. Environ. Pollut. 2018, 243, 228–237. [Google Scholar] [CrossRef]
- Bandyopadhyay, K.K.; Mohanty, M.; Painuli, D.K.; Misra, A.K.; Hati, K.M.; Mandal, K.G.; Ghosh, P.K.; Chaudhary, R.S.; Acharya, C.L. Influence of Tillage Practices and Nutrient Management on Crack Parameters in a Vertisol of Central India. Soil. Tillage Res. 2003, 71, 133–142. [Google Scholar] [CrossRef]
- Sharma, P.K.; Verma, T.S.; Bhagat, R.M. Soil Structural Improvements with the Addition of Lantana Camara Biomass in Rice-wheat Cropping. Soil. Use Manag. 1995, 11, 199–203. [Google Scholar] [CrossRef]
- Kalčíková, G.; Žgajnar Gotvajn, A.; Kladnik, A.; Jemec, A. Impact of Polyethylene Microbeads on the Floating Freshwater Plant Duckweed Lemna Minor. Environ. Pollut. 2017, 230, 1108–1115. [Google Scholar] [CrossRef]
- Sun, X.-D.; Yuan, X.-Z.; Jia, Y.; Feng, L.-J.; Zhu, F.-P.; Dong, S.-S.; Liu, J.; Kong, X.; Tian, H.; Duan, J.-L.; et al. Differentially Charged Nanoplastics Demonstrate Distinct Accumulation in Arabidopsis Thaliana. Nat. Nanotechnol. 2020, 15, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Wu, J.; Xiong, H.; Zeb, A.; Yang, T.; Su, X.; Su, L.; Liu, W. Impact of Polystyrene Nanoplastics (PSNPs) on Seed Germination and Seedling Growth of Wheat (Triticum aestivum L.). J. Hazard. Mater. 2020, 385, 121620. [Google Scholar] [CrossRef]
- Li, B.; Huang, S.; Wang, H.; Liu, M.; Xue, S.; Tang, D.; Cheng, W.; Fan, T.; Yang, X. Effects of Plastic Particles on Germination and Growth of Soybean (Glycine Max): A Pot Experiment under Field Condition. Environ. Pollut. 2021, 272, 116418. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Zhang, Y.; Chang, S.; Tao, L.; Su, G. Uptake, Accumulation and Translocation of Traditional and Novel Organophosphate Esters by Rice Seedlings in the Presence of Micro(Nano)-Polystyrene Plastics: Effects of Concentration and Size of Particles. Sci. Total Environ. 2023, 898, 165534. [Google Scholar] [CrossRef]
- Zang, H.; Zhou, J.; Marshall, M.R.; Chadwick, D.R.; Wen, Y.; Jones, D.L. Microplastics in the Agroecosystem: Are They an Emerging Threat to the Plant-Soil System? Soil. Biol. Biochem. 2020, 148, 107926. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Lehnert, T.; Linck, L.T.; Lehmann, A.; Rillig, M.C. Frontiers Production Office Erratum: Microplastic Shape, Polymer Type, and Concentration Affect Soil Properties and Plant Biomass. Front. Plant Sci. 2021, 12, 714541. [Google Scholar] [CrossRef]
- Leifheit, E.F.; Lehmann, A.; Rillig, M.C. Potential Effects of Microplastic on Arbuscular Mycorrhizal Fungi. Front. Plant Sci. 2021, 12, 626709. [Google Scholar] [CrossRef]
- Xiao, M.; Ding, J.; Luo, Y.; Zhang, H.; Yu, Y.; Yao, H.; Zhu, Z.; Chadwick, D.R.; Jones, D.; Chen, J.; et al. Microplastics Shape Microbial Communities Affecting Soil Organic Matter Decomposition in Paddy Soil. J. Hazard. Mater. 2022, 431, 128589. [Google Scholar] [CrossRef] [PubMed]
- Selvam, S.; Jesuraja, K.; Venkatramanan, S.; Roy, P.D.; Jeyanthi Kumari, V. Hazardous Microplastic Characteristics and Its Role as a Vector of Heavy Metal in Groundwater and Surface Water of Coastal South India. J. Hazard. Mater. 2021, 402, 123786. [Google Scholar] [CrossRef]
- Samandra, S.; Johnston, J.M.; Jaeger, J.E.; Symons, B.; Xie, S.; Currell, M.; Ellis, A.V.; Clarke, B.O. Microplastic Contamination of an Unconfined Groundwater Aquifer in Victoria, Australia. Sci. Total Environ. 2022, 802, 149727. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Gui, X.; Xu, X.; Zhao, L.; Qiu, H.; Cao, X. Microplastics in the Soil-Groundwater Environment: Aging, Migration, and Co-Transport of Contaminants—A Critical Review. J. Hazard. Mater. 2021, 419, 126455. [Google Scholar] [CrossRef]
- Luo, Z.; Zhu, J.; Yu, L.; Yin, K. Heavy Metal Remediation by Nano Zero-Valent Iron in the Presence of Microplastics in Groundwater: Inhibition and Induced Promotion on Aging Effects. Environ. Pollut. 2021, 287, 117628. [Google Scholar] [CrossRef]
- Gao, J.; Pan, S.; Li, P.; Wang, L.; Hou, R.; Wu, W.-M.; Luo, J.; Hou, D. Vertical Migration of Microplastics in Porous Media: Multiple Controlling Factors under Wet-Dry Cycling. J. Hazard. Mater. 2021, 419, 126413. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.; Pan, S.; Shen, Z.; Song, Y.; Jin, Y.; Wu, W.-M.; Hou, D. Microplastics Undergo Accelerated Vertical Migration in Sand Soil Due to Small Size and Wet-Dry Cycles. Environ. Pollut. 2019, 249, 527–534. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.F.; McKay, L.D. Colloid Transport in the Subsurface: Past, Present, and Future Challenges. Vadose Zone J. 2004, 3, 326–337. [Google Scholar] [CrossRef]
- DeNovio, N.M.; Saiers, J.E.; Ryan, J.N. Colloid Movement in Unsaturated Porous Media: Recent Advances and Future Directions. Vadose Zone J. 2004, 3, 338–351. [Google Scholar] [CrossRef]
- Chu, Y.; Jin, Y.; Flury, M.; Yates, M.V. Mechanisms of Virus Removal during Transport in Unsaturated Porous Media. Water Resour. Res. 2001, 37, 253–263. [Google Scholar] [CrossRef]
- Nicole, W. An Ill Wind? Growing Recognition of Airborne Nano- and Microplastic Exposures. Environ. Health Perspect 2023, 131, 042001. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, A.; Laborte, A.; Nelson, A.; Singh, R.K. Salinity Stress Detection in Rice Crops Using Time Series MODIS VI Data. Int. J. Remote Sens. 2019, 40, 8186–8202. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic Mulching in Agriculture. Trading Short-Term Agronomic Benefits for Long-Term Soil Degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef]
- Liuyue He, Z.L.J.X. Soil Microplastics Pollution in Agriculture. Science 2023, 379, 547. [Google Scholar] [CrossRef]
- Wei, Q.; Wei, Q.; Xu, J.; Liu, Y.; Wang, D.; Chen, S.; Qian, W.; He, M.; Chen, P.; Zhou, X.; et al. Nitrogen Losses from Soil as Affected by Water and Fertilizer Management under Drip Irrigation: Development, Hotspots and Future Perspectives. Agric. Water Manag. 2024, 296, 108791. [Google Scholar] [CrossRef]
- Qi, D.; Yan, J.; Zhu, J. Effect of a Reduced Fertilizer Rate on the Water Quality of Paddy Fields and Rice Yields under Fishpond Effluent Irrigation. Agric. Water Manag. 2020, 231, 105999. [Google Scholar] [CrossRef]
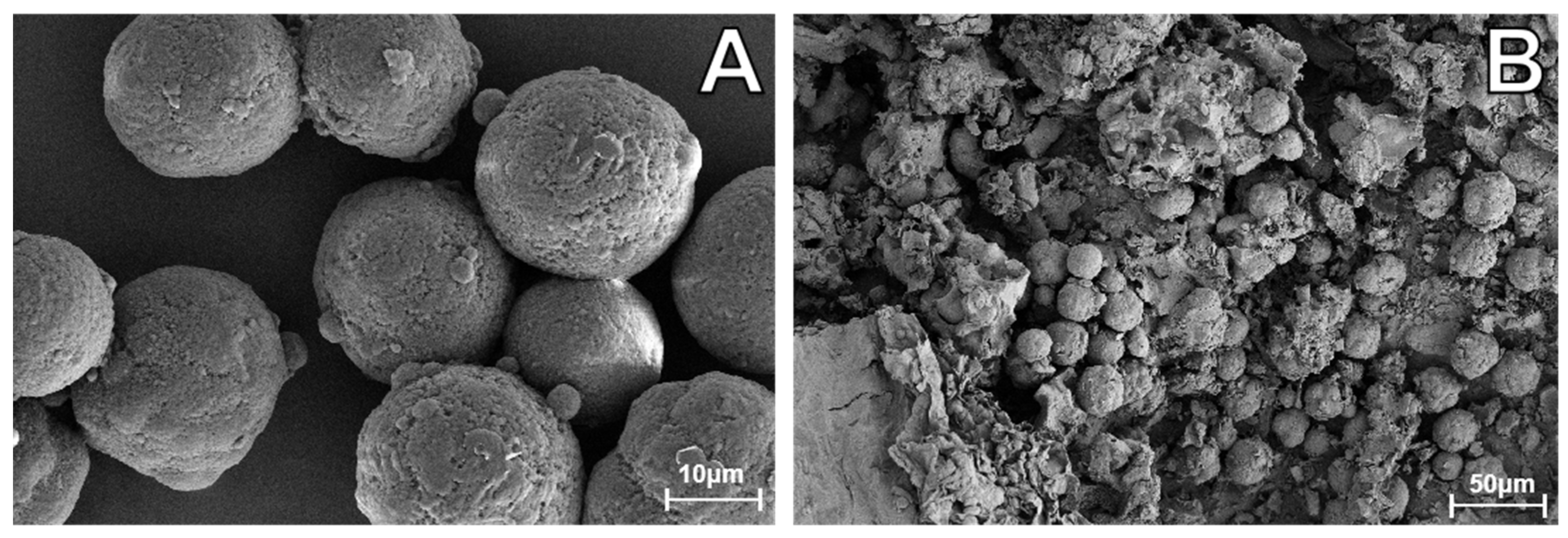

| Types of Microplastics | Size (µm) | Relative Frequency |
|---|---|---|
| PE | 1~2 | 0.16% |
| 2~5 | 4.80% | |
| 5~10 | 18.30% | |
| 10~25 | 40.57% | |
| 25~50 | 25.24% | |
| 50~100 | 10.94% |
| The RM of MPs | I | D | I × D |
|---|---|---|---|
| First soil layer | ** | * | NS |
| Second soil layer | NS | NS | NS |
| Third soil layer | NS | NS | NS |
| Fourth soil layer | NS | * | NS |
| Fifth soil layer | *** | NS | NS |
| The RM of MPs | C | De | C × De |
|---|---|---|---|
| All of soil block | *** | * | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, C.; Wang, Z.; Tian, M.; Wang, Y.; Liu, J.; Qiang, X.; Masharifov, U.; Chen, K. Migration of Microplastics in the Rice–Duckweed System under Different Irrigation Modes. Agriculture 2024, 14, 1460. https://doi.org/10.3390/agriculture14091460
Hong C, Wang Z, Tian M, Wang Y, Liu J, Qiang X, Masharifov U, Chen K. Migration of Microplastics in the Rice–Duckweed System under Different Irrigation Modes. Agriculture. 2024; 14(9):1460. https://doi.org/10.3390/agriculture14091460
Chicago/Turabian StyleHong, Cheng, Zhenchang Wang, Minghao Tian, Yuexiong Wang, Jinjing Liu, Xiaoman Qiang, Umidbek Masharifov, and Kexin Chen. 2024. "Migration of Microplastics in the Rice–Duckweed System under Different Irrigation Modes" Agriculture 14, no. 9: 1460. https://doi.org/10.3390/agriculture14091460
APA StyleHong, C., Wang, Z., Tian, M., Wang, Y., Liu, J., Qiang, X., Masharifov, U., & Chen, K. (2024). Migration of Microplastics in the Rice–Duckweed System under Different Irrigation Modes. Agriculture, 14(9), 1460. https://doi.org/10.3390/agriculture14091460






