Exploring the Factors Affecting Terrestrial Soil Respiration in Global Warming Manipulation Experiments Based on Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
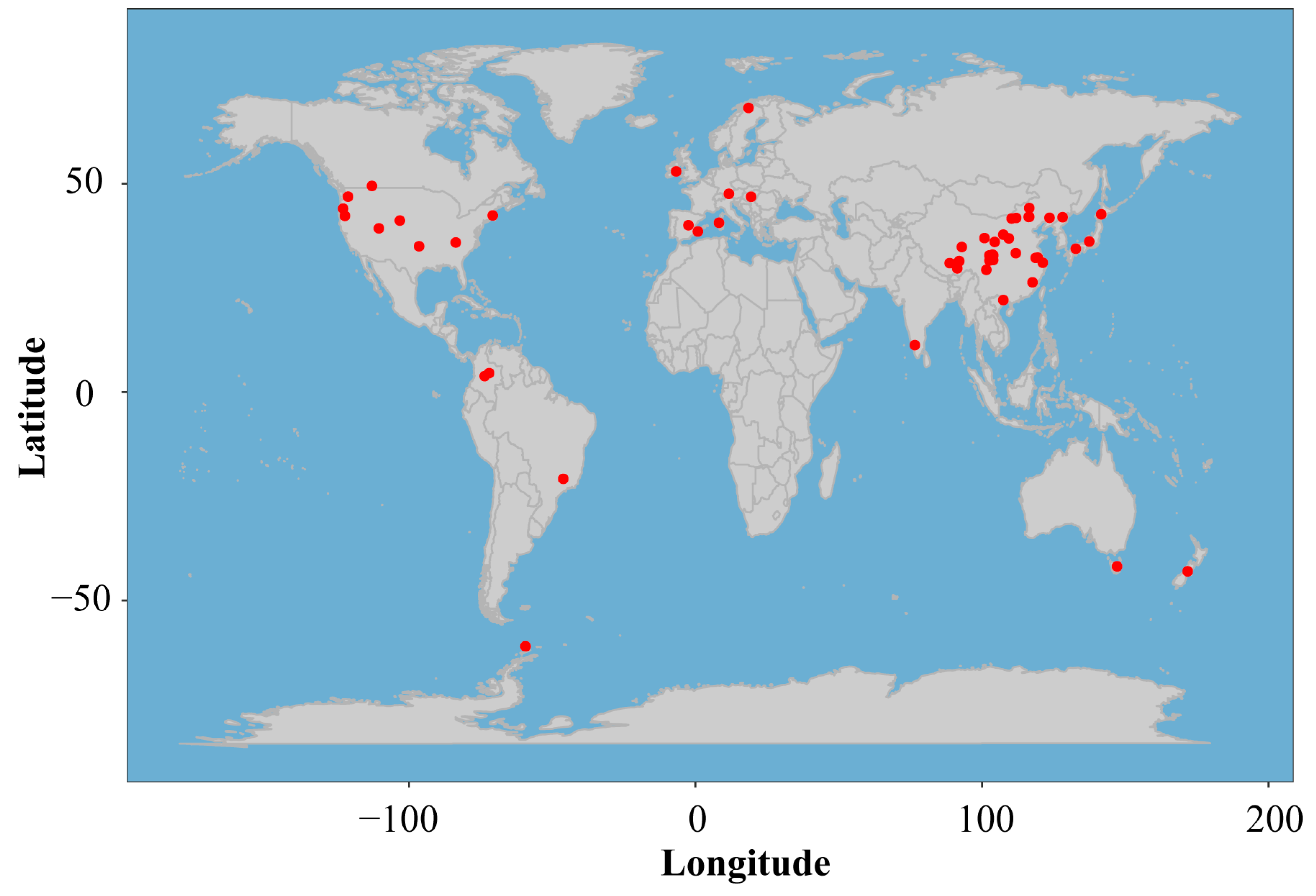
2.1. Search Strategy and Exclusion Criteria
- (1)
- Studies reported results of manipulative experiments conducted in the outdoor environment and having both control and treatment groups. Each group had at least three plots as replicates. In addition, the area of each plot was larger than 1 m2. The treatment groups were manipulated artificially to simulate global warming drivers.
- (2)
- Experiments were conducted in the field, while lab incubation, growth chamber, and translocation studies were not included in this synthesis.
- (3)
- Studies examined effects of simulated global warming drivers on soil respiration components, including Rs, Rh, and Ra. In a multi-factorial study, only control and changed warming treatment data were extracted, and interactions were excluded.
2.2. Data Extraction
2.3. Data Structure
2.4. Data Availability
2.5. Effect Size Metrics
2.6. Data Analyses
2.7. Potential Publication Bias Analyses
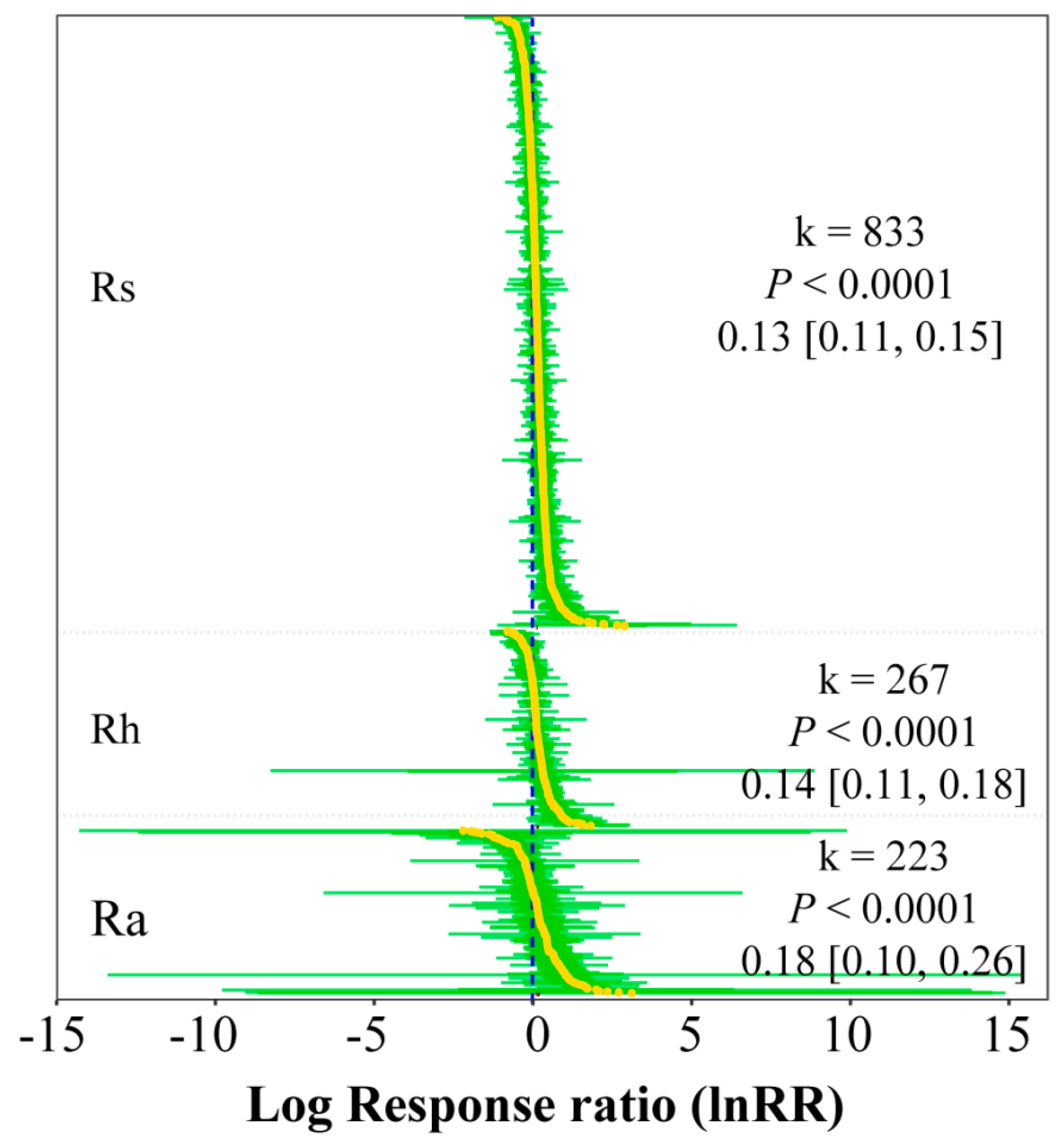
3. Results
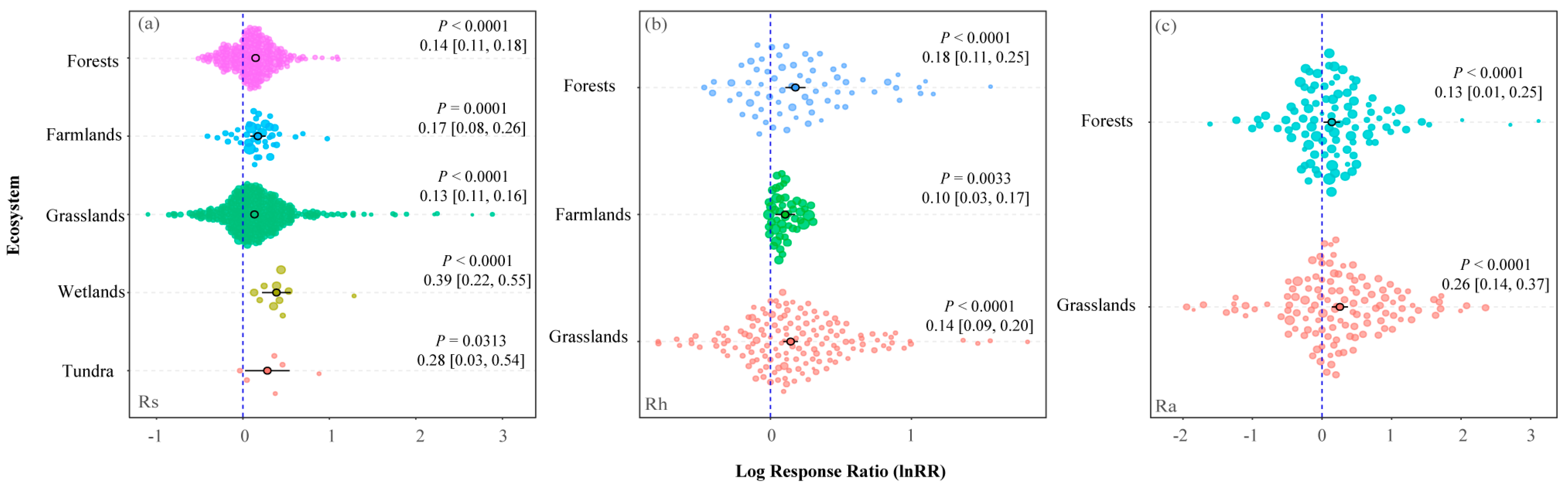
3.1. Response of Rs, Rh, and Ra to Warming in Different Ecosystems
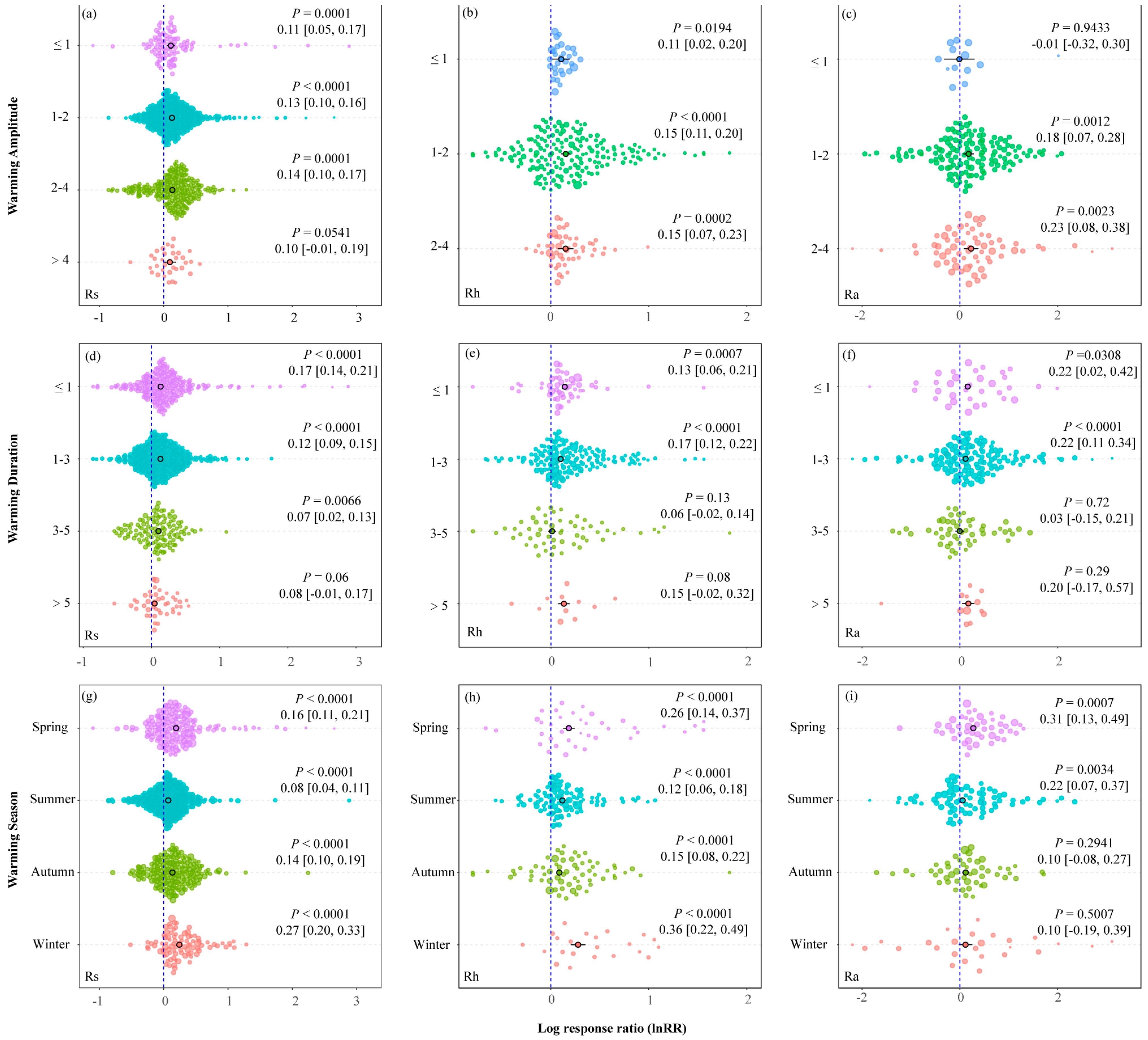
3.2. Soil Warming Amplitude, Warming Duration, and Sampling Season
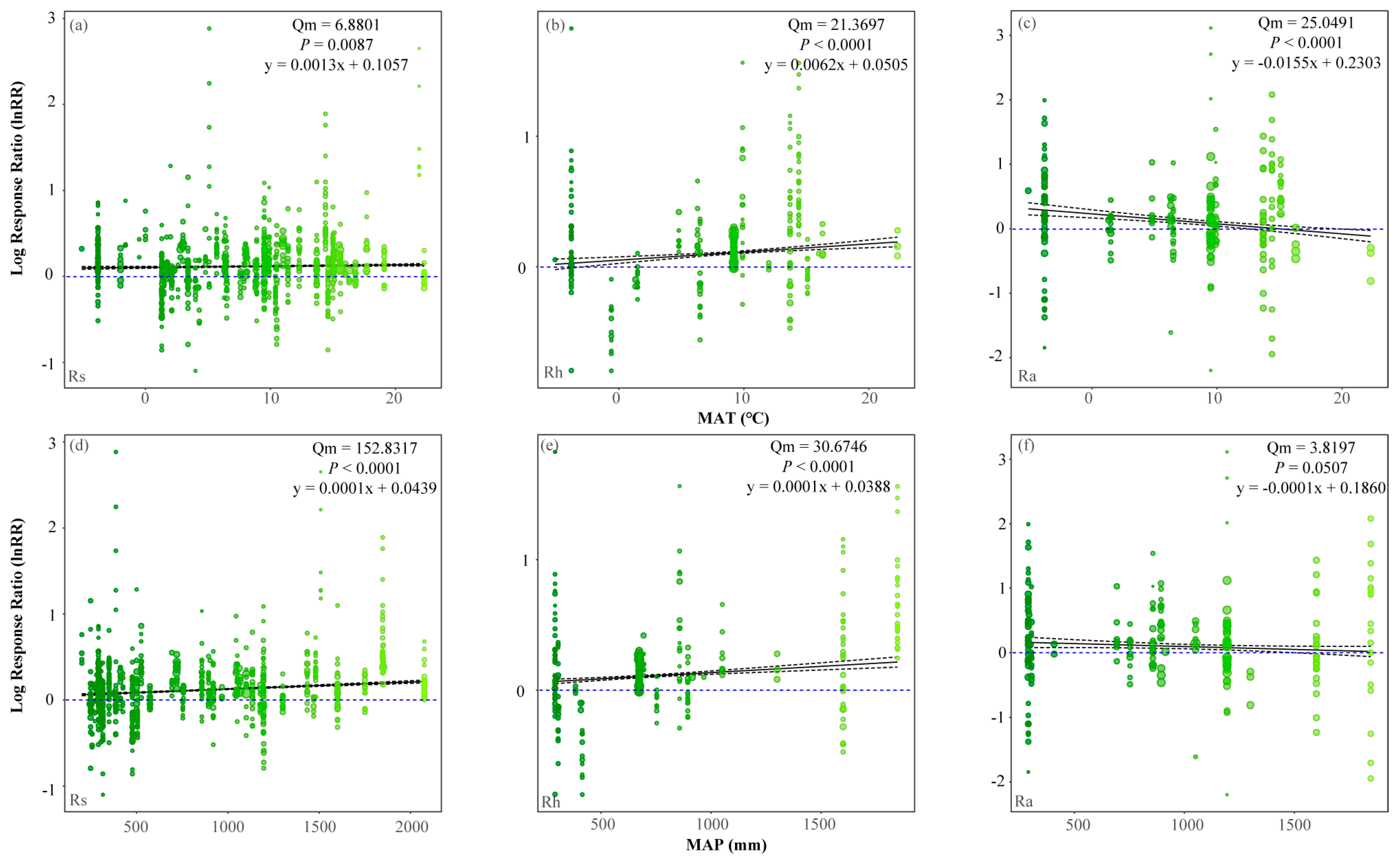
3.3. Environmental Factors Affecting the Response of Rs, Rh, and Ra to Warming
3.3.1. Climate Factors
3.3.2. Plant Pools
3.3.3. Soil Property
3.4. Optimal Model Selection
4. Discussion
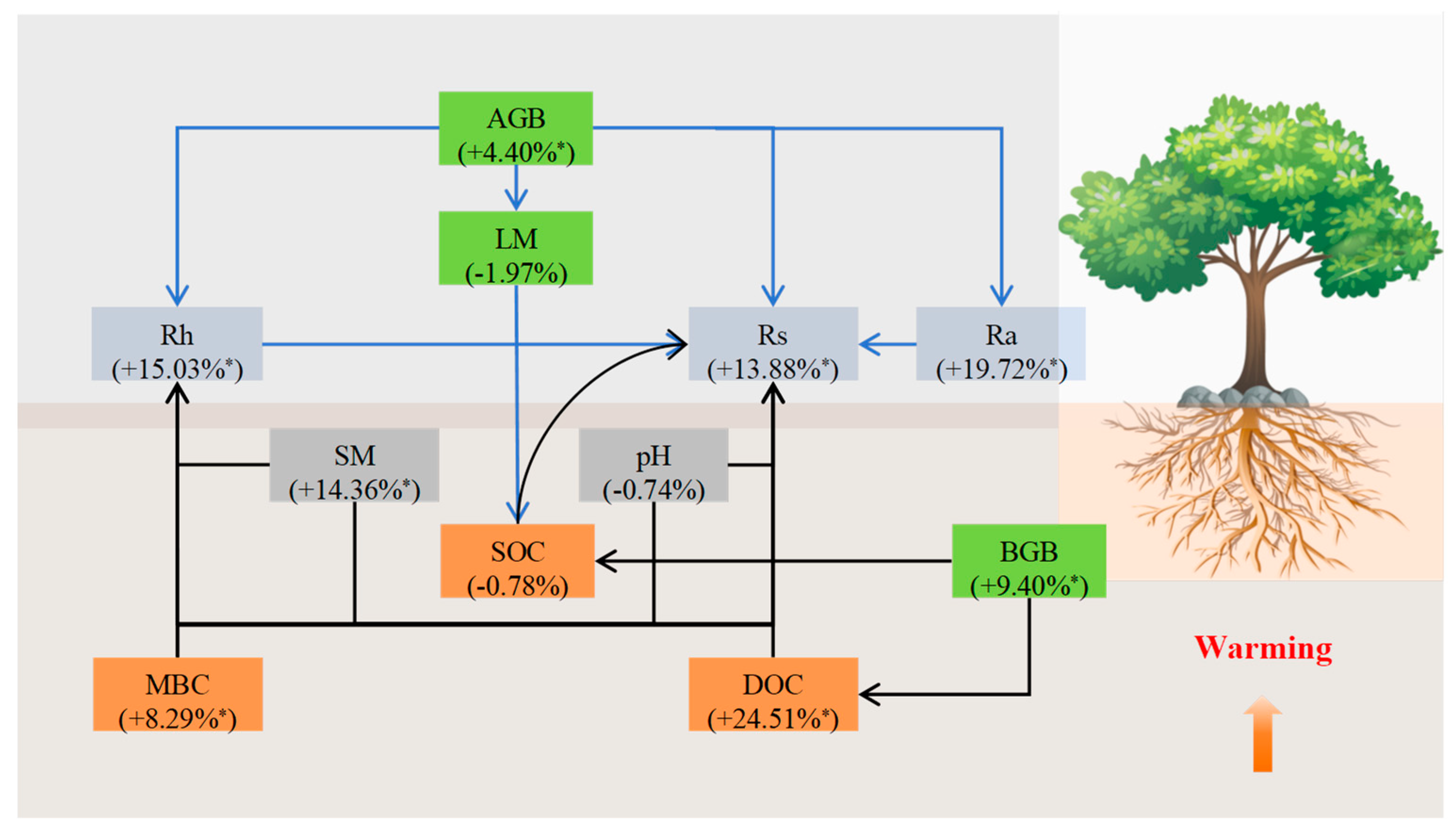
4.1. Category Moderators Affecting the Response of Rs, Rh, and Ra to Warming
4.2. Continuous Moderators Affecting the Response of Rs, Rh, and Ra to Warming
5. Limitations and Future Experiments
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- IPCC. Summary for policymakers. In Climate Change 2023: Synthesis Report. A Report of the Intergovernmental Panel on Climate Change. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Lee, H., Romero, J., Eds.; Core Writing Team, IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Lastra, M.; López, J.; Troncoso, J.S.; Sampedro, L. Warming and Wrack Supply Will Accelerate CO2 Emission and Nutrients Release on Antarctic Sedimentary Shores: A Case Study on a Volcanic Island. Ecosystems 2020, 24, 855–874. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, W.; Liu, M.; Liu, Q. Responses of soil respiration and its components to experimental warming in an alpine scrub ecosystem on the eastern Qinghai-Tibet Plateau. Sci. Total Environ. 2018, 643, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, G.X.; Yang, Y.; Gao, Y.H.; Liu, G.S. Plant production, and carbon and nitrogen source pools, are strongly intensified by experimental warming in alpine ecosystems in the Qinghai-Tibet Plateau. Soil. Biol. Biochem. 2011, 43, 942–953. [Google Scholar]
- Liberati, D.; Guidolotti, G.; de Dato, G.; De Angelis, P. Enhancement of ecosystem carbon uptake in a dry shrubland under moderate warming: The role of nitrogen-driven changes in plant morphology. Global Chang. Biol. 2021, 27, 5629–5642. [Google Scholar] [CrossRef]
- Liang, J.Y.; Xia, J.Y.; Liu, L.L.; Wan, S.Q. Global patterns of the responses of leaf-level photosynthesis and respiration in terrestrial plants to experimental warming. J. Plant Ecol. 2013, 6, 437–447. [Google Scholar] [CrossRef]
- Macdonald, C.A.; Anderson, I.C.; Khachane, A.; Singh, B.P.; Barton, C.V.M.; Duursma, R.A.; Ellsworth, D.S.; Singh, B.K. Plant productivity is a key driver of soil respiration response to climate change in a nutrient-limited soil. Basic. Appl. Ecol. 2021, 50, 155–168. [Google Scholar] [CrossRef]
- Zhou, L.Y. Interactive effects of global change factors on soil respiration and its components: A meta-analysis. Global Chang. Biol. 2016, 22, 3157–3169. [Google Scholar] [CrossRef] [PubMed]
- Pingintha, N.; Chayawat, C.; Hong, J.; Leclerc, M.Y.; Luo, Y.; Zhou, X. Soil Respiration and the Environment; An Imprint of Elsevier Science; Academic Press: London, UK, 2006; ISBN 0-12-088782-7. [Google Scholar]
- Schlesinger, W.H.; Andrews, J.A. Soil respiration and the global carbon cycle. Biogeochemistry 2000, 48, 7–20. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Thomson, A. Temperature-associated increases in the global soil respiration record. Nature 2010, 464, 579–582. [Google Scholar] [CrossRef]
- Yu, H.Y.; Liu, X.D.; Ma, Q.H.; Yin, Z.T.; Wang, Y.H.; Xu, Z.Z.; Zhou, G.S. Climatic warming enhances soil respiration resilience in an arid ecosystem. Sci. Total Environ. 2021, 756, 144005. [Google Scholar] [CrossRef]
- Zou, J.L.; Tobin, B.; Luo, Y.Q.; Osborne, B. Response of soil respiration and its components to experimental warming and water addition in a temperate Sitka spruce forest ecosystem. Agr. Forest Meteorol. 2018, 260, 204–215. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Bailey, V.L.; Chen, M.; Gough, C.M.; Vargas, R. Globally rising soil heterotrophic respiration over recent decades. Nature 2018, 560, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Melillo, J.M.; Frey, S.D.; DeAngelis, K.M.; Werner, W.J.; Bernard, M.J.; Bowles, F.P.; Pold, G.; Knorr, M.A.; Grandy, A.S. Long-term pattern and magnitude of soil carbon feedback to the climate system in a warming world. Science 2017, 358, 101–104. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.R.; Wang, J.X.; Li, D.J.; Shi, Z.M.; Liu, Y.C.; Xu, J.; Hong, P.Z.; Yu, H.L.; Zhao, Z.; et al. Contrasting responses of heterotrophic and root-dependent respiration to soil warming in a subtropical plantation. Agr. Forest Meteorol. 2017, 247, 221–228. [Google Scholar] [CrossRef]
- Yue, P.; Cui, X.Q.; Gong, Y.M.; Li, K.H.; Goulding, K.; Liu, X.J. Impact of elevated precipitation, nitrogen deposition and warming on soil respiration in a temperate desert. Biogeosciences 2018, 15, 2007–2019. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef]
- Pendall, E.; Heisler-White, J.L.; Williams, D.G.; Dijkstra, F.A.; Carrillo, Y.; Morgan, J.A.; LeCain, D.R. Warming Reduces Carbon Losses from Grassland Exposed to Elevated Atmospheric Carbon Dioxide. PLoS ONE 2013, 8, e71921. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.Q.; Hui, D.F.; Wallace, L.; Luo, Y.Q. Direct and indirect effects of experimental warming on ecosystem carbon processes in a tallgrass prairie. Glob. Biogeochem. Cycles 2005, 19, 1–13. [Google Scholar] [CrossRef]
- Lei, X.D.; Peng, C.H.; Tian, D.L.; Sun, J.F. Meta-analysis and its application in global change research. Chin. Sci. Bull. 2007, 52, 289–302. [Google Scholar] [CrossRef]
- Romero-Olivares, A.L.; Allison, S.D.; Treseder, K.K. Soil microbes and their response to experimental warming over time: A meta-analysis of field studies. Soil Biol. Biochem. 2017, 107, 32–40. [Google Scholar] [CrossRef]
- Song, J.; Wan, S.; Piao, S.; Knapp, A.K.; Classen, A.T.; Vicca, S.; Ciais, P.; Hovenden, M.J.; Leuzinger, S.; Beier, C.; et al. A meta-analysis of 1119 manipulative experiments on terrestrial carbon-cycling responses to global change. Nat. Ecol. Evol. 2019, 3, 1309–1320. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.W.; Zhao, D.S.; Zhu, Y.; Zhang, R.D.; Guo, C.Y. Response of grassland soil respiration to experimental warming: The long-term effects may be greater than we thought. Soil Biol. Biochem. 2022, 168, 108616. [Google Scholar] [CrossRef]
- Feng, J.G.; Wang, J.S.; Ding, L.B.; Yao, P.P.; Qiao, M.P.; Yao, S.C. Meta-analyses of the effects of major global change drivers on soil respiration across China. Atmos. Environ. 2017, 150, 181–186. [Google Scholar] [CrossRef]
- Ngaba, M.J.Y.; Uwiragiye, Y.; Bol, R.; de Vries, W.; Jian, J.S.; Zhou, J.B. Global cross-biome patterns of soil respiration responses to individual and interactive effects of nitrogen addition, altered precipitation, and warming. Sci. Total Environ. 2023, 858, 159808. [Google Scholar] [CrossRef]
- Ghorbani, M.; Konvalina, P.; Kopecky, M.; Kolář, L. A meta-analysis on the impacts of different oxidation methods on the surface area properities of biochar. Land Degrad. Dev. 2023, 34, 299–312. [Google Scholar] [CrossRef]
- Burda, B.U.; O’Connor, E.A.; Webber, E.M.; Redmond, N.; Perdue, L.A. Estimating data from figures with a Web-based program: Considerations for a systematic review. Res. Synth. Methods 2017, 8, 258–262. [Google Scholar] [CrossRef]
- Stevens, J.R.; Nicholas, G. Hierarchical dependence in meta-analysis. J. Educ. Behav. Stat. 2010, 34, 46–73. [Google Scholar] [CrossRef]
- Hedges, L.V.; Gurevitch, J.; Curtis, P.S. The Meta-Analysis of Response Ratios in Experimental Ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Speidel, B. Effective Care of the Newborn Infant. Arch. Dis. Child. 1992, 67, 1415–1416. [Google Scholar] [CrossRef]
- Lajeunesse, M.J.; Fitzjohn, R. Facilitating systematic reviews, data extraction and meta-analysis with the metagear package for R. Methods Ecol. Evol. 2015, 7, 323–330. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Calcagno, V.; Mazancourt, C.D. glmulti: AnRPackage for Easy Automated Model Selection with (Generalized) Linear Models. J. Stat. Softw. 2010, 34, 1–29. [Google Scholar] [CrossRef]
- Mallen-Cooper, M.; Nakagawa, S.; Eldridge, D.J. Global meta-analysis of soil-disturbing vertebrates reveals strong effects on ecosystem patterns and processes. Global Ecol. Biogeogr. 2019, 28, 661–679. [Google Scholar] [CrossRef]
- Vetter, D. Handbook of Meta-Analysis in Ecology and Evolution. Q. Rev. Biol. 2014, 89, 53–54. [Google Scholar] [CrossRef]
- Chatelain, M.; Drobniak, S.M.; Szulkin, M. The association between stressors and telomeres in non-human vertebrates: A meta-analysis. Ecol. Lett. 2020, 23, 381–398. [Google Scholar] [CrossRef]
- Luo, C.Y.; Xu, G.P.; Chao, Z.G.; Wang, S.P.; Lin, X.W.; Hu, Y.G.; Zhang, Z.H.; Duan, J.C.; Chang, X.F.; Su, A.L.; et al. Effect of warming and grazing on litter mass loss and temperature sensitivity of litter and dung mass loss on the Tibetan plateau. Global Chang. Biol. 2010, 16, 1606–1617. [Google Scholar] [CrossRef]
- Bronson, D.R.; Gower, S.T.; Tanner, M.; Linder, S.; Van Herk, I. Response of soil surface CO2 flux in a boreal forest to ecosystem warming. Global Chang. Biol. 2008, 14, 856–867. [Google Scholar] [CrossRef]
- Lu, M.; Zhou, X.H.; Yang, Q.; Li, H.; Luo, Y.Q.; Fang, C.M.; Chen, J.K.; Yang, X.; Li, B. Responses of ecosystem carbon cycle to experimental warming: A meta-analysis. Ecology 2013, 94, 726–738. [Google Scholar] [CrossRef]
- Yan, C.; Yuan, Z.; Shi, X.; Lock, T.R.; Kallenbach, R.L. A global synthesis reveals more response sensitivity of soil carbon flux than pool to warming. J. Soil. Sediment. 2019, 20, 1208–1221. [Google Scholar] [CrossRef]
- Karhu, K.; Auffret, M.D.; Dungait, J.A.J.; Hopkins, D.W.; Prosser, J.I.; Singh, B.K.; Subke, J.A.; Wookey, P.A.; Ågren, G.I.; Sebastià, M.T.; et al. Temperature sensitivity of soil respiration rates enhanced by microbial community response. Nature 2014, 513, 81–84. [Google Scholar] [CrossRef]
- Rustad, L.E.; Campbell, J.L.; Marion, G.M.; Norby, R.J.; Mitchell, M.J.; Hartley, A.E.; Cornelissen, J.H.C.; Gurevitch, J.; GCTE-News. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 2001, 126, 543–562. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Q.; Wu, Y.H.; Zhang, J.; Wang, G.X.; DeLuca, T.H.; Zhu, W.Z.; Li, A.D.; Duan, M.; He, L. Soil warming and nitrogen deposition alter soil respiration, microbial community structure and organic carbon composition in a coniferous forest on eastern Tibetan Plateau. Geoderma 2019, 353, 283–292. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, S.Q.; Li, C.L.; Zhang, J.J.; Wang, L.C. Effects of temperature on soil organic carbon fractions contents, aggregate stability and structural characteristics of humic substances in a Mollisol. J. Soil. Sediment. 2016, 16, 1849–1857. [Google Scholar] [CrossRef]
- Fang, X.; Zhou, G.Y.; Qu, C.; Huang, W.J.; Zhang, D.Q.; Li, Y.L.; Yi, Z.G.; Liu, J.X. Translocating subtropical forest soils to a warmer region alters microbial communities and increases the decomposition of mineral-associated organic carbon. Soil. Biol. Biochem. 2020, 142, 107707. [Google Scholar] [CrossRef]
- Ziegler, S.E.; Billings, S.A.; Lane, C.S.; Li, J.W.; Fogel, M.L. Warming alters routing of labile and slower-turnover carbon through distinct microbial groups in boreal forest organic soils. Soil Biol. Biochem. 2013, 60, 23–32. [Google Scholar] [CrossRef]
- Wang, X.; Hu, H.B.; Zheng, X.; Deng, W.B.; Chen, J.Y.; Zhang, S.; Cheng, C. Will climate warming of terrestrial ecosystem contribute to increase soil greenhouse gas fluxes in plot experiment? A global meta-analysis. Sci. Total Environ. 2022, 827, 154114. [Google Scholar] [CrossRef]
- Carey, J.C.; Tang, J.W.; Templer, P.H.; Kroeger, K.D.; Crowther, T.W.; Burton, A.J.; Dukes, J.S.; Emmett, B.; Frey, S.D.; Heskel, M.A.; et al. Temperature response of soil respiration largely unaltered with experimental warming. Proc. Natl. Acad. Sci. USA 2016, 113, 13797–13802. [Google Scholar] [CrossRef]
- Oechel, W.C.; Vourlitis, G.L.; Hastings, S.J.; Zulueta, R.C.; Hinzman, L.; Kane, D. Acclimation of ecosystem CO2 exchange in the Alaskan Arctic in response to decadal climate warming. Nature 2000, 406, 978–981. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, M.T.; Holthof, E.; Smith, A.R.; Koller, E.; Emmett, B.A. Contrasting response of summer soil respiration and enzyme activities to long-term warming and drought in a wet shrubland (NE Wales, UK). Appl. Soil. Ecol. 2017, 110, 151–155. [Google Scholar] [CrossRef]
- Ravn, N.R.; Ambus, P.; Michelsen, A. Impact of decade-long warming, nutrient addition and shading on emission and carbon isotopic composition of CO2 from two subarctic dwarf shrub heaths. Soil Biol. Biochem. 2017, 111, 15–24. [Google Scholar] [CrossRef]
- Fierer, N.; Craine, J.M.; McLauchlan, K.; Schimel, J.P. Litter quality and the temperature sensitivity of decomposition. Ecology 2005, 86, 320–326. [Google Scholar] [CrossRef]
- Escolar, C.; Maestre, F.T.; Rey, A. Biocrusts modulate warming and rainfall exclusion effects on soil respiration in a semi-arid grassland. Soil Biol. Biochem. 2015, 80, 9–17. [Google Scholar] [CrossRef] [PubMed]
- He, W.Q. Effects of altered litter input on soil physicochemical properties in forest ecosystems. J. Ecol. 2024, 44, 1755–1763. [Google Scholar]
- Lin, L.T.; Sun, X.K.; Yu, Z.Y.; Wang, K.L.; Zeng, D.H. Effects of nitrogen addition on microbial respiration and root respiration in a sandy grassland. J. Appl. Ecol. 2016, 27, 2189–2196. [Google Scholar]
- Lee, M.-S.; Nakane, K.; Nakatsubo, T.; Mo, W.-H.; Koizumi, H. Effects of rainfall events on soil CO2 flux in a cool temperate deciduous broad-leaved forest. Ecol. Res. 2002, 17, 401–409. [Google Scholar] [CrossRef]
- Wang, Y.H.; Song, C.; Liu, H.Y.; Wang, S.P.; Zeng, H.; Luo, C.Y.; He, J.S. Precipitation determines the magnitude and direction of interannual responses of soil respiration to experimental warming. Plant Soil. 2021, 458, 75–91. [Google Scholar] [CrossRef]
- Ding, J.P.; Luo, Y.Q.; Zhou, X.; Yue, X.F.; Lian, J. Progress in the study of plant root respiration research methods and influencing factors. Acta Prataculturae Sin. 2015, 24, 206–216. [Google Scholar]
- Yu, C.Q.; Wang, J.W.; Shen, Z.X.; Fu, G. Effects of experimental warming and increased precipitation on soil respiration in an alpine meadow in the Northern Tibetan Plateau. Sci. Total Environ. 2019, 647, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Schloss, A.L.; Melillo, J.M.; McGuire, A.D.; Kicklighter, D.W.; Moore, B.; Vorosmarty, C.J. Global climate change and terrestrial net primary production. Nature 1993, 363, 234–240. [Google Scholar]
- Xing, W.; Lu, X.M.; Ying, J.Y.; Lan, Z.C.; Chen, D.M.; Bai, Y.F. Disentangling the effects of nitrogen availability and soil acidification on microbial taxa and soil carbon dynamics in natural grasslands. Soil Biol. Biochem. 2022, 164, 108495. [Google Scholar] [CrossRef]
- Xu, M.; Shang, H. Contribution of soil respiration to the global carbon equation. J. Plant Physiol. 2016, 203, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Knorr, W.; Prentice, I.C.; House, J.I.; Holland, E.A. Long-term sensitivity of soil carbon turnover to warming. Nature 2005, 433, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Lie, Z.Y.; Lin, W.; Huang, W.J.; Fang, X.; Huang, C.M.; Wu, T.; Chu, G.W.; Liu, S.Z.; Meng, Z.; Zhou, G.Y.; et al. Warming changes soil N and P supplies in model tropical forests. Biol. Fert. Soils 2019, 55, 751–763. [Google Scholar] [CrossRef]
- Xu, X.; Shi, Z.; Li, D.J.; Zhou, X.H.; Sherry, R.A.; Luo, Y.Q. Plant community structure regulates responses of prairie soil respiration to decadal experimental warming. Global Chang. Biol. 2015, 21, 3846–3853. [Google Scholar] [CrossRef] [PubMed]
- Schimel, D.S.; Braswell, B.H.; Holland, E.A.; McKeown, R.; Ojima, D.S.; Painter, T.H.; Parton, W.J.; Townsend, A.R. Climatic, edaphic, and biotic controls over storage and turnover of carbon in soils. Global Biogeochem. Cycles 1994, 8, 279–293. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.L.; Piao, S.L.; Janssens, I.A.; Tang, J.W.; Liu, W.X.; Chi, Y.G.; Wang, J.; Xu, S. Soil respiration under climate warming: Differential response of heterotrophic and autotrophic respiration. Global Chang. Biol. 2014, 20, 3229–3237. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Hu, H.; Wang, Q.; Wang, X.; Ma, B. Exploring the Factors Affecting Terrestrial Soil Respiration in Global Warming Manipulation Experiments Based on Meta-Analysis. Agriculture 2024, 14, 1581. https://doi.org/10.3390/agriculture14091581
Chen X, Hu H, Wang Q, Wang X, Ma B. Exploring the Factors Affecting Terrestrial Soil Respiration in Global Warming Manipulation Experiments Based on Meta-Analysis. Agriculture. 2024; 14(9):1581. https://doi.org/10.3390/agriculture14091581
Chicago/Turabian StyleChen, Xue, Haibo Hu, Qi Wang, Xia Wang, and Bing Ma. 2024. "Exploring the Factors Affecting Terrestrial Soil Respiration in Global Warming Manipulation Experiments Based on Meta-Analysis" Agriculture 14, no. 9: 1581. https://doi.org/10.3390/agriculture14091581




