Varying Tolerance to Diesel Toxicity Revealed by Growth Response Evaluation of Petunia grandiflora Shoot Lines Regenerated after Diesel Fuel Treatment †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Setup and Glasshouse Conditions
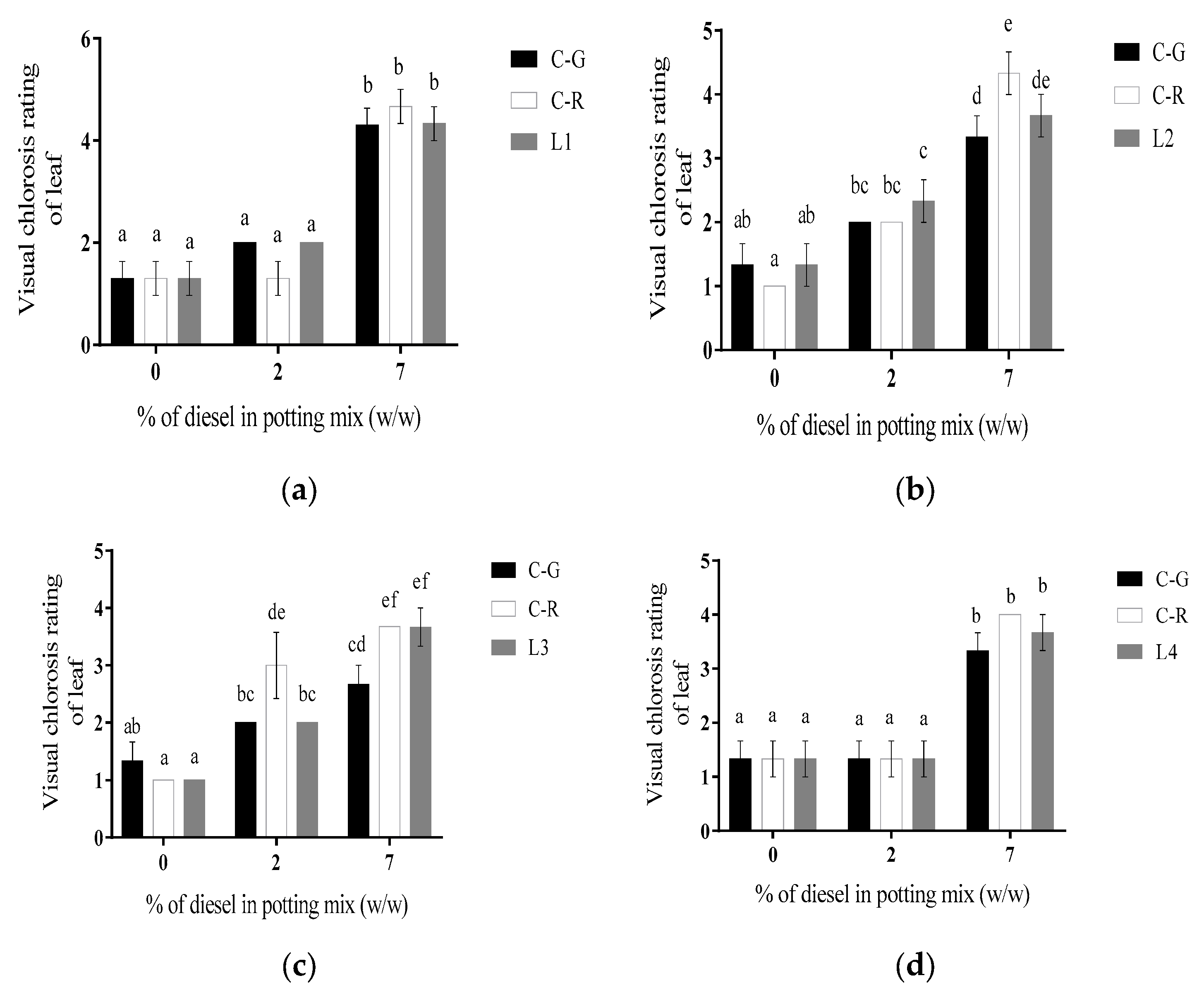
2.3. Number of Leaves and Leaf Chlorosis Rating
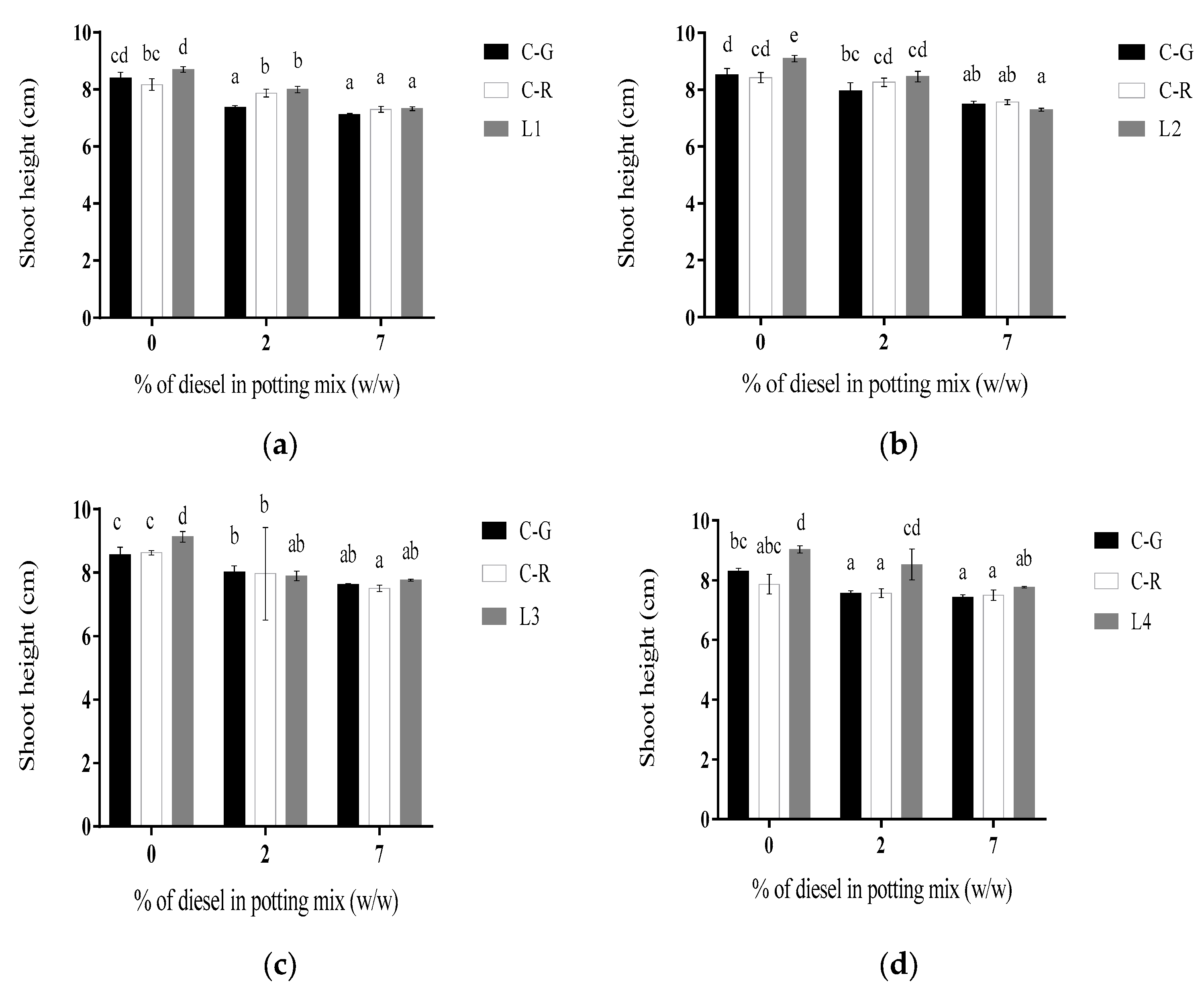
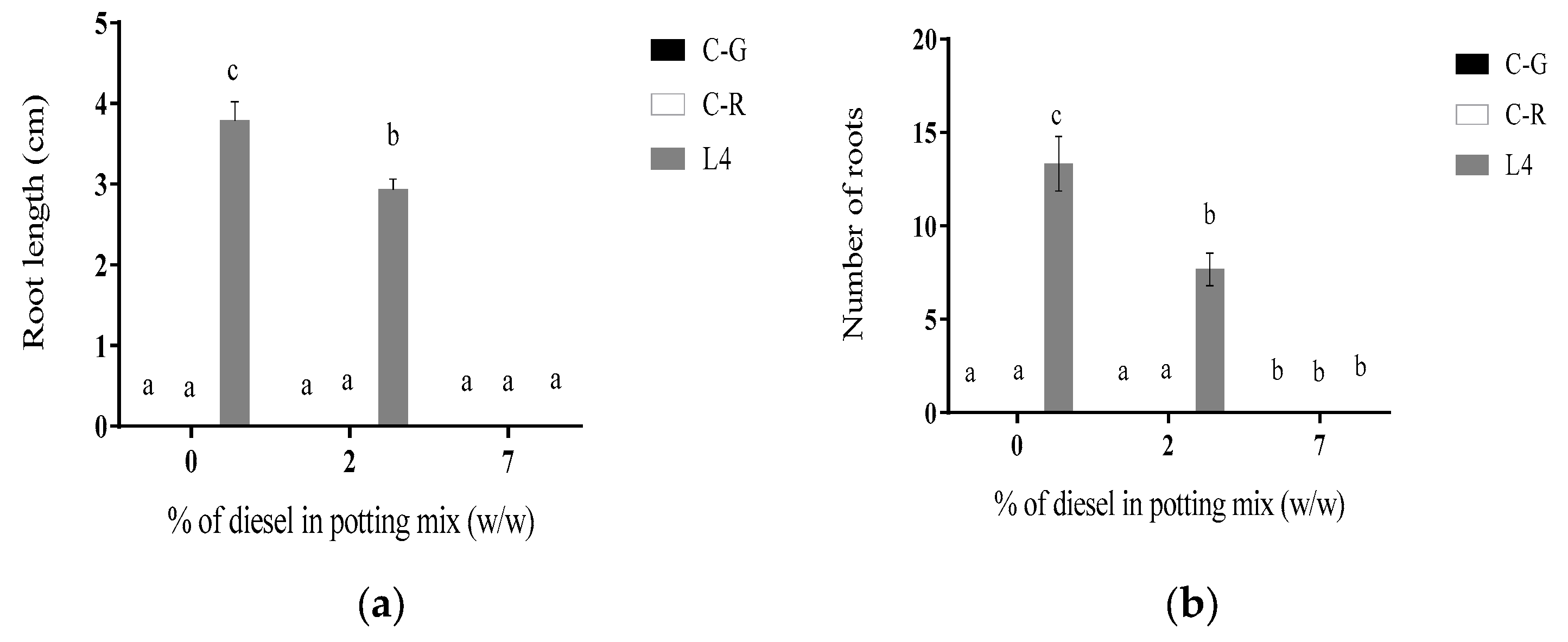
2.4. Shoot Growth
2.5. Microbial Plate Counts
2.6. Analysis of Diesel Content in Potting Mix
2.6.1. Ultrasonic Extraction (Method 3550C in [25])
2.6.2. Total Petroleum Hydrocarbon (TPH) Analysis
2.7. Statistical Analyses
3. Results
3.1. In Vitro Selected Versus Control Petunia Grandiflora Lines
3.1.1. Line L1
3.1.2. Line L2
3.1.3. Line L3
3.1.4. Line L4
3.2. Total Microbial Plate Count
3.3. Total Petroleum Hydrocarbon Analyses
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zheng, L.; Rosenzweig, R.; Chen, F.; Qin, J.; Li, T.; Han, J.; Istvan, P.; Diaz-Reck, D.; Gelman, F.; Arye, G. Bioremediation of Petroleum-Contaminated Soils with Biosurfactant-Producing Degraders Isolated from the Native Desert Soils. Microorganisms 2022, 10, 2267. [Google Scholar] [CrossRef] [PubMed]
- Ceccanti, B.; Masciandaro, G.; Garcia, C.; Macci, C.; Doni, S. Soil bioremediation: Combination of earthworms and compost for the ecological remediation of a hydrocarbon polluted soil. Water Air Soil Pollut. 2006, 177, 383–397. [Google Scholar] [CrossRef]
- Mitter, E.K.; Germida, J.J.; de Freitas, J.R. Impact of diesel and biodiesel contamination on soil microbial community activity and structure. Sci. Rep. 2021, 11, 10856. [Google Scholar] [CrossRef]
- Wante, S.P.; Leung, D.W. Influence of toxic diesel fuel on Petunia grandiflora calli and after plant regeneration. 3 Biotech 2022, 12, 179. [Google Scholar] [CrossRef]
- Jagtap, S.S.; Woo, S.M.; Kim, T.-S.; Dhiman, S.S.; Kim, D.; Lee, J.-K. Phytoremediation of diesel-contaminated soil and saccharification of the resulting biomass. Fuel 2014, 116, 292–298. [Google Scholar] [CrossRef]
- Palmroth MR, T.; Pichtel, J.; Puhakka, J.A. Phytoremediation of subarctic soil contaminated with diesel fuel. Bioresour. Technol. 2002, 84, 221–228. [Google Scholar] [CrossRef]
- Pittarello, M.; Busato, J.G.; Carletti, P.; Dobbss, L.B. Possible developments for ex situ phytoremediation of contaminated sediments, in tropical and subtropical regions—Review. Chemosphere 2017, 182, 707–719. [Google Scholar]
- Eze, M.O.; Thiel, V.; Hose, G.C.; George, S.C.; Daniel, R. Bacteria-plant interactions synergistically enhance biodegradation of diesel fuel hydrocarbons. Commun. Earth Environ. 2022, 3, 192. [Google Scholar]
- Shahsavari, E.; Adetutu, E.M.; Anderson, P.A.; Ball, A.S. Tolerance of selected plant species to petrogenic hydrocarbons and effect of plant rhizosphere on the microbial removal of hydrocarbons in contaminated soil. Water Air Soil Pollut. 2013, 224, 1495. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q.; Xu, Y.; Wang, L.; Liang, X. Phytoremediation for co-contaminated soils of benzo[a]pyrene (B[a]P) and heavy metals using ornamental plant Tagetes patula. J. Hazard. Mater. 2011, 186, 2075–2082. [Google Scholar]
- Peng, S.; Zhou, Q.; Cai, Z.; Zhang, Z. Phytoremediation of petroleum contaminated soils by Mirabilis jalapa L. in a greenhouse plot experiment. J. Hazard. Mater. 2009, 168, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Liu, R.; Jin, C.; Dai, Y. Efficiency of five ornamental plant species in the phytoremediation of polycyclic aromatic hydrocarbon (PAH)-contaminated soil. Ecol. Eng. 2015, 75, 384–391. [Google Scholar] [CrossRef]
- Ikeura, H.; Kawasaki, Y.; Kaimi, E.; Nishiwaki, J.; Noborio, K.; Tamaki, M. Screening of plants for phytoremediation of oil-contaminated soil. Int. J. Phytoremediation 2016, 18, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Wang, M.; Feng, X.; Liu, R.; Xue, Z.; Zhou, Q. Improving the uptake of PAHs by the ornamental plant Sedum spectabile using nano-SiO2 and nano-CeO2. Sci. Total Environ. 2023, 870, 161808. [Google Scholar] [CrossRef]
- Panwar, R.; Mathur, J. Comparative analysis of remediation efficiency and ultrastructural translocalization of polycyclic aromatic hydrocarbons in Medicago sativa, Helianthus annuus, and Tagetes erecta. Int. J. Phytoremediation 2023, 25, 1743–1761. [Google Scholar] [CrossRef]
- Wante, S.P.; Leung DW, M. Phytotoxicity testing of diesel-contaminated water using Petunia grandiflora Juss. Mix F1 and Marigold-Nemo Mix (Tagetes patula L.). Environ. Monit. Assess. 2018, 190, 408. [Google Scholar] [CrossRef]
- Liu, J.; Xin, X.; Zhou, Q. Phytoremediation of contaminated soils using ornamental plants. Environ. Rev. 2017, 26, 43–54. [Google Scholar] [CrossRef]
- Lin, Y.; Jones, M.L. Evaluating the growth-promoting effects of microbial biostimulants on greenhouse floriculture crops. HortScience 2022, 57, 97–109. [Google Scholar] [CrossRef]
- Phillips, L.A.; Greer, C.W.; Germida, J.J. Culture-based and culture-independent assessment of the impact of mixed and single plant treatments on rhizosphere microbial communities in hydrocarbon-contaminated flare-pit soil. Soil Biol. Biochem. 2006, 38, 2823–2833. [Google Scholar] [CrossRef]
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef]
- Scheres, B.; Van Der Putten, W.H. The plant perceptron connects environment to development. Nature 2017, 543, 337–345. [Google Scholar] [CrossRef] [PubMed]
- ShirzadianGilan, R.; Parvizi, Y.; Pazira, E.; Rejali, F. Remediation capacity of drought-tolerant plants and bacteria in petroleum hydrocarbon-contaminated soil in Iran. S. Afr. J. Bot. 2023, 153, 1–10. [Google Scholar] [CrossRef]
- Başar, H. Analytical methods for evaluating iron chlorosis in peach trees. Commun. Soil Sci. Plant Anal. 2003, 34, 327–341. [Google Scholar] [CrossRef]
- Euliss, K.; Ho, C.-H.; Schwab, A.P.; Rock, S.; Banks, M.K. Greenhouse and field assessment of phytoremediation for petroleum contaminants in a riparian zone. Bioresour. Technol. 2008, 99, 1961–1971. [Google Scholar] [CrossRef]
- USEPA. Method 3550C: Ultrasonic Extraction; Part of Test Methods for Evaluating Solid Waste, Physical/Chemical Method; United State Government: Washington, DC, USA, 2007.
- Hell, R. Transgenic plants and crops. J. Plant Physiol. 2003, 160, 718. [Google Scholar]
- Drizou, F.; Graham, N.S.; Bruce, T.J.; Ray, R.V. Development of high-throughput methods to screen disease caused by Rhizoctonia solani AG 2-1 in oilseed rape. Plant Methods 2017, 13, 45. [Google Scholar] [CrossRef]
- Liu, R.; Jadeja, R.N.; Zhou, Q.; Liu, Z. Treatment and remediation of petroleum-contaminated soils using selective ornamental plants. Environ. Eng. Sci. 2012, 29, 494–501. [Google Scholar] [CrossRef]
- Meng, Z.; Duan, A.; Chen, D.; Dassanayake, K.B.; Wang, X.; Liu, Z.; Liu, H.; Gao, S. Suitable indicators using stem diameter variation-derived indices to monitor the water status of greenhouse tomato plants. PLoS ONE 2017, 12, e0171423. [Google Scholar] [CrossRef]
- Santala, K.R.; Ryser, P. Influence of heavy-metal contamination on plant response to water availability in white birch, Betula papyrifera. Environ. Exp. Bot. 2009, 66, 334–340. [Google Scholar] [CrossRef]
- Allan, I.J.; Semple, K.T.; Hare, R.; Reid, B.J. Cyclodextrin enhanced biodegradation of polycyclic aromatic hydrocarbons and phenols in contaminated soil slurries. Environ. Sci. Technol. 2007, 41, 5498–5504. [Google Scholar] [CrossRef]
- Thavamani, P.; Megharaj, M.; Naidu, R. Multivariate analysis of mixed contaminants (PAHs and heavy metals) at manufactured gas plant site soils. Environ. Monit. Assess. 2012, 184, 3875–3885. [Google Scholar] [CrossRef] [PubMed]
- Rucińska-Sobkowiak, R.; Nowaczyk, G.; Krzesłowska, M.; Rabęda, I.; Jurga, S. Water status and water diffusion transport in lupine roots exposed to lead. Environ. Exp. Bot. 2013, 87, 100–109. [Google Scholar] [CrossRef]
- Henzler, T.; Ye, Q.; Steudle, E. Oxidative gating of water channels (aquaporins) in Chara by hydroxyl radicals. Plant Cell Environ. 2004, 27, 1184–1195. [Google Scholar] [CrossRef]
- Rucińska-Sobkowiak, R. Water relations in plants subjected to heavy metal stresses. Acta Physiol. Plant. 2016, 38, 257. [Google Scholar] [CrossRef]
- De Filippis, L.F.; Ziegler, H. Effect of sublethal concentrations of zinc, cadmium and mercury on the photosynthetic carbon reduction cycle of Euglena. J. Plant Physiol. 1993, 142, 167–172. [Google Scholar] [CrossRef]
- Burritt, D.J. The polycyclic aromatic hydrocarbon phenanthrene causes oxidative stress and alters polyamine metabolism in the aquatic liverwort Riccia fluitans L. Plant Cell Environ. 2008, 31, 1416–1431. [Google Scholar] [CrossRef]
- Alkio, M.; Tabuchi, T.M.; Wang, X.; Colon-Carmona, A. Stress responses to polycyclic aromatic hydrocarbons in Arabidopsis include growth inhibition and hypersensitive response-like symptoms. J. Exp. Bot. 2005, 56, 2983–2994. [Google Scholar] [CrossRef]
- Osuagwu, A.N.; Okigbo, A.U.; Ekpo, I.A.; Chukwurah, P.N.; Agbor, R.B. Effect of crude oil pollution on growth parameters, chlorophyll content and bulbils yield in air potato (Dioscorea bulbifera L.). Int. J. Appl. 2013, 3, 37–42. [Google Scholar]
- Lamb, C.; Dixon, R.A. The oxidative burst in plant disease resistance. Annu. Rev. Plant Biol. 1997, 48, 251–275. [Google Scholar] [CrossRef]
- Mellersh, D.G.; Heath, M.C. Plasma membrane–cell wall adhesion is required for expression of plant defense responses during fungal penetration. Plant Cell 2001, 13, 413–424. [Google Scholar]
- Fernández, V.; Eichert, T.; Del Río, V.; López-Casado, G.; Heredia-Guerrero, J.A.; Abadía, A.; Heredia, A.; Abadía, J. Leaf structural changes associated with iron deficiency chlorosis in field-grown pear and peach: Physiological implications. Plant Soil 2008, 311, 161. [Google Scholar] [CrossRef]
- Larbi, A.; Abadía, A.; Abadía, J.; Morales, F. Down co-regulation of light absorption, photochemistry, and carboxylation in Fe-deficient plants growing in different environments. Photosynth. Res. 2006, 89, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Gómez, S.; Petenello, M.C.; Feldman, S.R. Growth, nutrient status, and photosynthetic response to diesel-contaminated soil of a cordgrass, Spartina argentinensis. Mar. Pollut. Bull. 2014, 79, 34–38. [Google Scholar] [CrossRef]
- Pien, S.; Wyrzykowska, J.; McQueen-Mason, S.; Smart, C.; Fleming, A. Local expression of expansin induces the entire process of leaf development and modifies leaf shape. Proc. Natl. Acad. Sci. USA 2001, 98, 11812–11817. [Google Scholar] [CrossRef] [PubMed]
- Balliana, A.G.; Moura, B.B.; Inckot, R.C.; Bona, C. Development of Canavalia ensiformis in soil contaminated with diesel oil. Environ. Sci. Pollut. Res. 2017, 24, 979–986. [Google Scholar] [CrossRef]
- Thomma BP, H.J.; Eggermont, K.; Penninckx IA, M.A.; Mauch-Mani, B.; Vogelsang, R.; Cammue BP, A.; Broekaert, W.F. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc. Natl. Acad. Sci. USA 1998, 95, 15107–15111. [Google Scholar] [CrossRef]
- Hou FS, L.; Milke, M.W.; Leung DW, M.; MacPherson, D.J. Variations in phytoremediation performance with diesel-contaminated soil. Environ. Technol. 2001, 22, 215–222. [Google Scholar]
- Hernández-Ortega, H.A.; Alarcón, A.; Ferrera-Cerrato, R.; Zavaleta-Mancera, H.A.; López-Delgado, H.A.; Mendoza-López, M.R. Arbuscular mycorrhizal fungi on growth, nutrient status, and total antioxidant activity of Melilotus albus during phytoremediation of a diesel-contaminated substrate. J. Environ. Manag. 2012, 95, S319–S324. [Google Scholar] [CrossRef]
- Hutchinson, S.L.; Schwab, A.P.; Banks, M.K. Biodegradation of petroleum hydrocarbons in the rhizosphere. In Phytoremediation: Transformation and Control of Contaminants; McCutcheon, S.C., Schnoor, J.L., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 355–386. [Google Scholar]
- Gaffney, T.; Friedrich, L.; Vernooij, B.; Negrotto, D.; Nye, G.; Uknes, S.; Ward, E.; Kessmann, H.; Ryals, J. Requirement of salicylic acid for the induction of systemic acquired resistance. Science 1993, 261, 754–756. [Google Scholar] [CrossRef]
- Kamath, R.; Schnoor, J.L.; Alvarez PJ, J. Effect of root-derived substrates on the expression of nah-lux genes in Pseudomonas fluorescens HK44: Implications for PAH biodegradation in the rhizosphere. Environ. Sci. Technol. 2004, 38, 1740–1745. [Google Scholar] [CrossRef]
- Graber, E.R.; Harel, Y.M.; Kolton, M.; Cytryn, E.; Silber, A.; David, D.R.; Tsechansky, L.; Borenshtein, M.; Elad, Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Koohakan, P.; Ikeda, H.; Jeanaksorn, T.; Tojo, M.; Kusakari, S.-I.; Okada, K.; Sato, S. Evaluation of the indigenous microorganisms in soilless culture: Occurrence and quantitative characteristics in the different growing systems. Sci. Hortic. 2004, 101, 179–188. [Google Scholar] [CrossRef]
- Rodrigues, D.M.; Caballero-Villalobos, L.; Turchetto, C.; Assis Jacques, R.; Kuhlemeier, C.; Freitas, L.B. Do we truly understand pollination syndromes in Petunia as much as we suppose? AoB Plants 2018, 10, ply057. [Google Scholar] [CrossRef] [PubMed]


| % (w/w) of Diesel Contaminant in Potting Mix | Plant Lines | Total Counts (105 cfu/g) 5 Weeks | Plant Lines | Total Counts (105 cfu/g) 5 Weeks | Plant Lines | Total Counts (105 cfu/g) 5 Weeks | Plant Lines | Total Counts (105 cfu/g) 5 Weeks | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Planted | Unplanted | Planted | Unplanted | Planted | Unplanted | Planted | Unplanted | |||||
| 0 | C-G | 62 a | 57 a | C-G | 49 a | 49 a | C-G | 66 a | 53 a | C-G | 68 a | 50 a |
| C-R | 56 a | 51 a | C-R | 74 a | 59 a | C-R | 66 a | 53 a | C-R | 51 a | 51 a | |
| L1 | 66 a | 55 a | L2 | 58 a | 54 a | L3 | 67 a | 51 a | L4 | 96 b | 60 a | |
| 2 | C-G | 93 a | 84 a | C-G | 99 b | 94 b | C-G | 102 a | 80 a | C-G | 85 a | 75 a |
| C-R | 100 a | 78 a | C-R | 101 b | 87 b | C-R | 107 a | 95 b | C-R | 70 a | 67 a | |
| L1 | 88 a | 82 a | L2 | 69 a | 65 a | L3 | 99 a | 87 ab | L4 | 120 b | 83 a | |
| 7 | C-G | 146 a | 143 b | C-G | 138 ab | 116 a | C-G | 113 a | 123 a | C-G | 144 a | 137 a |
| C-R | 130 a | 123 a | C-R | 158 b | 141 b | C-R | 149 ab | 143 a | C-R | 124 a | 117 a | |
| L1 | 173 b | 129 a | L2 | 115 a | 111 a | L3 | 154 b | 139 a | L4 | 115 a | 137 a | |
| % (w/w) of Diesel Contaminant in Potting Mix | Plant Lines | % of Residual TPHs after 5 Weeks | Plant Lines | % of Residual TPHs after 5 Weeks | Plant Lines | % of Residual TPHs after 5 Weeks | Plant Lines | % of Residual TPHs after 5 Weeks | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Planted | Unplanted | Planted | Unplanted | Planted | Unplanted | Planted | Unplanted | |||||
| 2 | C-G | 14.2 (±0.8) a | 14.6 (±1.2) a | C-G | 17.3 (±1.0) a | 21.5(±2.9) a | C-G | 19.0 (±3.4) a | 20.1 (±2.1) a | C-G | 19.3 (±1.5) a | 19.7 (±1.4) a |
| C-R | 14.6 (±1.4) a | 18.3 (±1.0) a | C-R | 16.6 (±1.4) a | 18.1 (±1.4) a | C-R | 15.5 (±0.9) a | 17.1 (±2.5) a | C-R | 15.3 (±1.8) a | 16.5 (±0.9) a | |
| L1 | 16.5 (±1.0) a | 20.5 (±1.4) a | L2 | 18.8 (±0.8) a | 21.5 (±1.4) a | L3 | 16.0 (±2.7) a | 16.5 (±1.7) a | L4 | 17.2 (±2.8) a | 17.0 (±0.9) a | |
| 7 | C-G | 57.9 (±2.0) b | 57.2 (±3.7) b | C-G | 50.0 (±4.5) bc | 46.2 (±4.8) b | C-G | 50.9 (±4.2) b | 49.8 (±3.9) b | C-G | 51.4 (±1.4) b | 46.7(±2.8) b |
| C-R | 50.3 (4.1) b | 51.4 (±3.2) b | C-R | 46.8 (±1.4) b | 50.1 (±6.7) b | C-R | 50.4 (±1.8) b | 51.0 (±3.0) b | C-R | 49.8 (±2.1) b | 51.7 (±4) bc | |
| L1 | 56.6 (±3.4) b | 59.0 (±2.9) b | L2 | 55.4 (±2.3) c | 57.2 (±4.8) b | L3 | 49.2(±2.6) b | 49.4 (±1.8) b | L4 | 50.2 (±2.2) b | 55.5 (±2.2) c | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wante, S.P.; Leung, D.W.M.; Alizadeh, H. Varying Tolerance to Diesel Toxicity Revealed by Growth Response Evaluation of Petunia grandiflora Shoot Lines Regenerated after Diesel Fuel Treatment. Agriculture 2024, 14, 1562. https://doi.org/10.3390/agriculture14091562
Wante SP, Leung DWM, Alizadeh H. Varying Tolerance to Diesel Toxicity Revealed by Growth Response Evaluation of Petunia grandiflora Shoot Lines Regenerated after Diesel Fuel Treatment. Agriculture. 2024; 14(9):1562. https://doi.org/10.3390/agriculture14091562
Chicago/Turabian StyleWante, Solomon Peter, David W. M. Leung, and Hossein Alizadeh. 2024. "Varying Tolerance to Diesel Toxicity Revealed by Growth Response Evaluation of Petunia grandiflora Shoot Lines Regenerated after Diesel Fuel Treatment" Agriculture 14, no. 9: 1562. https://doi.org/10.3390/agriculture14091562







