Exploring the Role of Biostimulants in Sweet Cherry (Prunus avium L.) Fruit Quality Traits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Weather Conditions
2.2. Plant Material, Treatments, and Sampling
2.3. Physical Characteristics of Sweet Cherries
Weight, Dimensions, Texture, and Skin Color
2.4. Chemical Properties of Sweet Cherries
2.4.1. Total Soluble Solids, pH, and Titratable Acidity
2.4.2. Bioactive Compounds
2.5. Sensory Evaluation of Sweet Cherries
2.6. Statistical Analysis
3. Results
3.1. Weather Conditions
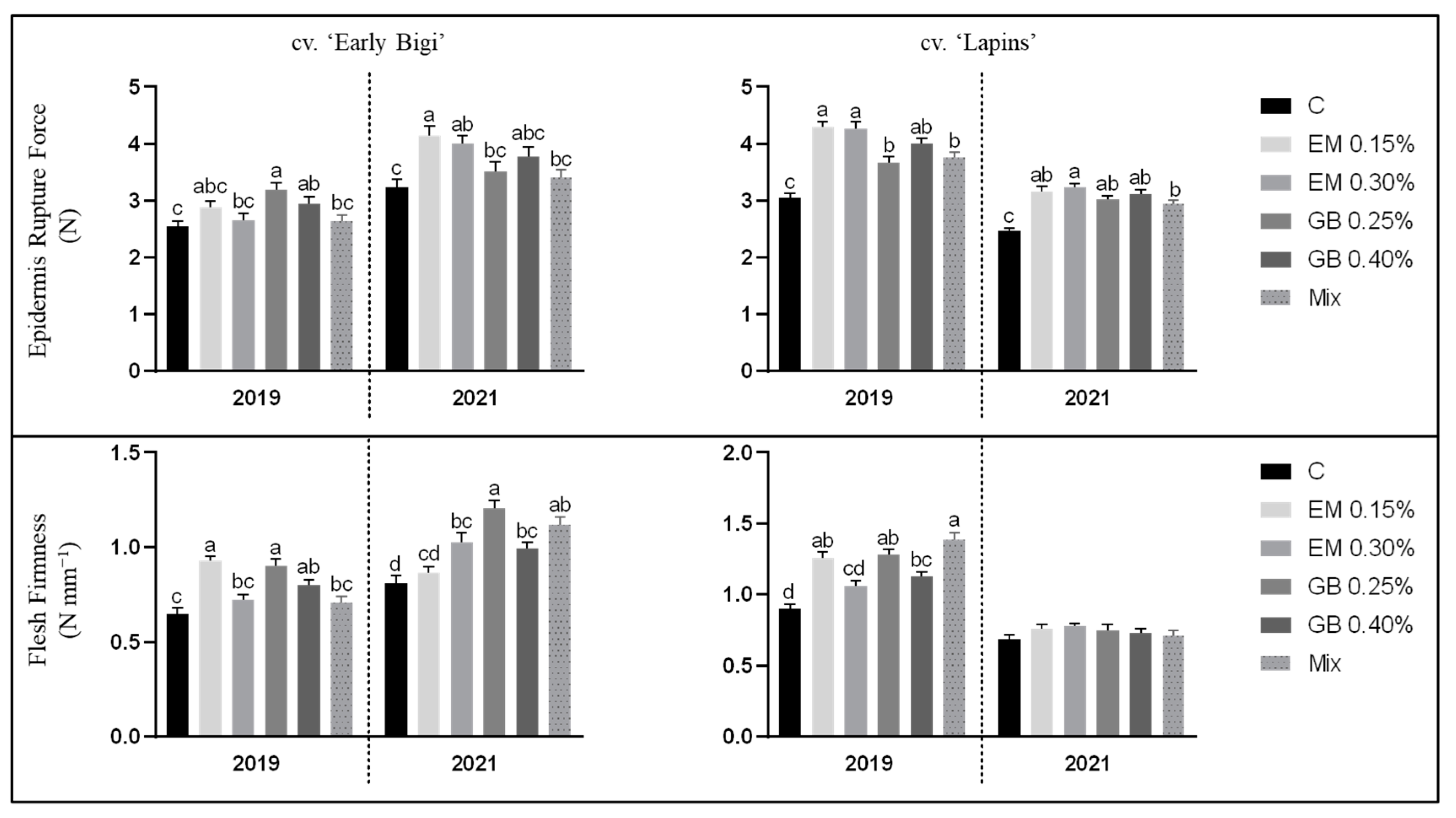
3.2. Biometric and Physical Characteristics of Sweet Cherries
3.3. Chemical Properties of Sweet Cherries
3.4. Bioactive Compounds
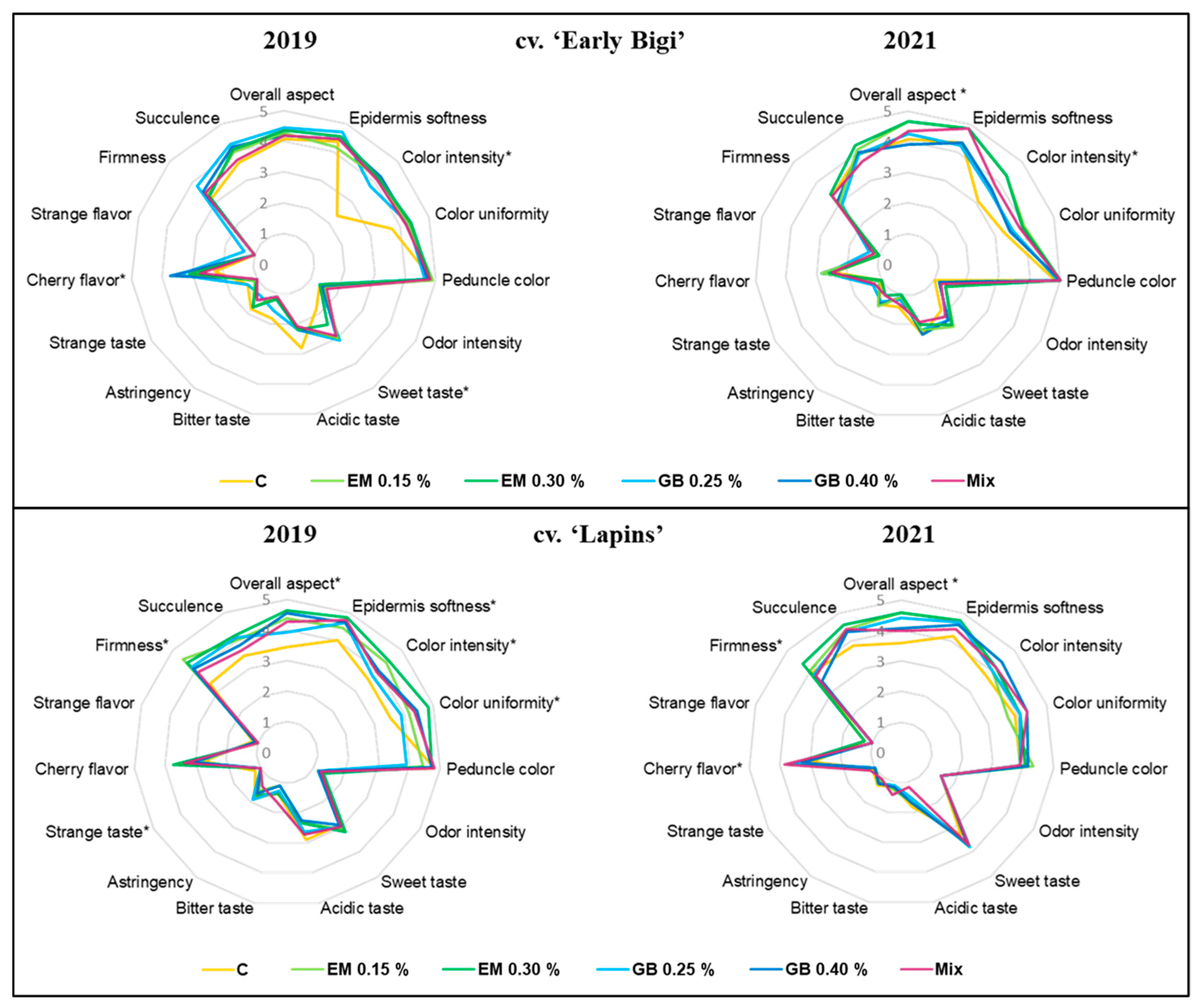
3.5. Sensory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basile, B.; Brown, N.; Valdes, J.M.; Cardarelli, M.; Scognamiglio, P.; Mataffo, A.; Rouphael, Y.; Bonini, P.; Colla, G. Plant-based biostimulant as sustainable alternative to synthetic growth regulators in two sweet cherry cultivars. Plants 2021, 10, 619. [Google Scholar] [CrossRef]
- Gonçalves, B.; Alfredo, A.; Oliveira, I.; Afonso, S.; Morais, M.C.; Correia, S.; Martins, S.; Silva, A.P. Sweet Cherry. In Temperate Fruits; Apple Academic Press: Cambridge, MA, USA, 2021; pp. 333–415. [Google Scholar]
- Afonso, S.; Oliveira, I.; Meyer, A.S.; Gonçalves, B. Biostimulants to improved tree physiology and fruit quality: A review with special focus on sweet cherry. Agronomy 2022, 12, 659. [Google Scholar] [CrossRef]
- Afonso, S.; Oliveira, I.; Ribeiro, C.; Vilela, A.; Meyer, A.M.; Gonçalves, B. Innovative edible coatings for postharvest storage of sweet cherries. Sci. Hortic. 2023, 310, 111738. [Google Scholar] [CrossRef]
- Romano, G.S.; Cittadini, E.D.; Pugh, B.; Schouten, R. Sweet cherry quality in the horticultural production chain. Stewart Postharvest Rev. 2006, 6, 1–9. [Google Scholar]
- FAOSTAT. Data. Available online: https://www.fao.org/faostat/en/#home (accessed on 11 July 2024).
- Gonçalves, B.; Silva, A.P.; Vilela, A.; Malheiro, A.; Ribeiro, C.; Bacelar, E.; Raimundo, F.; Guedes, F.; Cotez, I.; Sousa, J.R.; et al. Manual de Boas Práticas da Cultura da Cerejeira|Resende. Dolmen-Desenvolvimento Local e Regional, Crl. 2022. Available online: https://gocerejaresende.pt/manual.pdf (accessed on 8 July 2024).
- Wenden, B.; Campoy, J.; Lecourt, J. A collection of European sweet cherry phenology data for assessing climate change. Sci. Data 2016, 3, 160108. [Google Scholar] [CrossRef] [PubMed]
- Engin, H.; Sen, F.; Pamuk, G.; Gokbayrak, Z. Investigation of physiological disorders and fruit quality of sweet cherry. Eur. J. Hortic. Sci. 2009, 74, 118. [Google Scholar]
- Medda, S.; Fadda, A.; Mulas, M. Influence of Climate Change on Metabolism and Biological Characteristics in Perennial Woody Fruit Crops in the Mediterranean Environment. Horticulturae 2022, 8, 273. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef]
- Verma, N.; Sehrawat, K.D.; Mundlia, P.; Sehrawat, A.R.; Choudhary, R.; Rajput, V.D.; Minkina, T.; van Hullebusch, E.D.; Siddiqui, M.H.; Alamri, S. Potential use of ascophyllum nodosum as a biostimulant for improving the growth performance of Vigna aconitifolia (Jacq.) marechal. Plants 2021, 10, 2361. [Google Scholar] [CrossRef]
- Rojas, G.; Fernandez, E.; Whitney, C.; Luedeling, E.; Cuneo, I.F. Adapting sweet cherry orchards to extreme weather events–Decision Analysis in support of farmers’ investments in Central Chile. Agric. Syst. 2021, 187, 103031. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar applications of protein hydrolysate, plant and seaweed extracts increase yield but differentially modulate fruit quality of greenhouse tomato. HortScience 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Correia, S.; Oliveira, I.; Queirós, F.; Ribeiro, C.; Ferreira, L.; Luzio, A.; Silva, A.P.; Gonçalves, B. Preharvest application of seaweed based biostimulant reduced cherry (Prunus avium L.) cracking. Procedia Environ. Sci. 2015, 29, 251–252. [Google Scholar]
- Serapicos, M.; Afonso, S.; Gonçalves, B.; Silva, A.P. Exogenous application of glycine betaine on sweet cherry tree (Prunus avium L.): Effects on tree physiology and leaf properties. Plants 2022, 11, 3470. [Google Scholar] [CrossRef]
- Li, M.; Zhi, H.; Dong, Y. Influence of Preharvest and Postharvest Applications of Glycine Betaine on Fruit Quality Attributes and Storage Disorders of ‘Lapins’ and ‘Regina’ Cherries. HortScience Horts 2019, 54, 1540–1545. [Google Scholar] [CrossRef]
- Gonçalves, B.; Morais, M.C.; Sequeira, A.; Ribeiro, C.; Guedes, F.; Silva, A.P.; Aires, A. Quality preservation of sweet cherry cv.‘staccato’ by using glycine-betaine or Ascophyllum nodosum. Food Chem. 2020, 322, 126713. [Google Scholar]
- Correia, S.; Queirós, F.; Ribeiro, C.; Vilela, A.; Aires, A.; Barros, A.I.; Schouten, R.; Silva, A.P.; Gonçalves, B. Effects of calcium and growth regulators on sweet cherry (Prunus avium L.) quality and sensory attributes at harvest. Sci. Hortic. 2019, 248, 231–240. [Google Scholar]
- Ureta Ovalle, A.; Atenas, C.; Larraín, P. Application of an Ecklonia maxima seaweed product at two different timings can improve the fruit set and yield in ‘Bing’ sweet cherry trees. In VIII International Cherry Symposium; ISHS: Leuven, Belgium, 2017; pp. 319–326. [Google Scholar]
- Fadón, E.; Herrero, M.; Rodrigo, J. Flower development in sweet cherry framed in the BBCH scale. Sci. Hortic. 2015, 192, 141–147. [Google Scholar]
- Singleton, V.; Rossi, J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Dewanto, V.; Wu, X.; Adom, K.; Liu, R. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar]
- Nicoue, E.E.; Savard, S.; Belkacemi, K. Anthocyanins in wild blueberries of Quebec: Extraction and identification. J. Agric. Food Chem. 2007, 55, 5626–5635. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, M.A.; Whiting, M.; Ross, C.F. The influence of harvest time on sensory properties and consumer acceptance of sweet cherries. Horttechnology 2009, 19, 748–754. [Google Scholar] [CrossRef]
- ISO 6658; Sensory Analysis-Methodology—General Guidance. 3rd ed. International Organization for Standardization: Geneva, Switzerland, 2017; pp. 1–26.
- ISO 4121; Sensory Analysis-Guidelines for the Use of Quantitative Response Scales. 2nd ed. International Organization for Standardization: Geneva, Switzerland, 2003; pp. 1–9.
- Bund, S.; Norre, J. Seaweed extract improve cherry fruit quality. In Proceedings of the Australian Palnt Health Conference. Australian Society of Horticultural Science. New Zealand Institute of Agriculture and Horticuleture Science, Joint Conference, Lorne, Australia, 18–22 September 2011; pp. 18–22. [Google Scholar]
- Sajid, M.; Basit, A.; Ullah, Z.; Shah, S.T.; Ullah, I.; Mohamed, H.I.; Ullah, I. Chitosan-based foliar application modulated the yield and biochemical attributes of peach (Prunus persica L.) cv. Early Grand. Bull. Natl. Res. Cent. 2020, 44, 150. [Google Scholar]
- Esitken, A.; Pirlak, L.; Turan, M.; Sahin, F. Effects of floral and foliar application of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrition of sweet cherry. Sci. Hortic. 2006, 110, 324–327. [Google Scholar]
- Zhang, C.; Whiting, M. Plant growth regulators improve sweet cherry fruit quality without reducing endocarp growth. Sci. Hortic. 2013, 150, 73–79. [Google Scholar] [CrossRef]
- Stirk, W.A.; Van Staden, J. Comparison of cytokinin-and auxin-like activity in some commercially used seaweed extracts. J. Appl. Phycol. 1996, 8, 503–508. [Google Scholar]
- Pizzeghello, D.; Francioso, O.; Ertani, A.; Muscolo, A.; Nardi, S. Isopentenyladenosine and cytokinin-like activity of different humic substances. J. Geochem. Explor. 2013, 129, 70–75. [Google Scholar]
- Kocira, S.; Szparaga, A.; Kuboń, M.; Czerwińska, E.; Piskier, T. Morphological and biochemical responses of Glycine max (L.) Merr. to the use of seaweed extract. Agronomy 2019, 9, 93. [Google Scholar] [CrossRef]
- Baltazar, M.; Correia, S.; Guinan, K.J.; Sujeeth, N.; Bragança, R.; Gonçalves, B. Recent advances in the molecular effects of biostimulants in plants: An overview. Biomolecules 2021, 11, 1096. [Google Scholar] [CrossRef]
- Valenzuela-Soto, E.M.; Figueroa-Soto, C.G. Biosynthesis and Degradation of Glycine Betaine and Its Potential to Control Plant Growth and Development. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants; Hossain, M., Kumar, V., Burritt, D., Fujita, M., Mäkelä, P., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Stern, R.A.; Flaishman, M.; Applebaum, S.; Ben-Arie, R. Effect of synthetic auxins on fruit development of ‘Bing’cherry (Prunus avium L.). Sci. Hortic. 2007, 114, 275–280. [Google Scholar] [CrossRef]
- Clayton-Cuch, D.; Yu, L.; Shirley, N.; Bradley, D.; Bulone, V.; Böttcher, C. Auxin treatment enhances anthocyanin production in the non-climacteric sweet cherry (Prunus avium L.). Int. J. Mol. Sci. 2021, 22, 10760. [Google Scholar] [CrossRef]
- Flaishman, M.; Ben-Arie, R.; Stern, R.A.; Applebaum, S. Auxins increase fruit size of ‘bing’(Prunus avium L.) cherry in a warm climate. In XXVII International Horticultural Congress-IHC2006: International Symposium on Endogenous and Exogenous Plant Bioregulators; ISHS: Leuven, Belgium, 2006; pp. 243–250. [Google Scholar]
- Champa, W.H.; Gill MI, S.; Mahajan BV, C.; Arora, N.K. Preharvest salicylic acid treatments to improve quality and postharvest life of table grapes (Vitis vinifera L.) cv. Flame Seedless. J. Food Sci. Technol. 2015, 52, 3607–3616. [Google Scholar]
- Serradilla, M.J.; Martín, A.; Ruiz-Moyano, S.; Hernández, A.; López-Corrales, M.; de Guía Córdoba, M. Physicochemical and sensorial characterisation of four sweet cherry cultivars grown in Jerte Valley (Spain). Food Chem. 2012, 133, 1551–1559. [Google Scholar]
- Korley Kortei, N.; Tawia Odamtten, G.; Obodai, M.; Appiah, V.; Toah Akonor, P. Determination of color parameters of gamma irradiated fresh and dried mushrooms during storage. Croat. J. Food Technol. Biotechnol. Nutr. 2015, 10, 66–71. [Google Scholar]
- Giménez, M.J.; Valverde, J.M.; Valero, D.; Guillén, F.; Martínez-Romero, D.; Serrano, M.; Castillo, S. Quality and antioxidant properties on sweet cherries as affected by preharvest salicylic and acetylsalicylic acids treatments. Food Chem. 2014, 160, 226–232. [Google Scholar]
- Gonçalves, B.; Silva, A.P.; Moutinho-Pereira, J.; Bacelar, E.; Rosa, E.; Meyer, A.S. Effect of ripeness and postharvest storage on the evolution of colour and anthocyanins in cherries (Prunus avium L.). Food Chem. 2007, 103, 976–984. [Google Scholar]
- Kondo, S.; Inoue, K. Abscisic acid (ABA) and 1-aminocyclopropane-1-carboxylic acid (ACC) content during growth of ‘Satonishiki’ cherry fruit, and the effect of ABA and ethephon application on fruit quality. J. Hort. Sci. 1997, 72, 221–227. [Google Scholar]
- Kuhn, N.; Arellano, M.; Ponce, C.; Hodar, C.; Correa, F.; Multari, S.; Martens, S.; Carrera, E.; Donoso, J.M.; Meisel, L.A. RNA-Seq and WGBS Analyses During Fruit Ripening and in Response to ABA in Sweet Cherry (Prunus avium) Reveal Genetic and Epigenetic Modulation of Auxin and Cytokinin Genes. J. Plant Growth Regul. 2024, 1–23. [Google Scholar] [CrossRef]
- Ponce, C.; Kuhn, N.; Arellano, M.; Time, A.; Multari, S.; Martens, S.; Carreras, E.; Sagredo, B.; Donoso, J.; Meisel, L.A. Differential phenolic compounds and hormone accumulation patterns between early-and mid-maturing sweet cherry (Prunus avium L.) cultivars during fruit development and ripening. J. Agric. Food Chem. 2021, 69, 8850–8860. [Google Scholar]
- Zhang, C.; Whiting, M.D. Improving ‘Bing’ sweet cherry fruit quality with plant growth regulators. Sci. Hortic. 2011, 127, 341–346. [Google Scholar]
- Sabir, I.A.; Liu, X.; Jiu, S.; Whiting, M.; Zhang, C. Plant growth regulators modify fruit set, fruit quality, and return bloom in sweet cherry. HortScience 2021, 56, 922–931. [Google Scholar]
- Zhi, H.; Dong, Y. Seaweed-based biostimulants improves quality traits, postharvest disorders, and antioxidant properties of sweet cherry fruit and in response to gibberellic acid treatment. Sci. Hortic. 2024, 336, 113454. [Google Scholar]
- Hocking, B.; Tyerman, S.D.; Burton, R.A.; Gilliham, M. Fruit calcium: Transport and physiology. Front. Plant Sci. 2016, 7, 569. [Google Scholar]
- Dong, Y.; Zhi, H.; Wang, Y. Cooperative effects of pre-harvest calcium and gibberellic acid on tissue calcium content, quality attributes, and in relation to postharvest disorders of late-maturing sweet cherry. Sci. Hortic. 2019, 246, 123–128. [Google Scholar]
- Ricardo-Rodrigues, S.; Laranjo, M.; Agulheiro-Santos, A.C. Methods for quality evaluation of sweet cherry. J. Sci. Food Agric. 2023, 103, 463–478. [Google Scholar]
- Hampson, C.R.; Stanich, K.; McKenzie, D.L.; Herbert, L.; Lu, R.; Li, J.; Cliff, M.A. Determining the Optimum Firmness for Sweet Cherries Using Just-About-Right Sensory Methodology. Postharvest Biol. Technol. 2014, 91, 104–111. [Google Scholar]
- Wang, Y.; Long, L.E. Physiological and Biochemical Changes Relating to Postharvest Splitting of Sweet Cherries Affected by Calcium Application in Hydrocooling Water. Food Chem. 2015, 181, 241–247. [Google Scholar] [PubMed]
- Ballistreri, G.; Continella, A.; Gentile, A.; Amenta, M.; Fabroni, S.; Rapisarda, P. Fruit quality and bioactive compounds relevant to human health of sweet cherry (Prunus avium L.) cultivars grown in Italy. Food Chem. 2013, 140, 630–638. [Google Scholar]
- Hayaloglu, A.A.; Demir, N. Physicochemical characteristics, antioxidant activity, organic acid and sugar contents of 12 sweet cherry (Prunus avium L.) cultivars grown in Turkey. J. Food Sci. 2015, 80, C564–C570. [Google Scholar]
- Guyer, D.E.; Sinha, N.K.; Chang, T.-S.; Cash, J.N. Physicochemical and sensory characteristics of selected Michigan sweet cherry (Prunus avium L.) cultivars. J. Food Qual. 1993, 16, 355–370. [Google Scholar]
- Faniadis, D.; Drogoudi, P.D.; Vasilakakis, M. Effects of cultivar, orchard elevation, and storage on fruit quality characters of sweet cherry (Prunus avium L.). Sci. Hortic. 2010, 125, 301–304. [Google Scholar] [CrossRef]
- Pereira, S.; Silva, V.; Bacelar, E.; Guedes, F.; Silva, A.P.; Ribeiro, C.; Gonçalves, B. Cracking in Sweet Cherry Cultivars ‘Early Bigi’ and ‘Lapins’: Correlation with Quality Attributes. Plants 2020, 9, 1557. [Google Scholar] [CrossRef]
- López, L.; Larrigaudière, C.; Giné-Bordonaba, J.; Echeverria, G. Defining key parameters and predictive markers of ‘Early Bigi’cherry consumer satisfaction by means of differential storage scenarios. Postharvest Biol. Technol. 2023, 195, 112117. [Google Scholar]
- Martins, V.; Silva, V.; Pereira, S.; Afonso, S.; Oliveira, I.; Santos, M.; Ribeiro, C.; Vilela, A.; Bacelar, E.; Silva, A.P.; et al. Rootstock Affects the Fruit Quality of ‘Early Bigi’ Sweet Cherries. Foods 2021, 10, 2317. [Google Scholar] [CrossRef]
- García-Montiel, F.; López, D.; Guirao, P.; García, F.; Frutos, D.; López-Ortega, G.; Carrillo, A.; López, D.; Cos, J. Preliminary results of sweet cherry (Prunus avium L.) collection in Jumilla, Murcia, Spain. Acta Hortic. 2017, 1161, 281–286. [Google Scholar]
- Radičević, S.; Marić, S.; Milošević, N.; Glišić, I.; Đorđević, M. Phenological characteristics and fruit quality of introduced sweet cherry (Prunus avium L.) cultivars in agroecological conditions of Čačak. Voćarstvo 2022, 56, 93–99. [Google Scholar]
- Drake, S.R.; Fellman, J.K. Indicators of Maturity and Storage Quality of ‘Rainier’ Sweet Cherry. HortScience 1987, 22, 283–285. [Google Scholar] [CrossRef]
- Velardo-Micharet, B.; Peñas Díaz, L.; Tapia García, I.M.; Nieto Serrano, E.; Campillo Torres, C. Effect of irrigation on postharvest quality of two sweet cherry cultivars (Prunus avium L.). Acta Hortic. 2017, 1161, 667–672. [Google Scholar] [CrossRef]
- Usenik, V.; Fajt, N.; Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. Sweet cherry pomological and biochemical characteristics influenced by rootstock. J. Agric. Food Chem. 2010, 58, 4928–4933. [Google Scholar]
- Yao, L.; Liang, D.; Xia, H.; Pang, Y.; Xiao, Q.; Huang, Y.; Zhang, W.; Pu, C.; Wang, J.; Lv, X. Biostimulants promote the accumulation of carbohydrates and biosynthesis of anthocyanins in ‘Yinhongli’plum. Front. Plant Sci. 2023, 13, 1074965. [Google Scholar]
- Gupta, N.; Thind, S.K. Foliar Application of Glycine Betaine Alters Sugar Metabolism of Wheat Leaves Under Prolonged Field Drought Stress. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 89, 877–884. [Google Scholar] [CrossRef]
- Wang, L.I.; Shan, T.; Xie, B.; Ling, C.; Shao, S.; Jin, P.; Zheng, Y. Glycine betaine reduces chilling injury in peach fruit by enhancing phenolic and sugar metabolisms. Food Chem. 2019, 272, 530–538. [Google Scholar] [PubMed]
- Shukla, P.S.; Prithiviraj, B. Ascophyllum nodosum biostimulant improves the growth of Zea mays grown under phosphorus impoverished conditions. Front. Plant Sci. 2021, 11, 601843. [Google Scholar]
- Arteca, R.N. Plant Growth Substances: Principles and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Abou-Aly, H.E.; Mady, M.A. Complemented effect of glycine betaine and biofertilizers on growth and produtivity of sweet pepper (Capsicum annuum L.) plant under high temperature condition. J. Plant Prod. 2014, 5, 505–526. [Google Scholar]
- Ruiz-Aracil, M.C.; Valverde, J.M.; Lorente-Mento, J.M.; Carrión-Antolí, A.; Castillo, S.; Martínez-Romero, D.; Guillén, F. Sweet Cherry (Prunus avium L.) Cracking during Development on the Tree and at Harvest: The Impact of Methyl Jasmonate on Four Different Growing Seasons. Agriculture 2023, 13, 1244. [Google Scholar] [CrossRef]
- Ruiz-Aracil, M.C.; Valverde, J.M.; Beltrà, A.; Carrión-Antolí, A.; Lorente-Mento, J.M.; Nicolás-Almansa, M.; Guillén, F. Putrescine Increases Frost Tolerance and Effectively Mitigates Sweet Cherry (Prunus avium L.) Cracking: A Study of Four Different Growing Cycles. Agronomy 2024, 14, 23. [Google Scholar] [CrossRef]
- Palacios-Peralta, C.; Ruiz, A.; Ercoli, S.; Reyes-Díaz, M.; Bustamante, M.; Muñoz, A.; Osorio, P.; Ribera-Fonseca, A. Plastic Covers and Potassium Pre-Harvest Sprays and Their Influence on Antioxidant Properties, Phenolic Profile, and Organic Acids Composition of Sweet Cherry Fruits Cultivated in Southern Chile. Plants 2023, 12, 50. [Google Scholar] [CrossRef]
- Ozturk, B.; Akkaya, H.; Aglar, E.; Saracoglu, O. Effect of preharvest biofilm application regimes on cracking and fruit quality traits in ‘0900 Ziraat’ sweet cherry cultivar. BMC Plant Biol. 2024, 24, 574. [Google Scholar] [CrossRef]
- Souza, M.L.D.; Morgado, C.M.A.; Marques, K.M.; Mattiuz, C.F.M.; Mattiuz, B.H. Pós-colheita de mangas’ Tommy Atkins’ recobertas com quitosana. Rev. Bras. De Frutic. 2011, 33, 337–343. [Google Scholar]
- Luo, H.; Dai, S.; Ren, J.; Zhang, C.; Ding, Y.; Li, Z.; Sun, Y.; Ji, K.; Wang, Y.; Li, Q.; et al. The Role of ABA in the Maturation and Postharvest Life of a Nonclimacteric Sweet Cherry Fruit. J. Plant Growth Regul. 2014, 33, 373–383. [Google Scholar] [CrossRef]
- Ockun, M.A.; Gercek, Y.C.; Demirsoy, H.; Demirsoy, L.; Macit, I.; Oz, G.C. Comparative evaluation of phenolic profile and antioxidant activity of new sweet cherry (Prunus avium L.) genotypes in Turkey. Phytochem. Anal. 2022, 33, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Kelebek, H.; Selli, S. Evaluation of chemical constituents and antioxidant activity of sweet cherry (Prunus avium L.) cultivars. Int. J. Food Sci. Technol. 2011, 46, 2530–2537. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sanchez-Cortes, S.; Nardi, S. Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: Chemical and metabolomic approaches. Front. Plant Sci. 2014, 5, 375. [Google Scholar] [CrossRef]
- De Saeger, J.; Van Praet, S.; Vereecke, D.; Park, J.; Jacques, S.; Han, T.; Depuydt, S. Toward the molecular understanding of the action mechanism of Ascophyllum nodosum extracts on plants. J. Appl. Phycol. 2020, 32, 573–597. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants 2021, 10, 531. [Google Scholar] [CrossRef]
- Habibi, F.; Valero, D.; Serrano, M.; Guillén, F. Exogenous application of glycine betaine maintains bioactive compounds, antioxidant activity, and physicochemical attributes of blood orange fruit during prolonged cold storage. Front. Nutr. 2022, 9, 873915. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, E.; De Lorenzis, G.; Ricciardi, V.; Baltazar, M.; Pereira, S.; Correia, S.; Ferreira, H.; Alves, F.; Cortez, I.; Gonçalves, B.; et al. Exploring Seaweed and Glycine Betaine Biostimulants for Enhanced Phenolic Content, Antioxidant Properties, and Gene Expression of Vitis vinifera cv. “Touriga Franca” Berries. Int. J. Mol. Sci. 2024, 25, 5335. [Google Scholar] [CrossRef]
- Fazzari, M.; Fukumoto, L.; Mazza, G.; Livrea, M.A.; Tesoriere, L.; Marco, L.D. In vitro bioavailability of phenolic compounds from five cultivars of frozen sweet cherries (Prunus avium L.). J. Agric. Food Chem. 2008, 56, 3561–3568. [Google Scholar] [CrossRef]
- Clayton, M.; Biasi, W.V.; Agar, I.T.; Southwick, S.M.; Mitcham, E.J. Sensory quality of ‘Bing’ sweet cherries following preharvest treatment with hydrogen cyanamide, calcium ammonium nitrate, or gibberellic acid. HortScience 2006, 41, 745–748. [Google Scholar] [CrossRef]
- Soteriou, G.A.; Rouphael, Y.; Emmanouilidou, M.G.; Antoniou, C.; Kyratzis, A.C.; Kyriacou, M.C. Biostimulatory Action of Vegetal Protein Hydrolysate and the Configuration of Fruit Physicochemical Characteristics in Grafted Watermelon. Horticulturae 2021, 7, 313. [Google Scholar] [CrossRef]
- Rodrigues, M.; Baptistella, J.L.C.; Horz, D.C.; Bortolato, L.M.; Mazzafera, P. Organic Plant Biostimulants and Fruit Quality—A Review. Agronomy 2020, 10, 988. [Google Scholar] [CrossRef]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress—A Review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]

| Cultivars | Biostimulants | Concentrations | Sweet Cherries Developmental Stages | Date of Spraying | ||
|---|---|---|---|---|---|---|
| ‘Early Bigi’ | Glycine betaine (GB) Ecklonia maxima extract (EM) | GB 0.25% and GB 0.40% EM 0.15% and EM 0.30% | 2019 | 2020 | 2021 | |
| BBCH 77 | 11 April | 1 April | 6 April | |||
| BBCH 81 | 19 April | 11 April | 15 April | |||
| BBCH 86 | 30 April | 16 April | 24 April | |||
| ‘Lapins’ | Glycine betaine (GB) Ecklonia maxima extract (EM) | GB 0.25% and GB 0.40% EM 0.15% and EM 0.30% | BBCH 77 | 11 April | 1 April | 6 April |
| BBCH 81 | 16 May | 4 May | 11 May | |||
| BBCH 86 | 24 May | 12 May | 31 May | |||
| Cultivar | Parameter | Year | C | EM 0.15% | EM 0.30% | GB 0.25% | GB 0.40% | Mix | Treatment Effect |
|---|---|---|---|---|---|---|---|---|---|
| ‘Early Bigi’ | Weight (g) | 2019 | 9.44 ± 0.74 ab | 7.67 ± 0.74 c | 7.46 ± 0.62 c | 9.57 ± 0.83 a | 8.84 ± 0.89 b | 8.00 ± 0.70 c | *** |
| 2021 | 8.65 ± 0.69 c | 9.54 ± 0.70 ab | 9.06 ± 0.71 bc | 9.49 ± 0.87 ab | 8.83 ± 0.87 c | 9.81 ± 0.86 a | *** | ||
| Length (mm) | 2019 | 24.84 ± 0.58 a | 23.46 ± 0.85 cd | 22.83 ± 0.61 d | 24.47 ± 0.66 ab | 24.12 ± 0.71 bc | 23.67 ± 0.89 c | *** | |
| 2021 | 22.40 ± 1.46 c | 23.48 ± 0.78 b | 22.82 ± 0.94 bc | 23.35 ± 1.07 b | 23.26 ± 0.95 b | 24.33 ± 0.77 a | *** | ||
| Diameter (mm) | 2019 | 22.87 ± 0.76 ab | 21.40 ± 0.70 c | 20.60 ± 0.59 d | 23.18 ± 1.01 a | 22.14 ± 0.97 bc | 21.84 ± 0.96 c | *** | |
| 2021 | 22.11 ± 0.87 bc | 22.66 ± 1.08 ab | 21.69 ± 1.06 c | 22.40 ± 1.02 abc | 22.63 ± 0.84 ab | 23.02 ± 1.08 a | *** | ||
| Width (mm) | 2019 | 28.48 ± 0.98 ab | 26.07 ± 0.92 c | 26.48 ± 1.01 c | 28.79 ± 0.88 a | 27.66 ± 1.34 b | 26.47 ± 1.01 c | *** | |
| 2021 | 28.09 ± 1.21 | 28.13 ± 1.14 | 27.68 ± 1.12 | 28.57 ± 1.67 | 27.66 ± 0.98 | 28.09 ± 1.16 | n.s. | ||
| ‘Lapins’ | Weight (g) | 2019 | 8.41 ± 1.12 d | 11.12 ± 1.23 c | 12.00 ± 0.75 ab | 11.62 ± 1.12 bc | 12.76 ± 1.08 a | 11.82 ± 1.10b c | *** |
| 2021 | 7.50 ± 1.30 d | 10.37 ± 0.76 b | 11.04 ± 0.83 a | 10.35 ± 0.83 b | 9.25 ± 0.82 c | 8.89 ± 0.58 c | *** | ||
| Length (mm) | 2019 | 26.65 ± 0.99 ab | 23.41 ± 0.97 d | 26.03 ± 0.75 bc | 26.08 ± 1.33 bc | 26.84 ± 0.98 a | 25.81 ± 0.99 c | *** | |
| 2021 | 23.09 ± 1.30 d | 25.01 ± 0.81 ab | 25.31 ± 0.89 a | 25.36 ± 0.94 a | 24.26 ± 0.61 c | 24.34 ± 0.89b c | *** | ||
| Diameter (mm) | 2019 | 25.03 ± 1.16 a | 22.24 ± 1.06 d | 24.88 ± 0.67 ab | 24.18 ± 0.99 bc | 24.88 ± 0.91 ab | 24.00 ± 0.97 c | *** | |
| 2021 | 21.42 ± 1.22 d | 24.02 ± 1.06 a | 23.88 ± 0.86 a | 23.74 ± 0.64 ab | 23.12 ± 0.75 bc | 22.58 ± 0.70 c | *** | ||
| Width (mm) | 2019 | 29.00 ± 1.29 ab | 25.91 ± 1.45 c | 29.06 ± 1.03 ab | 28.68 ± 0.98 b | 29.71 ± 0.89 a | 29.10 ± 1.06 ab | *** | |
| 2021 | 24.64 ± 1.72 c | 27.61 ± 1.00 a | 28.25 ± 1.07 a | 27.89 ± 0.89 a | 26.63 ± 0.99 b | 26.53 ± 0.87 b | *** |
| Cultivar | Parameter | Year | C | EM 0.15% | EM 0.30% | GB 0.25% | GB 0.40% | Mix | Treatment Effect |
|---|---|---|---|---|---|---|---|---|---|
| ‘Early Bigi’ | L* | 2019 | 51.02 ± 2.89 a | 37.37 ± 1.81 c | 40.43 ± 4.42 bc | 43.16 ± 4.17 b | 43.25 ± 4.17 b | 40.33 ± 3.81 bc | *** |
| 2021 | 48.69 ± 2.27 a | 45.40 ± 2.97 c | 47.90 ± 2.85 cb | 46.52 ± 2.94 abc | 45.83 ± 4.36 bc | 42.84 ± 3.31 d | *** | ||
| C* | 2019 | 41.60 ± 1.65 ab | 34.92 ± 3.69 d | 37.20 ± 5.31 cd | 42.23 ± 3.09 a | 40.56 ± 4.32 abc | 37.98 ± 4.72 bcd | *** | |
| 2021 | 42.06 ± 2.19 b | 44.01 ± 1.95 a | 42.50 ± 2.01 ab | 42.02 ± 2.26 b | 41.86 ± 3.09 b | 41.77 ± 2.77 b | *** | ||
| Hue° | 2019 | 35.09 ± 1.14 a | 27.14 ± 2.61 c | 30.03 ± 2.13 b | 31.40 ± 2.13 b | 30.88 ± 3.06 b | 30.12 ± 2.32 b | *** | |
| 2021 | 33.97 ± 2.07 a | 33.39 ± 1.42 a | 33.83 ± 1.88 ª | 33.29 ± 1.82 a | 32.98 ± 2.27 a | 31.45 ± 2.28 b | *** | ||
| ‘Lapins’ | L* | 2019 | 41.58 ± 3.52 a | 36.89 ± 3.40 b | 34.99 ± 2.21bc | 33.47 ± 2.40 c | 34.17 ± 3.09 c | 33.85 ± 1.81 c | *** |
| 2021 | 35.46 ± 1.84 b | 38.40 ± 1.98 a | 38.18 ± 2.59 ª | 37.61 ± 1.89 a | 37.78 ± 2.54 a | 36.95 ± 2.28 ab | *** | ||
| C* | 2019 | 42.22 ± 3.57 a | 35.37 ± 4.96 b | 35.01 ± 3.52 b | 31.33 ± 4.47 c | 34.83 ± 4.95 b | 30.29 ± 4.05 c | *** | |
| 2021 | 38.58 ± 2.39 | 38.55 ± 2.31 | 39.69 ± 2.56 | 38.98 ± 2.48 | 38.78 ± 2.28 | 37.97 ± 2.73 | n.s. | ||
| Hue° | 2019 | 29.89 ± 2.08 a | 25.87 ± 3.82 b | 25.61 ± 1.96 b | 23.12 ± 2.76 c | 26.36 ± 2.01 b | 22.85 ± 2.34 c | *** | |
| 2021 | 26.79 ± 2.59 | 27.62 ± 2.03 | 28.09 ± 2.64 | 26.33 ± 2.58 | 26.81 ± 2.76 | 26.88 ± 2.62 | n.s. |
| Cultivar | Parameters | Year | C | EM 0.15% | EM 0.30% | GB 0.25% | GB 0.40% | Mix | Treatment Effect |
|---|---|---|---|---|---|---|---|---|---|
| ‘Early Bigi’ | TSS (°Brix) | 2019 | 10.38 ± 0.13 c | 12.50 ± 0.28 a | 11.60 ± 0.23 b | 12.38 ± 0.34 a | 11.65 ± 0.19 b | 12.03 ± 0.40 ab | *** |
| 2021 | 8.58 ± 0.03 c | 9.93 ± 0.12 ab | 9.53 ± 0.29 b | 9.50 ± 0.56 b | 10.17 ± 0.23 ab | 10.47 ± 0.32 a | * | ||
| TA (% malic acid) | 2019 | 0.58 ± 0.01 ab | 0.54 ± 0.06 ab | 0.49 ± 0.02 | 0.62 ± 0.03 a | 0.53 ± 0.06 b | 0.53 ± 0.01 b | *** | |
| 2021 | 0.36 ± 0.01 | 0.39 ± 0.01 | 0.39 ± 1.78 | 0.37 ± 0.01 | 0.48 ± 0.05 | 0.39 ± 0.03 | n.s. | ||
| MI (TSS/TA) | 2019 | 17.97 ± 0.09 b | 23.92 ± 1.34 a | 23.35 ± 0.92 a | 19.96 ± 0.99 b | 23.59 ± 0.81 a | 22.64 ± 0.47 a | *** | |
| 2021 | 23.89 ± 0.32 ab | 25.03 ± 0.50 a | 26.05 ± 0.59 a | 25.51 ± 1.53 a | 21.17 ± 2.32 b | 27.07 ± 1.36 a | * | ||
| ‘Lapins’ | TSS (°Brix) | 2019 | 15.13 ± 0.25 d | 19.37 ± 0.07 b | 18.18 ± 0.16 c | 19.32 ± 0.79 b | 19.42 ± 0.41 b | 21.20 ± 0.23 a | *** |
| 2021 | 14.28 ± 0.19 c | 16.13 ± 0.50 ab | 16.30 ± 0.36 a | 15.93 ± 0.42 ab | 15.23 ± 0.2 bc | 16.33 ± 0.38 a | *** | ||
| TA (% malic acid) | 2019 | 0.63 ± 0.02 bc | 0.70 ± 0.02 ab | 0.59 ± 0.04 c | 0.57 ± 0.04 c | 0.62 ± 0.02 c | 0.76 ± 0.02 a | *** | |
| 2021 | 0.49 ± 0.01 a | 0.46 ± 0.01 b | 0.45 ± 0.01 b | 0.50 ± 0.02 a | 0.46 ± 0.01 b | 0.45 ± 0.01 b | *** | ||
| MI (TSS/TA) | 2019 | 24.03 ± 0.56 b | 27.70 ± 0.68 bc | 31.00 ± 1.96 ab | 34.26 ± 2.34 a | 31.39 ± 1.39 ab | 27.80 ± 0.89 bc | *** | |
| 2021 | 28.59 ± 0.61 d | 34.91 ± 0.75 ab | 36.27 ± 1.18 a | 31.65 ± 0.31 c | 33.43 ± 1.14 bc | 36.25 ± 0.93 a | *** |
| Cultivar | Parameters | Year | C | EM 0.15% | EM 0.30% | GB 0.25% | GB 0.40% | Mix | Treatment Effect |
|---|---|---|---|---|---|---|---|---|---|
| ‘Early Bigi’ | Total phenolics (mg GAE g−1 DW) | 2019 | 7.19 ± 0.26 c | 8.22 ± 0.63 bc | 9.54 ± 0.71 a | 8.08 ± 0.38 bc | 9.09 ± 0.76 ab | 7.45 ± 0.84 c | ** |
| 2021 | 5.82 ± 0.35 d | 9.13 ± 0.27 a | 8.31 ± 0.28 b | 8.58 ± 0.31 ab | 7.95 ± 0.26 b | 6.89 ± 0.11 c | *** | ||
| Total anthocyanin (mg cy-3-rut g−1 DW) | 2019 | 14.18 ± 0.63 e | 37.77 ± 1.39 a | 38.52 ± 1.89 a | 25.04 ± 0.32 c | 31.32 ± 1.28 b | 18.88 ± 0.63 d | *** | |
| 2021 | 31.11 ± 2.93 c | 54.13 ± 2.29 ab | 58.57 ± 3.72 a | 47.26 ± 3.32 b | 58.42 ± 0.95 a | 33.35 ± 2.14 c | *** | ||
| ‘Lapins’ | Total phenolics (mg GAE g−1 DW) | 2019 | 5.96 ± 0.51 b | 7.77 ± 0.50 a | 6.78 ± 0.52 b | 6.28 ± 0.57 b | 8.07 ± 0.42 a | 6.45 ± 0.30 b | *** |
| 2021 | 8.20 ± 0.38 c | 10.04 ± 0.51 ab | 10.68 ± 0.89 a | 8.96 ± 0.15 bc | 9.68 ± 0.52 ab | 8.93 ± 0.91 bc | ** | ||
| Total anthocyanin (mg cy-3-rut g−1 DW) | 2019 | 64.03 ± 1.04 b | 80.15 ± 2.02 a | 84.35 ± 3.53 a | 81.95 ± 6.61 a | 82.92 ± 1.31 a | 76.03 ± 1.95 a | *** | |
| 2021 | 82.68 ± 2.59 c | 102.54 ± 4.60 b | 112.53 ± 3.64 a | 88.64 ± 1.74 c | 107.61 ± 2.89 b | 91.63 ± 2.22 c | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afonso, S.; Oliveira, I.; Ribeiro, C.; Vilela, A.; Meyer, A.S.; Gonçalves, B. Exploring the Role of Biostimulants in Sweet Cherry (Prunus avium L.) Fruit Quality Traits. Agriculture 2024, 14, 1521. https://doi.org/10.3390/agriculture14091521
Afonso S, Oliveira I, Ribeiro C, Vilela A, Meyer AS, Gonçalves B. Exploring the Role of Biostimulants in Sweet Cherry (Prunus avium L.) Fruit Quality Traits. Agriculture. 2024; 14(9):1521. https://doi.org/10.3390/agriculture14091521
Chicago/Turabian StyleAfonso, Sílvia, Ivo Oliveira, Carlos Ribeiro, Alice Vilela, Anne S. Meyer, and Berta Gonçalves. 2024. "Exploring the Role of Biostimulants in Sweet Cherry (Prunus avium L.) Fruit Quality Traits" Agriculture 14, no. 9: 1521. https://doi.org/10.3390/agriculture14091521








