Residues of Symbiont Cover Crops Improving Corn Growth and Soil-Dependent Health Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Growth Condition
2.2. Determination of Plant Biomass and Mycorrhiza Colonization
2.3. Determination of N Content in Leaf
2.4. Soil Sampling and Analysis
2.5. Statistical Analysis
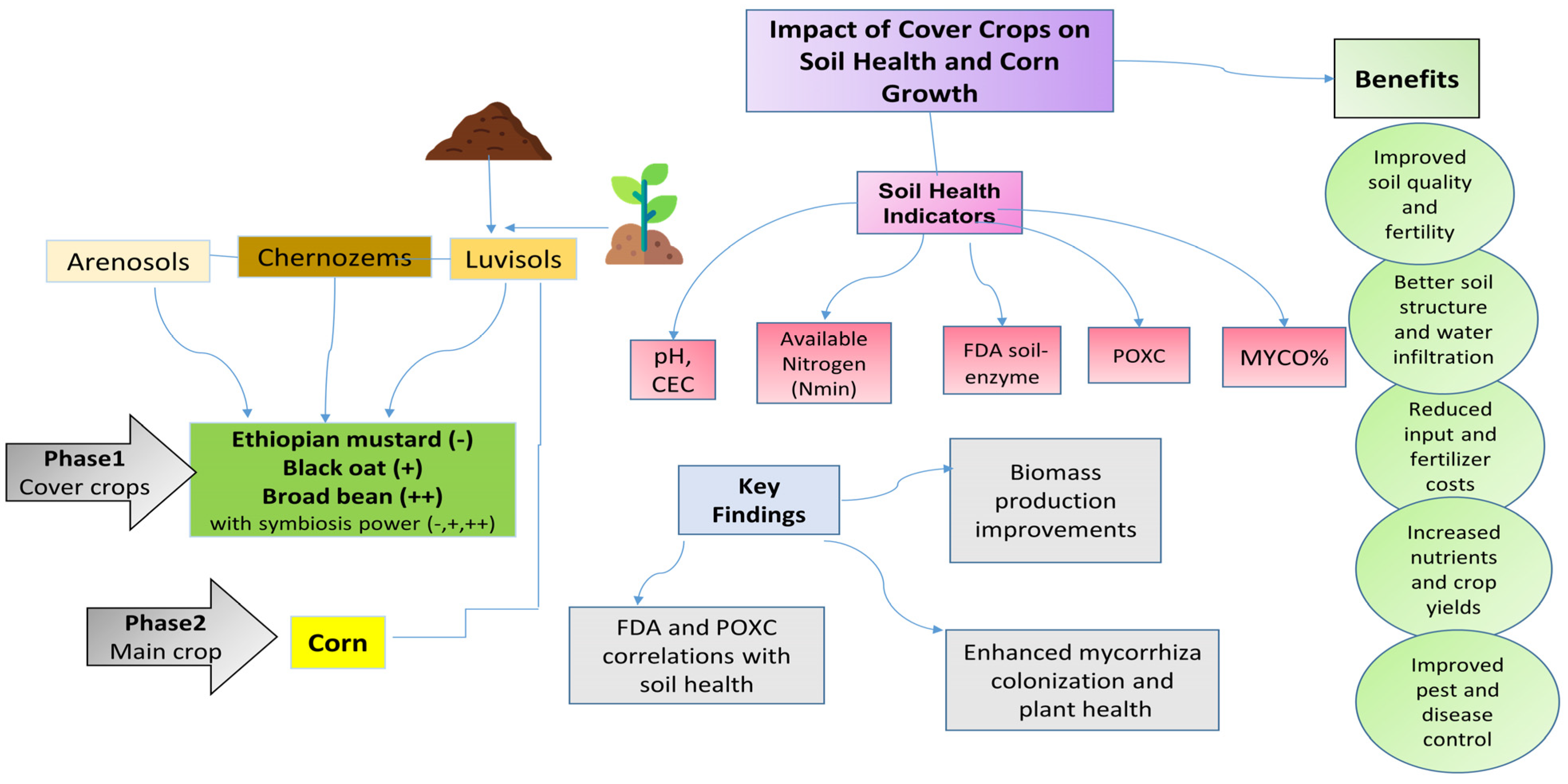
3. Results
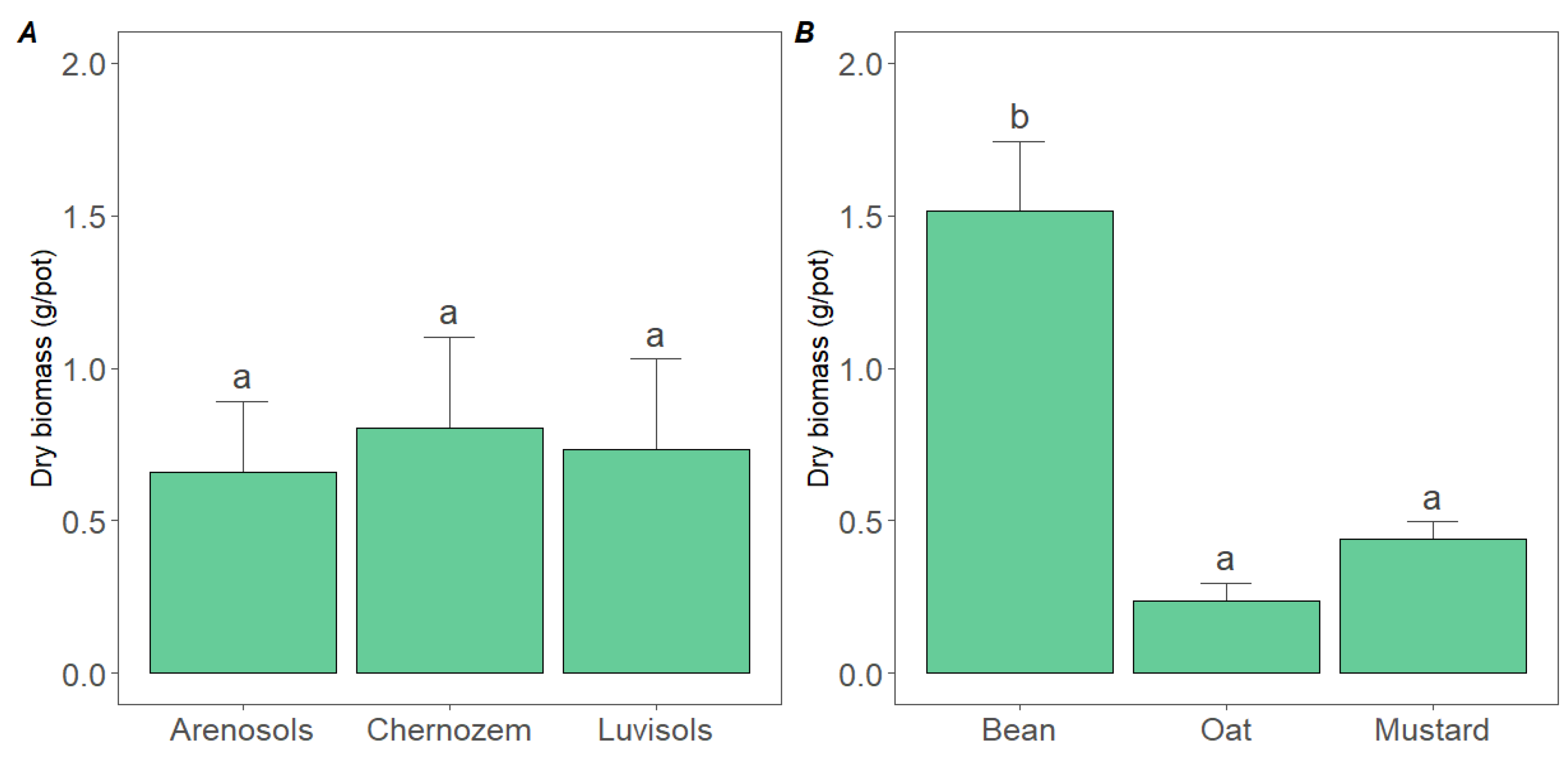
3.1. Growth Capacity of Cover Crops in the Used Soils
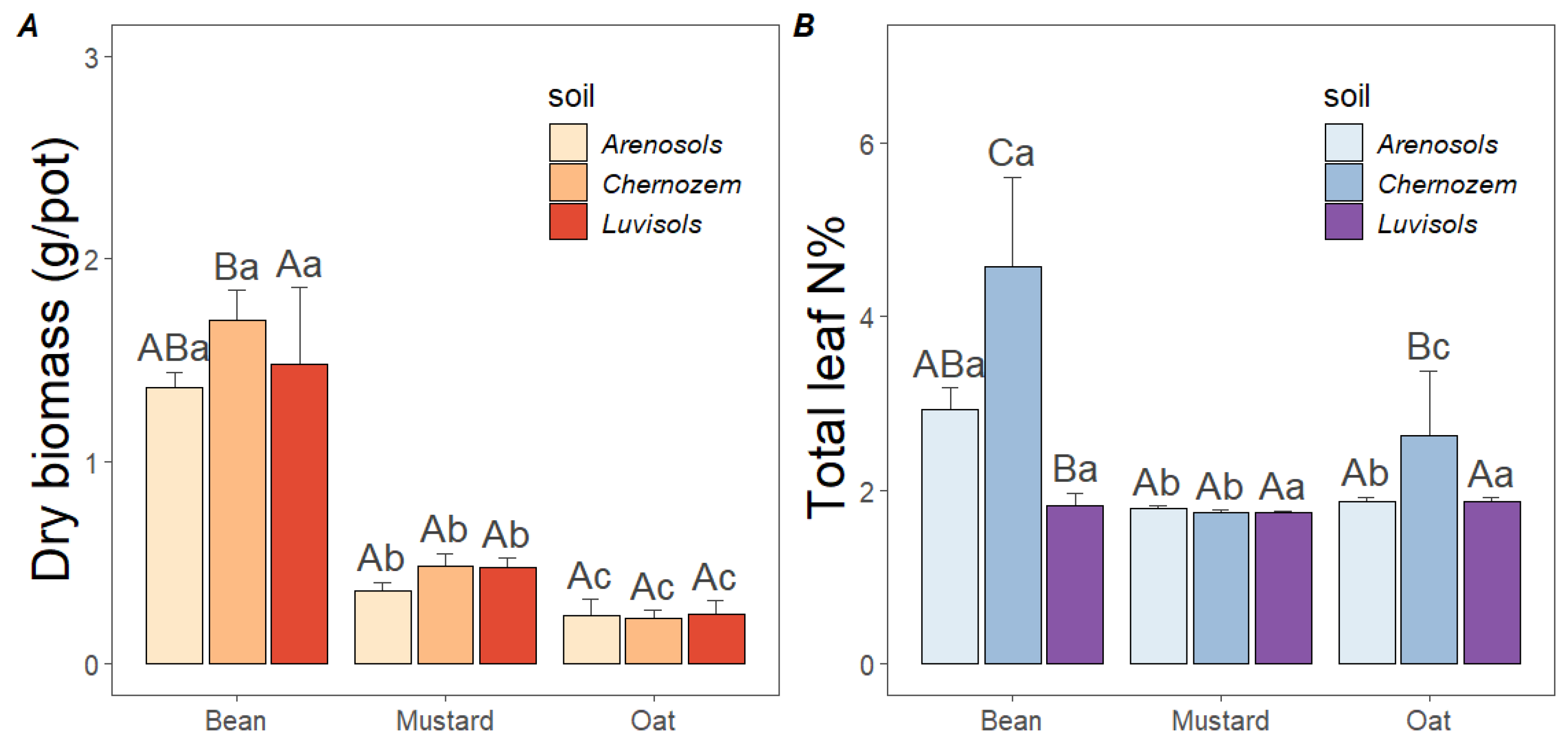
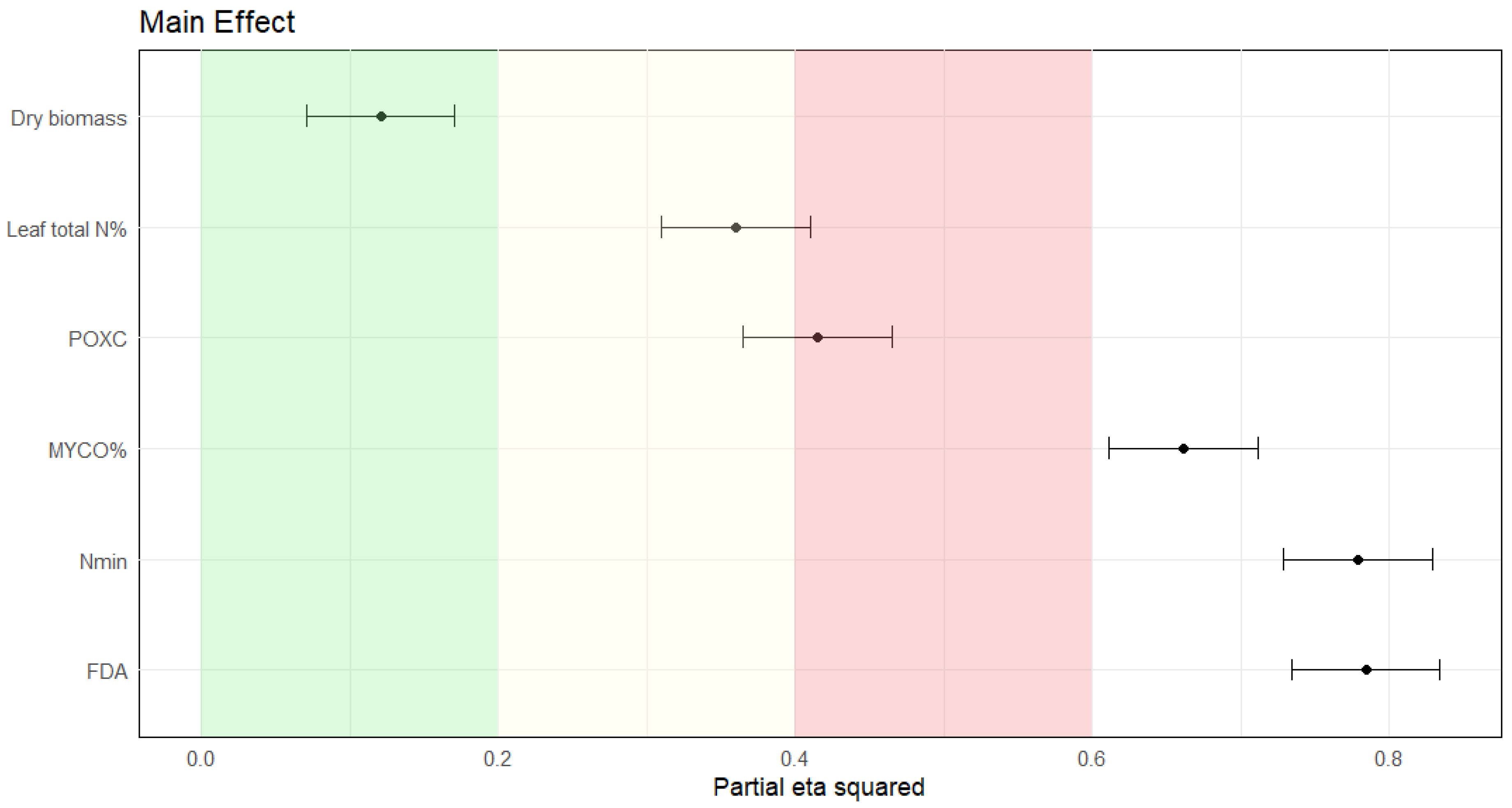
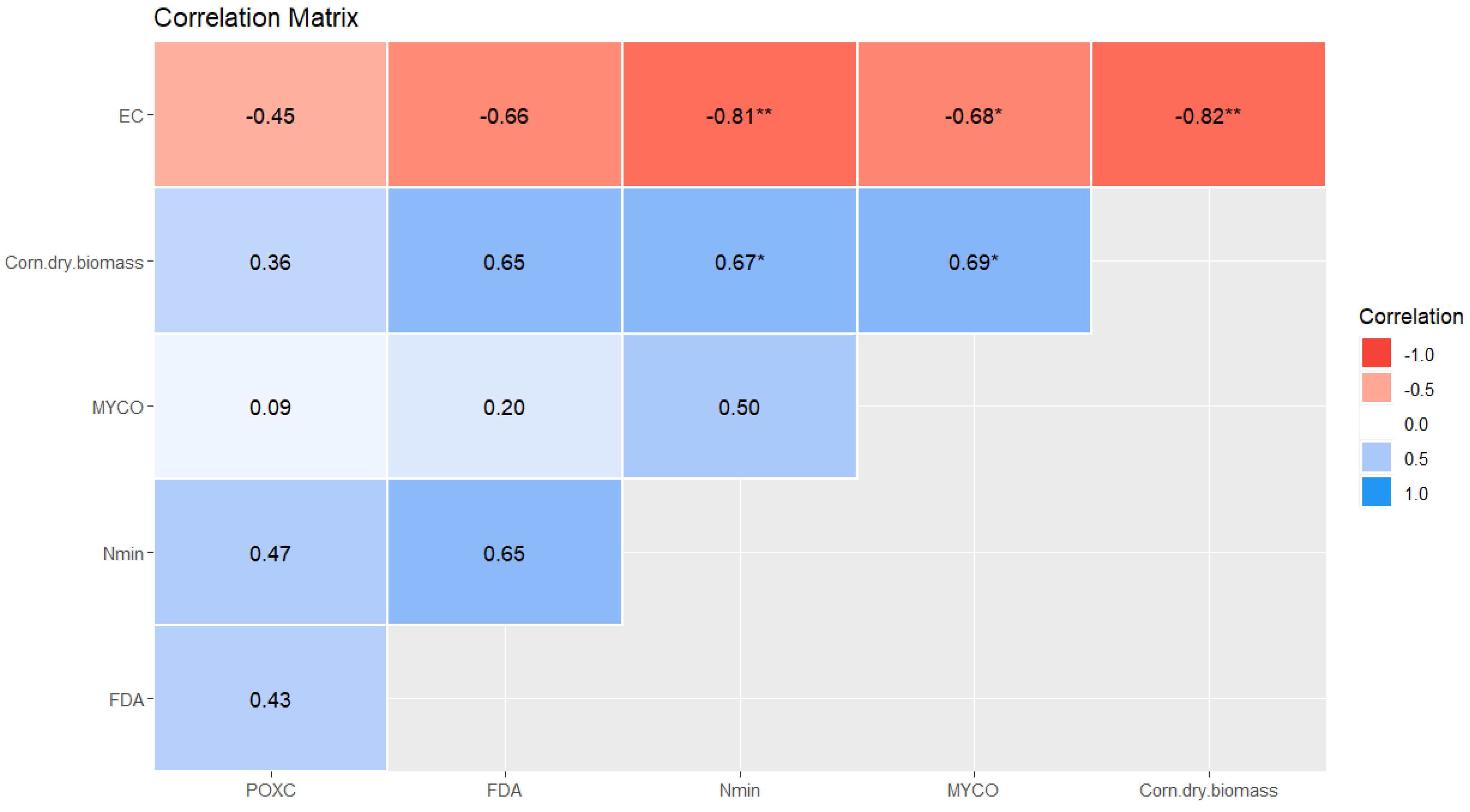
3.2. Potentials of Soil Biological Quality Indicators
3.3. Secondary Effect of Cover Crops on Corn Growth in Luvisols
4. Discussion
4.1. Cover Crop Species, Symbiosis Capacity and the Monitored Soil Characteristics
4.2. Cover Crop Biomass Influencing the Growth of Main Crop
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Bai, Z.; Caspari, T.; Gonzalez, M.R.; Batjes, N.H.; Mäder, P.; Bünemann, E.K.; de Goede, R.; Brussaard, L.; Xu, M.; Ferreira, C.S.S.; et al. Effects of agricultural management practices on soil quality: A review of long-term experiments for Europe and China. Agric. Ecosyst. Environ. 2018, 265, 1–7. [Google Scholar] [CrossRef]
- FAO. The Future of Food and Agriculture: Trends and Challenges; FAO: Rome, Italy, 2017; Volume 4.
- Fekete, I.; Varga, C.; Kotroczó, Z.; Tóth, J.A.; Várbiró, G. The relation between various detritus inputs and soil enzyme activities in a Central European deciduous forest. Geoderma 2011, 167–168, 15–21. [Google Scholar] [CrossRef]
- Kome, G.K.; Enang, R.K.; Tabi, F.O.; Yerima, B.P.K. Influence of Clay Minerals on Some Soil Fertility Attributes: A Review. Open J. Soil Sci. 2019, 09, 155–188. [Google Scholar] [CrossRef]
- Bergtold, J.S.; Ramsey, S.; Maddy, L.; Williams, J.R. A review of economic considerations for cover crops as a conservation practice. Renew. Agric. Food Syst. 2017, 34, 62–76. [Google Scholar] [CrossRef]
- Drinkwater, L.E.; Wagoner, P.; Sarrantonio, M. Legume-based cropping systems have reduced carbon and nitrogen losses. Nature 1998, 396, 262–265. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1694–1697. [Google Scholar] [CrossRef]
- Chen, M.; Arato, M.; Borghi, L.; Nouri, E.; Reinhardt, D. Beneficial services of arbuscular mycorrhizal fungi–from ecology to application. Front. Plant Sci. 2018, 9, 1270. [Google Scholar] [CrossRef]
- Weil, R.R.; Islam, K.R.; Stine, M.A.; Gruver, J.B.; Samson-Liebig, S.E. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Altern. Agric. 2003, 18, 3–17. [Google Scholar]
- Dabney, S.M.; Delgado, J.A.; Reeves, D.W. Using winter cover crops to improve soil and water quality. Commun. Soil Sci. Plant Anal. 2001, 32, 1221–1250. [Google Scholar] [CrossRef]
- Perrone, S.; Grossman, J.; Liebman, A.; Sooksa-nguan, T.; Gutknecht, J. Nitrogen fixation and productivity of winter annual legume cover crops in Upper Midwest organic cropping systems. Nutr. Cycl. Agroecosystems 2020, 117, 61–76. [Google Scholar] [CrossRef]
- Cumming, G. The New Statistics: Why and How. Psychol. Sci. 2014, 25, 7–29. [Google Scholar] [CrossRef] [PubMed]
- Shirale, A.O.; Kharche, V.K.; Zadode, R.S.; Meena, B.P.; Rajendiran, S. Soil biological properties and carbon dynamics subsequent to organic amendments addition in sodic black soils. Arch. Agron. Soil Sci. 2017, 63, 2023–2034. [Google Scholar] [CrossRef]
- Wang, S.; Srivastava, A.K.; Wu, Q.S.; Fokom, R. The effect of mycorrhizal inoculation on the rhizosphere properties of trifoliate orange (Poncirus trifoliata L. Raf.). Sci. Hortic. 2014, 170, 137–142. [Google Scholar] [CrossRef]
- Emnova, E. Biochemical Processes in Chernozem Soil under Different Fertilization Systems. Chem. J. Mold. 2021, 7, 110–114. [Google Scholar] [CrossRef]
- Barea, J.M.; Azcón-Aguilar, C.; Azcon, R.; Gange, A.C.; Brown, V.K. Interactions between mycorrhizal fungi and rhizosphere microorganisms within the context of sustainable soil-plant systems. In Multitrophic Interactions in Terrestrial Systems; Gange, A.C., Brown, V.K., Eds.; Blackwell Science: Cambridge, UK, 2002; pp. 65–77. [Google Scholar]
- Dudás, A.; Szalai, Z.M.; Vidéki, E.; Wass-Matics, H.; Kocsis, T.; Végvári, G.Y.; Kotroczó, Z.S.; Biró, B. Sporeforming bacillus bioeffectors for healthier fruit quality of tomato in pots and field. Appl. Ecol. Environ. Res. 2017, 15, 1399–1418. [Google Scholar] [CrossRef]
- Nelson, N.O.; Janke, R.R. Phosphorus sources and management in organic production systems. Horttechnology 2007, 17, 442–454. [Google Scholar] [CrossRef]
- Chețan, F.; Chețan, C.; Bogdan, I.; Moraru, P.I.; Pop, A.I.; Rusu, T. Use of Vegetable Residues and Cover Crops in the Cultivation of Maize Grown in Different Tillage Systems. Sustainability 2022, 14, 3609. [Google Scholar] [CrossRef]
- Monteiro, M.I.C.; Ferreira, F.N.; De Oliveira, N.M.M.; Ávila, A.K. Simplified version of the sodium salicylate method for analysis of nitrate in drinking waters. Anal. Chim. Acta 2003, 477, 125–129. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Singh, A.; Schöb, C.; Iannetta, P.P.M. Nitrogen fixation by common beans in crop mixtures is influenced by growth rate of associated species. BMC Plant Biol. 2023, 23, 253. [Google Scholar] [CrossRef]
- Gerke, J. The Central Role of Soil Organic Matter in Soil Fertility and Carbon Storage. Soil Syst. 2022, 6, 33. [Google Scholar] [CrossRef]
- Snapp, S.S.; Swinton, S.M.; Labarta, R.; Mutch, D.; Black, J.R.; Leep, R.; Nyiraneza, J.; O’Neil, K. Evaluating cover crops for benefits, costs and performance within cropping system niches. Agron. J. 2005, 97, 322–332. [Google Scholar] [CrossRef]
- Tawaraya, K. Arbuscular mycorrhizal dependency of different plant species and cultivars. Soil Sci. Plant Nutr. 2003, 49, 655–668. [Google Scholar] [CrossRef]
- Teasdale, J.R. Contribution of Cover Crops to Weed Management in Sustainable Agricultural Systems. J. Prod. Agric. 1996, 9, 475–479. [Google Scholar] [CrossRef]
- Alberti, P.K. Development of Best Management Practices for Production of Ethiopian Mustard (Brassica carinata) in South Dakota. 2017; p. 109. Available online: https://openprairie.sdstate.edu/cgi/viewcontent.cgi?article=3208&context=etd (accessed on 9 September 2024).
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular mycorrhizal fungi as natural biofertilizers: Let’s benefit from past successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.S.; Chase, C.A.; Stall, W.M.; Hutchinson, C.M. Competitiveness of Three Leguminous Cover Crops with Yellow Nutsedge (Cyperus esculentus) and Smooth Pigweed (Amaranthus hybridus). Weed Sci. 2007, 55, 613–618. [Google Scholar] [CrossRef]
- Sainju, U.M.; Senwo, Z.N.; Nyakatawa, E.Z.; Tazisong, I.A.; Reddy, K.C. Soil carbon and nitrogen sequestration as affected by long-term tillage, cropping systems, and nitrogen fertilizer sources. Agric. Ecosyst. Environ. 2008, 127, 234–240. [Google Scholar] [CrossRef]
- Rashid, M.; Hussain, Q.; Hayat, R.; Ahmed, M.; Riaz, M.; Khan, K.S.; Ashraf, M.I.; Alvi, S.; Basit, A.; Khalid, R. Soil carbon and legumes. Chapter 17. In Advances in Legumes for Sustainable Intensification; Academic Press: Cambridge, MA, USA, 2022; pp. 329–344. [Google Scholar] [CrossRef]
- Frankenberger, W.T.; Dick, W.A. Relationships between Enzyme Activities and Microbial Growth and Activity Indices in Soil. Soil Sci. Soc. Am. J. 1983, 47, 945–951. [Google Scholar] [CrossRef]
- Storkey, J.; Döring, T.; Baddeley, J.; Collins, R.; Roderick, S.; Jones, H.; Watson, C. Engineering a plant community to deliver multiple ecosystem services. Ecol. Appl. 2015, 25, 1034–1043. [Google Scholar] [CrossRef]
- Hamido, S.A.; Kpomblekou-A., K. Cover crop and tillage effects on soil enzyme activities following tomato. Soil Tillage Res. 2009, 105, 269–274. [Google Scholar] [CrossRef]
- Paolo, B.; Mazzoncini, M. Changes in weed community composition as influenced by cover crop and management system in continuous corn. Weed Sci. 2017, 49, 491–499. [Google Scholar] [CrossRef]
- Meetei, T.T.; Devi, Y.B.; Jackson, K. Effect of various enzymes on mineralization of soil organic matter. Plant Arch. 2020, 20, 3392–3395. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Nitrogen Analysis of Soil and Plant Tissues. J. AOAC Int. 1980, 63, 770–778. [Google Scholar] [CrossRef]
- Calderón, F.J.; Culman, S.; Six, J.; Franzluebbers, A.J.; Schipanski, M.; Beniston, J.; Grandy, S.; Kong, A.Y.Y. Quantification of Soil Permanganate Oxidizable C (POXC) Using Infrared Spectroscopy. Soil Sci. Soc. Am. J. 2017, 81, 277–288. [Google Scholar] [CrossRef]
- Van Eerd, L.L. Nitrogen dynamics and yields of fresh bean and sweet corn with different cover crops and planting dates. Nutr. Cycl. Agroecosystems 2018, 111, 33–46. [Google Scholar] [CrossRef]
- Cruz-Koizumi, Y.P.; Alayón-Gamboa, J.A.; Morón-Ríos, A.; Castellanos-Albores, J.; Aguilar-Chama, A.; Guevara, R.; Cruz-Koizumi, Y.P.; Alayón-Gamboa, J.A.; Morón-Ríos, A.; Castellanos-Albores, J.; et al. Effects of Organic and Chemical Agriculture Systems on Arbuscular Mycorrhizal Fungi and Green Tomato Production in Calakmul, Mexico. Agric. Sci. 2018, 9, 1145–1167. [Google Scholar] [CrossRef][Green Version]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef]
- Farzaneh, M.; Wichmann, S.; Gimplinger, D.M.; Kaul, H.P. Effects of mycorrhiza and nitrogen fertilizer on the biomass production of chickpea. Ital. J. Agron. 2008, (Suppl. S3), 523–524. [Google Scholar]
- Murphy-Bokern, D.; Stoddard, F.L.; Watson, C.A. Legumes in Cropping Systems; Murphy-Bokern, D., Stoddard, F.L., Watson, C.A., Eds.; CAB International: Wallingford, UK, 2017; pp. 1–256. [Google Scholar] [CrossRef]
- da Luz, J.H.S.; da Silva, M.B.; Barbosa, L.d.N.S.; de Souza, J.W.G.; Farias, M.R.d.S.; dos Santos, J.K.; De Gois, M.G.J.L.; Paulino, S.S.; Silva, R.B.; Silva, D.M.R.; et al. Short-Term Agronomic and Economic Responses to the Adoption of Cover Crops for Corn Rotation in the Brazilian Semiarid Region. Sustainability 2023, 15, 15091. [Google Scholar] [CrossRef]
- Cassman, K.G.; Dobermann, A.; Walters, D.T.; Yang, H. Meeting cereal demand while protecting natural resources and improving environmental quality. Annu. Rev. Environ. Resour. 2003, 28, 315–358. [Google Scholar] [CrossRef]
- Farzaneh, M.; Vierheilig, H.; Lössll, A.; Kaull, H.P. Arbuscular mycorrhiza enhances nutrient uptake in chickpea. Plant Soil Environ. 2011, 57, 465–470. [Google Scholar] [CrossRef]
- Augé, R.M. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar] [CrossRef]
- Johansen, A.; Finlay, R.D.; Olsson, P.A. Nitrogen metabolism of external hyphae of the arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 1996, 133, 705–712. [Google Scholar] [CrossRef]
- Lekberg, Y.; Rosendahl, S.; Olsson, P.A. The fungal perspective of arbuscular mycorrhizal colonization in “nonmycorrhizal” plants. New Phytol. 2015, 205, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Fekete, I.; Béni, Á.; Juhos, K.; Kotroczó, Z. The effects of a twenty-year litter manipulation experiment on the carbon content and water retention capacity of the examined Luvisols: Síkfőkút DIRT Project. Agrokémia és Talajt. 2022, 71, 239–253. (In Hungarian) [Google Scholar] [CrossRef]
- Gosling, P.; Hodge, A.; Goodlass, G.; Bending, G.D. Arbuscular mycorrhizal fungi and organic farming. Agric. Ecosyst. Environ. 2006, 113, 17–35. [Google Scholar] [CrossRef]
- Bogati, K.; Walczak, M. The Impact of Drought Stress on Soil Microbial Community, Enzyme Activities and Plants. Agronomy 2022, 12, 189. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158-IN18. [Google Scholar] [CrossRef]
- Kremen, C.; Iles, A.; Bacon, C. Diversified farming systems: An agroecological, systems-based alternative to modern industrial agriculture. Ecol. Soc. 2012, 17, 44. [Google Scholar] [CrossRef]
- Biró, B.; Köves-Péchy, K.; Vörös, I.; Takács, T.; Eggenberger, P.; Strasser, R.J. Interrelations between Azospirillum and Rhizobium nitrogen-fixers and arbuscular mycorrhizal fungi in the rhizosphere of alfalfa in sterile, AMF-free or normal soil conditions. Appl. Soil Ecol. 2000, 15, 159–168. [Google Scholar] [CrossRef]
- Thirkell, T.J.; Charters, M.D.; Elliott, A.J.; Sait, S.M.; Field, K.J. Are mycorrhizal fungi our sustainable saviours? Considerations for achieving food security. J. Ecol. 2017, 105, 921–929. [Google Scholar] [CrossRef]
- Abdalla, M.; Hastings, A.; Cheng, K.; Yue, Q.; Chadwick, D.; Espenberg, M.; Truu, J.; Rees, R.M.; Smith, P. A critical review of the impacts of cover crops on nitrogen leaching, net greenhouse gas balance and crop productivity. Glob. Chang. Biol. 2019, 25, 2530–2543. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Lutfalla, S.; Riley, W.J.; Torn, M.S.; Schmidt, M.W.I.; Soussana, J.F. The changing faces of soil organic matter research. Eur. J. Soil Sci. 2018, 69, 23–30. [Google Scholar] [CrossRef]
- Johnson, N.C.; Graham, J.H.; Smith, F.A. Functioning of mycorrhizal associations along the mutualism–parasitism continuum*. New Phytol. 1997, 135, 575–585. [Google Scholar] [CrossRef]
- Quintarelli, V.; Radicetti, E.; Allevato, E.; Stazi, S.R.; Haider, G.; Abideen, Z.; Bibi, S.; Jamal, A.; Mancinelli, R. Cover Crops for Sustainable Cropping Systems: A Review. Agriculture 2022, 12, 2076. [Google Scholar] [CrossRef]
- Kovács, F.; Papdi, E.; Veres, A.; Mohai, P.; Szegő, A.; Juhos, K. More efficient nitrogen utilization through wool pellet and soil inoculation. Agrosyst. Geosci. Environ. 2024, 7, e20534. [Google Scholar] [CrossRef]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 4th ed.; Allyn and Bacon: Boston, MA, USA, 2001. [Google Scholar]
- Barea, J.M.; Azcón, R.; Azcón-Aguilar, C. Mycorrhizosphere interactions to improve plant fitness and soil quality. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2002, 81, 343–351. [Google Scholar] [CrossRef]
- Villányi, I.; Füzy, A.; Biró, B. Non-target microorganisms affected in the rhizosphere of the transgenic Bt corn. Cereal Res. Commun. 2006, 34, 105–108. [Google Scholar]
- Bowman, M.; Poley, K.; McFarland, E. Farmers employ diverse cover crop management strategies to meet soil health goals. Agric. Environ. Lett. 2022, 7, e20070. [Google Scholar] [CrossRef]
- Biró, B.; Domonkos, M.; Kiss, E. Catabolic FDA microbial activity as site-dependent monitoring tool in soils of an industrial town. Int. Rev. Appl. Sci. Eng. 2012, 3, 41–46. [Google Scholar] [CrossRef]
- Sharma, P.; Leigh, L.; Chang, J.; Maimaitijiang, M.; Caffé, M. Above-Ground Biomass Estimation in Oats Using UAV Remote Sensing and Machine Learning. Sensors 2022, 22, 601. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Xiong, S.; You, C.; Zhang, L.; Li, Y.; Hong, Z.; Hu, Y.; Li, J.; Li, H.; Wang, L.; et al. Soil Microbial Biomass and Community Composition across a Chronosequence of Chinese Cedar Plantations. Forests 2023, 14, 470. [Google Scholar] [CrossRef]
- Vivas, A.; Biró, B.; Ruíz-Lozano, J.M.; Barea, J.M.; Azcón, R. Two bacterial strains isolated from a Zn-polluted soil enhance plant growth and mycorrhizal efficiency under Zn-toxicity. Chemosphere 2006, 62, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Kladivko, E. Cover Crops for Soil Nitrogen Cycling. Midwest Cover Crops Council. 2016. Available online: https://www.midwestcovercrops.org/2016/08/26/cover-crops-nitrogen-management/ (accessed on 30 March 2024).


| Soil Types | Texture | pH | SOM (%) | Bulk Density (g/cm) | Water-Holding Capacity (%) | Replication First/Second Experiment |
|---|---|---|---|---|---|---|
| Arenosols | Sand | 7.46 | 1.16 | 1.65 | 15 | 4/- |
| Chernozems | Loam | 7.35 | 2.81 | 1.35 | 40 | 4/- |
| Luvisols | Clay loam | 4.91 | 1.64 | 1.25 | 50 | 4/8 |
| Cover Crops | Soil Parameters | Type of Soils | Tukey’s Post Hoc Test |
|---|---|---|---|
| Broad Bean (Vicia faba) | FDA | Arenosol | 10.68 ± 1.10 ab |
| Chernozem | 12.29 ± 1 2.2 b | ||
| Luvisol | 8.87 ± 8.87 a | ||
| EC | Arenosol | 86.90 ± 6.15 a | |
| Chernozem | 122.25 ± 17.34 ab | ||
| Luvisol | 202.00 ± 82.51 b | ||
| POXC | Arenosol | 461.11 ± 107.29 b | |
| Chernozem | 187.16 ± 72.00 a | ||
| Luvisol | 90.72 ± 35.71 a | ||
| Ethiopian Mustard (Brassica carinata) | FDA | Arenosol | 6.79 ± 2.36 a |
| Chernozem | 10.64 ± 3.89 a | ||
| Luvisol | 5.84 ± 1.61 a | ||
| EC | Arenosol | 78.82 ± 7.33 ab | |
| Chernozem | 85.40 ± 6.69 b | ||
| Luvisol | 66.00 ± 10.62 a | ||
| POXC | Arenosol | 76.03 ± 56.69 a | |
| Chernozem | 373.28 ± 34.92 b | ||
| Luvisol | 254.73 ± 31.74 c | ||
| Black Oat (Avena strigosa) | FDA | Arenosol | 8.34 ± 2.56 a |
| Chernozem | 16.27 ± 3.79 b | ||
| Luvisol | 5.12 ± 0.28 a | ||
| EC | Arenosol | 70.75 ± 4.69 a | |
| Chernozem | 103.25 ± 8.14 b | ||
| Luvisol | 111.25 ± 23.22 b | ||
| POXC | Arenosol | 62.47 ± 31.62 a | |
| Chernozem | 459.27 ± 94.99 b | ||
| Luvisol | 52.03 ± 32.92 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabalan, S.; Kovács, F.; Papdi, E.; Tóth, E.; Juhos, K.; Biró, B. Residues of Symbiont Cover Crops Improving Corn Growth and Soil-Dependent Health Parameters. Agriculture 2024, 14, 1601. https://doi.org/10.3390/agriculture14091601
Kabalan S, Kovács F, Papdi E, Tóth E, Juhos K, Biró B. Residues of Symbiont Cover Crops Improving Corn Growth and Soil-Dependent Health Parameters. Agriculture. 2024; 14(9):1601. https://doi.org/10.3390/agriculture14091601
Chicago/Turabian StyleKabalan, Sundoss, Flórián Kovács, Enikő Papdi, Eszter Tóth, Katalin Juhos, and Borbála Biró. 2024. "Residues of Symbiont Cover Crops Improving Corn Growth and Soil-Dependent Health Parameters" Agriculture 14, no. 9: 1601. https://doi.org/10.3390/agriculture14091601
APA StyleKabalan, S., Kovács, F., Papdi, E., Tóth, E., Juhos, K., & Biró, B. (2024). Residues of Symbiont Cover Crops Improving Corn Growth and Soil-Dependent Health Parameters. Agriculture, 14(9), 1601. https://doi.org/10.3390/agriculture14091601






