Effects of Nitrogen Addition on Soil Microbial Biomass: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
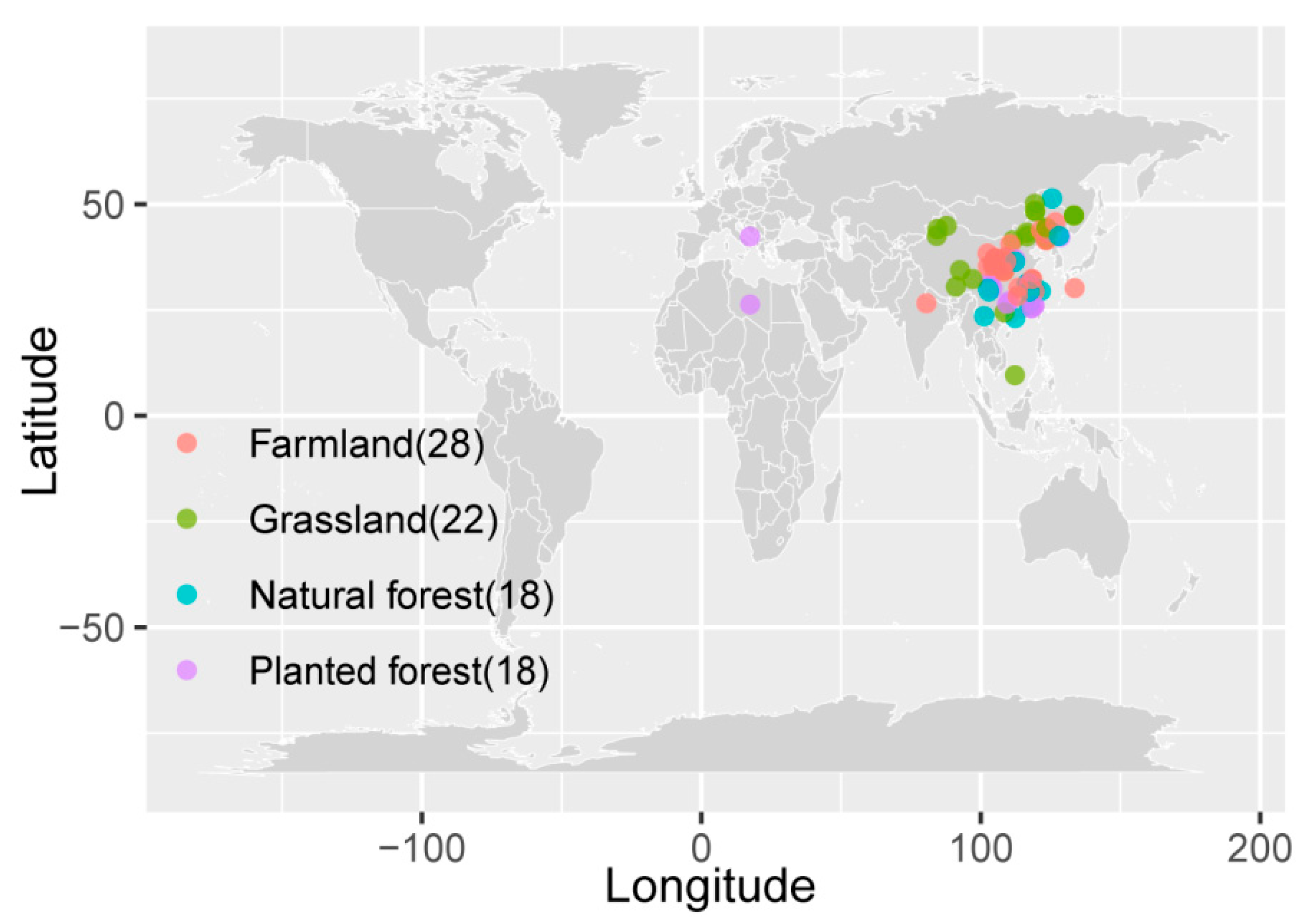
2.1. Literature Search and Screening
2.2. Compilation
2.3. Data Analysis
3. Results
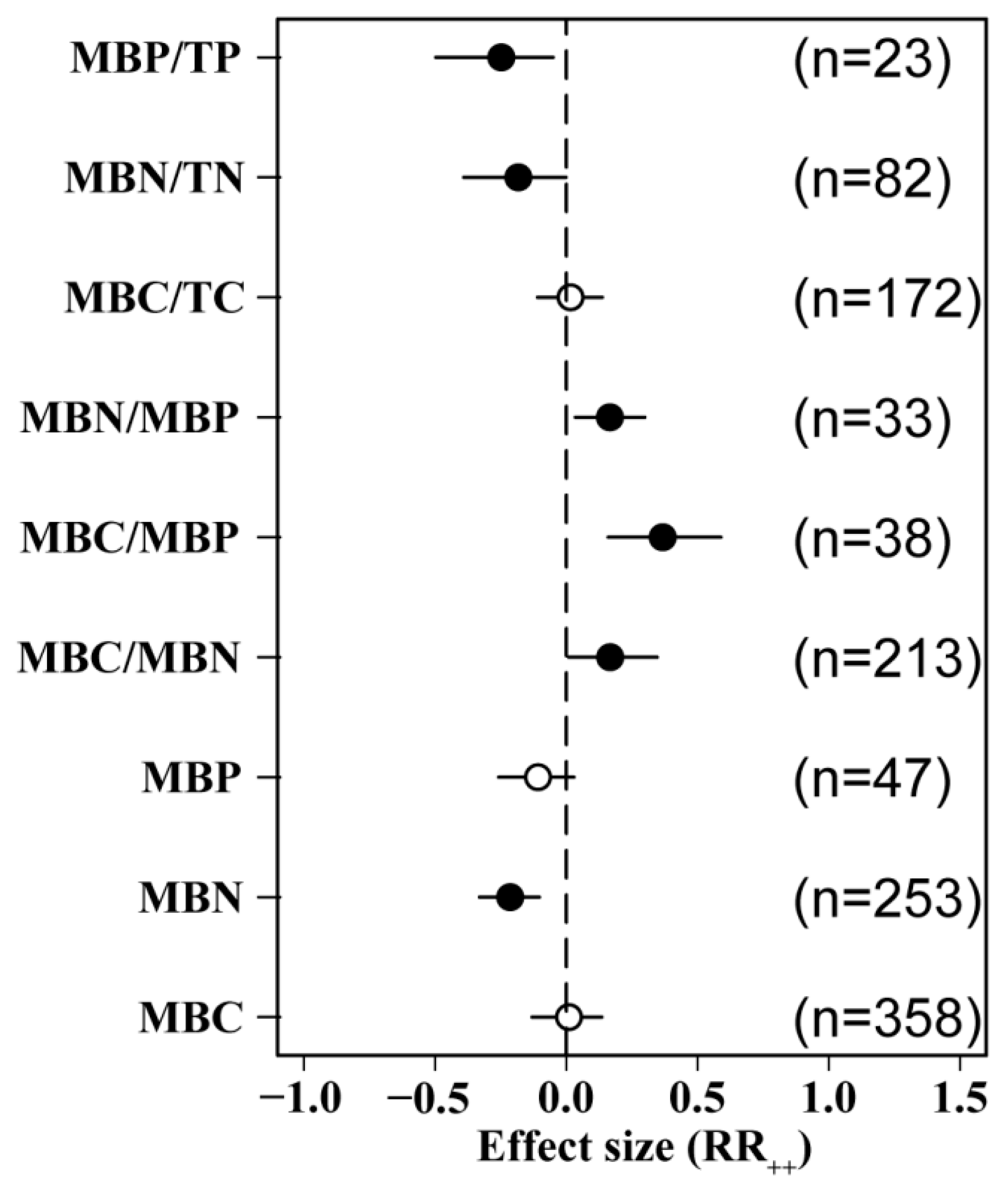
3.1. Effect of N Addition on Soil Microbial Biomass
3.2. Effects of N Addition Rates on Microbial Diversity and Plant Growth
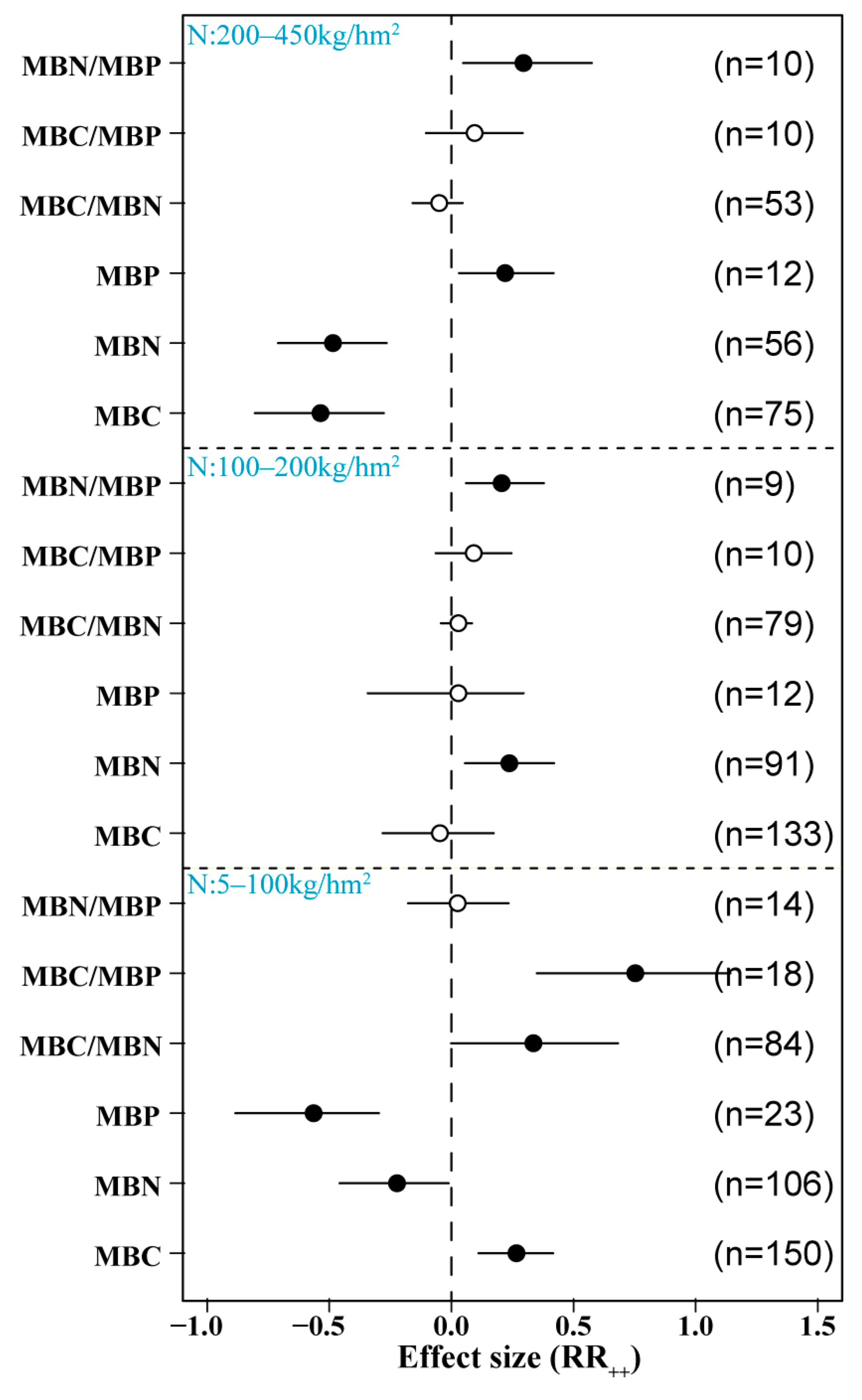
3.3. Effects of Different N Application Rates on Soil Microbial Biomass
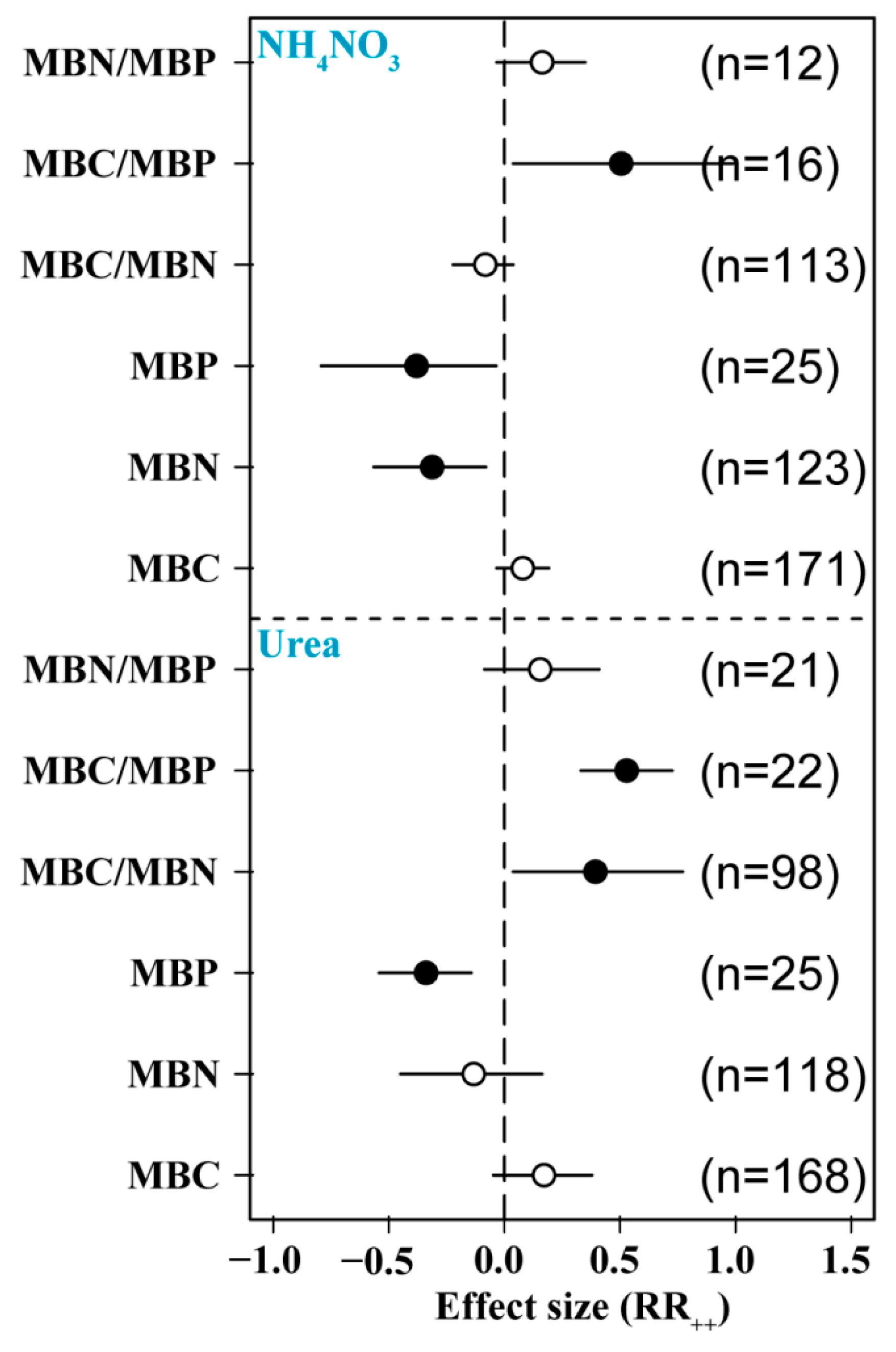
3.4. Effects of N Form on Soil Microbial Biomass
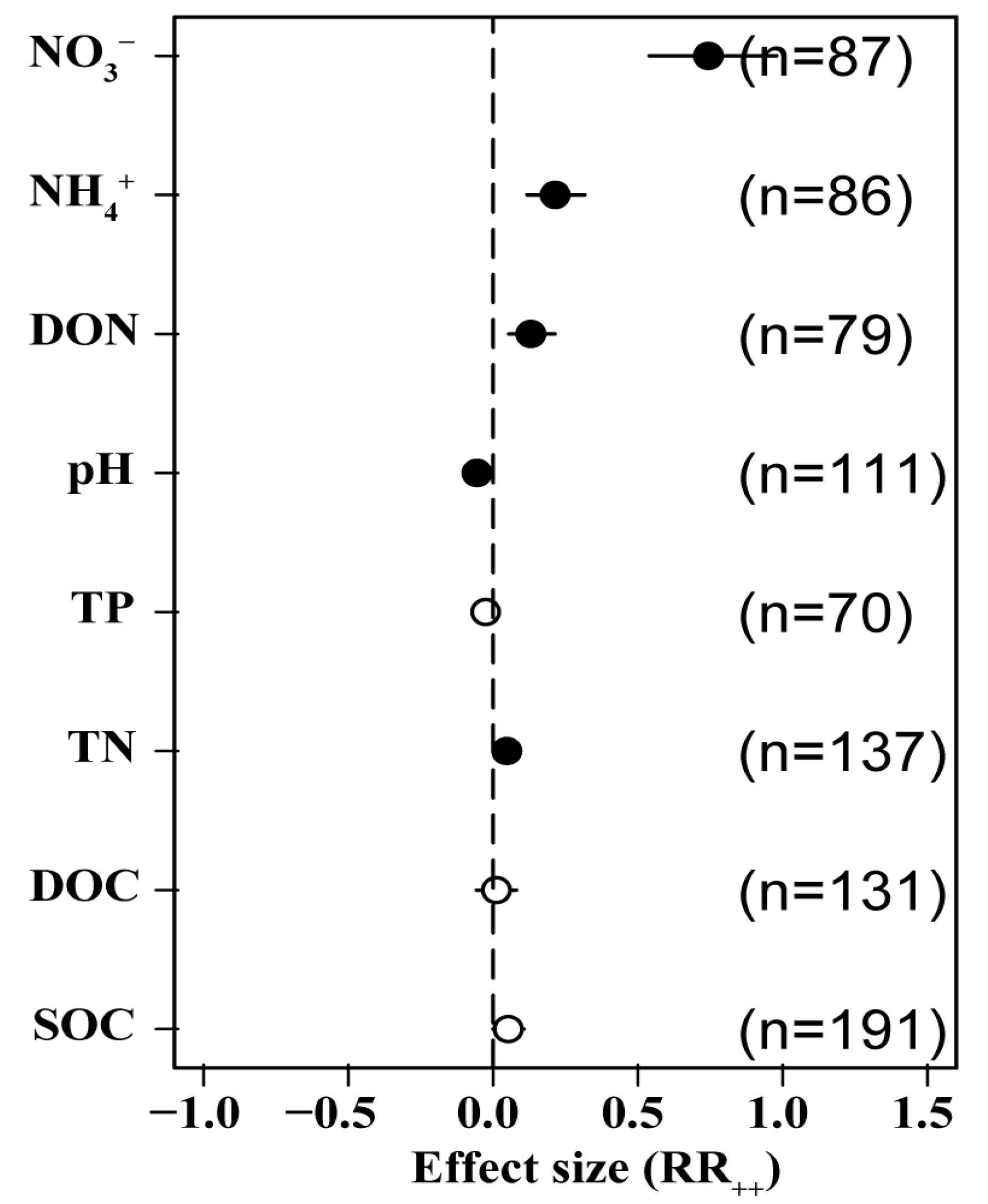
3.5. Effects of N Addition on Soil Chemical Properties
3.6. Effects of N Addition on Microbial Biomass in Different Ecosystems
3.7. Effects of N Addition on Soil Chemical Properties in Different Ecosystems
3.8. N Addition Affecting Soil Microbial Biomass Based on Random Forest Analysis
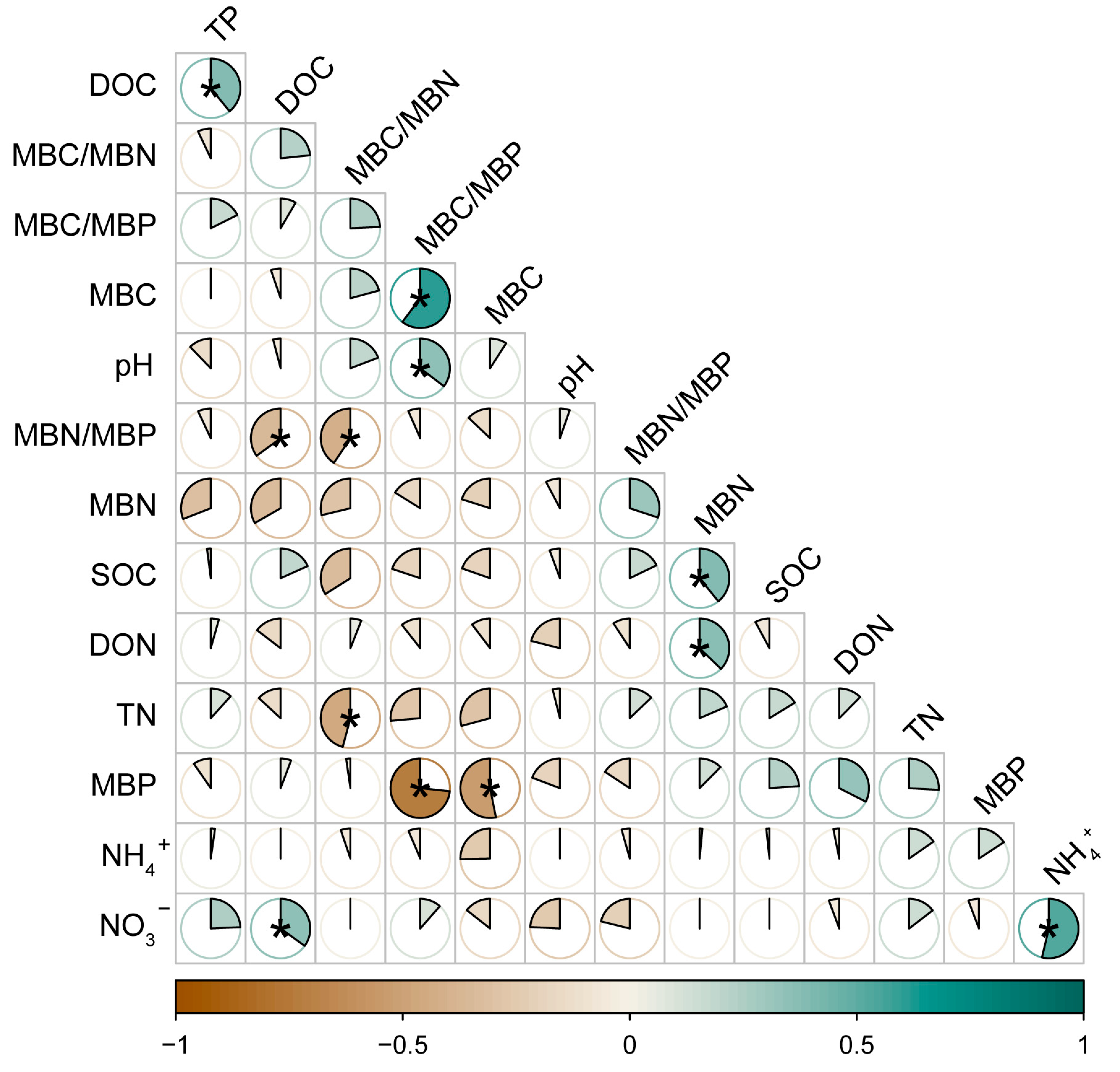
3.9. Correlation Analysis of Soil Physical and Chemical Factors
3.10. Linear Relationship between Environmental Factors and Soil Microbial Biomass
4. Discussion
4.1. Effect of N Addition on Soil Physical and Chemical Properties
4.2. Effects of N Addition on Positive and Negative Directions of Soil MBC and MBN
4.3. Effects of N Addition on MBP and MBC/MBN
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The Evolution and Future of Earth’s Nitrogen Cycle. Science 2010, 330, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Ti, C.; Gao, B.; Luo, Y.; Wang, S.; Chang, S.X.; Yan, X. Dry deposition of N has a major impact on surface water quality in the Taihu Lake region in southeast China. Atmos. Environ. 2018, 190, 1–9. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.J. Research progress on nitrogen deposition and grassland ecosystem response in Qinghai-Tibet Plateau. J. China Agric. Univ. 2018, 23, 151–158. [Google Scholar]
- Deng, S.P.; Moore, J.M.; Tabatabai, M.A. Characterization of active nitrogen pools in soils under different cropping systems. Biol. Fertil. Soils 2000, 32, 302–309. [Google Scholar] [CrossRef]
- Belay-Tedla, A.; Zhou, X.; Su, B.; Wan, S.; Luo, Y. Labile, recalcitrant, and microbial carbon and nitrogen pools of a tall grass prairie soil in the US Great Plains subjected to experimental warming and clipping. Soil Biol. Biochem. 2009, 41, 110–116. [Google Scholar] [CrossRef]
- Zhang, G.S.; Huang, G.B. Soil organic carbon sequestration potential in cropland. Acta Ecol. Sin. 2005, 25, 351–357. [Google Scholar]
- Sun, T.; Wang, Y.; Hui, D.; Jing, X.; Feng, W. Soil properties rather than climate and ecosystem type control the vertical variations of soil organic carbon, microbial carbon, and microbial quotient. Soil Biol. Biochem. 2020, 148, 107905. [Google Scholar] [CrossRef]
- Qualls, R.; Haines, B.; Swank, W.; Tyler, S. Retention of soluble organic nutrients by a forested ecosystem. Biogeochemistry 2002, 61, 135–171. [Google Scholar] [CrossRef]
- Hartmann, M.; Frey, B.; Mayer, J.; Mäder, P.; Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 2015, 9, 1177–1194. [Google Scholar] [CrossRef]
- Dou, X.L.; He, P.; Cheng, X.; Zhou, W. Long-term fertilization alters chemically-separated soil organic carbon pools: Based on stable C isotope analyses. Sci. Rep. 2016, 6, 19061. [Google Scholar] [CrossRef]
- Zhang, T.A.; Chen, H.Y.; Ruan, H. Global negative effects of nitrogen deposition on soil microbes. ISME J. 2018, 12, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.C.; Lin, W.S.; Pu, X.T.; Yang, Z.R.; Zheng, W.; Chen, Y.; Yang, Y. Effects of forest regeneration patterns on the quantity and chemical structure of soil solution dissolved organic carbon matter in subtropical forest. Chin. J. Appl. Ecol. 2016, 27, 1845–1852. [Google Scholar]
- Bargali, K.; Manral, V.; Padalia, K.; Bargali, S.S.; Upadhyay, V.P. Effect of vegetation type and season on microbial biomass carbon in Central Himalayan forest soils, India. Catena 2018, 171, 125–135. [Google Scholar] [CrossRef]
- Liu, Z.J.; Zhou, W.; Shen, J.B.; Li, S.T.; Ai, C. Soil quality assessment of yellow clayey paddy soils with different productivity. Biol. Fertil. Soils 2014, 50, 537–548. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Y.; Jiang, F.; Hu, Y.; Long, L.; Pei, L.; Li, J.; Xu, K. Responses of soil microbial biomass carbon, nitrogen and microbial entropy to different materials returned to corn fields. J. Soil Water Conserv. 2020, 34, 173–180. [Google Scholar]
- Kiboi, M.; Ngetich, K.; Mugendi, D.; Muriuki, A.; Adamtey, N.; Fliessbach, A. Microbial biomass and acid phosphomonoesterase activity in soils of the Central Highlands of Kenya. Geoderma Reg. 2018, 15, e00193. [Google Scholar] [CrossRef]
- Xu, Y.; Zhong, Z.; Zhang, W.; Han, X.; Yang, G.; Ren, C.; Feng, Y.; Ren, G.; Wang, X. Responses of soil nosZ–type denitrifying microbial communities to the various land–use types of the Loess Plateau, China. Soil Tillage Res. 2019, 195, 104378. [Google Scholar] [CrossRef]
- Zheng, J.S.; Hu, J.M.; Wei, X.H.; Wei, Y.; Su, S.; Li, T.; Xia, X.; Yu, Y.; Zhang, J. Effects of green manure returning on soil microbial biomass carbon and mineralization of organic carbon in smash ridging paddy field. Chin. J. Eco-Agric. 2021, 29, 691–703. [Google Scholar]
- Kuzyakov, Y.; Friedel, J.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Lin, M.; Deng, S.; Su, Y.; Liu, K.; Li, F. Effects of fertilization on soil active organic carbon and carbon sequestration of forage in Karst region. Plant Nutr. Fertil. Sci. 2012, 18, 1119–1126. [Google Scholar]
- Allison, S.D.; Lu, Y.; Weihe, C.; Goulden, M.L.; Martiny, A.C.; Treseder, K.K.; Martiny, J.B. Microbial abundanceand composition influence litter decomposition responseto environmental change. Ecology 2013, 94, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Wallenstein, M.D.; McNulty, S.; Fernandez, I.J.; Boggs, J.; Schlesinger, W.H. Nitrogen fertilization decreases forest soil fungal and bacterial biomass in three long-term experiments. For. Ecol. Manag. 2006, 222, 459–468. [Google Scholar] [CrossRef]
- Zhang, T.T.; Chen, S.T.; Wang, J.; Wang, Z.H.; Hu, Z.H. Effects of warming and straw application on soil microbial biomass carbon and nitrogen and bacterial community structure. Environ. Sci. 2019, 40, 4718–4724. [Google Scholar]
- Yan, G.; Yao, Z.; Zheng, X.; Liu, C. Characteristics of annual nitrous and nitri coxide emissions from majorce-realcropsinthe North China Pain under alternative fertilizer management. Agric. Eosc Ystems Environ. 2015, 207, 67–78. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Wu, J.; Huang, D.; Su, Y.; Wei, W. Spatial variability of soil microbial biomass carbon, nitrogen and phosphorus in a hilly red soil landscape in subtropical China. Soil Sci. Plant Nutr. 2010, 56, 693–704. [Google Scholar] [CrossRef]
- Li, T.T.; Nie, F.; Yang, W.; Yang, K.; He, R.Y.; Zhang, L.; Li, Z.J.; Xu, Z.F. Seasonal dynamics of labile soil nitrogen from four plantations at the western edge of the Sichuan basin. Chin. J. Appl. Environ. Biol. 2018, 24, 744–750. [Google Scholar]
- Hobbie, E.A.; Ouimette, A.P. Controls of nitrogen isotope patterns in soil profiles. Biogeochemistry 2009, 95, 355–371. [Google Scholar] [CrossRef]
- Iqbal, J.; Hu, R.; Feng, M.; Lin, S.; Malghani, S.; Ali, I.M. Microbial biomass, and dissolved organic carbon and nitrogen strongly affect soil respiration in different land uses: A case study at Three Gorges Reservoir Area, South China. Agric. Ecosyst. Environ. 2010, 137, 294–307. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, X.; Liao, Y.; Liu, Y. Effects of different amount of maize straw returning on soil fertility and yield of winter wheat. Plant Nutr. Fertil. Sci. 2010, 16, 612–619. [Google Scholar]
- Dempster, D.N.; Gleeson, D.; Solaiman, Z.; Jones, D.L.; Murphy, D. Decreased soil microbial biomass and nitrogen mineralisation with Eucalyptus biochar addition to a coarse textured soil. Plant Soil 2012, 354, 311–324. [Google Scholar] [CrossRef]
- Wang, R.; Filley, T.R.; Xu, Z.; Wang, X.; Li, M.-H.; Zhang, Y.; Luo, W.; Jiang, Y. Coupled response of soil carbon and nitrogen pools and enzyme activities to nitrogen and water addition in a semi-arid grassland of Inner Mongolia. Plant Soil 2014, 381, 323–336. [Google Scholar] [CrossRef]
- Wei, C.; Yu, Q.; Bai, E.; Lü, X.; Li, Q.; Xia, J.; Kardol, P.; Liang, W.; Wang, Z.; Han, X. Nitrogen deposition weakens plant–microbe interactions in grassland ecosystems. Glob. Chang. Biol. 2013, 19, 3688–3697. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.M. Interference of soil soluble inorganic phosphorus on the determination of microbial biomass phosphorus. Acta Ecol. Sin. 2001, 21, 993–996. [Google Scholar]
- Chen, G.C.; He, Z.L.; Wang, Y.J. Impact of pH on microbial biomass carbon and microbial biomass phosphorus in red soils. Pedosphere 2004, 14, 9–15. [Google Scholar]
- Parfitt, R.L. Phosphate adsorption on an oxisol. Soil Sci. Soc. Am. J. 1977, 41, 1064–1067. [Google Scholar] [CrossRef]
- Guo, Q. Soil acidification induced by nitrogen addition and its responses to water addition in Inner Mongolia Temperate Steppe, China. J. Appl. Ecol. 2019, 30, 3285–3291. [Google Scholar]
- Chen, D.D.; Zhang, S.H.; Dong, S.K.; Wang, X.T.; Du, G.Z. Effect of land-use on soil nutrients and microbial biomass of an alpine region on the northeastern Tibetan plateau, China. Land Degrad. Dev. 2010, 21, 446–452. [Google Scholar] [CrossRef]
- Li, Y.; Dong, S.; Wen, L.; Wang, X.; Wu, Y. The effects of fencing on carbon stocks in the degraded alpine grasslands of the Qinghai-Tibetan Plateau. J. Environ. Manag. 2013, 128, 393–399. [Google Scholar] [CrossRef]
- Nie, M.; Pendall, E.; Bell, C.; Gasch, C.K.; Raut, S.; Tamang, S.; Wallenstein, M.D. Positive climate feedbacks of soil microbial communities in a semi-arid grassland. Ecol. Lett. 2013, 16, 234–241. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z. Soil microbial biomass C and N along a climatic transect in the Mongolian steppe. Biol. Fertil. Soils 2004, 39, 344–351. [Google Scholar] [CrossRef]
- Qiu, L.; Wei, X.; Zhang, X.; Cheng, J.; Gale, W.; Guo, C.; Long, T. Soil organic carbon losses due to land use change in a semiarid grassland. Plant Soil 2012, 355, 299–309. [Google Scholar] [CrossRef]
- Qiao, N.; Xu, X.; Cao, G.; Ouyang, H.; Kuzyakov, Y. Land use change decreases soil carbon stocks in Tibetan grasslands. Plant Soil 2015, 395, 231–241. [Google Scholar] [CrossRef]
- Liu, S.; An, N.; Yang, J.; Dong, S.; Wang, C.; Yin, Y. Prediction of soil organic matter variability associated with different land use types in mountainous landscape in southwestern Yunnan province, China. Catena 2015, 133, 137–144. [Google Scholar] [CrossRef]
- Lu, X.; Yan, Y.; Sun, J.; Zhang, X.; Chen, Y.; Wang, X.; Cheng, G. Carbon, nitrogen, and phosphorus storage in alpine grassland ecosystems of Tibet: Effects of grazing exclusion. Ecol. Evol. 2015, 5, 4492–4504. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tian, D.; Wang, B.; Wang, J.; Wang, S.; Chen, H.Y.H.; Xu, X.; Wang, C.; He, N.; Niu, S. Microbes drive global soil nitrogen mineralization and availability. Glob. Chang. Biol. 2019, 25, 1078–1088. [Google Scholar] [CrossRef]
- Miller, K.S.; Geisseler, D. Temperature sensitivity of nitrogen mineralization in agricultural soils. Biol. Fertil. Soils 2018, 54, 853–860. [Google Scholar] [CrossRef]
- Taylor, A.E.; Myrold, D.D.; Bottomley, P.J. Temperature affects the kinetics of nitrite oxidation and nitrification coupling in four agricultural soils. Soil Biol. Biochem. 2019, 136, 107523. [Google Scholar] [CrossRef]
- Thangarajan, R.; Bolan, N.S.; Naidu, R.; Surapaneni, A. Effects of temperature and amendments on nitrogen mineralization in selected Australian soils. Environ. Sci. Pollut. Res. 2015, 22, 8843–8854. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Högberg, M.N.; Högberg, P.; Myrold, D.D. Is microbial community composition in boreal forest soils determined by pH, C-to-N ratio, the trees, or all three? Oecologia 2007, 150, 590–601. [Google Scholar] [CrossRef]
- Lebauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Yuan, M.; Duan, Q.; Sun, R.; Shen, Y.; Yu, Q.; Li, S. Influence of phosphorus fertilization patterns on the bacterial community in upland farmland. Ind. Crop. Prod. 2020, 155, 112761. [Google Scholar] [CrossRef]
- Liu, C.; Gong, X.; Dang, K.; Li, J.; Yang, P.; Gao, X.; Deng, X.; Feng, B. Linkages between nutrient ratio and the microbial community in rhizosphere soil following fertilizer management. Environ. Res. 2020, 184, 109261. [Google Scholar] [CrossRef] [PubMed]
- Vepsäläinen, M.; Kukkonen, S.; Vestberg, M.; Sirviö, H.; Niemi, R.M. Application of soilenzyme activity test kit in a field experiment. Soil Biol. Biochem. 2001, 33, 1665–1672. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Enhancing Nitrogen Use Efficiency in Crop Plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar]
- Changhui, W.; Feng, Z.; Xiang, Z.; Kuanhu, D. The effects of N and P additions on microbial N transformations and biomass on saline-alkaline grassland of loess plateau of northern china. Geoderma 2014, 213, 419–425. [Google Scholar] [CrossRef]
- Guo, T.C.; Song, X.; Ma, D.Y.; Cha, F.N.; Yue, Y.J.; Zhang, Y.; Li, Y.Z. Effect of nitrogen fertilizer on soil enzymatic activity and rhizosphere microorganisms of wheat. J. Soil Water Conserv. 2006, 20, 129–132. [Google Scholar]
- Liu, W.; Jiang, L.; Hu, S.; Li, L.; Liu, L.; Wan, S. Decoupling of soil microbes and plants with increasing anthropogenic nitrogen inputs in a temperate steppe. Soil Biol. Biochem. 2014, 72, 116–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, X.; He, N.; Long, M.; Huang, J.; Zhang, G.; Wang, Q.; Han, X. Increase in ammonia volatilization from soil in response to N deposition in Inner Mongolia grasslands. Atmos. Environ. 2014, 84, 156–162. [Google Scholar] [CrossRef]
- Mangalassery, S.; Kalaivanan, D.; Philip, P.S. Effect of inorganic fertilisers and organic amendments on soil aggregation and biochemical characteristics in a weathered tropical soil. Soil Tillage Res. 2019, 187, 144–151. [Google Scholar] [CrossRef]
- Ren, F.; Sun, N.; Xu, M.; Zhang, X.; Wu, L.; Xu, M. Changes in soil microbial biomass with manure application in cropping systems: A meta-analysis. Soil Tillage Res. 2019, 194, 104291. [Google Scholar] [CrossRef]
- Shen, S.M.; Hart, P.B.S.; Powlson, D.S.; Jenkinson, D.S. The nitrogen cycle in the Broadbalk wheat experiment: 15N-labeled fertilizer residues in soil and in the soil microbial biomass. Soil Biol. Biochem. 1989, 21, 529–533. [Google Scholar] [CrossRef]
- Qiu, S.; Peng, P.; Liu, Q.; Rong, X. Soil microbial biomass nitrogen and its role in nitrogen cycling. Chin. J. Ecol. 2006, 25, 443–448. [Google Scholar]
- Huang, G.; Li, Y.; Su, Y.G. Divergent responses of soil microbial communities to water and nitrogen addition in a temperate desert. Geoderma 2015, 251–252, 55–64. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Belnap, J.; Rudgers, J.; Kuske, C.R.; Martinez, N.; Sandquist, D. Soil microbial responses to nitrogen addition in arid ecosystems. Front. Microbiol. 2015, 6, 819. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms-A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Cusack, D.F.; Silver, W.L.; Torn, M.S.; Burton, S.D.; Firestone, M.K. Changes in microbial community characteristics and soil organic matter with nitrogen additions in two tropical forests. Ecology 2011, 92, 621–632. [Google Scholar] [CrossRef]
- Heinze, S.; Raupp, J.; Joergensen, R.G. Effects of fertilizer and spatial heterogeneity in soil pH on microbial biomass indices in a long-term field trial of organic agriculture. Plant Soil 2010, 328, 203–215. [Google Scholar] [CrossRef]
- Van Diepen, L.T.A.; Lilleskov, E.A.; Pregitzer, K.S.; Miller, R.M. Simulated Nitrogen Deposition Causes a Decline of Intra- and Extraradical Abundance of Arbuscular Mycorrhizal Fungi and Changes in Microbial Community Structure in Northern Hardwood Forests. Ecosystems 2010, 13, 683–695. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, W.; Sun, T.; Chen, L.; Pang, X.; Wang, Y.; Xiao, F. N and P fertilization reduced soil autotrophic and heterotrophic respiration in a young Cunninghamia lanceolata forest. Agric. For. Meteorol. 2017, 232, 66–73. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Burton, A.J.; Zak, D.R.; Talhelm, A.F. Stimulated chronic nitrogen deposition increase carbon storage in Northern Temperate forests. Glob. Change Biol. 2008, 14, 142–153. [Google Scholar] [CrossRef]
- Hooker, T.D.; Stark, J.M. Soil C and N cycling in three semiarid vegetation types: Response to an in situ pulse of plant detritus. Soil Biol. Biochem. 2008, 40, 2678–2685. [Google Scholar] [CrossRef]
- Luo, X.B.; Liang, R.B.; Wang, Y.X. Response of soil microbial carbon and nitrogen pools to increasing atmospheric nitrogen deposition in forest surface soil. Ecol. Environ. Sci. 2014, 23, 365–370. [Google Scholar]
- Zhang, W.; Parker, K.M.; Luo, Y.; Wan, S.; Wallace, L.L.; Hu, S. Soil microbial responses to experimental warming and clipping in a tallgrass prairie. Glob. Chang. Biol. 2007, 11, 27–28. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, W.; Yang, H.; Yu, X.; Gutknecht, J.L.M.; Zhang, Z.; Wan, S.; Ma, K. Soil microbial responses to warming and increased precipitation and their implications for ecosystem C cycling. Oecologia 2013, 173, 1125–1142. [Google Scholar] [CrossRef]
- Jian, S.; Li, J.; Chen, J.I.; Wang, G.; Mayes, M.A.; Dzantor, K.E.; Hui, D.; Luo, Y. Soil extracellular enzymc activities, soil carbon and nitrogen storage under nitrogen fertilization. A meta-analysis. Soil Biol. Biochem. 2016, 101, 32–43. [Google Scholar] [CrossRef]
- Chen, D.; Li, J.; Lan, Z.; Hu, S.; Bai, Y. Soil acidification exerts a greater control on soil respiration than soil nitrogen availability in grasslands subjected to long-term nitrogen enrichment. Funct. Ecol. 2016, 30, 658–669. [Google Scholar] [CrossRef]
- Li, H.; Yang, S.; Xu, Z.; Yan, Q.; Li, X.; van Nostrand, J.D.; He, Z.; Yao, F.; Han, X.; Zhou, J.; et al. Rsponses of soil microbial functional genes to global changes are indirectly influenced by aboveground plant biomass variation. Soil. Biol. Biochem. 2017, 104, 18–29. [Google Scholar] [CrossRef]
- Pietri JC, A.; Brookes, P.C. Relationships between soil pH and microbial properties in a UK arable soil. Soil Biol. Biochem. 2008, 40, 1856–1861. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Chen, G.T.; Liang, Z.; Li, R.H.; Ma, H.Y.; Tu, L.H. Ten-year nitrogen addition did not significantly affect soil phosphorus fractions in a Pleioblastus amarus plantation. Ecol. Environ. Sci. 2018, 27, 677–684. [Google Scholar]
- Deng, Q.; Hui, D.; Dennis, S.; Reddy, K.C. Responses of terrestrial ecosystem phosphorus cycling to nitrogen addition: A meta-analysis. Glob. Ecol. Biogeogr. 2017, 26, 713–728. [Google Scholar] [CrossRef]
- Cheng, L.; Zhou, J.C.; Lin, K.M.; Zhang, Q.F.; Zhou, J.R.; Lin, Q.Y.; Zheng, W.; Wang, T.; Chen, Y.M. Effects of nitrogen addition on soil microbial community structure in a subtropical Phyllostachy pubescens. Chin. J. Ecol. 2020, 39, 1929–1937. [Google Scholar]
- Fan, Y.; Zhong, X.; Lin, F.; Liu, C.; Yang, L.; Wang, M.; Chen, G.; Chen, Y.; Yang, Y. Responses of soil phosphorus fractions after nitrogen addition in a subtropical forest ecosystem: Insights from decreased Fe and Al oxides and increased plant roots. Geoderma 2019, 337, 246–255. [Google Scholar] [CrossRef]
- Ahmed, W.; Liu, K.; Qaswar, M.; Huang, J.; Huang, Q.; Xu, Y.; Ali, S.; Mehmood, S.; Asghar, R.M.A.; Mahmood, M.; et al. Long-Term Mineral Fertilization Improved the Grain Yield and Phosphorus Use Efficiency by Changing Soil P Fractions in Ferralic Cambisol. Agronomy 2019, 9, 784. [Google Scholar] [CrossRef]
- Wan, W.; Li, X.; Han, S.; Wang, L.; Luo, X.; Chen, W.; Huang, Q. Soil aggregate fractionation and phosphorus fraction driven by long-term fertilization regimes affect the abundance and composition of P-cycling-related bacteria. Soil Tillage Res. 2020, 196, 104475. [Google Scholar] [CrossRef]
- Chen, C.; Condron, L.; Davis, M.; Sherlock, R. Seasonal changes in soil phosphorus and associated microbial properties under adjacent grassland and forest in New Zealand. For. Ecol. Manag. 2003, 177, 539–557. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Spilles, A.; Bonkowski, M. C:N:P stoichiometry and nutrient limitation of the soil microbial biomass in a grazed grassland site under experimental P limitation or excess. Ecol. Process. 2012, 1, 6. [Google Scholar] [CrossRef]
- Foote, J.; Boutton, T.; Scott, D. Soil C and N storage and microbial biomass in US southern pine forests: Influence of forest management. For. Ecol. Manag. 2015, 355, 48–57. [Google Scholar] [CrossRef]
- Yang, J.; Yu, F.; Yu, Y.; Zhang, J.; Wang, R.; Srinivasulu, M.; Vasenev, V.I. Characterization, source apportionment, and risk assessment of polycyclic aromatic hydrocarbons in urban soil of Nanjing, China. J. Soils Sediments 2016, 17, 1116–1125. [Google Scholar] [CrossRef]
- Cao, C.; Jiang, S.; Ying, Z.; Zhang, F.; Han, X. Spatial variability of soil nutrients and microbiological properties after the establishment of leguminous shrub Caragana microphylla Lam. plantation on sand dune in the Horqin Sandy Land of Northeast China. Ecol. Eng. 2011, 37, 1467–1475. [Google Scholar] [CrossRef]
- Huang, G.; Li, Y.; Su, Y.G. Effects of increasing precipitation on soil microbial community composition and soil respiration in a temperate desert, Northwestern China. Soil Biol. Biochem. 2015, 83, 52–56. [Google Scholar] [CrossRef]
- Wu, J.P.; Han, X.H.; Xu, Y.D.; Ren, C.J.; Yang, G.H.; Ren, G.X. Ecological stoichiometry of soil end soil microbial biomass C, N, P under grain-to-green program in Loess Hilly region. Acta Agrestia Sin. 2016, 24, 783–792. [Google Scholar]
- Wei, S.Z.; Zhao, Q.; Liao, M.Q.; Zhou, S.X.; He, C.; Wang, L.; Huang, C. Effects of simulated nitrogen deposition on microbial biomass during litter decompositionin a natural evergreen broad-leaved forest in the rainy area of West China. Acta Ecol. Sin. 2018, 38, 8001–8007. [Google Scholar]
- Zhao, H.; Huang, G.; Li, Y.; Ma, J.; Sheng, J.; Jia, H.; Li, C. Effects of increased summer precipitation and nitrogen addition on root decomposition in a temperate desert. PLoS ONE 2015, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, S.; He, T.; Liu, L.; Wu, J. Response of organic carbon mineralization and microbial community to leaf litter and nutrient additions in subtropical forest soils. Soil Biol. Biochem. 2014, 71, 13–20. [Google Scholar] [CrossRef]
- Qiao, N.; Xu, X.; Hu, Y.; Blagodatskaya, E.; Liu, Y.; Schaefer, D.; Kuzyakov, Y. Carbon and nitrogen additions induce distinct priming effects along an organicmatter decay continuum. Sci. Rep. 2016, 6, 19865. [Google Scholar] [CrossRef]
- Micks, P.; Aber, J.D.; Boone, R.D.; Davidson, E.A. Short-term soil respiration and nitrogen immobilization response to nitrogen applications in control and nitrogen-enriched temperate forests. For. Ecol. Manag. 2004, 196, 57–70. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Sun, Z.L.; Wang, Q.K. Effects of biochar and nitrogen additions on soil organic carbon decomposition and balance in a subtropical forest. Chin. J. Ecol. 2020, 39, 2851–2859. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, C.; Ruan, Y.; Jia, Z. Effects of Nitrogen Addition on Soil Microbial Biomass: A Meta-Analysis. Agriculture 2024, 14, 1616. https://doi.org/10.3390/agriculture14091616
He C, Ruan Y, Jia Z. Effects of Nitrogen Addition on Soil Microbial Biomass: A Meta-Analysis. Agriculture. 2024; 14(9):1616. https://doi.org/10.3390/agriculture14091616
Chicago/Turabian StyleHe, Chen, Yunze Ruan, and Zhongjun Jia. 2024. "Effects of Nitrogen Addition on Soil Microbial Biomass: A Meta-Analysis" Agriculture 14, no. 9: 1616. https://doi.org/10.3390/agriculture14091616
APA StyleHe, C., Ruan, Y., & Jia, Z. (2024). Effects of Nitrogen Addition on Soil Microbial Biomass: A Meta-Analysis. Agriculture, 14(9), 1616. https://doi.org/10.3390/agriculture14091616




