Agronomic Strategies for Sustainable Cotton Production: A Systematic Literature Review
Abstract
1. Introduction
2. Materials and Methods
- Peer-reviewed articles written in English were included;
- Conference papers, conference reviews, and book chapters were not reviewed;
- The most recent and informative papers from the same experimental topic were given priority.
3. Results and Discussion
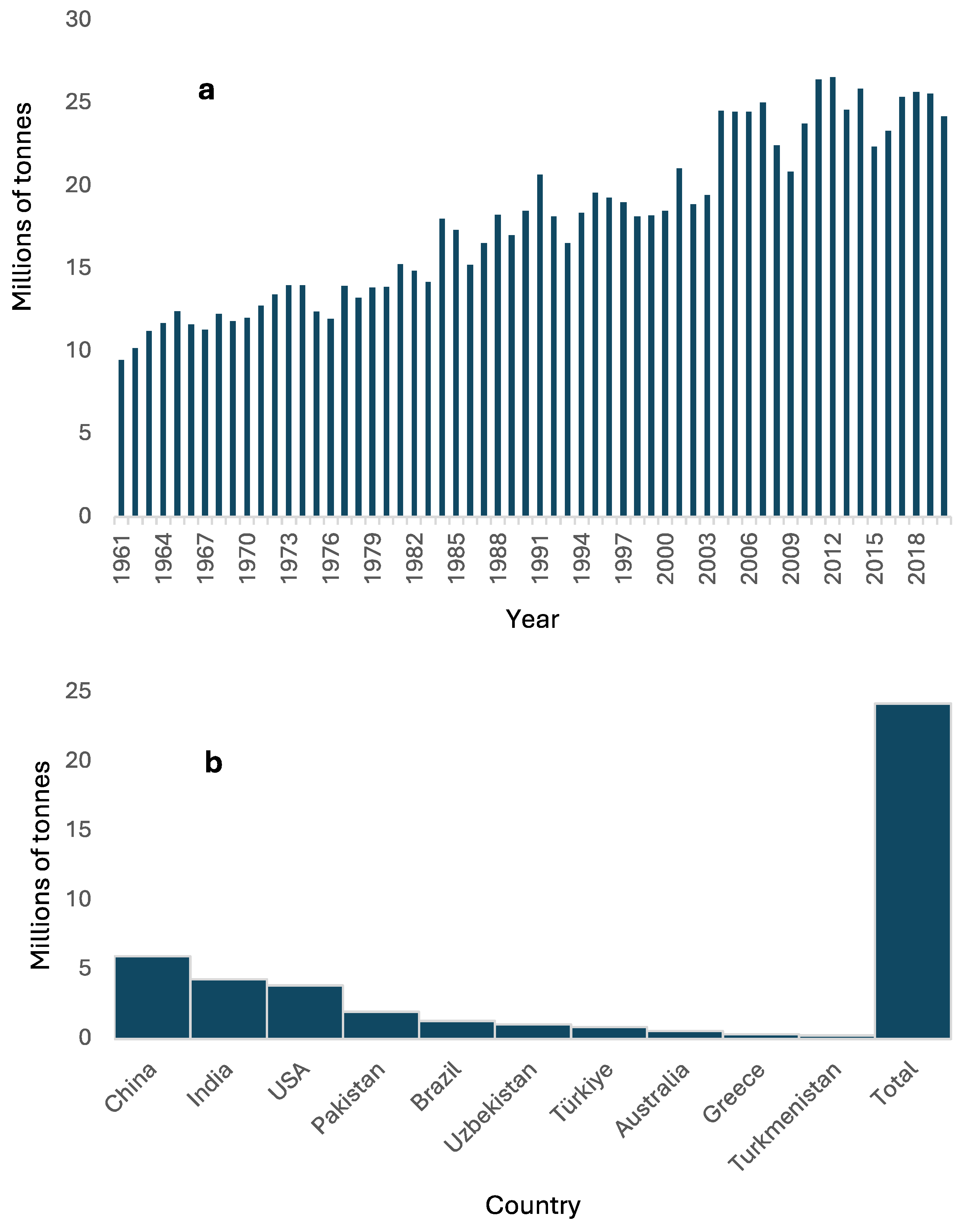
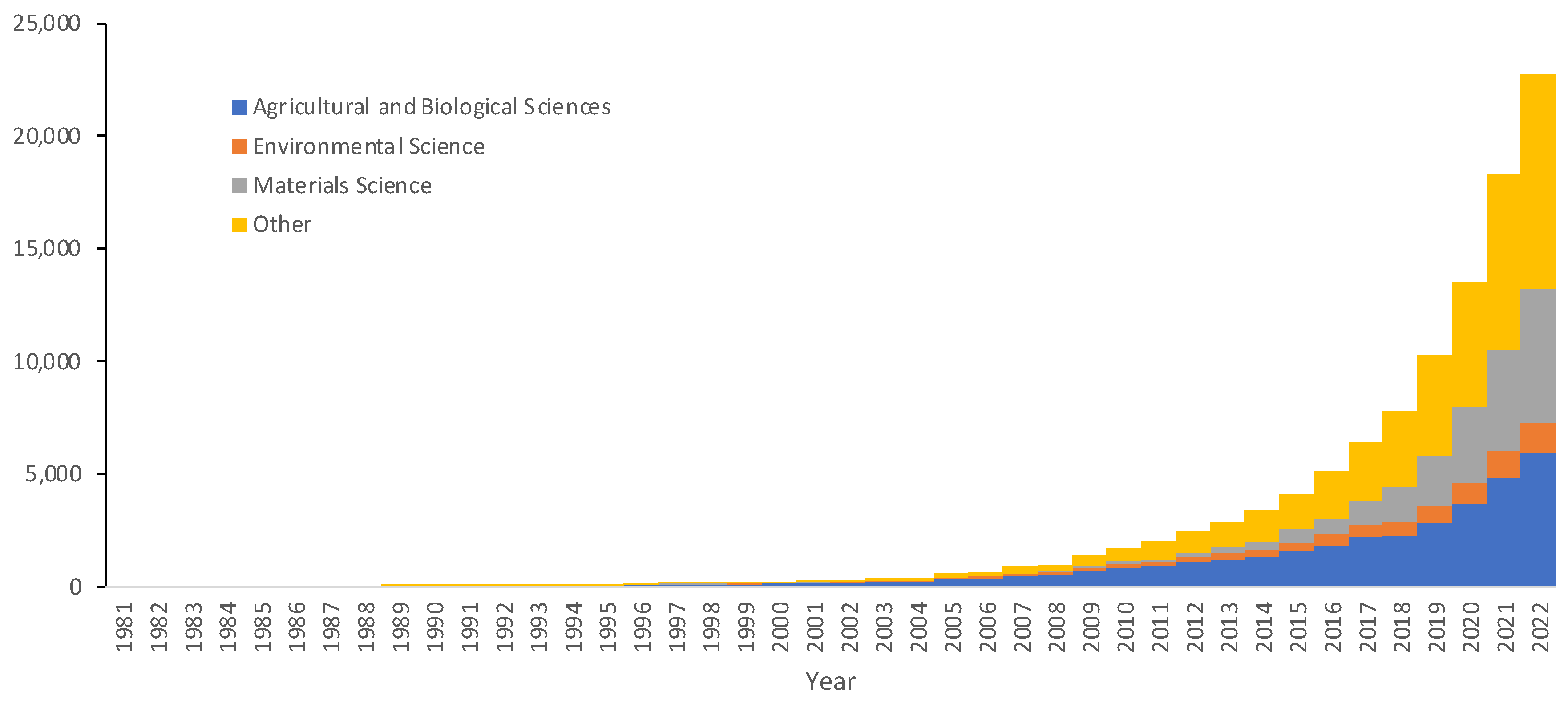
3.1. Cotton Production and Literature Database
3.2. Mineral Nutrients and Plant–Microorganism Relationships
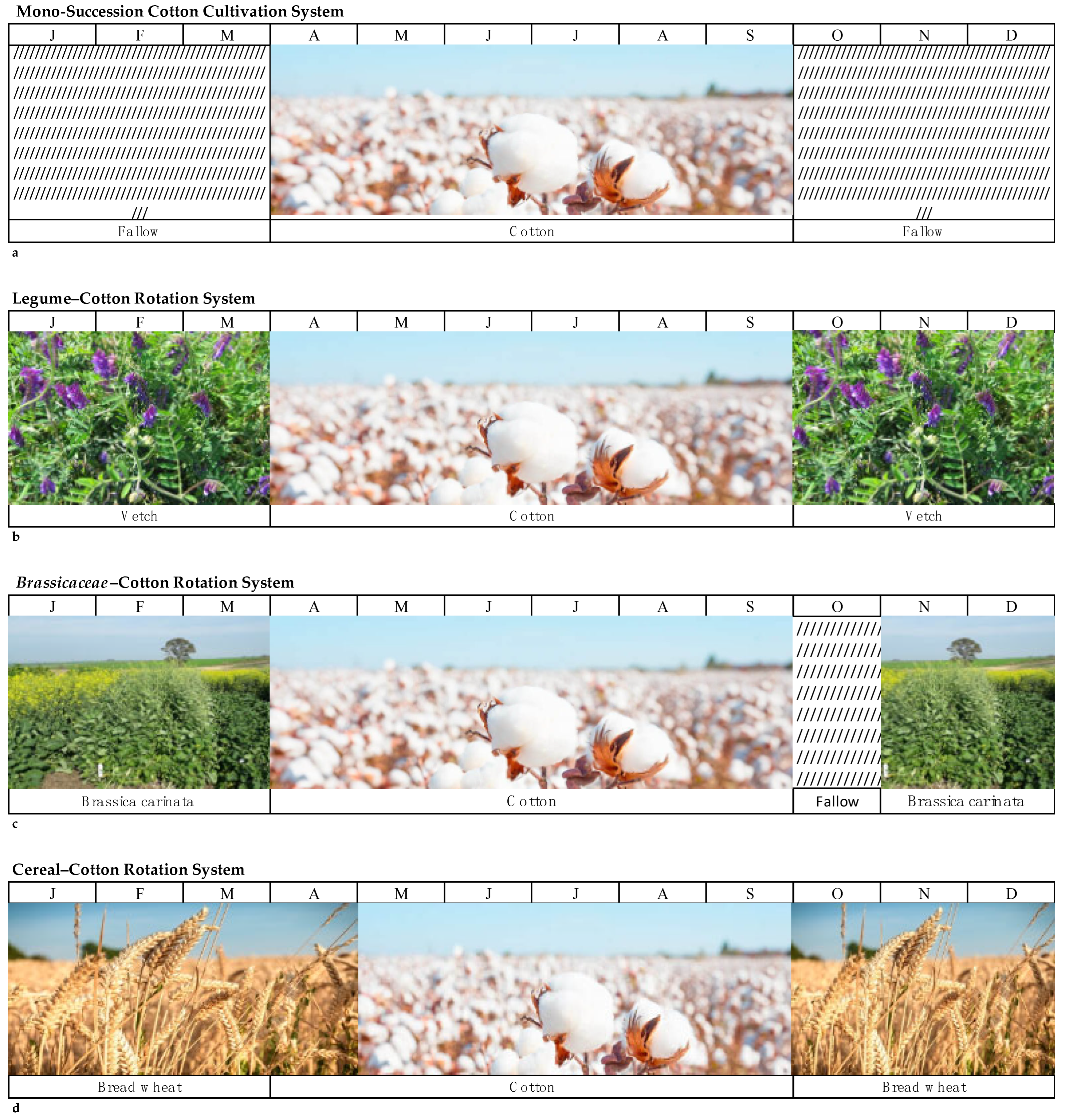
3.3. Monoculture and Polyculture Systems
3.3.1. Legumes
3.3.2. Brassicas
3.3.3. Cereals
3.4. Water Management
3.4.1. Irrigation Systems
3.4.2. Irrigation Termination Strategies
4. Final Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Wakelyn, P.J.; Chaudhry, M.R. Organic cotton: Production practices and post-harvest considerations. Sustain. Text. Life Cycle Environ. Impact 2009, 11, 231–301. [Google Scholar] [CrossRef]
- EPA’s Report on the Environment (ROE) (2008 Final Report) (2017) EPA. Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=190806 (accessed on 7 August 2024).
- USDA—National Agricultural Statistics Service—Surveys—Agricultural Chemical Use Program. Available online: https://www.nass.usda.gov/Surveys/Guide_to_NASS_Surveys/Chemical_Use/ (accessed on 7 February 2023).
- Reisig, D.D.; Huseth, A.S.; Bacheler, J.S.; Aghaee, M.A.; Braswell, L.; Burrack, H.J.; Flanders, K.; Greene, J.K.; Herbert, D.A.; Jacobson, A.; et al. Long-term empirical and observational evidence of practical Helicoverpa zea resistance to cotton with pyramided bt toxins. J. Econ. Entomol. 2018, 111, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xu, Z.; Zhu, Y.C.; Huang, F.; Wang, Y.; Li, H.; Li, H.; Gao, C.; Zhou, W.; Shen, J. Evidence of field-evolved resistance to cry1ac-expressing bt cotton in Helicoverpa armigera (Lepidoptera: Noctuidae) in northern China. Pest. Manag. Sci. 2010, 66, 155–161. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, W.; Zhao, J.; Jin, L.; Yang, J.; Liu, C.; Yang, Y.; Wu, S.; Wu, K.; Cui, J.; et al. Diverse genetic basis of field-evolved resistance to bt cotton in cotton bollworm from China. Proc. Natl. Acad. Sci. USA 2012, 109, 10275–10280. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Kamath, S.P.; Mohan, K.S.; Head, G.; Sumerford, D.V. Inheritance of field-relevant resistance to the Bacillus thuringiensis protein Cry1Ac in Pectinophora gossypiella (Lepidoptera: Gelechiidae) collected from India. Pest. Manag. Sci. 2016, 72, 558–565. [Google Scholar] [CrossRef]
- Mathew, L.G.; Ponnuraj, J.; Mallappa, B.; Chowdary, L.R.; Zhang, J.; Tay, W.T.; Walsh, T.K.; Gordon, K.H.J.; Heckel, D.G.; Downes, S.; et al. ABC transporter mis-splicing associated with resistance to Bt toxin Cry2Ab in laboratory- and field-selected pink bollworm. Sci. Rep. 2018, 8, 13531. [Google Scholar] [CrossRef]
- Kruger, M.; Van Rensburg, J.; Berg, J.V.D. Resistance to Bt maize in Busseola Fusca (Lepidoptera: Noctuidae) from Vaalharts, South Africa. Environ. Entomol. 2011, 40, 477–483. [Google Scholar] [CrossRef]
- Van den Berg, J.; Hilbeck, A.; Bøhn, T. Pest resistance to Cry1Ab Bt maize: Field resistance, contributing factors and lessons from South Africa. Crop Prot. 2013, 54, 154–160. [Google Scholar] [CrossRef]
- Grimi, D.A.; Parody, B.; Ramos, M.L.; Machado, M.; Ocampo, F.; Willse, A.; Martinelli, S.; Head, G. Field-evolved resistance to Bt maize in sugarcane borer (Diatraea Saccharalis) in Argentina. Pest. Manag. Sci. 2018, 74, 905–913. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Carrière, Y. Surge in Insect Resistance to Transgenic Crops and Prospects for Sustainability. Nat. Biotechnol. 2017, 35, 926–935. [Google Scholar] [CrossRef]
- Huff, J.A.; Reynolds, D.B.; Dodds, D.M.; Irby, J.T. Glyphosate tolerance in enhanced glyphosate-resistant cotton (Gossypium Hirsutum). Weed Technol. 2010, 24, 289–294. [Google Scholar] [CrossRef]
- Gimsing, A.L.; Borggaard, O.K.; Jacobsen, O.S.; Aamand, J.; Sørensen, J. Chemical and microbiological soil characteristics controlling glyphosate mineralisation in danish surface Soils. Appl. Soil Ecol. 2004, 27, 233–242. [Google Scholar] [CrossRef]
- Rolando, C.A.; Baillie, B.R.; Thompson, D.G.; Little, K.M. The risks associated with glyphosate-based herbicide use in planted forests. Forests 2017, 8, 208. [Google Scholar] [CrossRef]
- Altieri, M.A. The ecological role of biodiversity in agroecosystems. Agric. Ecosyst. Environ. 1999, 74, 19–31. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A.; Jakobsen, I. Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol. 2003, 133, 16–20. [Google Scholar] [CrossRef]
- Mai, W.; Xue, X.; Feng, G.; Yang, R.; Tian, C. Arbuscular mycorrhizal fungi—15-fold enlargement of the soil volume of cotton roots for phosphorus uptake in intensive planting conditions. Eur. J. Soil Biol. 2019, 90, 31–35. [Google Scholar] [CrossRef]
- Osteen, C.; Gottlieb, J.; Vasavada, U. Agricultural Resources and Environmental Indicators, 2012 Edition; USDA-ERS Economic Information Bulletin No. 98; USDA: Washington, DC, USA, 2012; Available online: https://ssrn.com/abstract=2141408 (accessed on 7 August 2024).
- Adeli, A.; Brooks, J.P.; Read, J.J.; Miles, D.M.; Shankle, M.W.; Jenkins, J.N. Impact of cover crop on nutrient losses in an upland soil. Commun. Soil Sci. Plant Anal. 2021, 52, 536–550. [Google Scholar] [CrossRef]
- Ashworth, A.J.; Allen, F.L.; Saxton, A.M.; Tyler, D.D. Long-term cotton yield impacts from cropping rotations and biocovers under no-tillage. J. Cotton Sci. 2016, 20, 95–102. [Google Scholar] [CrossRef]
- Wu, B.; Tian, F.; Zhang, M.; Piao, S.; Zeng, H.; Zhu, W.; Liu, J.; Elnashar, A.; Lu, Y. Quantifying global agricultural water appropriation with data derived from earth observations. J. Clean. Prod. 2022, 358, 131891. [Google Scholar] [CrossRef]
- Kebede, H.; Fisher, D.K.; Sui, R.; Reddy, K.N.; Kebede, H.; Fisher, D.K.; Sui, R.; Reddy, K.N. Irrigation methods and scheduling in the delta region of mississippi: Current status and strategies to improve irrigation efficiency. Am. J. Plant Sci. 2014, 5, 2917–2928. [Google Scholar] [CrossRef]
- Roth, G.; Harris, G.; Gillies, M.; Montgomery, J.; Wigginton, D. Water-use efficiency and productivity trends in australian irrigated cotton: A review. Crop Pasture Sci. 2013, 64, 1033–1048. [Google Scholar] [CrossRef]
- Barnes, E.M.; Campbell, B.T.; Vellidis, G.; Porter, W.M.; Payero, J.O.; Leib, B.G.; Sui, R.; Fisher, D.K.; Anapalli, S.; Colaizzi, P.D.; et al. Forty years of increasing cotton’s water productivity and why the trend will continue. Appl. Eng. Agric. 2020, 36, 457–478. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; Clark, J.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, 7. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 30 January 2023).
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef]
- Tong, X.; Xu, M.; Wang, X.; Bhattacharyya, R.; Zhang, W.; Cong, R. Long-term fertilization effects on organic carbon fractions in a red soil of China. CATENA 2014, 113, 251–259. [Google Scholar] [CrossRef]
- Tao, L.; Li, F.-B.; Liu, C.-S.; Feng, X.-H.; Gu, L.-L.; Wang, B.-R.; Wen, S.-L.; Xu, M.-G. Mitigation of soil acidification through changes in soil mineralogy due to long-term fertilization in Southern China. CATENA 2019, 174, 227–234. [Google Scholar] [CrossRef]
- Martínez-Dalmau, J.; Berbel, J.; Ordóñez-Fernández, R. Nitrogen Fertilization. A review of the risks associated with the inefficiency of its use and policy responses. Sustainability 2021, 13, 5625. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Monaco, A.L.; Ruta, C.; Mauromicale, G. Mycorrhizal inoculation improves plant growth and yield of micropropagated early globe artichoke under field conditions. Agriculture 2022, 12, 114. [Google Scholar] [CrossRef]
- Lombardo, S.; Abbate, C.; Pandino, G.; Parisi, B.; Scavo, A.; Mauromicale, G. Productive and physiological response of organic potato grown under highly calcareous soils to fertilization and mycorrhization management. Agronomy 2020, 10, 1200. [Google Scholar] [CrossRef]
- Badda, N.; Yadav, K.; Aggarwal, A.; Kadian, N. Consortium Effect of Arbuscular Mycorrhizal fungi and pseudomonas fluorescens with various levels of superphosphate on growth improvement of cotton (G. arboreum L.). J. Nat. Fibers 2014, 12, 12–25. [Google Scholar] [CrossRef]
- Rochester, I.J.; Peoples, M.B.; Hulugalle, N.R.; Gault, R.R.; Constable, G.A. Using legumes to enhance nitrogen fertility and improve soil condition in cotton cropping systems. Field Crop. Res. 2001, 70, 27–41. [Google Scholar] [CrossRef]
- Eskandari, S.; Guppy, C.N.; Knox, O.G.G.; Flavel, R.J.; Backhouse, D.; Haling, R.E. Mycorrhizal contribution to phosphorus nutrition of cotton in low and highly sodic soils using dual isotope labelling (32P and 33P). Soil Biol. Biochem. 2017, 105, 37–44. [Google Scholar] [CrossRef]
- Thompson, J.P.; Seymour, N.P.; Clewett, T.G. Stunted cotton (Gossypium Hirsutum L.) fully recovers biomass and yield of seed cotton after delayed root inoculation with spores of an arbuscular mycorrhizal fungus (Glomus Mosseae). Australas. Plant Pathol. 2012, 41, 431–437. [Google Scholar] [CrossRef]
- Ortas, İ.; Akpinar, C.; Demirbas, A. Sour Orange (Citrus Aurantium L.) Growth is strongly mycorrhizal dependent in terms of phosphorus (P) nutrition rather than zinc (Zn). Commun. Soil Sci. Plant Anal. 2016, 47, 2514–2527. [Google Scholar] [CrossRef]
- Zhou, J.; Chai, X.; Zhang, L.; George, T.S.; Wang, F.; Feng, G. Different arbuscular mycorrhizal fungi cocolonizing on a single plant root system recruit distinct microbiomes. mSystems 2020, 5, 10. [Google Scholar] [CrossRef]
- Lynch, J.P.; Ho, M.D. Rhizoeconomics: Carbon costs of phosphorus acquisition. Plant Soil 2005, 269, 45–56. [Google Scholar] [CrossRef]
- Ortas, I.; Iqbal, M.T. Mycorrhizal Inoculation enhances growth and nutrition of cotton plant. J. Plant Nutr. 2019, 42, 2043–2056. [Google Scholar] [CrossRef]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Update on arbuscular mycorrhizas and phosphorus nutrition roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition 1. Plant Physiol. Ò 2011, 156, 1050–1057. [Google Scholar] [CrossRef]
- Nagy, R.; Drissner, D.; Amrhein, N.; Jakobsen, I.; Bucher, M. Mycorrhizal phosphate uptake pathway in tomato is phosphorus-repressible and transcriptionally regulated. New Phytol. 2009, 181, 950–959. [Google Scholar] [CrossRef]
- Mai, W.; Xue, X.; Feng, G.; Tian, C. Simultaneously maximizing root/mycorrhizal growth and phosphorus uptake by cotton plants by optimizing water and phosphorus management. BMC Plant Biol. 2018, 18, 334. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M. Arbuscular mycorrhizal isolate and phosphogypsum effects on growth and nutrients acquisition of cotton (Gossypium Hirsutum L.). Adv. Hortic. Sci. 2016, 30, 121–128. [Google Scholar] [CrossRef]
- Salgado, F.H.M.; Moreira, F.M.d.S.; Siqueira, J.O.; Barbosa, R.H.; Paulino, H.B.; Carneiro, M.A.C. Fungos micorrízicos arbusculares e estimulante da colonização na cultura do algodoeiro e do milho. Cienc. Rural. 2017, 47, 6. [Google Scholar] [CrossRef]
- Korejo, A.A.; Shah, A.N.; Sial, T.A.; Ali, S.; Lahori, A.H.; Narej, A.M.; Channo, Z.A.; Aneel, C.; Korejo, A.; Sial, A.; et al. Growth yield and yield components of cotton as influenced by NPK ratios in combination of foliar application of zinc levels under tandojam conditions. Pure Appl. Biol. 2015, 4, 268–274. [Google Scholar] [CrossRef]
- Cely, M.V.T.; de Oliveira, A.G.; de Freitas, V.F.; de Luca, M.B.; Barazetti, A.R.; dos Santos, I.M.O.; Gionco, B.; Garcia, G.V.; Prete, C.E.C.; Andrade, G. Inoculant of arbuscular mycorrhizal fungi (Rhizophagus clarus) Increase yield of soybean and cotton under field conditions. Front. Microbiol. 2016, 7, 720. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, A.J.; Owens, P.R.; Allen, F.L. Long-term cropping systems management influences soil strength and nutrient cycling. Geoderma 2020, 361, 114062. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Huang, W.; Chen, J.; Wu, F.; Jia, Y.; Han, Y.; Wang, G.; Feng, L.; Li, X.; et al. Cover crops and N fertilization affect soil ammonia volatilization and N2O emission by regulating the soil labile carbon and nitrogen fractions. Agric. Ecosyst. Environ. 2022, 340, 108188. [Google Scholar] [CrossRef]
- Li, L.; Konkel, J.; Jin, V.L.; Schaeffer, S.M. Conservation management improves agroecosystem function and resilience of soil nitrogen cycling in response to seasonal changes in climate. Sci. Total Environ. 2021, 779, 146457. [Google Scholar] [CrossRef]
- Tiwari, R.; Reinhardt Piskáčková, T.A.; Devkota, P.; Mulvaney, M.J.; Ferrell, J.A.; Leon, R.G. Growing winter Brassica carinata as part of a diversified crop rotation for integrated weed management. GCB Bioenergy 2021, 13, 425–435. [Google Scholar] [CrossRef]
- Chatterjee, A.; Clay, D.E. Cover crops impacts on nitrogen scavenging, nitrous oxide emissions, nitrogen fertilizer replacement, erosion, and soil health. In Soil Fertility Management in Agroecosystems; ACSESS: Madison, WI, USA, 2017; pp. 76–88. [Google Scholar] [CrossRef]
- Scavo, A.; Fontanazza, S.; Restuccia, A.; Pesce, G.R.; Abbate, C.; Mauromicale, G. The role of cover crops in improving soil fertility and plant nutritional status in temperate climates. A review. Agron. Sustain. Dev. 2022, 42, 93. [Google Scholar] [CrossRef]
- Lemessa, F.; Wakjira, M. Cover Crops as a means of ecological weed management in agroecosystems. J. Crop Sci. Biotechnol. 2015, 18, 133–145. [Google Scholar] [CrossRef]
- Scavo, A.; Restuccia, A.; Abbate, C.; Lombardo, S.; Fontanazza, S.; Pandino, G.; Anastasi, U.; Mauromicale, G. Trifolium subterraneum cover cropping enhances soil fertility and weed seedbank dynamics in a mediterranean apricot orchard. Agron. Sustain. Dev. 2021, 41, 70. [Google Scholar] [CrossRef]
- Restuccia, A.; Scavo, A.; Lombardo, S.; Pandino, G.; Fontanazza, S.; Anastasi, U.; Abbate, C.; Mauromicale, G. Long-term effect of cover crops on species abundance and diversity of weed flora. Plants 2020, 9, 1506. [Google Scholar] [CrossRef] [PubMed]
- Korres, N.E.; Norsworthy, J.K. Influence of a rye cover crop on the critical period for weed control in cotton. Weed Sci. 2015, 63, 346–352. [Google Scholar] [CrossRef]
- Abdalla, M.; Hastings, A.; Cheng, K.; Yue, Q.; Chadwick, D.; Espenberg, M.; Truu, J.; Rees, R.M.; Smith, P. A critical review of the impacts of cover crops on nitrogen leaching, net greenhouse gas balance and crop productivity. Glob. Chang. Biol. 2019, 25, 2530–2543. [Google Scholar] [CrossRef]
- Liang, J.; He, Z.; Shi, W. Cotton/Mung Bean Intercropping Improves Crop Productivity, Water Use Efficiency, Nitrogen Uptake, and Economic Benefits in the Arid Area of Northwest China. Agric. Water Manag. 2020, 240, 106277. [Google Scholar] [CrossRef]
- Touchton, J.T.; Rickerl, D.H.; Walker, R.H.; Snipes, C.E. Winter legumes as a nitrogen source for no-tillage cotton. Soil Tillage Res. 1984, 4, 391–401. [Google Scholar] [CrossRef]
- Zhang, T.; Zhai, Y.; Ma, X.; Shen, X.; Bai, Y.; Zhang, R.; Ji, C.; Hong, J. Towards environmental sustainability: Life Cycle Assessment-based water footprint analysis on China’s cotton production. J. Clean. Prod. 2021, 313, 127925. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, X.; Wei, T.; Yang, Z.; Jia, Z.; Yang, B.; Han, Q.; Ren, X. Effects of straw incorporation on the soil nutrient contents, enzyme activities, and crop yield in a semiarid region of China. Soil Tillage Res. 2016, 160, 65–72. [Google Scholar] [CrossRef]
- Kliebenstein, D.J.; Kroymann, J.; Mitchell-Olds, T. The glucosinolate–myrosinase system in an ecological and evolutionary context. Curr. Opin. Plant Biol. 2005, 8, 264–271. [Google Scholar] [CrossRef]
- Scavo, A.; Mauromicale, G. Crop allelopathy for sustainable weed management in agroecosystems: Knowing the present with a view to the future. Agronomy 2021, 11, 2104. [Google Scholar] [CrossRef]
- Decker, H.L.; Gamble, A.V.; Balkcom, K.S.; Johnson, A.M.; Hull, N.R. Cover crop monocultures and mixtures affect soil health indicators and crop yield in the southeast United States. Soil Sci. Soc. Am. J. 2022, 86, 1312–1326. [Google Scholar] [CrossRef]
- Patra, R.; Saha, D.; Jagadamma, S. Winter Wheat cover crop increased subsoil organic carbon in a long-term cotton cropping system in Tennessee. Soil Tillage Res. 2022, 224, 105521. [Google Scholar] [CrossRef]
- Finney, D.M.; White, C.M.; Kaye, J.P. Biomass production and carbon/nitrogen ratio influence ecosystem services from cover crop mixtures. Agron. J. 2016, 108, 39–52. [Google Scholar] [CrossRef]
- Novara, A.; Cerda, A.; Barone, E.; Gristina, L. Cover crop management and water conservation in vineyard and olive orchards. Soil Tillage Res. 2021, 208, 104896. [Google Scholar] [CrossRef]
- Zhou, X.V.; Larson, J.A.; Sykes, V.R.; Ashworth, A.J.; Allen, F.L. Crop rotation, cover crop, and poultry litter effects on no-tillage cotton profitability. Agron. J. 2021, 113, 2648–2663. [Google Scholar] [CrossRef]
- Lebeau, S.G.; Brye, K.R.; Daniels, M.; Wood, L.S. Cover Crop and Wheel-Track Effects on Soil Properties under Cotton Production in Eastern Arkansas. Agrosys. Geosci. Environ. 2024, 7, e20549. [Google Scholar] [CrossRef]
- Cetin, O.; Bilgel, L. Effects of different irrigation methods on shedding and yield of cotton. Agric. Water Manag. 2002, 54, 1–15. [Google Scholar] [CrossRef]
- Pinnamaneni, S.R.; Anapalli, S.S.; Sui, R.; Bellaloui, N.; Reddy, K.N. Effects of irrigation and planting geometry on cotton (Gossypium hirsutum L.) fiber quality and seed composition. J. Cotton Res. 2021, 4, 2. [Google Scholar] [CrossRef]
- Bordovsky, J.P.; Mustian, J.T.; Ritchie, G.L.; Lewis, K.L. Cotton irrigation timing with variable seasonal irrigation capacities in the Texas South Plains. Appl. Eng. Agric. 2015, 31, 883–897. [Google Scholar] [CrossRef]
- Koudahe, K.; Sheshukov, A.Y.; Aguilar, J.; Djaman, K. Irrigation-water management and productivity of cotton: A review. Sustainability 2021, 13, 10070. [Google Scholar] [CrossRef]
- Hunsaker, D.J. Basal crop coefficients and water use for early maturity cotton. Trans. ASAE 1999, 42, 927–936. [Google Scholar] [CrossRef]
- Kumar, V.; Udeigwe, T.K.; Clawson, E.L.; Rohli, R.V.; Miller, D.K. Crop water use and stage-specific crop coefficients for irrigated cotton in the mid-south, United States. Agric. Water Manag. 2015, 156, 63–69. [Google Scholar] [CrossRef]
- Farahani, H.J.; Oweis, T.Y.; Izzi, G. Crop coefficient for drip-irrigated cotton in a Mediterranean environment. Irrig. Sci. 2008, 26, 375–383. [Google Scholar] [CrossRef]
- Ayars, J.E.; Hutmacher, R.B. Crop coefficients for irrigating cotton in the presence of groundwater. Irrig. Sci. 1994, 15, 45–52. [Google Scholar] [CrossRef]
- Grismer, M.E. Regional cotton lint yield, ETc and water value in Arizona and California. Agric. Water Manag. 2002, 54, 227–242. [Google Scholar] [CrossRef]
- Evett, S.R.; Baumhardt, R.L.; Howell, T.A.; Ibragimov, N.M.; Hunsaker, D.J. Cotton. In Crop Yield Response to Water; FAO: Rome, Italy, 2012; ISSN 0254-5284. [Google Scholar]
- Howell, T.A.; Davis, K.R.; McCormick, R.L.; Yamada, H.; Walhood, V.T.; Meek, D.W. Water use efficiency of narrow row cotton. Irrig. Sci. 1984, 5, 195–214. [Google Scholar] [CrossRef]
- Ibragimov, N.; Evett, S.R.; Esanbekov, Y.; Kamilov, B.S.; Mirzaev, L.; Lamers, J.P.A. Water use efficiency of irrigated cotton in uzbekistan under drip and furrow irrigation. Agric. Water Manag. 2007, 90, 112–120. [Google Scholar] [CrossRef]
- Mchugh, A.D.; Bhattarai, S.; Lotz, G.; Midmore, D.J. Effects of subsurface drip irrigation rates and furrow irrigation for cotton grown on a vertisol on off-site movement of sediments, nutrients and pesticides. Agron. Sustain. Dev. 2008, 28, 507–519. [Google Scholar] [CrossRef]
- Yazar, A.; Sezen, S.M.; Sesveren, S. LEPA and trickle irrigation of cotton in the southeast Anatolia project (GAP) area in Turkey. Agric. Water Manag. 2002, 54, 189–203. [Google Scholar] [CrossRef]
- Goebel, T.S.; Lascano, R.J. Rainwater use by cotton under subsurface drip and center pivot irrigation. Agric. Water Manag. 2019, 215, 1–7. [Google Scholar] [CrossRef]
- Wanjura, D.F.; Upchurch, D.R.; Mahan, J.R.; Burke, J.J. Cotton yield and applied water relationships under drip irrigation. Agric. Water Manag. 2002, 55, 217–237. [Google Scholar] [CrossRef]
- Shroff, S.; Miglani, V. Water-Saving and Economic Gains of Micro Irrigation Adoption Scheme “Per Drop More Crop”: A Case of Sugarcane, Banana and Cotton Cultivation in Maharashtra. Econ. Aff. 2024, 69, 487–502. [Google Scholar] [CrossRef]
- Mai, W.X.; Xue, X.R.; Azeem, A. Growth of cotton crop (Gossypium hirsutum L.) higher under drip irrigation because of better phosphorus uptake. Appl. Ecol. Environ. Res. 2022, 20, 4865–4878. [Google Scholar] [CrossRef]
- Cotton—Land & Water. Available online: https://www.fao.org/land-water/databases-and-software/crop-information/cotton/en/ (accessed on 30 January 2023).
- Çetin, O.; Kara, A. Assesment of water productivity using different drip irrigation systems for cotton. Agric. Water Manag. 2019, 223, 105693. [Google Scholar] [CrossRef]
- Whitaker, J.R.; Ritchie, G.L.; Bednarz, C.W.; Mills, C.I. Cotton subsurface drip and overhead irrigation efficiency, maturity, yield, and quality. Agron. J. 2008, 100, 1763–1768. [Google Scholar] [CrossRef]
- Wang, J.; Du, G.; Tian, J.; Zhang, Y.; Jiang, C.; Zhang, W. Effect of irrigation methods on root growth, root-shoot ratio and yield components of cotton by regulating the growth redundancy of root and shoot. Agric. Water Manag. 2020, 234, 106120. [Google Scholar] [CrossRef]
- Lu, W.; Ren, A.; Yang, J.; Yu, L.; Ma, C.; Zhang, Q. Soil water and salt movement and spatial distribution of fine alfalfa roots under drip irrigation. Nongye Gongcheng Xuebao/Trans. Chin. Soc. Agric. Eng. 2014, 30, 128–137. [Google Scholar] [CrossRef]
- Zong, R.; Wang, Z.; Wu, Q.; Guo, L.; Lin, H. Characteristics of carbon emissions in cotton fields under mulched drip irrigation. Agric. Water Manag. 2020, 231, 105992. [Google Scholar] [CrossRef]
- Tan, M.; Li, W.; Zong, R.; Li, X.; Han, Y.; Luo, P.; Dhital, Y.P.; Lin, H.; Li, H.; Wang, Z. Long-Term Mulched Drip Irrigation Enhances the Stability of Soil Aggregates by Increasing Organic Carbon Stock and Reducing Salinity. Soil Tillage Res. 2024, 240, 106069. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, J.; Wen, Y.; Li, W.; Zhu, Y.; Song, L.; Li, Y.; Liang, Y.; Wang, Z. Effects of Different Film Types on Cotton Growth and Yield under Drip Irrigation. Sustainability 2024, 16, 4173. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, J.; Dhital, Y.; Wu, X.; Song, L.; Zhu, Y.; Chen, P.; Li, W.; Wang, Z. Integrated Effects of Plastic Film Residues on Cotton Growth and Field Carbon Sequestration under Drip Irrigation in Arid Oasis Regions. Agric. Ecosyst. Environ. 2022, 339, 108131. [Google Scholar] [CrossRef]
- Cao, J.; Chen, P.; Li, Y.; Fang, H.; Gu, X.; Li, Y. Effect of Plastic Film Residue on Vertical Infiltration Under Different Initial Soil Moisture Contents and Dry Bulk Densities. Water 2020, 12, 1346. [Google Scholar] [CrossRef]
- Jiang, X.J.; Liu, W.; Wang, E.; Zhou, T.; Xin, P. Residual Plastic Mulch Fragments Effects on Soil Physical Properties and Water Flow Behavior in the Minqin Oasis, Northwestern China. Soil Tillage Res. 2017, 166, 100–107. [Google Scholar] [CrossRef]
- Can, H.; Wang, X.; Wang, S.; Lu, B.; Guo, W.; Liu, C.; Tang, X. Impact of Agricultural Residual Plastic Film on the Growth and Yield of Drip-Irrigated Cotton in Arid Region of Xinjiang, China. Int. J. Agric. Biol. Eng. 2020, 13, 160–169. [Google Scholar] [CrossRef]
- Delate, K.; Heller, B.; Shade, J. Organic cotton production may alleviate the environmental impacts of intensive conventional cotton production. Renew. Agric. Food Syst. 2021, 36, 405–412. [Google Scholar] [CrossRef]
- Grimes, D.W.; Dickens, W.L. Dating termination of cotton irrigation from soil water-retention characteristics. Agron. J. 1974, 66, 403–404. [Google Scholar] [CrossRef]
- Fletcher, R.S.; Showler, A.T.; Funk, P.A. Employing broadband spectra and cluster analysis to assess thermal defoliation of cotton. Comput. Electron. Agric. 2014, 105, 103–110. [Google Scholar] [CrossRef]
- Pelletier, M.G.; Wanjura, J.D.; Holt, G.A. Chemical-free cotton defoliation by; mechanical, flame and laser girdling. Agronomy 2017, 7, 9. [Google Scholar] [CrossRef]
- Vories, E.D.; Greene, J.K.; Teague, T.G.; Stewart, J.H.; Phipps, B.J.; Pringle, H.C.; Clawson, E.L.; Hogan, R.J.; O’Leary, P.F.; Griffin, T.W. Determining the optimum timing for the final furrow irrigation on mid-south cotton. Appl. Eng. Agric. 2011, 27, 737–745. [Google Scholar] [CrossRef]
- Reba, M.L.; Teague, T.G.; Vories, E.D. A retrospective review of cotton irrigation on a production farm in the mid-south. J. Cotton Sci. 2014, 18, 137–144. [Google Scholar] [CrossRef]
- Karam, F.; Lahoud, R.; Masaad, R.; Daccache, A.; Mounzer, O.; Rouphael, Y. Water use and lint yield response of drip irrigated cotton to the length of irrigation season. Agric. Water Manag. 2006, 85, 287–295. [Google Scholar] [CrossRef]
- Masasi, B.; Taghvaeian, S.; Boman, R.; Datta, S. Impacts of irrigation termination date on cotton yield and irrigation requirement. Agriculture 2019, 9, 39. [Google Scholar] [CrossRef]
- Buttar, G.S.; Aujla, M.S.; Thind, H.S.; Singh, C.J.; Saini, K.S. Effect of timing of first and last irrigation on the yield and water use efficiency in cotton. Agric. Water Manag. 2007, 89, 236–242. [Google Scholar] [CrossRef]


| AMF Species | Benefits | Target Parameter | Effect Compared to Uninoculated Control | Reference |
|---|---|---|---|---|
| Acaulospora scrobiculata | Nutrient uptake, increase in the surface area explored by roots and shoots | Root dry matter | +75% | [47] |
| P uptake | +59% | |||
| Claroideoglomus etunicatum | Nutrient uptake, increase in the surface area explored by roots and shoots | Root dry matter | +56% | [47] |
| P uptake | +76% | |||
| Funneliformis mosseae | Zn and P uptake (less than C. etunicatum) | P uptake | +110% | [42] |
| Effect on the soil microbiome | Higher abundance of Actinobacteria and Gemmatimonadetes | [37] | ||
| Gigaspora margarita | Nutrient uptake, increase in the surface area explored by roots and shoots | Ca, Zn, P uptake | +68% Ca, +69% Zn, +76% P | [38] |
| Effect on the soil microbiome | Higher abundance of Proteobacteria, Cyanobacteria, and Fusobacteria | [37] | ||
| Glomus intraradices, G. viscosum, and G. mosseae | Growth response of cotton nutrient uptake | N, P, K, Ca, Mn, Fe, Cu, Zn uptake | +65% N, +148% P, +92% K, +65% Ca, +129% Mn, +73% Fe, +91% Cu, +85% Zn | [45] |
| Rhizophagus clarus | Increase in P and nitrogen content in inoculated plants, root colonization | Effect on plant biomass | +81% | [47] |
| Shoot N uptake | +75% in N uptake | [47] |
| Irrigation System | Effects | Climate | Location | Reference | |||
|---|---|---|---|---|---|---|---|
| Arid | Arid Continental | Mediterranean | Semi-Arid | ||||
| DI | 2 | 1 | 2 | ||||
| Less efficient in the germination phase compared to SI | 1 | Şanlıurfa, Turkey | [92] | ||||
| +14% seed-cotton production compared to FI | 1 | Uzbekistan | [84] | ||||
| +19.8% shoot biomass compared to FLI | 1 | Urumqi, China | [90] | ||||
| +20% cotton yield compared to FI and +29% than SI | 1 | Anatolia, Turkey | [73] | ||||
| +25% WUE compared to FI and +35% compared to SI | |||||||
| +30% seed-cotton production compared to FI and +21% compared to SI | |||||||
| +12.10% shoot P content vs. FLI | 1 | Urumqi, China | [90] | ||||
| LEPA | 1 | ||||||
| −5.75% seed cotton yields compared to DI | 1 | Southeastern Anatolia, Turkey | [86] | ||||
| +1.61% D3 water consumption compared to DI | |||||||
| MDI | 2 | ||||||
| +61.49% biomass production compared to DI | 1 | Xinjiang, China | [96] | ||||
| +12.84% cotton yield vs. DI | |||||||
| +4.80%–12.87% soil moisture content (from full flowering to the bolls open stage) vs. DI | 1 | Xinjiang, China | [94] | ||||
| SSD | 1 | ||||||
| Irrigation water saving compared to LEPA | 1 | Texas, United States | [87] | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitale, G.S.; Scavo, A.; Zingale, S.; Tuttolomondo, T.; Santonoceto, C.; Pandino, G.; Lombardo, S.; Anastasi, U.; Guarnaccia, P. Agronomic Strategies for Sustainable Cotton Production: A Systematic Literature Review. Agriculture 2024, 14, 1597. https://doi.org/10.3390/agriculture14091597
Vitale GS, Scavo A, Zingale S, Tuttolomondo T, Santonoceto C, Pandino G, Lombardo S, Anastasi U, Guarnaccia P. Agronomic Strategies for Sustainable Cotton Production: A Systematic Literature Review. Agriculture. 2024; 14(9):1597. https://doi.org/10.3390/agriculture14091597
Chicago/Turabian StyleVitale, Giuseppe Salvatore, Aurelio Scavo, Silvia Zingale, Teresa Tuttolomondo, Carmelo Santonoceto, Gaetano Pandino, Sara Lombardo, Umberto Anastasi, and Paolo Guarnaccia. 2024. "Agronomic Strategies for Sustainable Cotton Production: A Systematic Literature Review" Agriculture 14, no. 9: 1597. https://doi.org/10.3390/agriculture14091597
APA StyleVitale, G. S., Scavo, A., Zingale, S., Tuttolomondo, T., Santonoceto, C., Pandino, G., Lombardo, S., Anastasi, U., & Guarnaccia, P. (2024). Agronomic Strategies for Sustainable Cotton Production: A Systematic Literature Review. Agriculture, 14(9), 1597. https://doi.org/10.3390/agriculture14091597












