Effects of Dietary Inclusion of Saccharina latissima and Ulva lactuca on Growth Performance and Gut Health in Growing Rabbits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Trial
2.2. Performance Parameters
2.3. Sample Collection
2.4. Histopathological Study and Morphometric Measures
2.5. Coccidia Counts
2.6. E. coli Counts, EPEC and Antimicrobial Susceptibility Testing
2.7. Determination of Clostridium Perfringens and Clostridioides Difficile by qPCR
2.8. Microbiome Analysis
2.9. Crude Mucin Determination
2.10. Quantitative PCR Analysis of Gene Expression
2.11. Statistical Analysis
3. Results
3.1. Growth Performance and Mortality
3.2. Effects of Macroalgae on Gut Health Parameters
3.2.1. Populations of Selected Bacterial Species
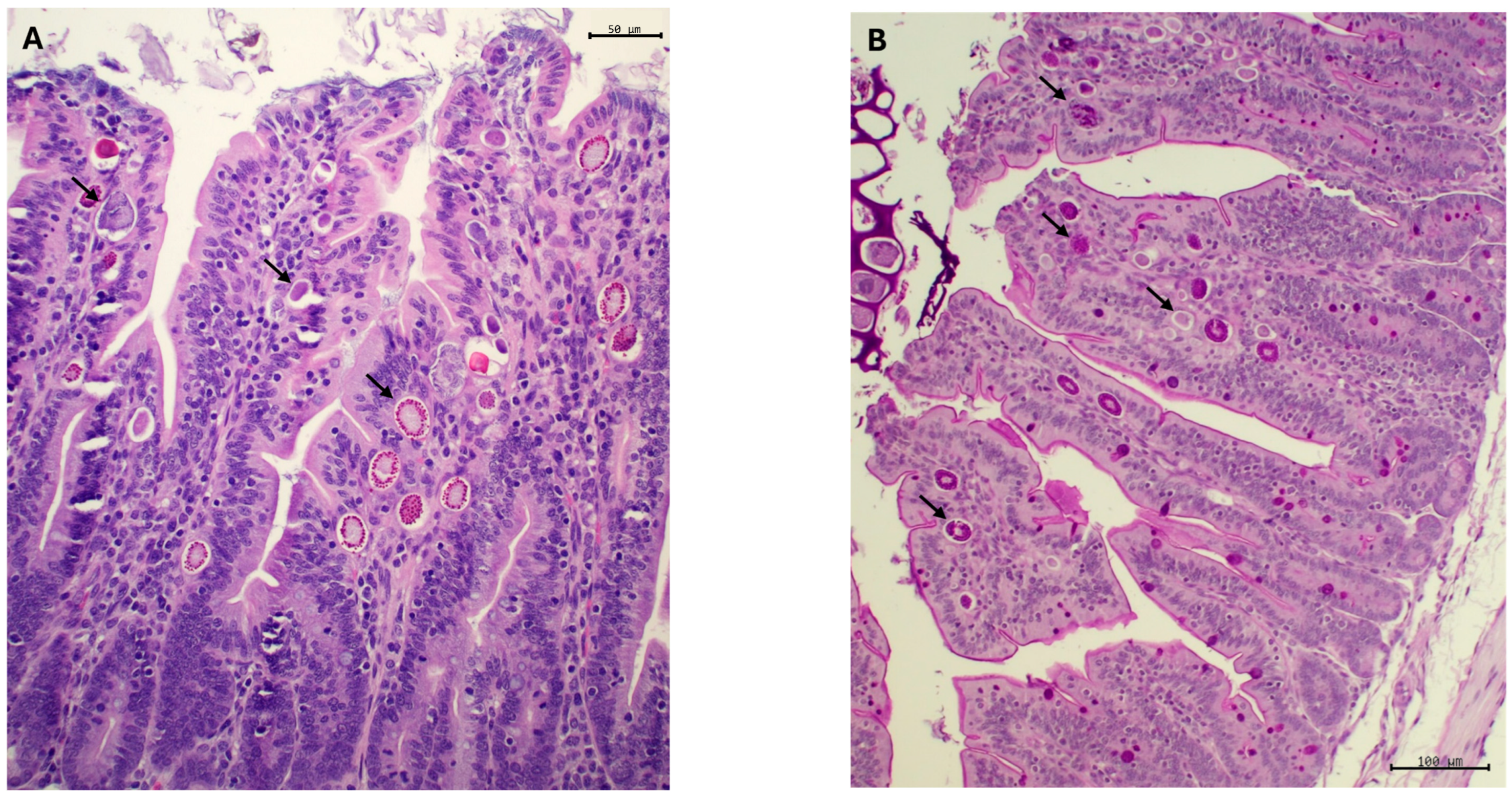
3.2.2. Coccidia
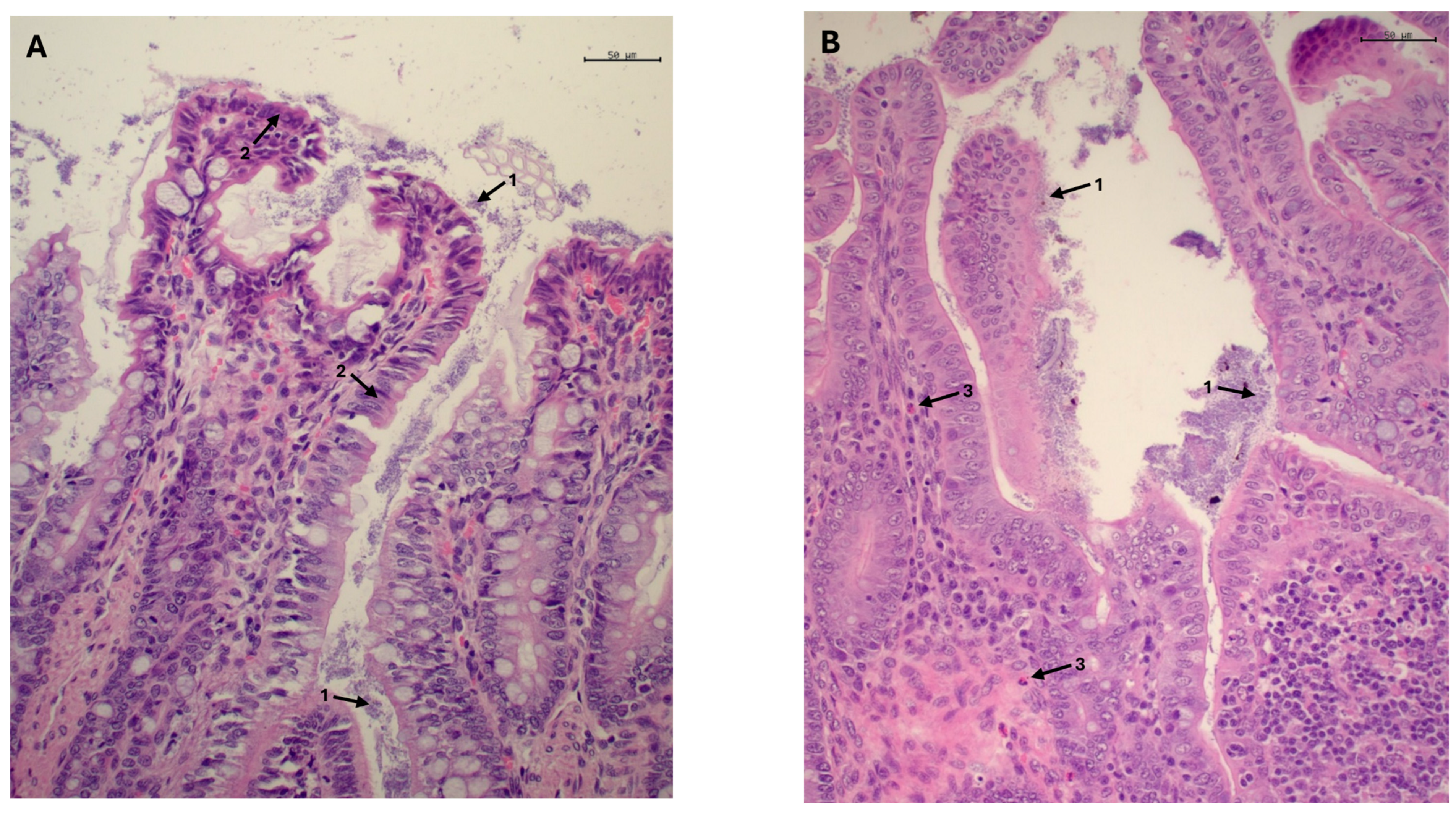
3.2.3. Histological Examination, Morphometric Study and Crude Mucin Content in Ileum
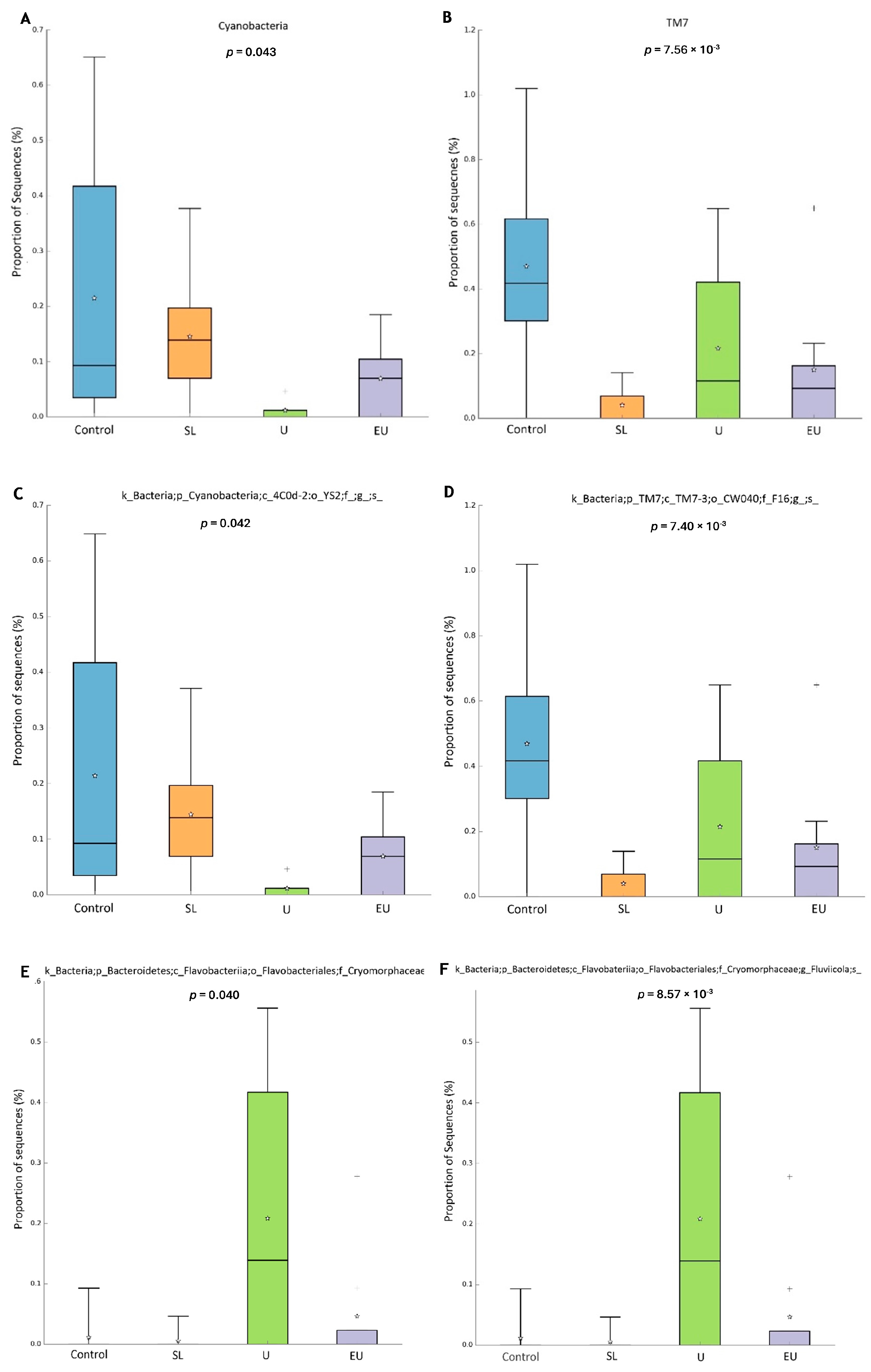
3.2.4. Microbiome
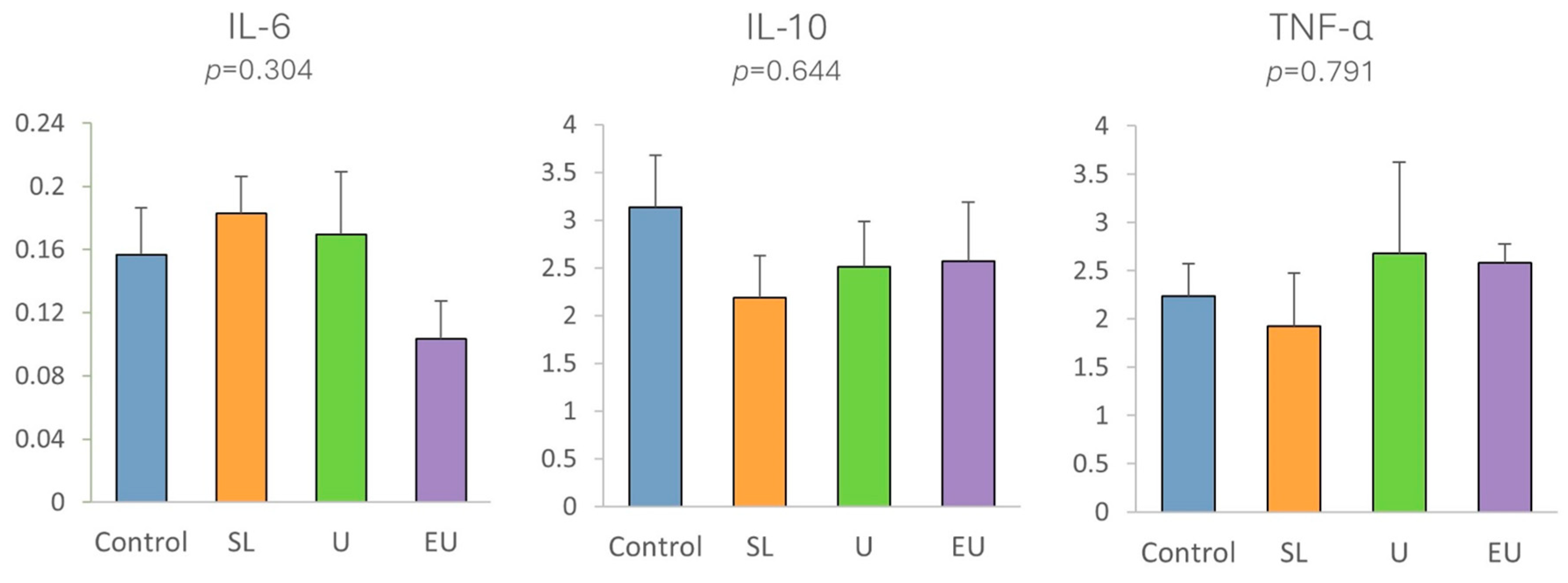
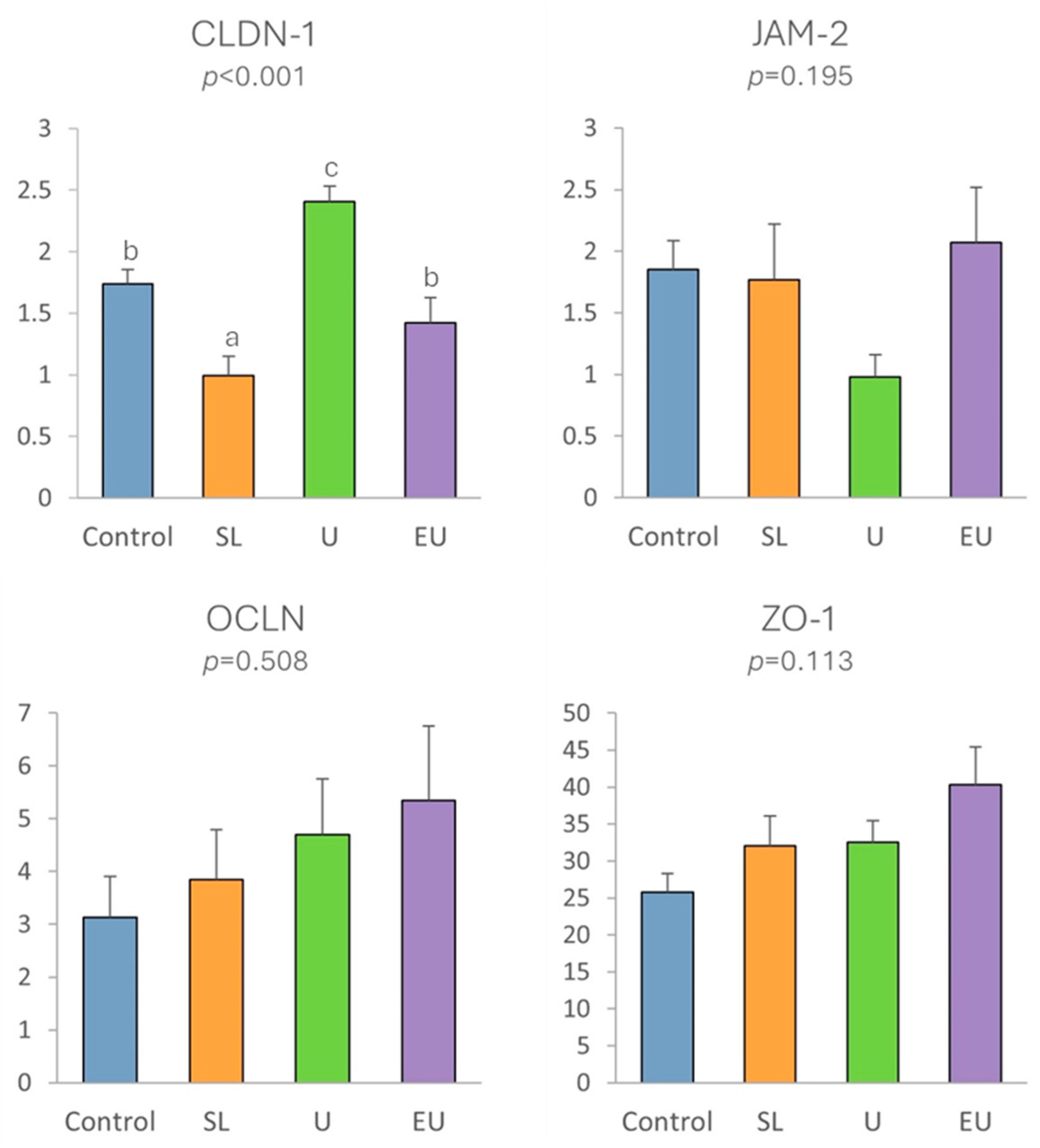
3.2.5. Immune-Related and Tight Junction Protein Gene Expression in Cecal Appendix
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solans, L.; Arnal, J.L.; Sanz, C.; Benito, A.; Chacón, G.; Alzuguren, O.; Fernández, A.B. Rabbit Enteropathies on Commercial Farms in the Iberian Peninsula: Etiological Agents Identified in 2018–2019. Animals 2019, 9, 1142. [Google Scholar] [CrossRef] [PubMed]
- El-Ashram, S.; Aboelhadid, S.M.; Abdel-Kafy, E.S.M.; Hashem, S.A.; Mahrous, L.N.; Farghly, E.M.; Kamel, A.A. Investigation of Pre- and Post-Weaning Mortalities in Rabbits Bred in Egypt, with Reference to Parasitic and Bacterial Causes. Animals 2020, 10, 537. [Google Scholar] [CrossRef] [PubMed]
- European Union. Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on Additives for Use in Animal Nutrition. Off. J. Eur. Union 2003, L268, 29–43. [Google Scholar]
- Evans, F.D.; Critchley, A.T. Seaweeds for Animal Production Use. J. Appl. Phycol. 2014, 26, 891–899. [Google Scholar] [CrossRef]
- Sweeney, T.; O’Doherty, J.V. Marine Macroalgal Extracts to Maintain Gut Homeostasis in the Weaning Piglet. Domest. Anim. Endocrinol. 2016, 56, S84–S89. [Google Scholar] [CrossRef]
- McDonnell, P.; Figat, S.; Odoherty, J.V. The Effect of Dietary Laminarin and Fucoidan in the Diet of the Weanling Piglet on Performance, Selected Faecal Microbial Populations and Volatile Fatty Acid Concentrations. Animal 2010, 4, 579–585. [Google Scholar] [CrossRef]
- Walsh, A.M.; Sweeney, T.; O’Shea, C.J.; Doyle, D.N.; O’Doherty, J.V. Effect of Supplementing Varying Inclusion Levels of Laminarin and Fucoidan on Growth Performance, Digestibility of Diet Components, Selected Faecal Microbial Populations and Volatile Fatty Acid Concentrations in Weaned Pigs. Anim. Feed Sci. Technol. 2013, 183, 151–159. [Google Scholar] [CrossRef]
- El-banna, S.G.; Hassan, A.A.; Okab, A.B.; Koriem, A.A.; Ayoub, M.A. Effect of Feeding Diets Supplemented with Seaweed on Growth Performance and Some Blood Hematological and Biochemical Characteristics of Male Baladi Rabbits. In Proceedings of the 4th International Conference on Rabbit Production in Hot Climates, Sharm El-Shiekh, Egypt, 24–27 February 2005; Volume 382, pp. 373–382. [Google Scholar]
- Rossi, R.; Vizzarri, F.; Chiapparini, S.; Ratti, S.; Casamassima, D.; Palazzo, M.; Corino, C. Effects of Dietary Levels of Brown Seaweeds and Plant Polyphenols on Growth and Meat Quality Parameters in Growing Rabbit. Meat Sci. 2020, 161, 107987. [Google Scholar] [CrossRef] [PubMed]
- Al-Soufi, S.; Nicodemus, N.; Carro, M.D.; López-Alonso, M.; Miranda, M.; Muíños, A.; Cegarra, E.; Vázquez-Belda, B.; Domínguez, H.; Torres, M.D.; et al. Marine Macroalgae in Rabbit Nutrition: In Vitro Digestibility, Caecal Fermentability, and Microbial Inhibitory Activity of Seven Macroalgae Species from Galicia (NW Spain). Agriculture 2023, 13, 1995. [Google Scholar] [CrossRef]
- Alfonzo, R.; Pérez, E.; Al-Soufi, S.; de la Cruz, P.; Silva, V.; Martín, L.; del Pozo, R.; Rybicka, A.; Buján, M.; Domínguez, H.; et al. Efecto de la Inclusión de Productos de Algas Sobre la Digestibilidad Fecal y Otros Parámetros Digestivos en Gazapos en Crecimiento. In Proceedings of the XLVII Symposium de Cunicultura de ASESCU, León, Spain, 31 May–1 June 2023; pp. 77–81. [Google Scholar]
- Hardouin, K.; Bedoux, G.; Burlot, A.S.; Donnay-Moreno, C.; Bergé, J.P.; Nyvall-Collén, P.; Bourgougnon, N. Enzyme-Assisted Extraction (EAE) for the Production of Antiviral and Antioxidant Extracts from the Green Seaweed Ulva Armoricana (Ulvales, Ulvophyceae). Algal Res. 2016, 16, 233–239. [Google Scholar] [CrossRef]
- de Blas, C.; Mateos, G.G. Feed Formulation. In Nutrition of the Rabbit; CABI: Wallingford, UK, 2020. [Google Scholar]
- Ocasio-Vega, C.; Delgado, R.; Abad-Guamán, R.; Carabaño, R.; Carro, M.D.; Menoyo, D.; García, J. The Effect of Cellobiose on the Health Status of Growing Rabbits Depends on the Dietary Level of Soluble Fiber. J. Anim. Sci. 2018, 96, 1806–1817. [Google Scholar] [CrossRef] [PubMed]
- Heim, G.; Walsh, A.M.; Sweeney, T.; Doyle, D.N.; O’Shea, C.J.; Ryan, M.T.; O’Doherty, J.V. Effect of Seaweed-Derived Laminarin and Fucoidan and Zinc Oxide on Gut Morphology, Nutrient Transporters, Nutrient Digestibility, Growth Performance and Selected Microbial Populations in Weaned Pigs. Br. J. Nutr. 2014, 111, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- MAFF. Manual de Técnicas de Laboratorio Parasitolóxico Veterinario; Boletín Técnico N° 18; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 1986; pp. 2–67. [Google Scholar]
- Gómez-Duarte, O.G.; Arzuza, O.; Urbina, D.; Bai, J.; Guerra, J.; Montes, O.; Puello, M.; Mendoza, K.; Castro, G.Y. Detection of Escherichia coli Enteropathogens by Multiplex Polymerase Chain Reaction from Children’s Diarrheal Stools in Two Caribbean-Colombian Cities. Foodborne Pathog. Dis. 2010, 7, 199–206. [Google Scholar] [CrossRef]
- Díaz-Jiménez, D.; García-Meniño, I.; Herrera, A.; Lestón, L.; Mora, A. Microbiological Risk Assessment of Turkey and Chicken Meat for Consumer: Significant Differences Regarding Multidrug Resistance, Mcr or Presence of Hybrid AEPEC/ExPEC Pathotypes of E. coli. Food Control 2021, 123, 107713. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- García, V.; García-Meniño, I.; Gómez, V.; Jiménez-Orellana, M.; Méndez, A.; Aguarón, A.; Roca, E.; Mora, A. Mobile Colistin Resistance (MCR), Extended-Spectrum Beta-Lactamase (ESBL) and Multidrug Resistance Monitoring in Escherichia coli (Commensal and Pathogenic) in Pig Farming: Need of Harmonized Guidelines and Clinical Breakpoints. Front. Microbiol. 2022, 13, 1042612. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.; Shi, Q.; Huang, S.; Yu, T.; Zhang, L.; Yang, H. Multiplex PCR Method for Simultaneous Detection of Five Pathogenic Bacteria Closely Related to Foodborne Diseases. 3 Biotech 2021, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Leterme, P.; Froidmont, E.; Rossi, F.; Théwis, A. The High Water-Holding Capacity of Pea Inner Fibers Affects the Ileal Flow of Endogenous Amino Acids in Pigs. J. Agric. Food Chem. 1998, 46, 1927–1934. [Google Scholar] [CrossRef]
- Romero, C.; Nicodemus, N.; Rodríguez, J.D.; García, A.I.; de Blas, C.D. Effect of Type of Grinding of Barley and Dehydrated Alfalfa on Performance, Digestion, and Crude Mucin Ileal Concentration in Growing Rabbits. J. Anim. Sci. 2011, 89, 2472–2484. [Google Scholar] [CrossRef]
- Abad, R.; Ibáñez, M.A.; Carabaño, R.; García, J. Quantification of Soluble Fibre in Feedstuffs for Rabbits and Evaluation of the Interference between the Determinations of Soluble Fibre and Intestinal Mucin. Anim. Feed Sci. Technol. 2013, 182, 61–70. [Google Scholar] [CrossRef]
- Li, S.; Liu, T.; Wang, K.; Li, C.; Wu, F.; Yang, X.; Zhao, M.; Chen, B.; Chen, X. The Ratios of Dietary Non-Fibrous Carbohydrate (NFC) to Neutral Detergent Fiber (NDF) Influence Intestinal Immunity of Rabbits by Regulating Gut Microbiota Composition and Metabolites. Front. Microbiol. 2023, 14, 1146787. [Google Scholar] [CrossRef] [PubMed]
- Delgado, R.; Menoyo, D.; Abad-Guamán, R.; Nicodemus, N.; Carabaño, R.; García, J. Effect of Dietary Soluble Fibre Level and N-6/n-3 Fatty Acid Ratio on Digestion and Health in Growing Rabbits. Anim. Feed Sci. Technol. 2019, 255, 114222. [Google Scholar] [CrossRef]
- Khalid, A.R.; Yasoob, T.B.; Zhang, Z.; Zhu, X.; Hang, S. Dietary Moringa Oleifera Leaf Powder Improves Jejunal Permeability and Digestive Function by Modulating the Microbiota Composition and Mucosal Immunity in Heat Stressed Rabbits. Environ. Sci. Pollut. Res. 2022, 29, 80952–80967. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical Analysis of Taxonomic and Functional Profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef]
- Heim, G.; Sweeney, T.; O’Shea, C.J.; Doyle, D.N.; O’Doherty, J.V. Effect of Maternal Dietary Supplementation of Laminarin and Fucoidan, Independently or in Combination, on Pig Growth Performance and Aspects of Intestinal Health. Anim. Feed Sci. Technol. 2015, 204, 28–41. [Google Scholar] [CrossRef]
- Walsh, A.M.; Sweeney, T.; O’Shea, C.J.; Doyle, D.N.; O’Doherty, J.V. Effect of Dietary Laminarin and Fucoidan on Selected Microbiota, Intestinal Morphology and Immune Status of the Newly Weaned Pig. Br. J. Nutr. 2013, 110, 1630–1638. [Google Scholar] [CrossRef]
- Coudert, P.; Jobert, J.L.; Larour, G.; Guittet, M. Relation Entre l’ Entéropathie Épizootique Du Lapin (EEL) et l’infestation Par Les Coccidies: Enquête Épidémiologique. In Proceedings of the 10émes Journées de la Recherche Cunicole, Paris, France, 19 November 2003; pp. 239–242. [Google Scholar]
- Fortun-Lamothe, L.; Boullier, S. A Review on the Interactions between Gut Microflora and Digestive Mucosal Immunity. Possible Ways to Improve the Health of Rabbits. Livest. Sci. 2007, 107, 1–18. [Google Scholar] [CrossRef]
- Gidenne, T.; Fortun-Lamothe, L. Feeding Strategy for Young Rabbits around Weaning: A Review of Digestive Capacity and Nutritional Needs. Anim. Sci. 2002, 75, 169–184. [Google Scholar] [CrossRef]
- Milon, A.; Oswald, E.; De Rycke, J. Rabbit EPEC: A Model for the Study of Enteropathogenic Escherichia coli. Vet. Res. 1999, 30, 203–219. [Google Scholar]
- Blanco, J.E.; Blanco, M.; Blanco, J.; Mora, A.; Balaguer, L.; Cuervo, L.; Balsalobre, C.; Muñoa, F. Prevalence and Characteristics of Enteropathogenic Escherichia coli with the Eae Gene in Diarrhoeic Rabbits. Microbiol. Immunol. 1997, 41, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Maxie, M.G. Chapter 1: Alimentary System. In Jubb, Kennedy & Palmer´s Pathology of Domestic Animals; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015; Volume 2, pp. 60–242. [Google Scholar]
- Percy, D.H.; Barthold, S.W. Chapter 6. Rabbit. In Pathology of Laboratory Rodents and Rabbits; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 253–307. [Google Scholar]
- Choi, Y.; Hosseindoust, A.; Goel, A.; Lee, S.; Jha, P.K.; Kwon, I.K.; Chae, B.J. Effects of Ecklonia Cava as Fucoidan-Rich Algae on Growth Performance, Nutrient Digestibility, Intestinal Morphology and Caecal Microflora in Weanling Pigs. Asian-Australas. J. Anim. Sci. 2017, 30, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Luo, S.; Yan, C. Gut Microbiota Implications for Health and Welfare in Farm Animals: A Review. Animals 2022, 12, 93. [Google Scholar] [CrossRef]
- Hu, X.; Wang, F.; Yang, S.; Yuan, X.; Yang, T.; Zhou, Y.; Li, Y. Rabbit Microbiota across the Whole Body Revealed by 16S RRNA Gene Amplicon Sequencing. BMC Microbiol. 2021, 21, 312. [Google Scholar] [CrossRef]
- Combes, S.; Michelland, R.J.; Monteils, V.; Cauquil, L.; Soulié, V.; Tran, N.U.; Gidenne, T.; Fortun-Lamothe, L. Postnatal Development of the Rabbit Caecal Microbiota Composition and Activity. FEMS Microbiol. Ecol. 2011, 77, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Brinig, M.M.; Lepp, P.W.; Ouverney, C.C.; Armitage, G.C.; Relman, D.A. Prevalence of Bacteria of Division TM7 in Human Subgingival Plaque and Their Association with Disease. Appl. Environ. Microbiol. 2003, 69, 1687–1694. [Google Scholar] [CrossRef]
- Hugenholtz, P.; Tyson, G.W.; Webb, R.I.; Wagner, A.M.; Blackall, L.L. Investigation of Candidate Division TM7, a Recently Recognized Major Lineage of the Domain Bacteria, with No Known Pure-Culture Representatives. Appl. Environ. Microbiol. 2001, 67, 411–419. [Google Scholar] [CrossRef]
- Kumar, P.S.; Griffen, A.L.; Barton, J.A.; Paster, B.J.; Moeschberger, M.L.; Leys, E.J. New Bacterial Species Associated with Chronic Periodontitis. J. Dent. Res. 2003, 82, 338–344. [Google Scholar] [CrossRef]
- Ouverney, C.C.; Armitage, G.C.; Relman, D.A. Single-Cell Enumeration of an Uncultivated TM7 Subgroup in the Human Subgingival Crevice. Appl. Environ. Microbiol. 2003, 69, 6294–6298. [Google Scholar] [CrossRef]
- Kuehbacher, T.; Rehman, A.; Lepage, P.; Hellmig, S.; Fölsch, U.R.; Schreiber, S.; Ott, S.J. Intestinal TM7 Bacterial Phylogenies in Active Inflammatory Bowel Disease. J. Med. Microbiol. 2008, 57, 1569–1576. [Google Scholar] [CrossRef]
- Hu, C.; Rzymski, P. Non-Photosynthetic Melainabacteria (Cyanobacteria) in Human Gut: Characteristics and Association with Health. Life 2022, 12, 476. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse Taxa of Cyanobacteria Produce-N-Methylamino-L-Alanine, a Neurotoxic Amino Acid. Proc. Natl. Acad. Sci. USA 2005, 102, 5074–5078. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Sukhchuluun, G.; Bo, T.B.; Chi, Q.S.; Yang, J.J.; Chen, B.; Zhang, L.; Wang, D.H. Huddling Remodels Gut Microbiota to Reduce Energy Requirements in a Small Mammal Species during Cold Exposure. Microbiome 2018, 6, 103. [Google Scholar] [CrossRef]
- Shi, T.T.; Xin, Z.; Hua, L.; Wang, H.; Zhao, R.X.; Yang, Y.L.; Xie, R.R.; Liu, H.Y.; Yang, J.K. Comparative Assessment of Gut Microbial Composition and Function in Patients with Graves’ Disease and Graves’ Orbitopathy. J. Endocrinol. Investig. 2021, 44, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Loviselli, A.; Manzin, A.; et al. Gut Microbiota Markers Associated with Obesity and Overweight in Italian Adults. Sci. Rep. 2021, 11, 5532. [Google Scholar] [CrossRef] [PubMed]
- Chase, C.C.L. Enteric Immunity: Happy Gut, Healthy Animal. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 1–18. [Google Scholar] [CrossRef]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of Tight Junction Permeability by Intestinal Bacteria and Dietary Components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as Nutritional and Functional Food Sources: Revisiting Our Understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, B.; Xue, H.; Liu, C.; Song, W. Blidingia sp. Extracts Improve Intestinal Health and Reduce Diarrhoea in Weanling Piglets. J. Anim. Physiol. Anim. Nutr. 2023, 107, 1198–1205. [Google Scholar] [CrossRef]
| Ingredient | % |
|---|---|
| Alfalfa meal | 15 |
| Wheat bran | 10 |
| Beet pulp | 15 |
| Sunflower meal | 19 |
| Wheat | 17 |
| Palm kernel flour | 4 |
| Oat hulls | 5 |
| Straw | 10 |
| Beet molasses | 4 |
| Corn DDGS | 1 |
| Soybean oil | 0.5 |
| Sodium chloride | 0.3 |
| Vitamin and mineral premix 1 | 0.3 |
| Diclazuril 0.5% | 1 ppm |
| L-Lysine sulphate | 0.21 |
| L-Threonine | 0.084 |
| Chemical Composition | Control | SL | U | EU |
|---|---|---|---|---|
| Moisture | 10.7 | 10.6 | 11.0 | 10.4 |
| Ash | 6.93 | 6.85 | 6.98 | 7.08 |
| Crude protein 1 | 13.9 | 13.8 | 13.9 | 14.1 |
| Neutral detergent fiber | 32.9 | 33.0 | 34.1 | 34.6 |
| Acid detergent fiber | 20.2 | 19.9 | 20.0 | 19.9 |
| Iodine (mg/kg) | 1.12 | 7.41 | 1.20 | 1.14 |
| Gene | Accession Number | Primer Sequences (5′→3′) | Size (bp) | Reference |
|---|---|---|---|---|
| GAPDH | NM_001082253.1 | F: GCCGTGGGTGGAATCATACT R: GCCGTGGGTGGAATCATACT | 165 | [26] |
| IL-6 | DQ680161 | F: GAGCATCCTGGAGACCATCAA R: CCAGTGCCTCCTTTCTGTTCA | 83 | [27] |
| IL-10 | D84217 | F: GAGAACCACAGTCCAGCCAT R: CATGGCTTTGTAGACGCTT | 179 | [27] |
| TNF-α | NM_001082263.1 | F: CTCAGGAGGAAGAGTCCCCAA R: GCTACTACGTGGGCTAGAGG | 115 | [26] |
| CLDN-1 | NM_001089316.1 | F: CGTGCCTTGATGGTGATTGG R: TCCGCATCTTTTGCTCCTCA | 112 | [26] |
| OCLN | XM_017344772.1 | F: TCCGACTTCGTGGAGAGAGT R: AAGCTCATGAACCACCTCGG | 126 | [26] |
| JAM-2 | XM_017346699.1 | F: GGGAGCGGATACCTTTCCTG R: GTATTGCCACCTTCCCACCA | 128 | [28] |
| ZO-1 | XM_017348360.1 | F: AGAGGATTTGTCAGCCCAGC R: TTTCTCTGGCAACATCGGCT | 125 | [26] |
| Control | SL | U | EU | SEM | p-Cov | p-Treat | |
|---|---|---|---|---|---|---|---|
| 33–54 days of age | |||||||
| Average weight 33 d (g) | 925 | 900 | 909 | 886 | 15.7 | --- | 0.37 |
| ADG (g) | 37.1 | 38.6 | 38.4 | 37.7 | 0.98 | 0.27 | 0.69 |
| ADFI (g) | 96.9 | 95.9 | 94.6 | 95.6 | 1.92 | 0.29 | 0.87 |
| FCR (g/g) | 2.62 | 2.48 | 2.46 | 2.55 | 0.056 | 0.89 | 0.17 |
| Mortality (%) | 9.24 | 7.07 | 7.07 | 7.61 | --- | --- | 0.85 |
| Untransformed mortality estimates | −2.28 ±0.25 | −2.58 ±0.29 | −2.58 ±0.29 | −2.50 ±0.28 | --- | --- | 0.85 |
| 54–61 days of age | |||||||
| Average weight 54 d (g) | 1684 | 1715 | 1712 | 1696 | 20.4 | <0.001 | 0.69 |
| ADG (g) | 47.3 | 42.9 | 44.4 | 45.2 | 1.27 | 0.27 | 0.10 |
| Mortality (%) | 1.60 | 1.09 | 1.09 | 0.54 | --- | --- | 0.81 |
| Untransformed mortality estimates | −4.10 ±0.58 | −4.51 ±0.71 | −4.51 ±0.71 | −5.21 ±1.00 | --- | --- | 0.81 |
| 33–61 days of age | |||||||
| Average weight 61 d (g) | 2015 | 2015 | 2023 | 2013 | 20.8 | <0.001 | 0.99 |
| ADG (g) | 39.7 | 39.6 | 39.9 | 39.6 | 0.74 | 0.12 | 0.99 |
| Mortality (%) | 10.9 | 8.20 | 8.20 | 8.20 | --- | --- | 0.74 |
| Untransformed mortality estimates | −2.10 ±0.24 | −2.42 ±0.27 | −2.42 ±0.27 | −2.42 ±0.27 | --- | --- | 0.74 |
| Variable | Control | SL | U | EU | p-Value |
|---|---|---|---|---|---|
| E. coli count (cfu/g) | 50 × 10 6 | 25 × 10 6 | 37 × 10 6 | 50 × 10 6 | 0.75 |
| Untransformed E. coli count | 17.73 ± 0.41 | 17.03 ± 0.58 | 17.44 ± 0.48 | 17.73 ± 0.41 | 0.75 |
| Rabbit, % | |||||
| E. coli growth | 50 | 37.5 | 62.5 | 75 | 0.48 |
| Untransformed E. coli growth | 0.00 ± 0.71 | −0.51 ± 0.73 | 0.51 ± 0.73 | 1.10 ± 0.82 | 0.48 |
| E. coli > 100 cfu/g | 50 | 25 | 50 | 62.5 | 0.53 |
| Untransformed E. coli overgrowth | 0.00 ± 0.71 | −1.10 ± 0.82 | 0.00 ± 0.71 | 0.51 ± 0.73 | 0.53 |
| Enteropathogenic E. coli | 0 | 25 | 0 | 25 | - |
| Multidrug-resistant E. coli | 37.5 | 12.5 | 37.5 | 37.5 | 0.67 |
| Untransformed multi-resistant E. coli | −0.51 ± 0.73 | −1.95 ± 1.07 | −0.51 ± 0.73 | −0.51 ± 0.73 | 0.67 |
| Clostridium perfringens | nd | nd | nd | Nd | - |
| Clostridioides difficile | nd | nd | nd | Nd | - |
| Variable | Control | SL | U | EU | p-Value |
|---|---|---|---|---|---|
| Recount of coccidia, opg | 6756 | 3211 | 9100 | 20,088 | 0.10 |
| Untransformed coccidia count estimates | 8.82 ± 0.57 | 8.07 ± 0.83 | 9.12 ± 0.53 | 9.91 ± 0.33 | 0.10 |
| Variable | Control | SL | U | EU | SEM | p-Value |
|---|---|---|---|---|---|---|
| Villi height | 335 bc | 391 c | 280 b | 195 a | 9.19 | <0.001 |
| Crypt depth | 119 a | 141 b | 106 a | 102 a | 2.19 | <0.001 |
| VH:CD | 2.82 | 2.77 | 2.64 | 1.91 | 0.053 | 0.55 |
| Crude mucin (%) | 8.14 | 9.43 | 8.78 | 7.77 | 0.489 | 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Soufi, S.; Losada, A.P.; López-Alonso, M.; Cardelle-Cobas, A.; Mora, A.; Lamas, A.; Panadero, R.; Miranda, M.; Muíños, A.; Cegarra, E.; et al. Effects of Dietary Inclusion of Saccharina latissima and Ulva lactuca on Growth Performance and Gut Health in Growing Rabbits. Agriculture 2024, 14, 1605. https://doi.org/10.3390/agriculture14091605
Al-Soufi S, Losada AP, López-Alonso M, Cardelle-Cobas A, Mora A, Lamas A, Panadero R, Miranda M, Muíños A, Cegarra E, et al. Effects of Dietary Inclusion of Saccharina latissima and Ulva lactuca on Growth Performance and Gut Health in Growing Rabbits. Agriculture. 2024; 14(9):1605. https://doi.org/10.3390/agriculture14091605
Chicago/Turabian StyleAl-Soufi, Sabela, Ana Paula Losada, Marta López-Alonso, Alejandra Cardelle-Cobas, Azucena Mora, Alexandre Lamas, Rosario Panadero, Marta Miranda, Antonio Muíños, Eugenio Cegarra, and et al. 2024. "Effects of Dietary Inclusion of Saccharina latissima and Ulva lactuca on Growth Performance and Gut Health in Growing Rabbits" Agriculture 14, no. 9: 1605. https://doi.org/10.3390/agriculture14091605
APA StyleAl-Soufi, S., Losada, A. P., López-Alonso, M., Cardelle-Cobas, A., Mora, A., Lamas, A., Panadero, R., Miranda, M., Muíños, A., Cegarra, E., & García, J. (2024). Effects of Dietary Inclusion of Saccharina latissima and Ulva lactuca on Growth Performance and Gut Health in Growing Rabbits. Agriculture, 14(9), 1605. https://doi.org/10.3390/agriculture14091605












