Microbiological Soil Quality Indicators Associated with Long-Term Agronomical Management of Mediterranean Fruit Orchards
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cases
- Plum BioDynamic (Pl-BioD): the soil was not tilled but covered by seeded grasses—mowed in April, June, and July—and by shredded pruning material (September). Plant residues were left on the ground as mulch;
- Peach Organic system (Pe-Org): the soil was managed as above, except for the time of grass mowing (May and June) and pruning shredding (January);
- Apricot Conventional Tillage (Ap-CT): the soil was tilled at 20–25 cm depth throughout the year by a milling machine with the aim of maintaining it free from weeds. Pruning material was left on the ground as mulch in September;
- Plum Conventional Tillage (Pl-CM): the soil of inter-rows was temporarily covered by spontaneous grasses—from autumn until spring—and tilled at least twice a year by means of a motor hoe operating at 10 cm depth. Plant residues were left on the ground as mulching. Weeds in the row under the trees were managed by mechanical weeding. Pruning residues were shredded and left on the ground as mulch in September;
- Kiwi Conventional Management (Ki-CM): the soil was managed according to the local best management practices (BMPs) adopted within Piana del Sele. In particular, soil between the rows was covered by natural grasses which were mowed three times per year and left on the ground as mulching. In the rows under the trees, weeds were controlled by chemical weeding (Glyphosate). Pruning material was shredded and left in the rows as mulch;
- Peach Conventional Management (Pe-CM): the soil was managed following the same criteria used for Ki-CM.
2.2. Electromagnetic Induction Acquisitions and Identification of Sampling Zones within the Experimental Fruit Orchards
2.3. Soil Sampling
2.4. General Soil Analysis
2.5. Microbiological Activity Analysis
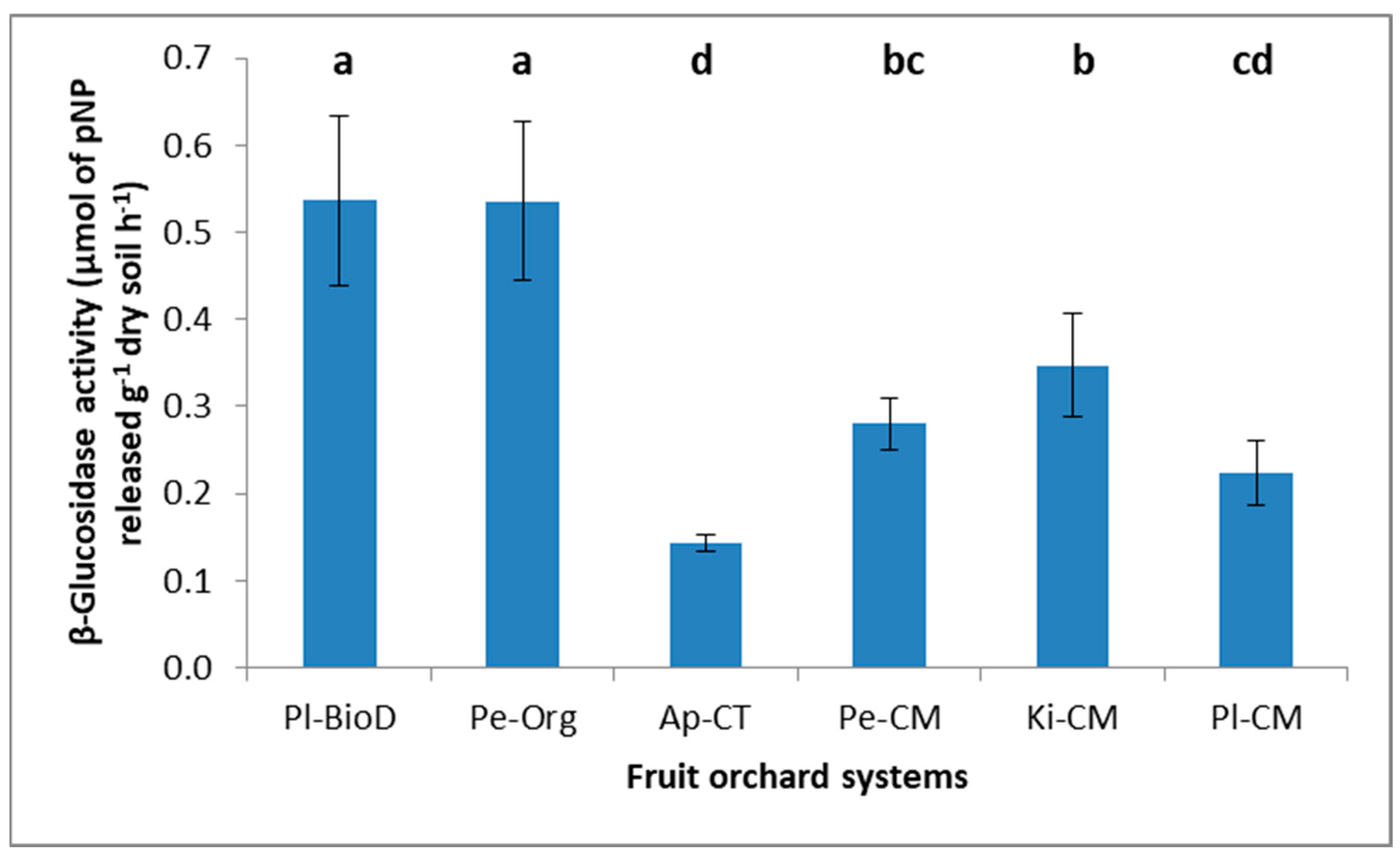
2.5.1. β-Glucosidase Assay
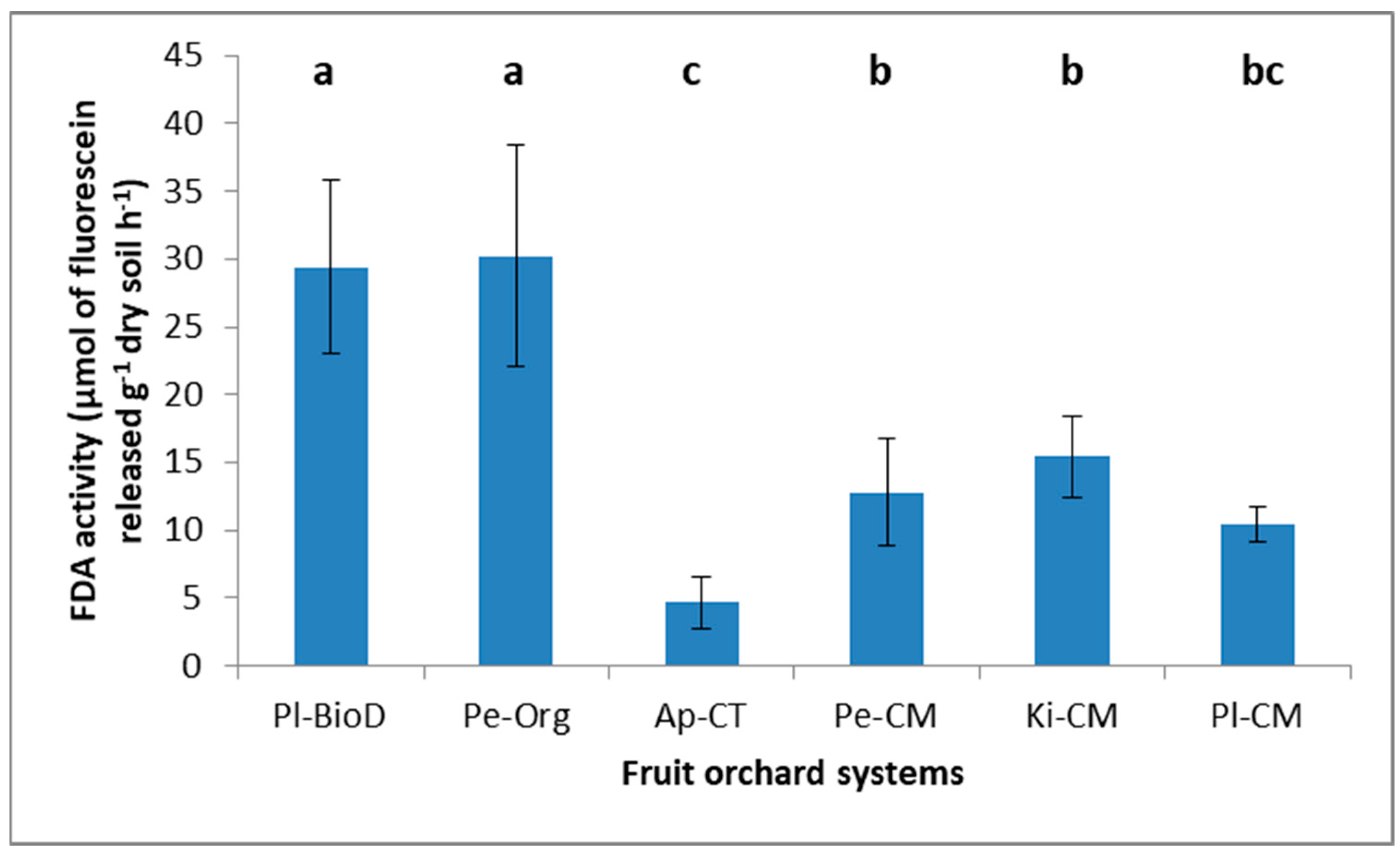
2.5.2. Fluorescein Diacetate Hydrolysis Assay
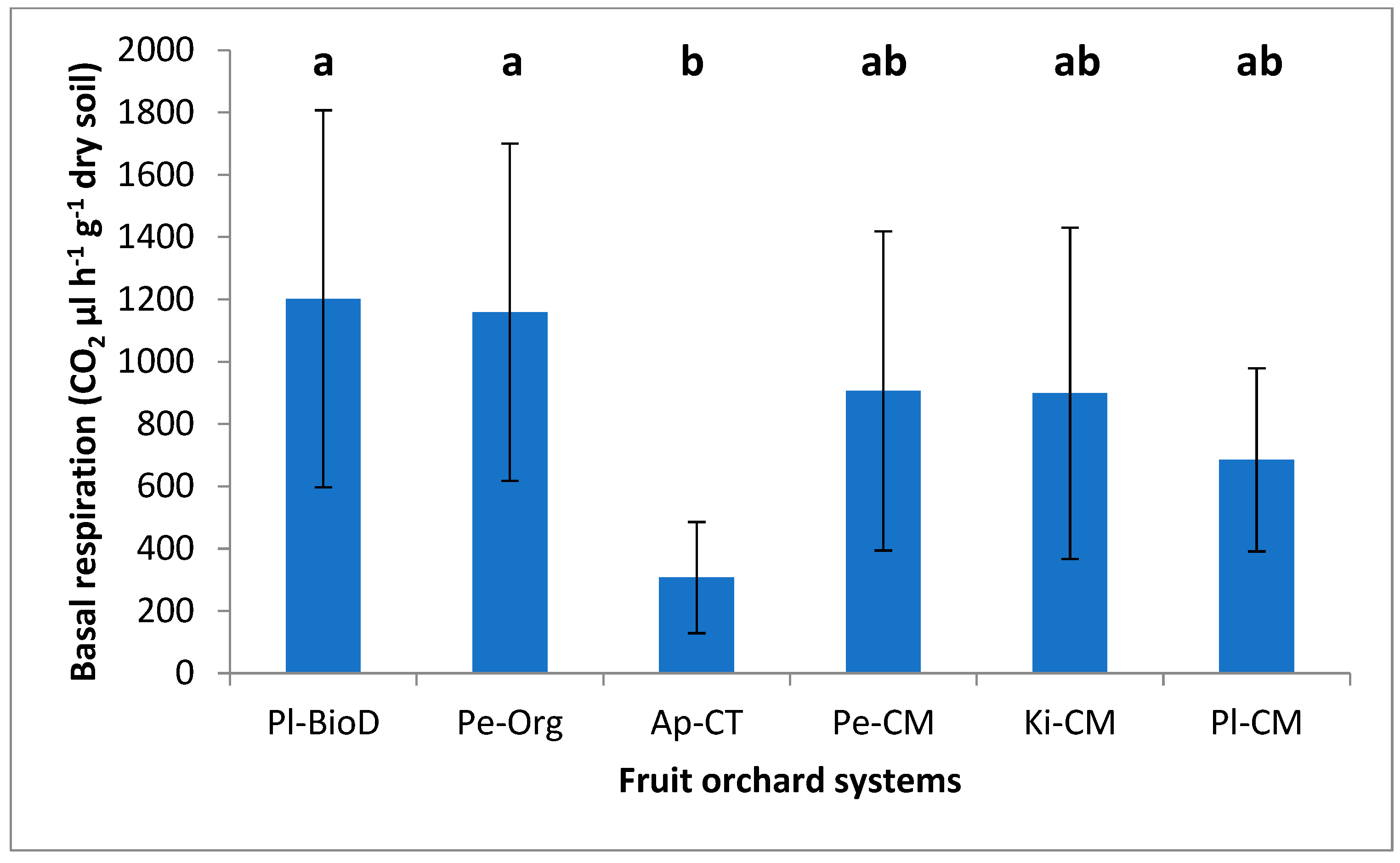
2.5.3. Soil Basal Respiration
2.5.4. Community-Level Physiological Profiling (CLPP) Analysis
2.6. Statistical Analysis
3. Results
3.1. ECa Values
3.2. Soil Parameters
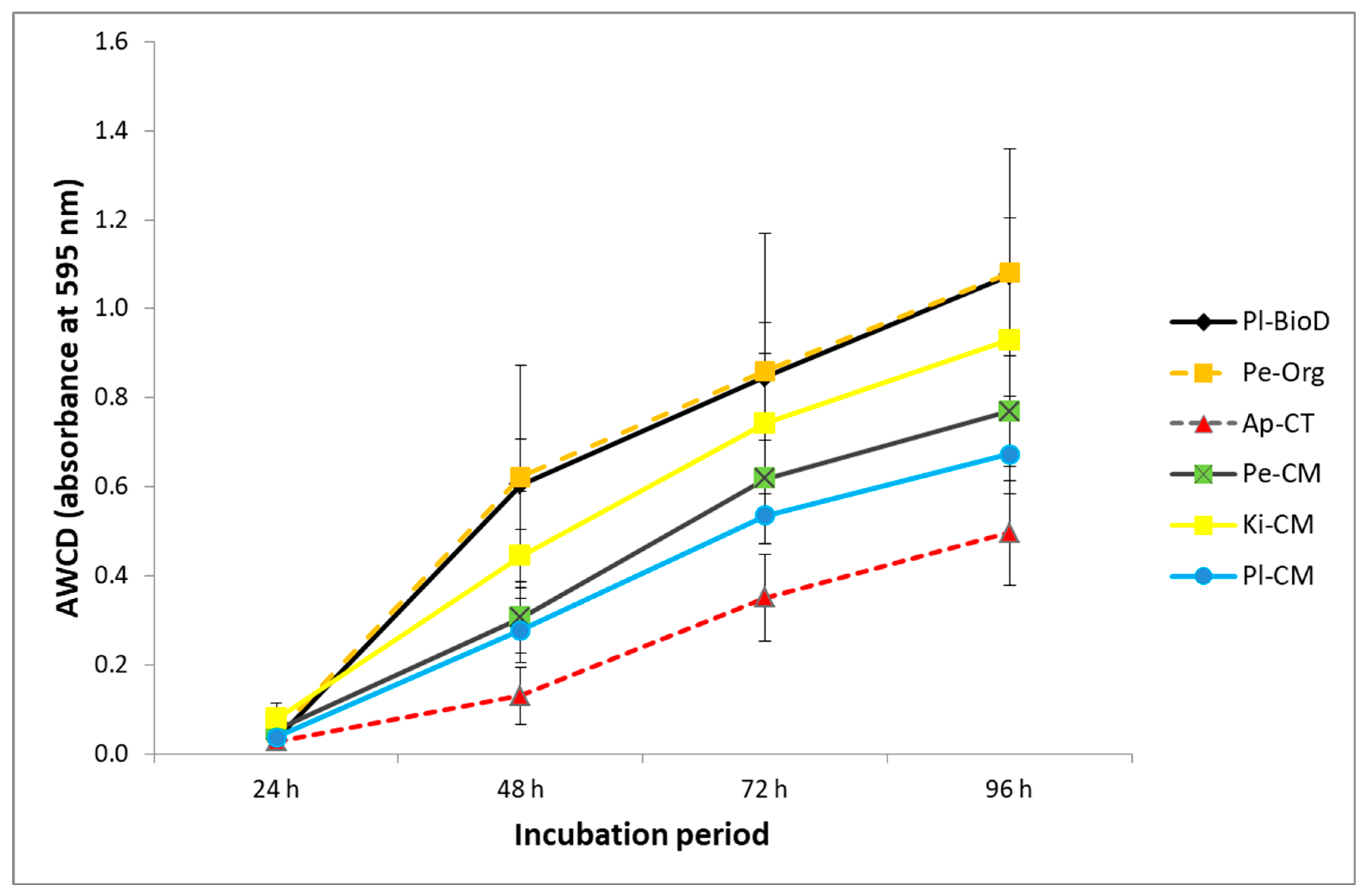
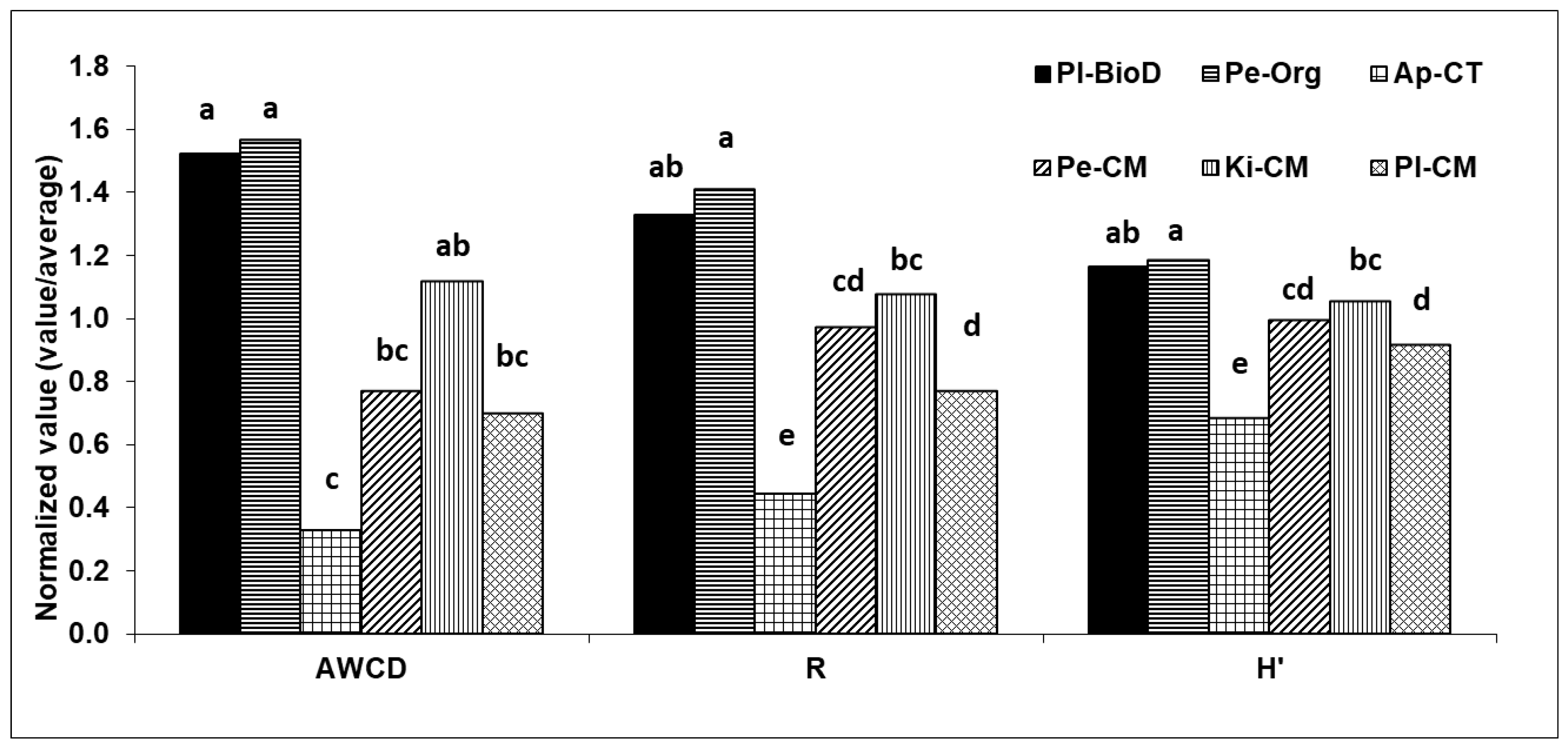
3.3. Microbial Activities
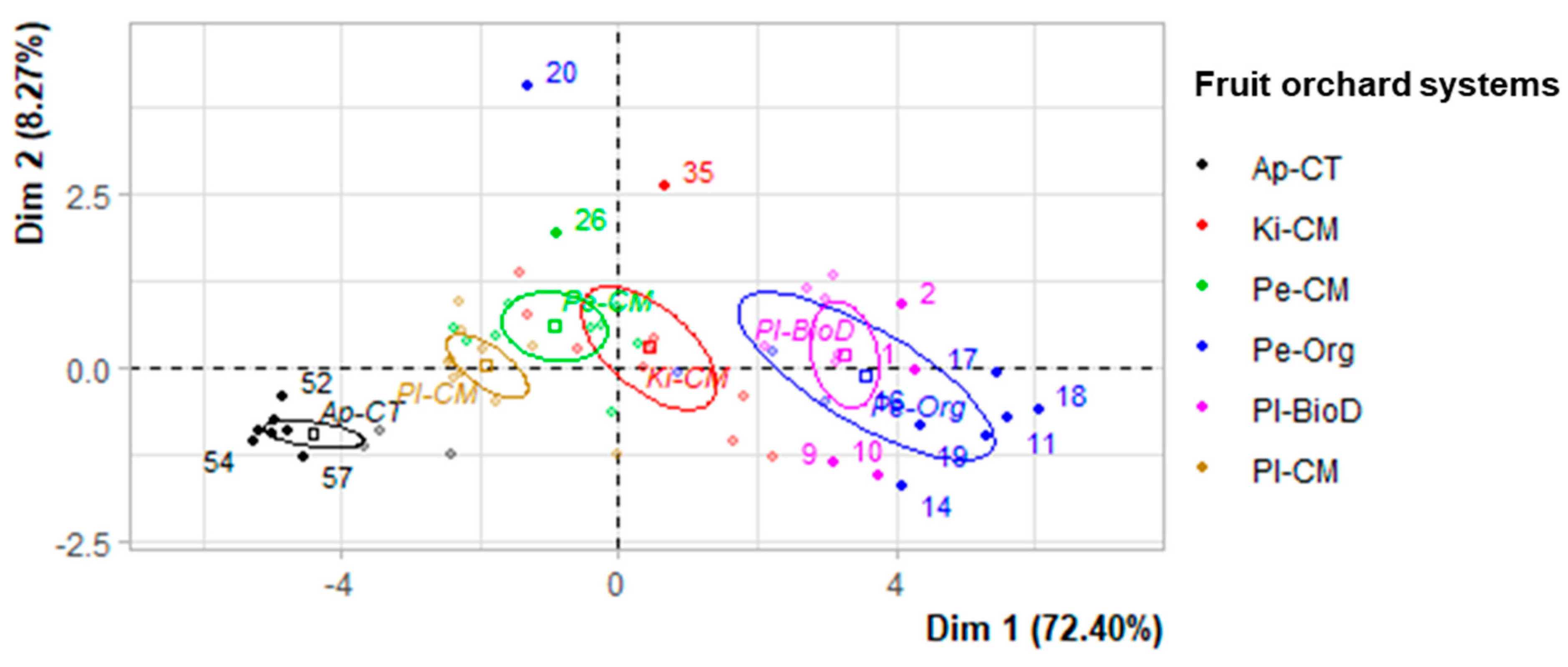
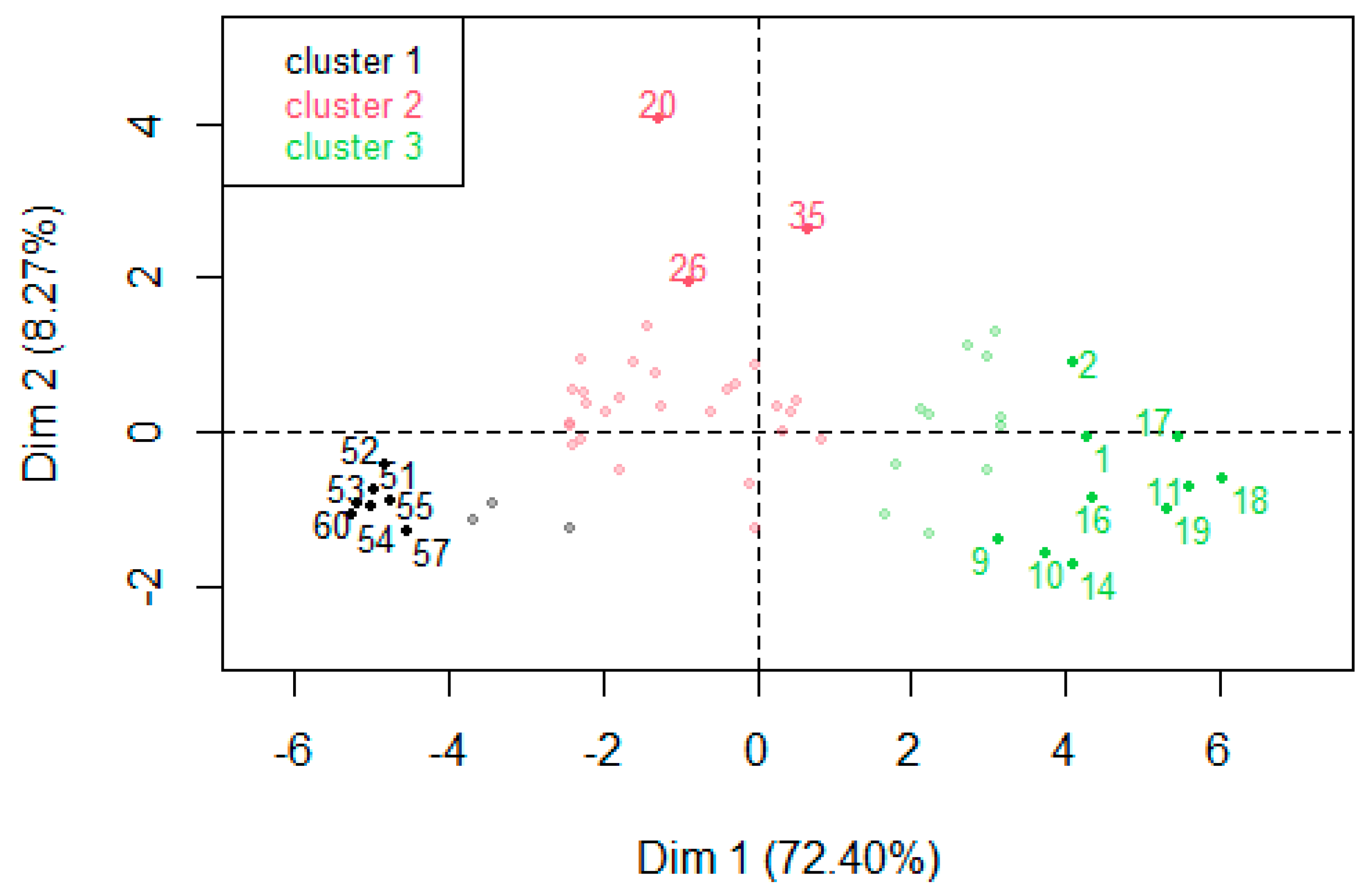
3.4. PCA Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bloem, J.; Schouten, A.J.; Sørensen, S.J.; Rutger, M.; van der Werf, A.; Breure, A.M. Monitoring and evaluating soil quality. In Microbiological Methods for Assessing Soil Quality; Bloem, J., Hopkins, D.W., Benedetti, A., Eds.; CABI Publishing: Oxfordshire, UK, 2006. [Google Scholar]
- Marzaioli, R.; D’Ascoli, R.; De Pascale, R.A.; Rutigliano, F.A. Soil quality in a Mediterranean area of Southern Italy as related to different land use types. Appl. Soil Ecol. 2010, 44, 205–212. [Google Scholar] [CrossRef]
- Schloter, M.; Dilly, O.; Munch, J.C. Indicators for Evaluating Soil Quality. Agric. Ecosyst. Environ. 2003, 98, 255–262. [Google Scholar] [CrossRef]
- Dick, W.A. Influence of long-term tillage and crop rotation combinations on soil enzyme activities. Soil Sci. Soc. Am. J. 1984, 48, 569–574. [Google Scholar] [CrossRef]
- Dick, R.P. Soil enzyme activities as indicators of soil quality. In Defining Soil Quality for a Sustainable Environment; Doran, J.W., Coleman, D.C., Bezdicek, D.F., Stewart, B.A., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 107–124. [Google Scholar]
- Doran, J.W.; Parkin, T.B. Defining and Assessing Soil Quality. In Defining Soil Quality for a Sustainable Environment; SSSA Special Publication no. 35; Soil Science Society of America: Madison, WI, USA, 1994. [Google Scholar]
- Karlen, D.L.; Mausbach, M.J.; Doran, J.W.; Cline, R.G.; Harris, R.F.; Schuman, G.E. Soil quality: A concept, definition, and framework for evaluation. Soil Sci. Soc. Am. J. 1997, 61, 4–10. [Google Scholar] [CrossRef]
- Doran, J.W.; Fraser, D.G.; Culik, M.N.; Liebhard, W.C. Influence of alternative and conventional agricultural management on soil microbial processes and nitrogen availability. Am. J. Altern. Agric. 1987, 2, 99–106. [Google Scholar] [CrossRef]
- Powlson, D.S.; Brookes, P.C.; Christensen, B.T. Measurement of soil microbial biomass provides an early indication of changes in total soil organic matter due to straw incorporation. Soil Biol. Biochem. 1987, 19, 159–164. [Google Scholar] [CrossRef]
- Werner, M.R.; Dindal, D.L. Effects of conversion to organic agricultural practices on soil biota. Am. J. Altern. Agric. 1990, 5, 24–32. [Google Scholar] [CrossRef]
- Fauci, M.F.; Dick, R.P. Soil microbial dynamics: Short- and long-term effects of inorganic and organic nitrogen. Soil Sci. Soc. Am. J. 1994, 58, 801–806. [Google Scholar] [CrossRef]
- Reganold, J.P. Comparison of soil properties as influenced by organic and conventional farming systems. Am. J. Altern. Agric. 1988, 3, 144–155. [Google Scholar] [CrossRef]
- Fraser, D.G.; Doran, J.W.; Sahs, W.W.; Lesoing, G.W. Soil microbial populations and activities under conventional and organic management. J. Environ. Qual. 1988, 17, 585–590. [Google Scholar] [CrossRef]
- Rastin, N.; Rosenplanter, K.; Huttermann, A. Seasonal variation of enzyme activity and their dependence on certain soil factors in a beech forest soil. Soil Biol. Biochem. 1988, 20, 637–642. [Google Scholar] [CrossRef]
- Criquet, S.; Vogt, G.; Le Petit, J. Endoglucasase and -glycosidase activities in an evergreen oak litter: Annual variation and regulating factors. Soil Biol. Biochem. 2002, 34, 1111–1120. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Garcia-Manzanedo, A.M.; Acosta-Martinez, V. Substrate specificity of arylamidase in soils. Soil Biol. Biochem. 2002, 34, 103–110. [Google Scholar] [CrossRef]
- Andersson, M.; Kjoller, A.; Struwe, S. Microbial enzyme activities in leaf litter, humus and mineral soil layers of European forests. Soil Biol. Biochem. 2004, 36, 1527–1537. [Google Scholar] [CrossRef]
- Niemi, R.M.; Vepsalainen, M.; Wallenius, K.; Simpanen, S.; Alakukku, L.; Pietola, L. Temporal and soil depth-related variation in soil enzyme activities and in root growth of red clover (Trifolium pratense) and timothy (Phleum pratense) in the field. Appl. Soil Ecol. 2005, 30, 113–125. [Google Scholar] [CrossRef]
- Nguyen, M.L.; Haynes, R.J.; Goh, K.M. Nutrient budgets and status in three pairs of conventional and alternative mixed cropping farms in Canterbury, New Zealand. Agric. Ecosyst. Environ. 1995, 52, 149–162. [Google Scholar] [CrossRef]
- Lehmann, J.; Hansel, C.M.; Kaiser, C.; Kleber, M.; Maher, K.; Manzoni, S.; Nunan, N.; Reichstein, J.P.; Schimel, J.P.; Torn, M.S.; et al. Persistence of soil organic carbon caused by functional complexity. Nat. Geosci. 2020, 13, 529–534. [Google Scholar] [CrossRef]
- Calbrix, R.; Laval, K.; Barray, R. Analysis of the potential functional diversity of the bacterial community in soil: A reproducible procedure using sole-carbon source utilization profiles. Eur. J. Soil Biol. 2005, 41, 11–20. [Google Scholar] [CrossRef]
- Doolittle, J.A.; Brevik, E.C. The use of electromagnetic induction techniques in soils studies. Geoderma 2014, 223–225, 33–45. [Google Scholar] [CrossRef]
- Greenhouse, J.P.; Slaine, D.D. The use of reconnaissance electromagnetic methods to map contaminant migration. Ground Water Monit. Rev. 1993, 3, 47–59. [Google Scholar] [CrossRef]
- Rhoades, J.D.; Chanduvi, F.; Lesch, S.M. Soil Salinity Assessment: Methods and Interpretation of Electrical Conductivity Measurements. FAO Irrigation and Drainage Paper 57; Food and Agricultural Organization of the United Nations: Rome, Italy, 1999. [Google Scholar]
- Corwin, D.L.; Kaffka, S.R.; Hopmans, J.W.; Mori, Y.; Lesch, S.M.; Oster, J.D. Assessment and field-scale mapping of soil quality properties of a saline-sodic soil. Geoderma 2003, 114, 231–259. [Google Scholar] [CrossRef]
- Chen, L.F.; Ong, C.K.; Neo, C.P.; Varadan, V.V.; Varadan, V.K. Microwave Electronics: Measurement and Material Characterization; John Wiley & Sons, Ltd.: Chichester, UK, 2004; p. 537. [Google Scholar]
- Domsch, H.; Giebel, A. Estimation of soil textural features from soil electrical conductivity recorded using the EM38. Precis. Agric. 2004, 5, 389–409. [Google Scholar] [CrossRef]
- Bronson, K.F.; Booker, J.D.; Officer, S.J.; Lascano, R.J.; Maas, S.J.; Searcy, S.W.; Booker, J. Apparent electrical conductivity, soil property and spatial covariance in the U.S. Southern high plains. Precis. Agric. 2005, 6, 297–311. [Google Scholar] [CrossRef]
- Friedman, S.P. Soil properties influencing apparent electrical conductivity: A review. Comput. Electron. Agric. 2005, 46, 45–70. [Google Scholar] [CrossRef]
- Regione Basilicata, Dipartimento Agricoltura, Sviluppo Rurale, Economia montana. I suoli Della Basilicata. Carta pedologica della Regione Basilicata in scala 1:250.000; Note illustrative; Ufficio Risorse Naturali in Agricoltura: Potenza, Italy, 2006. [Google Scholar]
- Regione Campania. I Suoli della Piana in Destra Sele. Progetto Carta dei Suoli della Campania 1:50.000; Assessorato all’Agricoltura—SeSIRCA: Caserta, Italy, 2004. [Google Scholar]
- Allen, D.; Clarke, J.; Lawrie, K.; Fitzpatrick, A.; Apps, H.; Lowis, W.; Hatch, M.; Price, A.; Wilkes, P.; Dore, D.; et al. Geophysics for the Irrigation Industry, Irrigation Insights n. 7; Land & Water Australia: Malaga, Australia, 2007; p. 180. [Google Scholar]
- McNeil, J.D. Geonics EM38 Ground Conductivity Meter: EM38 Operating Manual; Geonics Limited: Mississauga, ON, Canada, 1990. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Pauwels, J.M.; Van Ranst, E.; Verloo, M.; Mvondo Ze, A. Méthode d’analyse de Sols et de Plantes, Gestion de Stock de Verrerie et de Produits Chimiques. Manuel de Laboratoire de Pédologie. Publications Agricoles; AGCD: Bruxelles, Belgium, 1992; 28p. [Google Scholar]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and Galacosidases in Soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Vuorinen, A.H. Requirement of p-nitrophenol standard for each soil. Soil Biol. Biochem. 1993, 25, 295–296. [Google Scholar] [CrossRef]
- Ntougias, S.; Ehaliotis, C.; Papadopoulou, K.K.; Zervakis, G. Application of respiration and FDA hydrolysis measurements for estimating microbial activity during composting processes. Biol. Fertil. Soils 2006, 42, 330–337. [Google Scholar] [CrossRef]
- Perez-Piqueres, A.; Edel-Hermann, V.; Alabouvette, C.; Steinberg, C. Response of soil microbial communities to compost amendments. Soil Biol. Biochem. 2006, 38, 460–470. [Google Scholar] [CrossRef]
- Weber, K.P.; Legge, R.L. One-dimensional metric for tracking bacterial community divergence using sole carbon source utilization patterns. J. Microbiol. Methods 2009, 79, 55–61. [Google Scholar] [CrossRef]
- Garland, J.L.; Mills, A.L. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sol carbon- source utilization. Appl. Environ. Microbiol. 1991, 57, 2351–2359. [Google Scholar] [CrossRef]
- Preston-Malfham, J.; Boddy, L.; Randerson, P.F. Analysis of microbial functional diversity using sole-carbon-source utilization profiles e a critique. FEMS Microbiol. Ecol. 2002, 42, 1–14. [Google Scholar] [CrossRef]
- Garland, J.L. Analysis and interpretation of community-level physiological profiles in microbial ecology. FEMS Microbiol. Ecol. 1997, 24, 289–300. [Google Scholar] [CrossRef]
- Chodak, M.; Niklińska, M. Effect of texture and tree species on microbial properties of mine soils. Appl. Soil Ecol. 2010, 46, 268–275. [Google Scholar] [CrossRef]
- Carroll, Z.L.; Oliver, M.A. Exploring the spatial relations between soil physical properties and apparent electrical conductivity. Geoderma 2005, 128, 354–374. [Google Scholar] [CrossRef]
- King, J.A.; Dampney, P.M.R.; Lark, R.M.; Wheeler, H.C.; Bradley, R.I.; Mayr, T.R. Mapping Potential Crop Management Zones within Fields: Use of Yield-map Series and Patterns of Soil Physical Properties Identified by Electromagnetic Induction Sensing. Precis. Agric. 2005, 6, 167–181. [Google Scholar] [CrossRef]
- Lardo, E.; Palese, A.M.; Nuzzo, V.; Xiloyannis, C.; Celano, G. Variability of total soil respiration in a Mediterranean vineyard. Soil Res. 2015, 53, 541–551. [Google Scholar] [CrossRef]
- Lardo, E.; Arous, A.; Palese, A.M.; Nuzzo, V.; Celano, G. Electromagnetic induction: A support tool for the evaluation of soil CO2 emissions and soil organic carbon content in olive orchards under semi-arid conditions. Geoderma 2016, 264, 188–194. [Google Scholar] [CrossRef]
- Peralta, N.R.; Costa, J.L. Delineation of management zones with soil apparent electrical conductivity to improve nutrient management. Comput. Electron. Agric. 2013, 99, 218–226. [Google Scholar] [CrossRef]
- de Assis Silva, S.; dos Santos, R.O.; de Queiroz, D.M.; de Souza Lima, J.S.; Pajehú, L.F.; Carvalho Medauar, C. Apparent soil electrical conductivity in the delineation of management zones for cocoa cultivation. Inf. Process. Agric. 2022, 9, 443–455. [Google Scholar] [CrossRef]
- Tully, K.L.; McAskill, C. Promoting soil health in organically managed systems: A review. Org. Agric. 2019, 10, 339–358. [Google Scholar] [CrossRef]
- Gomiero, T.; Pimentel, D.; Paoletti, M.G. Environmental Impact of Different Agricultural Management Practices: Conventional vs. Organic Agriculture. Crit. Rev. Plant Sci. 2011, 30, 95–124. [Google Scholar] [CrossRef]
- Bai, Z.; Caspari, T.; Gonzalez, M.R.; Batjes, N.H.; Mäder, P.; Bünemann, E.K.; de Goede, R.; Brussaard, L.; Xu, M.; Ferreira, C.S.S.; et al. Effects of agricultural management practices on soil quality: A review of long-term experiments for Europe and China. Agric. Ecosyst. Environ. 2018, 265, 1–7. [Google Scholar] [CrossRef]
- Stewart, C.E.; Paustian, K.; Conant, R.T.; Plante, A.F.; Six, J. Soil carbon saturation: Concept, evidence and evaluation. Biogeochemistry 2007, 86, 19–31. [Google Scholar] [CrossRef]
- Quintarelli, V.; Radicetti, E.; Allevato, E.; Stazi, S.R.; Haider, G.; Abideen, Z.; Bibi, S.; Jamal, A.; Mancinelli, R. Cover Crops for Sustainable Cropping Systems: A Review. Agriculture 2022, 12, 2076. [Google Scholar] [CrossRef]
- García-González, I.; Hontoria, C.; Gabriel, J.L.; Alonso-Ayuso, M.; Quemada, M. Cover crops to mitigate soil degradation and enhance soil functionality in irrigated land. Geoderma 2018, 322, 81–88. [Google Scholar] [CrossRef]
- Degens, B.P.; Schipper, L.A.; Sparling, G.P.; Vojvodic-Vukovic, M. Decreases in Organic C Reserves in Soils Can Reduce the Catabolic Diversity of Soil Microbial Communities. Soil Biol. Biochem. 2000, 32, 189–196. [Google Scholar] [CrossRef]
- Bandick, A.P.; Dick, R.P. Field management effects on soil enzyme activities. Soil Biol. Biochem. 1999, 31, 1471–1479. [Google Scholar] [CrossRef]
- Vukicevich, E.; Lowery, T.; Bowen, P.; Úrbez-Torres, J.R.; Hart, M. Cover crops to increase soil microbial diversity and mitigate decline in perennial agriculture. A review. Agron. Sustain. Dev. 2016, 36, 48. [Google Scholar] [CrossRef]
- Praveen-Kumar, T.; Jagadish, C.; Panwar, J.; Shyam, K. A rapid method for assessment of plant residue quality. J. Plant Nutr. Soil Sci. 2003, 166, 662–666. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Jørgensen, H.B. Carbon quality rather than stoichiometry controls litter decomposition in a tropical rain forest. J. Ecol. 2010, 98, 754–763. [Google Scholar] [CrossRef]
- Finney, D.M.; Mirsky, S.B.; Ackroyd, V.J. Cover Crop Mixture Selection and Management. 2016 Southern SARE Cover Crop Conference. p. 3. Available online: https://southerncovercrops.org/wp-content/uploads/2019/03/Cover-Crop-Mixture-Selection-and-Management.pdf (accessed on 10 July 2024).
- Adam, G.; Duncan, H. Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef]
- Taylor, J.P.; Wilson, B.; Mills, M.S.; Burns, R.G. Comparison of microbial numbers and enzymatic activities in surface soils and subsoils using various techniques. Soil Biol. Biochem. 2002, 34, 387–401. [Google Scholar] [CrossRef]
- Ndiaye, E.L.; Sandeno, J.M.; McGrath, D.; Dick, R.P. Integrative biological indicators for detecting change in soil quality. Am. J. Altern. Agric. 2000, 15, 26–36. [Google Scholar] [CrossRef]
- Fisk, M.C.; Fahey, T.J. Microbial biomass and nitrogen cycling responses to fertilization and litter removal in young northern hardwood forests. Biogeochemistry 2001, 53, 201–223. [Google Scholar] [CrossRef]
- Dick, R.P.; Breakwell, D.P.; Turco, R.F. Soil enzyme activities and biodiversity measurements as integrative microbiological indicators. In Methods of Assessing Soil Quality; Doran, J.W., Jones, A.J., Eds.; SSSA: Madison, WI, USA, 1996; Volume 49, pp. 247–271. [Google Scholar]
- Frankenberger, W.T.; Dick, W.A. Relationship between enzyme activities and microbial growth and activity indices in soil. Soil Sci. Soc. Am. J. 1983, 47, 945–951. [Google Scholar] [CrossRef]
- Perez-Guzman, L.; Phillips, L.A.; Seuradge, B.J.; Agomoh, I.; Drury, C.F. An evaluation of biological soil health indicators in four long-term continuous agroecosystems in Canada. Agrosyst. Geosci. Environ. 2021, 4, e20164. [Google Scholar] [CrossRef]
- Sainju, U.M.; Liptzin, D.; Dangi, S.M. Enzyme activities as soil health indicators in relation to soil characteristics and crop production. Agrosyst. Geosci. Environ. 2022, 5, e20297. [Google Scholar] [CrossRef]
- Perez-Bejarano, A.; Mataix-Solera, J.; Zornoza, R.; Guerrero, C.; Arcenegui, V.; Mataix-Beneyto, J.; Cano-Amat, S. Influence of plant species on physical, chemical and biological soil properties in a Mediterranean forest soil. Eur. J. For. Res. 2010, 129, 15–24. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Z.; Zha, T.; Lu, Z.; Zheng, J.; Sun, O.J. Predicting soil respiration using carbon stock in roots, litter and soil organic matter in forests of Loess Plateau in China. Soil Biol. Biochem. 2013, 57, 135–143. [Google Scholar] [CrossRef]
- Schmidt, R.; Gravuer, K.; Bossange, A.V.; Mitchell, J.; Scow, K. Long-term use of cover crops and no-till shift soil microbial community life strategies in agricultural soil. PLoS ONE 2018, 13, e0192953. [Google Scholar] [CrossRef] [PubMed]
- Bertin, C.; Yang, X.; Weston, L.A. The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 2003, 256, 67–83. [Google Scholar] [CrossRef]
- De Graaff, M.A.È.; Classen, A.T.; Castro, H.F.; Schadt, C.W. Labile soil carbon inputs mediate the soil microbial community composition and plant residue decomposition rates. New Phytol. 2010, 188, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Noya, Y.E.; Gomez-Acata, S.; Montoya-Ciriaco, N.; Rojas-Valdez, A.; Suárez-Arriaga, M.C.; Valenzuela-Encinas, C.; Jiménez-Bueno, N.; Verhulst, N.; Govaerts, B.; Dendooven, L. Relative impacts of tillage, residue management and crop-rotation on soil bacterial communities in a semi-arid agroecosystem. Soil Biol Biochem. 2013, 65, 86–95. [Google Scholar] [CrossRef]
- Ramirez-Villanueva, D.A.; Bello-Lopez, J.M.; Navarro-Noya, Y.E.; Luna-Guido, M.; Verhulst, N.; Govaerts, B.; Dendooven, L. Bacterial community structure in maize residue amended soil with contrasting management practices. Appl. Soil Ecol. 2015, 90, 49–59. [Google Scholar] [CrossRef]
- Balkcom, K.; Schomberg, H.; Dewey Lee, R. Cover Crop Management. In Conservation Tillage Systems in the Southeast Production, Profitability and Stewardship; Bergtold, J., Sailus, M., Eds.; SARE Handbook Series 15 Sustainable Agriculture Research and Education (SARE); U.S. Department of Agriculture: Washington, DC, USA, 2020; pp. 56–76. [Google Scholar]
- Jenkinson, D.S.; Hart, P.B.S.; Rayner, J.H.; Parry, L.C. Modelling the turnover of organic matter in long-term experiments at Rothamsted. INTECOL Bull. 1987, 15, 1–8. [Google Scholar]
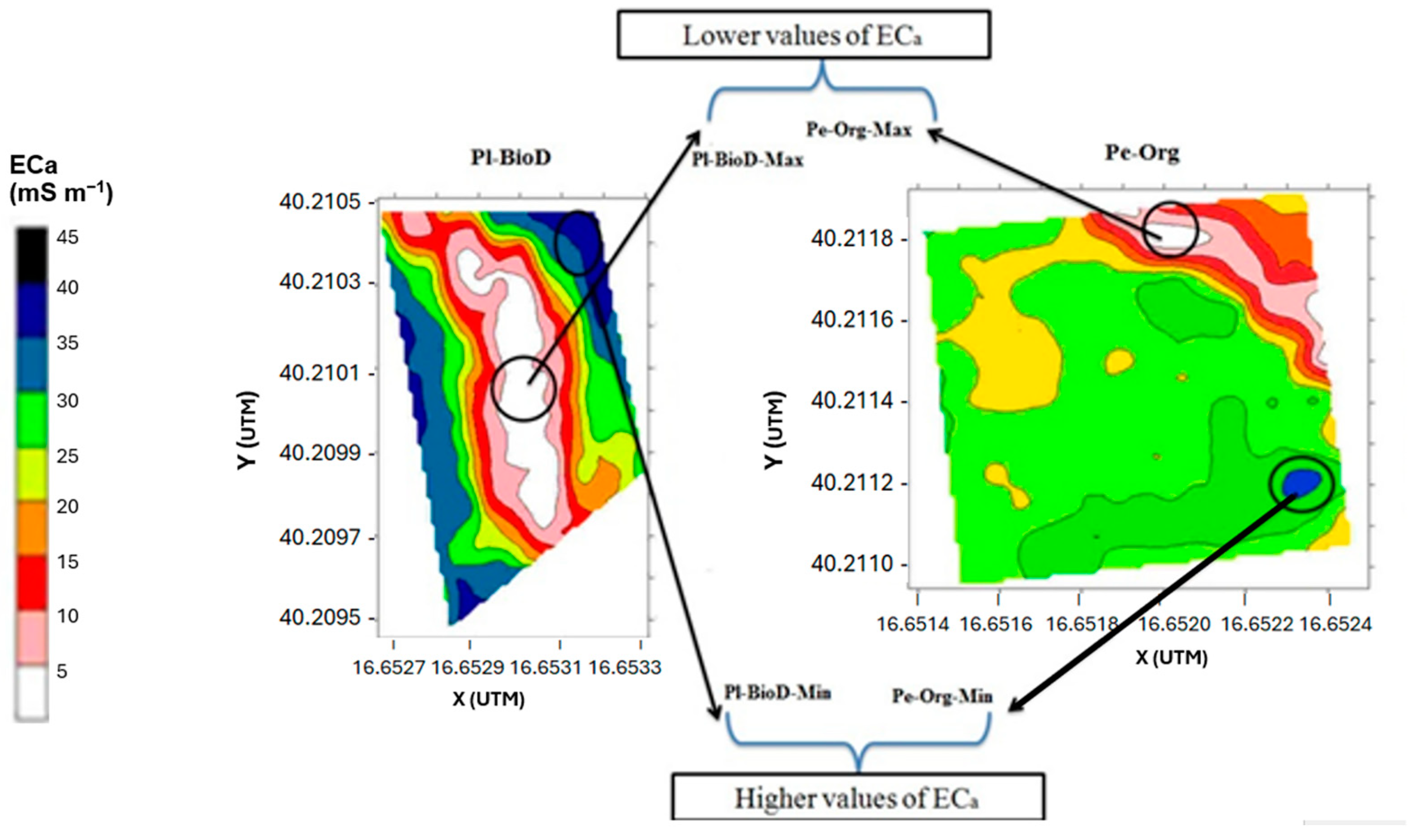


| Pl-BioD | Before 1996: Plum | 1996–2006: Plum | 2006–2014: Plum |
| Tilled | Organic management—spontaneous grass cover mowed and left on the ground, together with shredded pruning material as mulching | BioDynamic management—seeded grass cover mowed and left on the ground, together with shredded pruning material as mulching | |
| Pe-Org | Before 1999: Peach | 1999–2008: other Peach | 2008–2014: other Peach |
| Tilled | Organic management—spontaneous grass cover mowed and left on the ground, together with shredded pruning material as mulching | Organic management—spontaneous grass cover mowed and left on the ground, together with shredded pruning material as mulching | |
| Ap-CT | Before 2001: Peach | 2001–2014: Apricot | |
| Tilled (rotary tillers—20–25 cm depth) Pruning material shredded in the field | Tilled (rotary tillers—20–25 cm depth) Pruning material shredded in the field | ||
| Pe-CM | 1987–2011: Kiwi | 2011–2012: Grass | 2012–2014: Peach |
| With mulch: spontaneous grass cover between rows—chemical weeding (glyphosate) under the trees in the row | Tilled | Conventional Management –soil of inter-rows covered by spontaneous grasses mowed and left on the ground as mulching—chemical weeding (Glyphosate) under the trees on the row—Pruning material shredded in the field and left as mulching | |
| Ki-CM | Before 2013: Peach | 2013–2014: Kiwi | |
| Conventional Management—soil of inter-rows covered by spontaneous grasses mowed and left on the ground as mulching—chemical weeding (Glyphosate) under the trees on the row—Pruning material shredded in the field and left as mulching | Conventional Management –soil of inter-rows covered by spontaneous grasses mowed and left on the ground as mulching—chemical weeding (Glyphosate) under the trees on the row—Pruning material shredded in the field and left as mulching | ||
| Pl-CM | Before 1999: Peach | 1999–2014: Plum | |
| Tilled | Conventional Management - soil of inter-rows temporary covered by spontaneous grasses and tilled (motor hoe operating at 10 cm depth)—mechanical weeding in the row under the trees—Grass residues and shredded pruning material left on the ground as mulching | ||
| Fruit Orchard System | Species | Rootstock/Cultivar | Plants Distance (m) | Fruit Tree Shape |
|---|---|---|---|---|
| Pl-BioD | Plum (25 years) | Mirabolane/Black diamond | 4 × 4.5 | Vase |
| Pe-Org | Peach (6 years) | GF677/Sagitaria | 4.5 × 3.5 | Delayed vase |
| Ap-CT | Apricot (13 years) | Mirabolane/Ninfa | 5 × 5 | Vase |
| Pl-CM | Plum (20 years) | Mirabolane/Angeleno | 4.5 × 1.5 | Y transverse |
| Pe-CM | Peach (2 years) | Puebla de soto/Sweet | 4.5 × 1.5 | Y transverse |
| Ki-CM | Kiwi (2 years) | Bruno/Hayward | 4.5 × 2 | T-bar |
| ECa (mS m−1) | SOC (g kg−1) | pH | Electrical Conductivity (mS m−1) | Skeleton (g kg−1) | Sand (g kg−1) | Silt (g kg−1) | Clay (g kg−1) | |
|---|---|---|---|---|---|---|---|---|
| Orchard System | ECa range (ECa-min—ECa-max) | |||||||
| Pl-BioD | 0.3–40.0 | 17.9–18.0 | 7.2–7.2 | 301.5–310.5 | 14.1–132.2 | 285.4–313.8 | 309.6–326.9 | 261.9–373.8 |
| Pe-Org | 9.1–36.4 | 15.6–19.5 | 7.2–7.2 | 318.5–350.5 | 11.3–49.8 | 260.6–333.9 | 326.6–377.5 | 293.3–350.8 |
| Ap-CT | 0.5–38.8 | 6.1–10.2 | 7.2–7.4 | 335.5–615.0 | 22.9–272.9 | 316.2–406.4 | 190.9–296.1 | 188.8–365.3 |
| Pl-CM | 75.6–106.1 | 14.5–14.7 | 7.1–7.2 | 165.5–166.5 | 26.0–41.5 | 371.7–386.4 | 215.9–217.3 | 397.7–411.0 |
| Pe-CM | 50.0–89.5 | 16.2–19.7 | 7.8–8.0 | 160.7–171.3 | 15.0–29.0 | 335.1–362.3 | 251.0–275.2 | 362.5–413.9 |
| Ki-CM | 30.1–94.4 | 18.0–18.6 | 7.9–8.0 | 110.7–206.0 | 112.0–129.0 | 390.5–394.9 | 181.9–201.1 | 404.4–427.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arous, A.; Gargouri, K.; Palese, A.M.; Pane, C.; Scotti, R.; Zaccardelli, M.; Altieri, G.; Celano, G. Microbiological Soil Quality Indicators Associated with Long-Term Agronomical Management of Mediterranean Fruit Orchards. Agriculture 2024, 14, 1527. https://doi.org/10.3390/agriculture14091527
Arous A, Gargouri K, Palese AM, Pane C, Scotti R, Zaccardelli M, Altieri G, Celano G. Microbiological Soil Quality Indicators Associated with Long-Term Agronomical Management of Mediterranean Fruit Orchards. Agriculture. 2024; 14(9):1527. https://doi.org/10.3390/agriculture14091527
Chicago/Turabian StyleArous, Aissa, Kamel Gargouri, Assunta Maria Palese, Catello Pane, Riccardo Scotti, Massimo Zaccardelli, Gessica Altieri, and Giuseppe Celano. 2024. "Microbiological Soil Quality Indicators Associated with Long-Term Agronomical Management of Mediterranean Fruit Orchards" Agriculture 14, no. 9: 1527. https://doi.org/10.3390/agriculture14091527








