The Continuous Improvement of the Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)–CRISPR-Associated Protein System Has Led to Its Highly Efficient Application in Plants
Abstract
1. Introduction
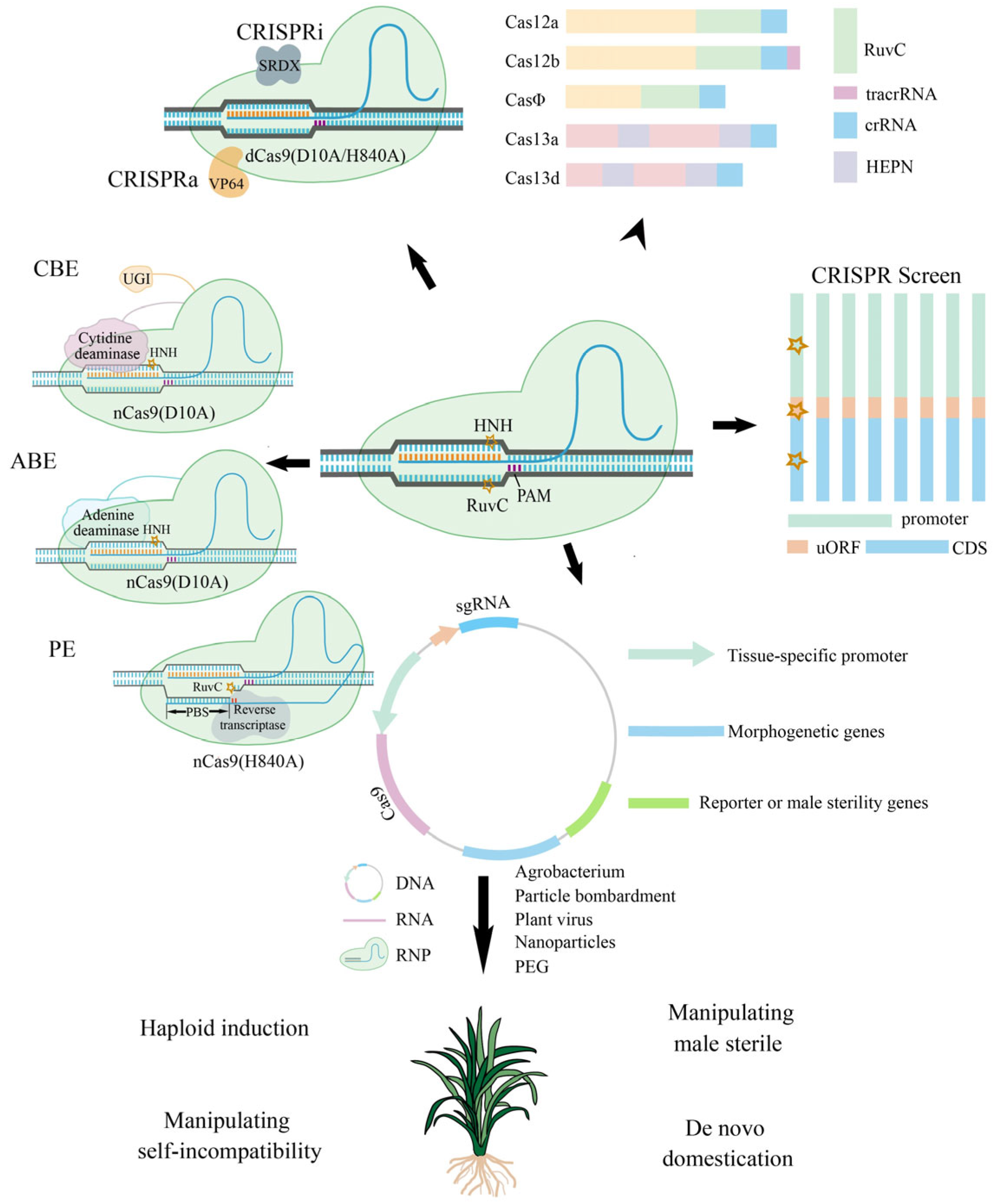
2. Diversity and Modification of Cas Proteins
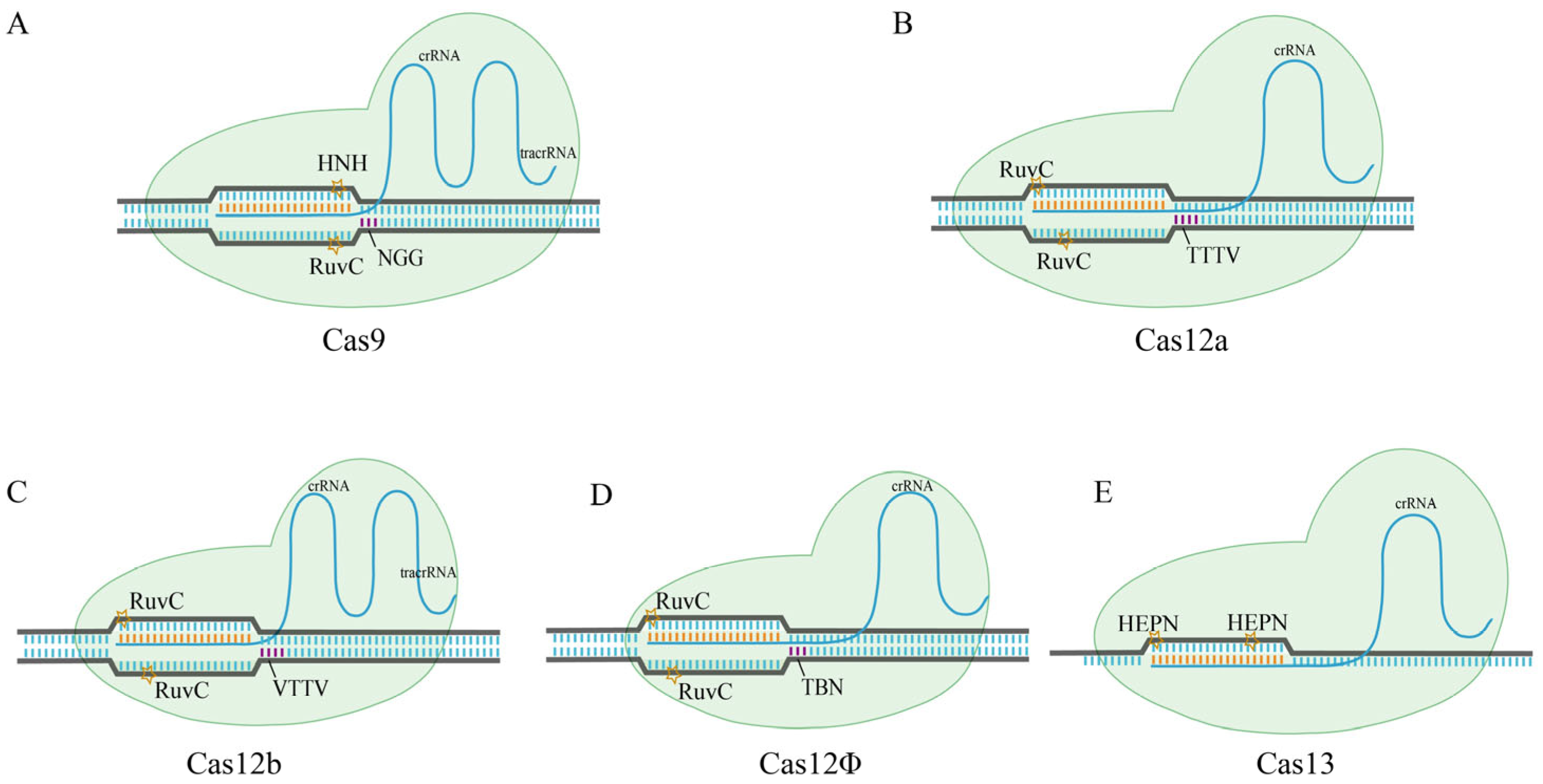
2.1. Class II Cas Proteins
2.1.1. Cas12a
2.1.2. Cas12b
2.1.3. CasΦ
2.1.4. Cas13
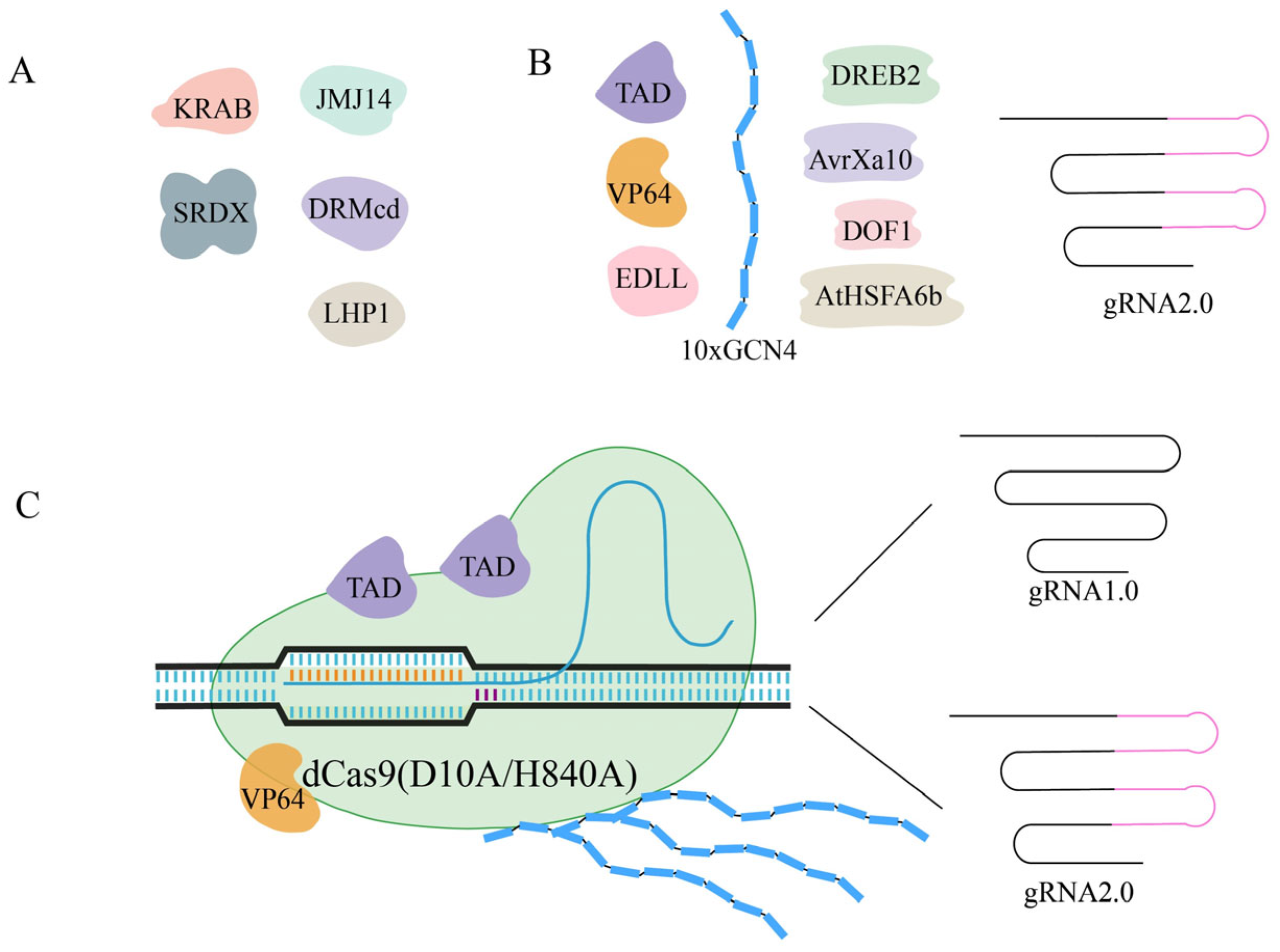
2.2. dCas9 and Transcriptional Regulation
2.3. Precise Editing
2.3.1. Cytosine Base Editing
2.3.2. Adenine Base Editing
2.3.3. Dual-Base Editing
2.3.4. Transversion Editing
2.3.5. Prime Editing
2.4. Cas9 Protein Variants
3. CRISPR–Cas Systems Targeting Different Locations with Different Breadths
3.1. Targeting the CDS
3.2. CRISPR Screen
| Species | Description | Number | Mutation Efficiency | Refs | ||
|---|---|---|---|---|---|---|
| Gene | sgRNA | Plant | ||||
| Rice | genome-scale | 34,234 | 88,541 | 91,004 | 83.90% | [171] |
| highly expressed genes in shoot base tissue | 12,802 | 25,604 | 14,000 | 65.30% | [172] | |
| anther-specific genes | 73 | 73 | 333 | 82.30% | [178] | |
| receptor-like kinase (RLK) family | 1072 | 1166 | 5039 | 92.10% | [179] | |
| seed-preferred genes | 310 | 375 | 2688 | 84.06% | [180] | |
| Tomato | LRR-XII gene family | 54 | 165 | 31 | 62.50% | [175] |
| TFs | 990 | 4379 | 487 | 23% | [173] | |
| Soybean | genes with potential functions in nodulation and seeds | 102 | 70 | 407 | 59.20% | [176] |
| Maize | genes related to agronomy and nutrition traits | 1244 | 1368 | 4356 | 81% | [177] |
| Cotton | plant development-related genes | 112 | 116 | 718 | 75% | [181] |
| endogenous insect-resistant genes | 502 | 969 | 2000 | 97.29% | [182] | |
| CDPK gene family | 82 | 246 | 518 | 89.49% | [183] | |
| Rapeseed | genes related to the reproductive organs | 10,480 | 18,414 | 1104 | 55.80% | [174] |
3.3. Targeting CRE in the Promoter and uORFs
4. Optimization of CRISPR–Cas Vectors
4.1. Application of Tissue-Specific Promoters
4.2. Acceleration of the Generation of Transgene-Free Plants
4.3. Application of Morphogenetic Genes
5. CRISPR–Cas System and Plant Breeding Practices
5.1. Bypassing Tissue Culture or Transgene-Free
5.2. Haploid Induction
5.3. MS and Self-Incompatibility Manipulation
5.4. De Novo Domestication
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, H.; Li, C.; Gao, C. Applications of CRISPR–Cas in agriculture and plant biotechnology. Nat. Rev. Mol. Cell Biol. 2020, 21, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Teng, F.; Li, T.; Zhou, Q. Simultaneous generation and germline transmission of multiple gene mutations in rat using CRISPR-Cas systems. Nat. Biotechnol. 2013, 31, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Wang, Y.; Li, J.; Zhang, Y.; Chen, K.; Liang, Z.; Zhang, K.; Liu, J.; Xi, J.J.; Qiu, J.-L.; et al. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 686–688. [Google Scholar] [CrossRef]
- Cardi, T.; Murovec, J.; Bakhsh, A.; Boniecka, J.; Bruegmann, T.; Bull, S.E.; Eeckhaut, T.; Fladung, M.; Galovic, V.; Linkiewicz, A.; et al. CRISPR/Cas-mediated plant genome editing: Outstanding challenges a decade after implementation. Trends Plant Sci. 2023, 28, 1144–1165. [Google Scholar] [CrossRef]
- Zhang, Y.; Malzahn, A.A.; Sretenovic, S.; Qi, Y. The emerging and uncultivated potential of CRISPR technology in plant science. Nat. Plants 2019, 5, 778–794. [Google Scholar] [CrossRef]
- Yan, W.X.; Hunnewell, P.; Alfonse, L.E.; Carte, J.M.; Keston-Smith, E.; Sothiselvam, S.; Garrity, A.J.; Chong, S.; Makarova, K.S.; Koonin, E.V.; et al. Functionally diverse type V CRISPR-Cas systems. Science 2019, 363, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Osakabe, K.; Osakabe, Y. Expanding the plant genome editing toolbox with recently developed CRISPR–Cas systems. Plant Physiol. 2022, 188, 1825–1837. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 2019, 576, 149–157. [Google Scholar] [CrossRef]
- Li, B.; Sun, C.; Li, J.; Gao, C. Targeted genome-modification tools and their advanced applications in crop breeding. Nat. Rev. Genet. 2024, 25, 603–622. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Sretenovic, S.; Ren, Q.; Yang, L.; Bao, Y.; Qi, C.; Yuan, M.; He, Y.; Liu, S.; Liu, X.; et al. Improving Plant Genome Editing with High-Fidelity xCas9 and Non-canonical PAM-Targeting Cas9-NG. Mol. Plant 2019, 12, 1027–1036. [Google Scholar] [CrossRef]
- Ren, B.; Liu, L.; Li, S.; Kuang, Y.; Wang, J.; Zhang, D.; Zhou, X.; Lin, H.; Zhou, H. Cas9-NG Greatly Expands the Targeting Scope of the Genome-Editing Toolkit by Recognizing NG and Other Atypical PAMs in Rice. Mol. Plant 2019, 12, 1015–1026. [Google Scholar] [CrossRef]
- Walton, R.T.; Christie, K.A.; Whittaker, M.N.; Kleinstiver, B.P. Unconstrained genome targeting with near-PAMless engineered CRISPR-Cas9 variants. Science 2020, 368, 290–296. [Google Scholar] [CrossRef]
- Xu, Z.; Kuang, Y.; Ren, B.; Yan, D.; Yan, F.; Spetz, C.; Sun, W.; Wang, G.; Zhou, X.; Zhou, H. SpRY greatly expands the genome editing scope in rice with highly flexible PAM recognition. Genome Biol. 2021, 22, 6. [Google Scholar] [CrossRef]
- Zinselmeier, M.H.; Casas-Mollano, J.A.; Cors, J.; Sychla, A.; Heinsch, S.C.; Voytas, D.F.; Smanski, M.J. Optimized dCas9 programmable transcriptional activators for plants. Plant Biotechnol. J. 2024, 22, 3202–3204. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Qi, Y. CRISPR-Combo–mediated orthogonal genome editing and transcriptional activation for plant breeding. Nat. Protoc. 2023, 18, 1760–1794. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Wu, X.; Markel, K.; Malzahn, A.A.; Kundagrami, N.; Sretenovic, S.; Zhang, Y.; Cheng, Y.; Shih, P.M.; Qi, Y. CRISPR–Act3.0 for highly efficient multiplexed gene activation in plants. Nat. Plants 2021, 7, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhong, Z.; Gallego-Bartolomé, J.; Li, Z.; Feng, S.; Kuo, H.Y.; Kan, R.L.; Lam, H.; Richey, J.C.; Tang, L.; et al. A gene silencing screen uncovers diverse tools for targeted gene repression in Arabidopsis. Nat. Plants 2023, 9, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, R.J.; Grotewold, E.; Stam, M. Cis-regulatory sequences in plants: Their importance, discovery, and future challenges. Plant Cell 2022, 34, 718–741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-X.; Li, B.-B.; Yang, Z.-G.; Huang, J.-Q.; Sun, W.-H.; Bhanbhro, N.; Liu, W.-T.; Chen, K.-M. Dissecting Plant Gene Functions Using CRISPR Toolsets for Crop Improvement. J. Agric. Food Chem. 2022, 70, 7343–7359. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, X.; Li, K.; Yao, Q.; Zhong, D.; Deng, Q.; Lu, Y. Large-scale genome editing in plants: Approaches, applications, and future perspectives. Curr. Opin. Biotechnol. 2023, 79, 102875. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Li, G.; Bandyopadhyay, A.; Qi, Y. Guide RNA library-based CRISPR screens in plants: Opportunities and challenges. Curr. Opin. Biotechnol. 2023, 79, 102883. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Mahfouz, M.M.; Mansoor, S. CRISPR-TSKO: A Tool for Tissue-Specific Genome Editing in Plants. Trends Plant Sci. 2020, 25, 123–126. [Google Scholar] [CrossRef]
- Singha, D.L.; Das, D.; Sarki, Y.N.; Chowdhury, N.; Sharma, M.; Maharana, J.; Chikkaputtaiah, C. Harnessing tissue-specific genome editing in plants through CRISPR/Cas system: Current state and future prospects. Planta 2021, 255, 28. [Google Scholar] [CrossRef]
- Li, J.; Pan, W.; Zhang, S.; Ma, G.; Li, A.; Zhang, H.; Liu, L. A rapid and highly efficient sorghum transformation strategy using GRF4-GIF1/ternary vector system. Plant J. 2023, 117, 1604–1613. [Google Scholar] [CrossRef]
- Wang, K.; Shi, L.; Liang, X.; Zhao, P.; Wang, W.; Liu, J.; Chang, Y.; Hiei, Y.; Yanagihara, C.; Du, L.; et al. The gene TaWOX5 overcomes genotype dependency in wheat genetic transformation. Nat. Plants 2022, 8, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qi, X.; Zhu, J.; Liu, C.; Fan, H.; Zhang, X.; Li, X.; Yang, Q.; Xie, C. Pollen self-elimination CRISPR–Cas genome editing prevents transgenic pollen dispersal in maize. Plant Commun. 2023, 4, 100637. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhou, T.; Xu, N.; Sun, X.; Song, S.; Liu, H.; Sun, Z.; Lv, Q.; Chen, J.; Tan, Y.; et al. Novel CRISPR/Cas9 system assisted by fluorescence marker and pollen killer for high-efficiency isolation of transgene-free edited plants in rice. Plant Biotechnol. J. 2024, 22, 1649–1651. [Google Scholar] [CrossRef]
- Demirer, G.S.; Silva, T.N.; Jackson, C.T.; Thomas, J.B.; Ehrhardt, D.W.; Rhee, S.Y.; Mortimer, J.C.; Landry, M.P. Nanotechnology to advance CRISPR–Cas genetic engineering of plants. Nat. Nanotechnol. 2021, 16, 243–250. [Google Scholar] [CrossRef]
- Shen, Y.; Ye, T.; Li, Z.; Kimutai, T.H.; Song, H.; Dong, X.; Wan, J. Exploiting viral vectors to deliver genome editing reagents in plants. aBIOTECH 2024, 5, 247–261. [Google Scholar] [CrossRef]
- Pacesa, M.; Pelea, O.; Jinek, M. Past, present, and future of CRISPR genome editing technologies. Cell 2024, 187, 1076–1100. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P.; et al. Evolutionary classification of CRISPR–Cas systems: A burst of class 2 and derived variants. Nat. Rev. Microbiol. 2019, 18, 67–83. [Google Scholar] [CrossRef]
- Piatek, A.; Ali, Z.; Baazim, H.; Li, L.; Abulfaraj, A.; Al-Shareef, S.; Aouida, M.; Mahfouz, M.M. RNA-guided transcriptional regulation in planta via synthetic dCas9-based transcription factors. Plant Biotechnol. J. 2014, 13, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Lowder, L.G.; Zhang, D.; Baltes, N.J.; Paul, J.W.; Tang, X.; Zheng, X.; Voytas, D.F.; Hsieh, T.-F.; Zhang, Y.; Qi, Y. A CRISPR/Cas9 Toolbox for Multiplexed Plant Genome Editing and Transcriptional Regulation. Plant Physiol. 2015, 169, 971–985. [Google Scholar] [CrossRef] [PubMed]
- Gaudelli, N.M.; Komor, A.C.; Rees, H.A.; Packer, M.S.; Badran, A.H.; Bryson, D.I.; Liu, D.R. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 2017, 551, 464–471. [Google Scholar] [CrossRef]
- Li, C.; Zong, Y.; Jin, S.; Zhu, H.; Lin, D.; Li, S.; Qiu, J.-L.; Wang, Y.; Gao, C. SWISS: Multiplexed orthogonal genome editing in plants with a Cas9 nickase and engineered CRISPR RNA scaffolds. Genome Biol. 2020, 21, 141. [Google Scholar] [CrossRef]
- Kavuri, N.R.; Ramasamy, M.; Qi, Y.; Mandadi, K. Applications of CRISPR/Cas13-Based RNA Editing in Plants. Cells 2022, 11, 2665. [Google Scholar] [CrossRef] [PubMed]
- Zetsche, B.; Gootenberg, J.S.; Abudayyeh, O.O.; Slaymaker, I.M.; Makarova, K.S.; Essletzbichler, P.; Volz, S.E.; Joung, J.; van der Oost, J.; Regev, A.; et al. Cpf1 Is a Single RNA-Guided Endonuclease of a Class 2 CRISPR-Cas System. Cell 2015, 163, 759–771. [Google Scholar] [CrossRef]
- Wang, M.; Mao, Y.; Lu, Y.; Tao, X.; Zhu, J.-K. Multiplex Gene Editing in Rice Using the CRISPR-Cpf1 System. Mol. Plant 2017, 10, 1011–1013. [Google Scholar] [CrossRef]
- Fonfara, I.; Richter, H.; Bratovič, M.; Le Rhun, A.; Charpentier, E. The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA. Nature 2016, 532, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Zetsche, B.; Heidenreich, M.; Mohanraju, P.; Fedorova, I.; Kneppers, J.; DeGennaro, E.M.; Winblad, N.; Choudhury, S.R.; Abudayyeh, O.O.; Gootenberg, J.S.; et al. Multiplex gene editing by CRISPR–Cpf1 using a single crRNA array. Nat. Biotechnol. 2016, 35, 31–34. [Google Scholar] [CrossRef]
- Yamano, T.; Nishimasu, H.; Zetsche, B.; Hirano, H.; Slaymaker, I.M.; Li, Y.; Fedorova, I.; Nakane, T.; Makarova, K.S.; Koonin, E.V.; et al. Crystal Structure of Cpf1 in Complex with Guide RNA and Target DNA. Cell 2016, 165, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Lowder, L.G.; Zhang, T.; Malzahn, A.A.; Zheng, X.; Voytas, D.F.; Zhong, Z.; Chen, Y.; Ren, Q.; Li, Q.; et al. A CRISPR–Cpf1 system for efficient genome editing and transcriptional repression in plants. Nat. Plants 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Mallon, J.; Poddar, A.; Wang, Y.; Tippana, R.; Yang, O.; Bailey, S.; Ha, T. Real-time observation of DNA target interrogation and product release by the RNA-guided endonuclease CRISPR Cpf1 (Cas12a). Proc. Natl. Acad. Sci. USA 2018, 115, 5444–5449. [Google Scholar] [CrossRef]
- Malzahn, A.A.; Tang, X.; Lee, K.; Ren, Q.; Sretenovic, S.; Zhang, Y.; Chen, H.; Kang, M.; Bao, Y.; Zheng, X.; et al. Application of CRISPR-Cas12a temperature sensitivity for improved genome editing in rice, maize, and Arabidopsis. BMC Biol. 2019, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Meng, X.; Li, J.; Wang, K.; Yu, H. Improving the efficiency of the CRISPR-Cas12a system with tRNA-crRNA arrays. Crop J. 2020, 8, 403–407. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Y.; Dailey, M.; Qi, Y. Hs1Cas12a and Ev1Cas12a confer efficient genome editing in plants. Front. Genome Ed. 2023, 5, 1251903. [Google Scholar] [CrossRef]
- Zhang, L.; Li, G.; Zhang, Y.; Cheng, Y.; Roberts, N.; Glenn, S.E.; DeZwaan-McCabe, D.; Rube, H.T.; Manthey, J.; Coleman, G.; et al. Boosting genome editing efficiency in human cells and plants with novel LbCas12a variants. Genome Biol. 2023, 24, 102. [Google Scholar] [CrossRef] [PubMed]
- Shmakov, S.; Abudayyeh, O.O.; Makarova, K.S.; Wolf, Y.I.; Gootenberg, J.S.; Semenova, E.; Minakhin, L.; Joung, J.; Konermann, S.; Severinov, K.; et al. Discovery and Functional Characterization of Diverse Class 2 CRISPR-Cas Systems. Mol. Cell 2015, 60, 385–397. [Google Scholar] [CrossRef]
- Yang, H.; Gao, P.; Rajashankar, K.R.; Patel, D.J. PAM-Dependent Target DNA Recognition and Cleavage by C2c1 CRISPR-Cas Endonuclease. Cell 2016, 167, 1814–1828. [Google Scholar] [CrossRef]
- Wu, D.; Guan, X.; Zhu, Y.; Ren, K.; Huang, Z. Structural basis of stringent PAM recognition by CRISPR-C2c1 in complex with sgRNA. Cell Res. 2017, 27, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Cui, T.; Feng, G.; Guo, L.; Xu, K.; Gao, Q.; Li, T.; Li, J.; Zhou, Q.; Li, W. Repurposing CRISPR-Cas12b for mammalian genome engineering. Cell Discov. 2018, 4, 63. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, J.; Sun, L.; Ma, Y.; Xu, J.; Liang, S.; Deng, J.; Tan, J.; Zhang, Q.; Tu, L.; et al. High efficient multisites genome editing in allotetraploid cotton (Gossypium hirsutum) using CRISPR/Cas9 system. Plant Biotechnol. J. 2017, 16, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Alariqi, M.; Wang, F.; Li, B.; Ding, X.; Rui, H.; Li, Y.; Xu, Z.; Qin, L.; Sun, L.; et al. The application of a heat-inducible CRISPR/Cas12b (C2c1) genome editing system in tetraploid cotton (G. hirsutum) plants. Plant Biotechnol. J. 2020, 18, 2436–2443. [Google Scholar] [CrossRef]
- Ming, M.; Ren, Q.; Pan, C.; He, Y.; Zhang, Y.; Liu, S.; Zhong, Z.; Wang, J.; Malzahn, A.A.; Wu, J.; et al. CRISPR–Cas12b enables efficient plant genome engineering. Nat. Plants 2020, 6, 202–208. [Google Scholar] [CrossRef]
- Gurel, F.; Wu, Y.; Pan, C.; Cheng, Y.; Li, G.; Zhang, T.; Qi, Y. On- and Off-Target Analyses of CRISPR-Cas12b Genome Editing Systems in Rice. CRISPR J. 2023, 6, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Qiao, X.; Zhao, Y.; Zhang, Z.; Gao, Y.; Shi, L.; Du, H.; Wang, L.; Zhang, Y.J.; Zhang, Y.; et al. Targeted mutagenesis in Arabidopsis thaliana using CRISPR-Cas12b/C2c1. J. Integr. Plant Biol. 2020, 62, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Su, F.; Chen, F.; Yan, C.; Xia, D.; Sun, H.; Li, S.; Duan, Z.; Ma, C.; Zhang, H.; et al. Genome editing in rice using CRISPR/Cas12i3. Plant Biotechnol. J. 2023, 22, 379–385. [Google Scholar] [CrossRef]
- Pausch, P.; Al-Shayeb, B.; Bisom-Rapp, E.; Tsuchida, C.A.; Li, Z.; Cress, B.F.; Knott, G.J.; Jacobsen, S.E.; Banfield, J.F.; Doudna, J.A. CRISPR-CasΦ from huge phages is a hypercompact genome editor. Science 2020, 369, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sretenovic, S.; Fan, T.; Cheng, Y.; Li, G.; Qi, A.; Tang, X.; Xu, Y.; Guo, W.; Zhong, Z.; et al. Hypercompact CRISPR–Cas12j2 (CasΦ) enables genome editing, gene activation, and epigenome editing in plants. Plant Commun. 2022, 3, 100453. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, Z.; Wu, Z.; Pausch, P.; Al-Shayeb, B.; Amerasekera, J.; Doudna, J.A.; Jacobsen, S.E. Genome editing in plants using the compact editor CasΦ. Proc. Natl. Acad. Sci. USA 2023, 120, e2216822120. [Google Scholar] [CrossRef]
- Ali, Z.; Mahas, A.; Mahfouz, M. CRISPR/Cas13 as a Tool for RNA Interference. Trends Plant Sci. 2018, 23, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Shmakov, S.; Smargon, A.; Scott, D.; Cox, D.; Pyzocha, N.; Yan, W.; Abudayyeh, O.O.; Gootenberg, J.S.; Makarova, K.S.; Wolf, Y.I.; et al. Diversity and evolution of class 2 CRISPR–Cas systems. Nat. Rev. Microbiol. 2017, 15, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhou, Y.; Xiao, Q.; He, B.; Geng, G.; Wang, Z.; Cao, B.; Dong, X.; Bai, W.; Wang, Y.; et al. Programmable RNA editing with compact CRISPR–Cas13 systems from uncultivated microbes. Nat. Methods 2021, 18, 499–506. [Google Scholar] [CrossRef]
- Abudayyeh, O.O.; Gootenberg, J.S.; Essletzbichler, P.; Han, S.; Joung, J.; Belanto, J.J.; Verdine, V.; Cox, D.B.T.; Kellner, M.J.; Regev, A.; et al. RNA targeting with CRISPR–Cas13. Nature 2017, 550, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Wolter, F.; Puchta, H. The CRISPR/Cas revolution reaches the RNA world: Cas13, a new Swiss Army knife for plant biologists. Plant J. 2018, 94, 767–775. [Google Scholar] [CrossRef]
- Aman, R.; Mahas, A.; Butt, H.; Ali, Z.; Aljedaani, F.; Mahfouz, M. Engineering RNA Virus Interference via the CRISPR/Cas13 Machinery in Arabidopsis. Viruses 2018, 10, 732. [Google Scholar] [CrossRef] [PubMed]
- Aman, R.; Ali, Z.; Butt, H.; Mahas, A.; Aljedaani, F.; Khan, M.Z.; Ding, S.; Mahfouz, M. RNA virus interference via CRISPR/Cas13a system in plants. Genome Biol. 2018, 19, 1. [Google Scholar] [CrossRef]
- Zhan, X.; Zhang, F.; Zhong, Z.; Chen, R.; Wang, Y.; Chang, L.; Bock, R.; Nie, B.; Zhang, J. Generation of virus-resistant potato plants by RNA genome targeting. Plant Biotechnol. J. 2019, 17, 1814–1822. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.; Ye, J.; Cao, X.; Xu, C.; Chen, B.; An, H.; Jiao, Y.; Zhang, F.; Yang, X.; et al. Establishing CRISPR/Cas13a immune system conferring RNA virus resistance in both dicot and monocot plants. Plant Biotechnol. J. 2019, 17, 1185–1187. [Google Scholar] [CrossRef]
- Yu, Y.; Pan, Z.; Wang, X.; Bian, X.; Wang, W.; Liang, Q.; Kou, M.; Ji, H.; Li, Y.; Ma, D.; et al. Targeting of SPCSV-RNase3 via CRISPR-Cas13 confers resistance against sweet potato virus disease. Mol. Plant Pathol. 2021, 23, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Konermann, S.; Lotfy, P.; Brideau, N.J.; Oki, J.; Shokhirev, M.N.; Hsu, P.D. Transcriptome Engineering with RNA-Targeting Type VI-D CRISPR Effectors. Cell 2018, 173, 665–676. [Google Scholar] [CrossRef]
- Mahas, A.; Aman, R.; Mahfouz, M. CRISPR-Cas13d mediates robust RNA virus interference in plants. Genome Biol. 2019, 20, 263. [Google Scholar] [CrossRef]
- Zhan, X.; Liu, W.; Nie, B.; Zhang, F.; Zhang, J. Cas13d-mediated multiplex RNA targeting confers a broad-spectrum resistance against RNA viruses in potato. Commun. Biol. 2023, 6, 855. [Google Scholar] [CrossRef]
- Sarkar, J.; Jyoti, T.P.; Sahana, S.; Bhattacharya, A.; Chandel, S.; Singh, R. CRISPR–Cas13d in plant biology: An insight. Plant Biotechnol. Rep. 2024, 18, 301–311. [Google Scholar] [CrossRef]
- Sharma, V.K.; Marla, S.; Zheng, W.; Mishra, D.; Huang, J.; Zhang, W.; Morris, G.P.; Cook, D.E. CRISPR guides induce gene silencing in plants in the absence of Cas. Genome Biol. 2022, 23, 6. [Google Scholar] [CrossRef] [PubMed]
- Abudayyeh, O.O.; Gootenberg, J.S.; Kellner, M.J.; Zhang, F. Nucleic Acid Detection of Plant Genes Using CRISPR-Cas13. CRISPR J. 2019, 2, 165–171. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, D.; Xiong, X.; Yan, B.; Xie, W.; Sheen, J.; Li, J.-F. A potent Cas9-derived gene activator for plant and mammalian cells. Nat. Plants 2017, 3, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Papikian, A.; Liu, W.; Gallego-Bartolomé, J.; Jacobsen, S.E. Site-specific manipulation of Arabidopsis loci using CRISPR-Cas9 SunTag systems. Nat. Commun. 2019, 10, 729. [Google Scholar] [CrossRef]
- Lowder, L.G.; Zhou, J.; Zhang, Y.; Malzahn, A.; Zhong, Z.; Hsieh, T.-F.; Voytas, D.F.; Zhang, Y.; Qi, Y. Robust Transcriptional Activation in Plants Using Multiplexed CRISPR-Act2.0 and mTALE-Act Systems. Mol. Plant 2018, 11, 245–256. [Google Scholar] [CrossRef]
- Pan, C.; Li, G.; Malzahn, A.A.; Cheng, Y.; Leyson, B.; Sretenovic, S.; Gurel, F.; Coleman, G.D.; Qi, Y. Boosting plant genome editing with a versatile CRISPR-Combo system. Nat. Plants 2022, 8, 513–525. [Google Scholar] [CrossRef]
- Morffy, N.; Van den Broeck, L.; Miller, C.; Emenecker, R.J.; Bryant, J.A.; Lee, T.M.; Sageman-Furnas, K.; Wilkinson, E.G.; Pathak, S.; Kotha, S.R.; et al. Identification of plant transcriptional activation domains. Nature 2024, 632, 166–173. [Google Scholar] [CrossRef]
- Selma, S.; Sanmartín, N.; Espinosa-Ruiz, A.; Gianoglio, S.; Lopez-Gresa, M.P.; Vázquez-Vilar, M.; Flors, V.; Granell, A.; Orzaez, D. Custom-made design of metabolite composition in N. benthamiana leaves using CRISPR activators. Plant Biotechnol. J. 2022, 20, 1578–1590. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Li, H.; Liu, Y.; Li, S.; Liang, Z. Highly efficient activation of endogenous gene in grape using CRISPR/dCas9-based transcriptional activators. Hortic. Res. 2022, 9, uhab037. [Google Scholar] [CrossRef]
- Ming, M.; Long, H.; Ye, Z.; Pan, C.; Chen, J.; Tian, R.; Sun, C.; Xue, Y.; Zhang, Y.; Li, J.; et al. Highly efficient CRISPR systems for loss-of-function and gain-of-function research in pear calli. Hortic. Res. 2022, 9, uhac148. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Yuan, G.; Lu, H.; Liu, Y.; Zhang, J.; Tuskan, G.A.; Muchero, W.; Chen, J.-G.; Yang, X. CRISPR/Cas9-based gene activation and base editing in Populus. Hortic. Res. 2023, 10, uhad085. [Google Scholar] [CrossRef] [PubMed]
- García-Murillo, L.; Valencia-Lozano, E.; Priego-Ranero, N.A.; Cabrera-Ponce, J.L.; Duarte-Aké, F.P.; Vizuet-de-Rueda, J.C.; Rivera-Toro, D.M.; Herrera-Ubaldo, H.; de Folter, S.; Alvarez-Venegas, R. CRISPRa-mediated transcriptional activation of the SlPR-1 gene in edited tomato plants. Plant Sci. 2023, 329, 111617. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Gao, H.; Lv, R.; Mao, W.; Zhu, J.; Liu, C.; Mao, L.; Li, X.; Xie, C. CRISPR/dCas-mediated gene activation toolkit development and its application for parthenogenesis induction in maize. Plant Commun. 2023, 4, 100449. [Google Scholar] [CrossRef]
- Yu, L.; Li, Z.; Ding, X.; Alariqi, M.; Zhang, C.; Zhu, X.; Fan, S.; Zhu, L.; Zhang, X.; Jin, S. Developing an efficient CRISPR–dCas9–TV-derived transcriptional activation system to create three novel cotton germplasm materials. Plant Commun. 2023, 4, 100600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tang, Y.; Tang, S.; Chen, L.; Li, T.; Yuan, H.; Xu, Y.; Zhou, Y.; Zhang, S.; Wang, J.; et al. An inducible CRISPR activation tool for accelerating plant regeneration. Plant Commun. 2024, 5, 100823. [Google Scholar] [CrossRef]
- Qi, L.S.; Larson, M.H.; Gilbert, L.A.; Doudna, J.A.; Weissman, J.S.; Arkin, A.P.; Lim, W.A. Repurposing CRISPR as an RNA-Guided Platform for Sequence-Specific Control of Gene Expression. Cell 2013, 152, 1173–1183. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Larson, M.H.; Morsut, L.; Liu, Z.; Brar, G.A.; Torres, S.E.; Stern-Ginossar, N.; Brandman, O.; Whitehead, E.H.; Doudna, J.A.; et al. CRISPR-Mediated Modular RNA-Guided Regulation of Transcription in Eukaryotes. Cell 2013, 154, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.; Kathiresan, V.; Kumari, P.; Newsom, S.; Parameshwaran, H.P.; Chen, X.; Liu, J.; Qin, P.Z.; Rajan, R. Coordinated Actions of Cas9 HNH and RuvC Nuclease Domains Are Regulated by the Bridge Helix and the Target DNA Sequence. Biochemistry 2021, 60, 3783–3800. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Kosicki, M.; Tomberg, K.; Bradley, A. Repair of double-strand breaks induced by CRISPR–Cas9 leads to large deletions and complex rearrangements. Nat. Biotechnol. 2018, 36, 765–771. [Google Scholar] [CrossRef]
- Zong, Y.; Wang, Y.; Li, C.; Zhang, R.; Chen, K.; Ran, Y.; Qiu, J.-L.; Wang, D.; Gao, C. Precise base editing in rice, wheat and maize with a Cas9-cytidine deaminase fusion. Nat. Biotechnol. 2017, 35, 438–440. [Google Scholar] [CrossRef]
- Shimatani, Z.; Kashojiya, S.; Takayama, M.; Terada, R.; Arazoe, T.; Ishii, H.; Teramura, H.; Yamamoto, T.; Komatsu, H.; Miura, K.; et al. Targeted base editing in rice and tomato using a CRISPR-Cas9 cytidine deaminase fusion. Nat. Biotechnol. 2017, 35, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Song, Q.; Li, C.; Jin, S.; Zhang, D.; Wang, Y.; Qiu, J.-L.; Gao, C. Efficient C-to-T base editing in plants using a fusion of nCas9 and human APOBEC3A. Nat. Biotechnol. 2018, 36, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Fei, H.; Zhu, Z.; Luo, Y.; Liu, J.; Gao, S.; Zhang, F.; Chen, Y.-H.; Wang, Y.; Gao, C. Rationally Designed APOBEC3B Cytosine Base Editors with Improved Specificity. Mol. Cell 2020, 79, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Sretenovic, S.; Liu, G.; Zhong, Z.; Wang, J.; Huang, L.; Tang, X.; Guo, Y.; Liu, L.; Wu, Y.; et al. Improved plant cytosine base editors with high editing activity, purity, and specificity. Plant Biotechnol. J. 2021, 19, 2052–2068. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Huang, J.; Lin, Q.; Fei, H.; He, Z.; Xu, H.; Li, Y.; Qu, K.; Han, P.; Gao, Q.; Li, B.; et al. Discovery of deaminase functions by structure-based protein clustering. Cell 2023, 186, 3182–3195. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zong, Y.; Lin, Q.; Zhang, H.; Chai, Z.; Zhang, D.; Chen, K.; Qiu, J.-L.; Gao, C. Precise, predictable multi-nucleotide deletions in rice and wheat using APOBEC–Cas9. Nat. Biotechnol. 2020, 38, 1460–1465. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zong, Y.; Wang, Y.; Jin, S.; Zhang, D.; Song, Q.; Zhang, R.; Gao, C. Expanded base editing in rice and wheat using a Cas9-adenosine deaminase fusion. Genome Biol. 2018, 19, 59. [Google Scholar] [CrossRef]
- Kang, B.-C.; Yun, J.-Y.; Kim, S.-T.; Shin, Y.; Ryu, J.; Choi, M.; Woo, J.W.; Kim, J.-S. Precision genome engineering through adenine base editing in plants. Nat. Plants 2018, 4, 427–431. [Google Scholar] [CrossRef]
- Jin, S.; Zong, Y.; Gao, Q.; Zhu, Z.; Wang, Y.; Qin, P.; Liang, C.; Wang, D.; Qiu, J.-L.; Zhang, F.; et al. Cytosine, but not adenine, base editors induce genome-wide off-target mutations in rice. Science 2019, 364, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Sun, Y.; Yan, R.; Liu, Y.; Zuo, E.; Gu, C.; Han, L.; Wei, Y.; Hu, X.; Zeng, R.; et al. Off-target RNA mutation induced by DNA base editing and its elimination by mutagenesis. Nature 2019, 571, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Hua, K.; Tao, X.; Liang, W.; Zhang, Z.; Gou, R.; Zhu, J.K. Simplified adenine base editors improve adenine base editing efficiency in rice. Plant Biotechnol. J. 2019, 18, 770–778. [Google Scholar] [CrossRef]
- Lapinaite, A.; Knott, G.J.; Palumbo, C.M.; Lin-Shiao, E.; Richter, M.F.; Zhao, K.T.; Beal, P.A.; Liu, D.R.; Doudna, J.A. DNA capture by a CRISPR-Cas9–guided adenine base editor. Science 2020, 369, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.F.; Zhao, K.T.; Eton, E.; Lapinaite, A.; Newby, G.A.; Thuronyi, B.W.; Wilson, C.; Koblan, L.W.; Zeng, J.; Bauer, D.E.; et al. Phage-assisted evolution of an adenine base editor with improved Cas domain compatibility and activity. Nat. Biotechnol. 2020, 38, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Wang, C.; Jia, M.; Guo, H.X.; Luo, P.Y.; Wang, M.G.; Zhu, J.K.; Zhang, H. Efficient generation of homozygous substitutions in rice in one generation utilizing an rABE8e base editor. J. Integr. Plant Biol. 2021, 63, 1595–1599. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Ren, B.; Liu, L.; Yan, F.; Li, S.; Wang, G.; Sun, W.; Zhou, X.; Zhou, H. High-efficiency and multiplex adenine base editing in plants using new TadA variants. Mol. Plant 2021, 14, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Grünewald, J.; Zhou, R.; Lareau, C.A.; Garcia, S.P.; Iyer, S.; Miller, B.R.; Langner, L.M.; Hsu, J.Y.; Aryee, M.J.; Joung, J.K. A dual-deaminase CRISPR base editor enables concurrent adenine and cytosine editing. Nat. Biotechnol. 2020, 38, 861–864. [Google Scholar] [CrossRef]
- Li, C.; Zhang, R.; Meng, X.; Chen, S.; Zong, Y.; Lu, C.; Qiu, J.-L.; Chen, Y.-H.; Li, J.; Gao, C. Targeted, random mutagenesis of plant genes with dual cytosine and adenine base editors. Nat. Biotechnol. 2020, 38, 875–882. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, T.; Chai, N.; Zeng, D.; Zhang, R.; Wu, Y.; Hang, J.; Liu, Y.; Deng, Q.; Tan, J.; et al. PhieDBEs: A DBD-containing, PAM-flexible, high-efficiency dual base editor toolbox with wide targeting scope for use in plants. Plant Biotechnol. J. 2024, 22, 3164–3174. [Google Scholar] [CrossRef]
- Zhang, A.; Shan, T.; Sun, Y.; Chen, Z.; Hu, J.; Hu, Z.; Ming, Z.; Zhu, Z.; Li, X.; He, J.; et al. Directed evolution rice genes with randomly multiplexed sgRNAs assembly of base editors. Plant Biotechnol. J. 2023, 21, 2597–2610. [Google Scholar] [CrossRef]
- Fan, T.; Cheng, Y.; Wu, Y.; Liu, S.; Tang, X.; He, Y.; Liao, S.; Zheng, X.; Zhang, T.; Qi, Y.; et al. High performance TadA-8e derived cytosine and dual base editors with undetectable off-target effects in plants. Nat. Commun. 2024, 15, 5103. [Google Scholar] [CrossRef]
- Chen, L.; Park, J.E.; Paa, P.; Rajakumar, P.D.; Prekop, H.-T.; Chew, Y.T.; Manivannan, S.N.; Chew, W.L. Programmable C:G to G:C genome editing with CRISPR-Cas9-directed base excision repair proteins. Nat. Commun. 2021, 12, 1384. [Google Scholar] [CrossRef] [PubMed]
- Kurt, I.C.; Zhou, R.; Iyer, S.; Garcia, S.P.; Miller, B.R.; Langner, L.M.; Grünewald, J.; Joung, J.K. CRISPR C-to-G base editors for inducing targeted DNA transversions in human cells. Nat. Biotechnol. 2020, 39, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Li, J.; Li, S.; Xin, X.; Hu, M.; Price, M.A.; Rosser, S.J.; Bi, C.; Zhang, X. Glycosylase base editors enable C-to-A and C-to-G base changes. Nat. Biotechnol. 2020, 39, 35–40. [Google Scholar] [CrossRef]
- Tian, Y.; Shen, R.; Li, Z.; Yao, Q.; Zhang, X.; Zhong, D.; Tan, X.; Song, M.; Han, H.; Zhu, J.K.; et al. Efficient C-to-G editing in rice using an optimized base editor. Plant Biotechnol. J. 2022, 20, 1238–1240. [Google Scholar] [CrossRef]
- Sretenovic, S.; Liu, S.; Li, G.; Cheng, Y.; Fan, T.; Xu, Y.; Zhou, J.; Zheng, X.; Coleman, G.; Zhang, Y.; et al. Exploring C-To-G Base Editing in Rice, Tomato, and Poplar. Front. Genome Ed. 2021, 3, 756766. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hong, M.; Luan, C.; Gao, H.; Ru, G.; Guo, X.; Zhang, D.; Zhang, S.; Li, C.; Wu, J.; et al. Adenine transversion editors enable precise, efficient A•T-to-C•G base editing in mammalian cells and embryos. Nat. Biotechnol. 2023, 42, 638–650. [Google Scholar] [CrossRef]
- Wu, X.; Ren, B.; Liu, L.; Qiu, S.; Li, X.g.; Li, P.; Yan, F.; Lin, H.; Zhou, X.; Zhang, D.; et al. Adenine base editor incorporating the N-methylpurine DNA glycosylase MPGv3 enables efficient A-to-K base editing in rice. Plant Commun. 2023, 4, 100668. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Wang, X.; Liu, Y.; Liu, N.; Li, Y.; Luo, J.; Ma, Q.; Wu, D.; Li, J.; Xu, C.; et al. Programmable A-to-Y base editing by fusing an adenine base editor with an N-methylpurine DNA glycosylase. Nat. Biotechnol. 2023, 41, 1080–1084. [Google Scholar] [CrossRef]
- Lin, Q.; Zong, Y.; Xue, C.; Wang, S.; Jin, S.; Zhu, Z.; Wang, Y.; Anzalone, A.V.; Raguram, A.; Doman, J.L.; et al. Prime genome editing in rice and wheat. Nat. Biotechnol. 2020, 38, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, C.; Yang, Y.; Zhao, S.; Kang, G.; He, X.; Song, J.; Yang, J. Versatile Nucleotides Substitution in Plant Using an Improved Prime Editing System. Mol. Plant 2020, 13, 675–678. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Chai, Y.P.; Lu, M.H.; Han, X.L.; Lin, Q.; Zhang, Y.; Zhang, Q.; Zhou, Y.; Wang, X.-C.; Gao, C.; et al. Prime editing efficiently generates W542L and S621I double mutations in two ALS genes in maize. Genome Biol. 2020, 21, 257. [Google Scholar] [CrossRef]
- Lu, Y.; Tian, Y.; Shen, R.; Yao, Q.; Zhong, D.; Zhang, X.; Zhu, J.K. Precise genome modification in tomato using an improved prime editing system. Plant Biotechnol. J. 2020, 19, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Li, J.; Liu, X.; Shan, T.; Qin, R.; Wei, P. Development of Plant Prime-Editing Systems for Precise Genome Editing. Plant Commun. 2020, 1, 100043. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Jin, S.; Zong, Y.; Yu, H.; Zhu, Z.; Liu, G.; Kou, L.; Wang, Y.; Qiu, J.-L.; Li, J.; et al. High-efficiency prime editing with optimized, paired pegRNAs in plants. Nat. Biotechnol. 2021, 39, 923–927. [Google Scholar] [CrossRef]
- Jin, S.; Lin, Q.; Luo, Y.; Zhu, Z.; Liu, G.; Li, Y.; Chen, K.; Qiu, J.-L.; Gao, C. Genome-wide specificity of prime editors in plants. Nat. Biotechnol. 2021, 39, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Liu, Y.; Xue, C.; Li, B.; Li, X.; Wang, Y.; Li, J.; Liu, G.; Huang, X.; Cao, X.; et al. An engineered prime editor with enhanced editing efficiency in plants. Nat. Biotechnol. 2022, 40, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Ni, P.; Zhao, Y.; Zhou, X.; Liu, Z.; Huang, Z.; Ni, Z.; Sun, Q.; Zong, Y. Efficient and versatile multiplex prime editing in hexaploid wheat. Genome Biol. 2023, 24, 156. [Google Scholar] [CrossRef] [PubMed]
- Doman, J.L.; Pandey, S.; Neugebauer, M.E.; An, M.; Davis, J.R.; Randolph, P.B.; McElroy, A.; Gao, X.D.; Raguram, A.; Richter, M.F.; et al. Phage-assisted evolution and protein engineering yield compact, efficient prime editors. Cell 2023, 186, 3983–4002. [Google Scholar] [CrossRef]
- Gupta, A.; Liu, B.; Raza, S.; Chen, Q.-J.; Yang, B. Modularly assembled multiplex prime editors for simultaneous editing of agronomically important genes in rice. Plant Commun. 2024, 5, 100741. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.-H.; Jeong, Y.K.; Habib, O.; Hong, S.-A.; Lim, K.; Kim, J.-S.; Bae, S. PE-Designer and PE-Analyzer: Web-based design and analysis tools for CRISPR prime editing. Nucleic Acids Res. 2021, 49, W499–W504. [Google Scholar] [CrossRef]
- Chen, P.J.; Hussmann, J.A.; Yan, J.; Knipping, F.; Ravisankar, P.; Chen, P.-F.; Chen, C.; Nelson, J.W.; Newby, G.A.; Sahin, M.; et al. Enhanced prime editing systems by manipulating cellular determinants of editing outcomes. Cell 2021, 184, 5635–5652. [Google Scholar] [CrossRef]
- Jiang, Y.; Chai, Y.; Qiao, D.; Wang, J.; Xin, C.; Sun, W.; Cao, Z.; Zhang, Y.; Zhou, Y.; Wang, X.-C.; et al. Optimized prime editing efficiently generates glyphosate-resistant rice plants carrying homozygous TAP-IVS mutation in EPSPS. Mol. Plant 2022, 15, 1646–1649. [Google Scholar] [CrossRef]
- Liu, X.; Gu, D.; Zhang, Y.; Jiang, Y.; Xiao, Z.; Xu, R.; Qin, R.; Li, J.; Wei, P. Conditional knockdown of OsMLH1 to improve plant prime editing systems without disturbing fertility in rice. Genome Biol. 2024, 25, 131. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Chen, W.; Suiter, C.C.; Lee, C.; Chardon, F.M.; Yang, W.; Leith, A.; Daza, R.M.; Martin, B.; Shendure, J. Precise genomic deletions using paired prime editing. Nat. Biotechnol. 2021, 40, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhang, X.-O.; Weng, Z.; Xue, W. Deletion and replacement of long genomic sequences using prime editing. Nat. Biotechnol. 2021, 40, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Lei, Y.; Li, B.; Gao, Q.; Li, Y.; Cao, W.; Yang, C.; Li, H.; Wang, Z.; Li, Y.; et al. Precise integration of large DNA sequences in plant genomes using PrimeRoot editors. Nat. Biotechnol. 2023, 42, 316–327. [Google Scholar] [CrossRef]
- Nishimasu, H.; Shi, X.; Ishiguro, S.; Gao, L.; Hirano, S.; Okazaki, S.; Noda, T.; Abudayyeh, O.O.; Gootenberg, J.S.; Mori, H.; et al. Engineered CRISPR-Cas9 nuclease with expanded targeting space. Science 2018, 361, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Hua, K.; Tao, X.; Han, P.; Wang, R.; Zhu, J.-K. Genome Engineering in Rice Using Cas9 Variants that Recognize NG PAM Sequences. Mol. Plant 2019, 12, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Mikami, M.; Endo, A.; Kaya, H.; Itoh, T.; Nishimasu, H.; Nureki, O.; Toki, S. Genome editing in plants by engineered CRISPR–Cas9 recognizing NG PAM. Nat. Plants 2018, 5, 14–17. [Google Scholar] [CrossRef]
- Ren, Q.; Sretenovic, S.; Liu, S.; Tang, X.; Huang, L.; He, Y.; Liu, L.; Guo, Y.; Zhong, Z.; Liu, G.; et al. PAM-less plant genome editing using a CRISPR–SpRY toolbox. Nat. Plants 2021, 7, 25–33. [Google Scholar] [CrossRef]
- Ren, J.; Meng, X.; Hu, F.; Liu, Q.; Cao, Y.; Li, H.; Yan, C.; Li, J.; Wang, K.; Yu, H.; et al. Expanding the scope of genome editing with SpG and SpRY variants in rice. Sci. China Life Sci. 2021, 64, 1784–1787. [Google Scholar] [CrossRef]
- Li, J.; Xu, R.; Qin, R.; Liu, X.; Kong, F.; Wei, P. Genome editing mediated by SpCas9 variants with broad non-canonical PAM compatibility in plants. Mol. Plant 2021, 14, 352–360. [Google Scholar] [CrossRef]
- Hu, Y.; Patra, P.; Pisanty, O.; Shafir, A.; Belew, Z.M.; Binenbaum, J.; Ben Yaakov, S.; Shi, B.; Charrier, L.; Hyams, G.; et al. Multi-Knock—A multi-targeted genome-scale CRISPR toolbox to overcome functional redundancy in plants. Nat. Plants 2023, 9, 572–587. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Q.; Zhu, Q.; Liu, W.; Chen, Y.; Qiu, R.; Wang, B.; Yang, Z.; Li, H.; Lin, Y.; et al. A Robust CRISPR/Cas9 System for Convenient, High-Efficiency Multiplex Genome Editing in Monocot and Dicot Plants. Mol. Plant 2015, 8, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Minkenberg, B.; Yang, Y. Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA-processing system. Proc. Natl. Acad. Sci. USA 2015, 112, 3570–3575. [Google Scholar] [CrossRef]
- Čermák, T.; Curtin, S.J.; Gil-Humanes, J.; Čegan, R.; Kono, T.J.Y.; Konečná, E.; Belanto, J.J.; Starker, C.G.; Mathre, J.W.; Greenstein, R.L.; et al. A Multipurpose Toolkit to Enable Advanced Genome Engineering in Plants. Plant Cell 2017, 29, 1196–1217. [Google Scholar] [CrossRef]
- Ding, D.; Chen, K.; Chen, Y.; Li, H.; Xie, K. Engineering Introns to Express RNA Guides for Cas9- and Cpf1-Mediated Multiplex Genome Editing. Mol. Plant 2018, 11, 542–552. [Google Scholar] [CrossRef]
- Zhang, Z.; Mao, Y.; Ha, S.; Liu, W.; Botella, J.R.; Zhu, J.-K. A multiplex CRISPR/Cas9 platform for fast and efficient editing of multiple genes in Arabidopsis. Plant Cell Rep. 2015, 35, 1519–1533. [Google Scholar] [CrossRef]
- Wang, C.; Shen, L.; Fu, Y.; Yan, C.; Wang, K. A Simple CRISPR/Cas9 System for Multiplex Genome Editing in Rice. J. Genet. Genom. 2015, 42, 703–706. [Google Scholar] [CrossRef]
- Hao, Y.; Zong, W.; Zeng, D.; Han, J.; Chen, S.; Tang, J.; Zhao, Z.; Li, X.; Ma, K.; Xie, X.; et al. Shortened snRNA promoters for efficient CRISPR/Cas-based multiplex genome editing in monocot plants. Sci. China Life Sci. 2020, 63, 933–935. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Liu, M.; Zhou, Y.; Li, F. Guide RNA scaffold variants enabled easy cloning of large gRNA cluster for multiplexed gene editing. Plant Biotechnol. J. 2023, 22, 460–471. [Google Scholar] [CrossRef]
- Liu, H.; Ding, Y.; Zhou, Y.; Jin, W.; Xie, K.; Chen, L.-L. CRISPR-P 2.0: An Improved CRISPR-Cas9 Tool for Genome Editing in Plants. Mol. Plant 2017, 10, 530–532. [Google Scholar] [CrossRef]
- Sun, J.; Liu, H.; Liu, J.; Cheng, S.; Peng, Y.; Zhang, Q.; Yan, J.; Liu, H.-J.; Chen, L.-L.; Birol, I. CRISPR-Local: A local single-guide RNA (sgRNA) design tool for non-reference plant genomes. Bioinformatics 2019, 35, 2501–2503. [Google Scholar] [CrossRef]
- Xie, X.; Ma, X.; Zhu, Q.; Zeng, D.; Li, G.; Liu, Y.-G. CRISPR-GE: A Convenient Software Toolkit for CRISPR-Based Genome Editing. Mol. Plant 2017, 10, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bae, S.; Kim, J.-S. Cas-Designer: A web-based tool for choice of CRISPR-Cas9 target sites. Bioinformatics 2015, 31, 4014–4016. [Google Scholar] [CrossRef]
- Concordet, J.-P.; Haeussler, M. CRISPOR: Intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res. 2018, 46, W242–W245. [Google Scholar] [CrossRef]
- Hassan, M.M.; Zhang, Y.; Yuan, G.; De, K.; Chen, J.-G.; Muchero, W.; Tuskan, G.A.; Qi, Y.; Yang, X. Construct design for CRISPR/Cas-based genome editing in plants. Trends Plant Sci. 2021, 26, 1133–1152. [Google Scholar] [CrossRef]
- Sun, T.; Liu, Q.; Chen, X.; Hu, F.; Wang, K. Hi-TOM 2.0: An improved platform for high-throughput mutation detection. Sci. China Life Sci. 2024, 67, 1532–1534. [Google Scholar] [CrossRef] [PubMed]
- Clement, K.; Rees, H.; Canver, M.C.; Farouni, R.; Gehrke, J.M.; Hsu, J.Y.; Cole, M.A.; Liu, D.R.; Joung, J.K.; Bauer, D.E.; et al. CRISPResso2 provides accurate and rapid genome editing sequence analysis. Nat. Biotechnol. 2019, 37, 215–216. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Shan, C.; Kang, F.; Yu, L.; Li, Z.; Yin, Y. CRISPR-GRANT: A cross-platform graphical analysis tool for high-throughput CRISPR-based genome editing evaluation. BMC Bioinform. 2023, 24, 219. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, G.; Wu, Y.; Bao, Y.; Zhang, Y.; Zhang, T. CrisprStitch: Fast evaluation of the efficiency of CRISPR editing systems. Plant Commun. 2024, 5, 100783. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shang, M.; Liu, T.; Wang, K. High-throughput methods for genome editing: The more the better. Plant Physiol. 2022, 188, 1731–1745. [Google Scholar] [CrossRef]
- Gaillochet, C.; Develtere, W.; Jacobs, T.B. CRISPR screens in plants: Approaches, guidelines, and future prospects. Plant Cell 2021, 33, 794–813. [Google Scholar] [CrossRef]
- Lu, Y.; Ye, X.; Guo, R.; Huang, J.; Wang, W.; Tang, J.; Tan, L.; Zhu, J.-K.; Chu, C.; Qian, Y. Genome-wide Targeted Mutagenesis in Rice Using the CRISPR/Cas9 System. Mol. Plant 2017, 10, 1242–1245. [Google Scholar] [CrossRef]
- Meng, X.; Yu, H.; Zhang, Y.; Zhuang, F.; Song, X.; Gao, S.; Gao, C.; Li, J. Construction of a Genome-Wide Mutant Library in Rice Using CRISPR/Cas9. Mol. Plant 2017, 10, 1238–1241. [Google Scholar] [CrossRef] [PubMed]
- Bi, M.; Wang, Z.; Cheng, K.; Cui, Y.; He, Y.; Ma, J.; Qi, M. Construction of transcription factor mutagenesis population in tomato using a pooled CRISPR/Cas9 plasmid library. Plant Physiol. Biochem. 2023, 205, 108094. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, K.; Yan, S.; Tang, M.; Zhou, W.; Yin, Y.; Chen, K.; Zhang, C.; Li, M. Genome-scale targeted mutagenesis in Brassica napus using a pooled CRISPR library. Genome Res. 2023, 33, 798–809. [Google Scholar] [CrossRef]
- Jacobs, T.B.; Zhang, N.; Patel, D.; Martin, G.B. Generation of a Collection of Mutant Tomato Lines Using Pooled CRISPR Libraries. Plant Physiol. 2017, 174, 2023–2037. [Google Scholar] [CrossRef]
- Bai, M.; Yuan, J.; Kuang, H.; Gong, P.; Li, S.; Zhang, Z.; Liu, B.; Sun, J.; Yang, M.; Yang, L.; et al. Generation of a multiplex mutagenesis population via pooled CRISPR-Cas9 in soya bean. Plant Biotechnol. J. 2019, 18, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-J.; Jian, L.; Xu, J.; Zhang, Q.; Zhang, M.; Jin, M.; Peng, Y.; Yan, J.; Han, B.; Liu, J.; et al. High-Throughput CRISPR/Cas9 Mutagenesis Streamlines Trait Gene Identification in Maize. Plant Cell 2020, 32, 1397–1413. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Han, J.; Hao, Y.; Yang, Z.; Chen, J.; Liu, Y.-G.; Zhu, Q.; Chen, L. An effective strategy to establish a male sterility mutant mini-library by CRISPR/Cas9-mediated knockout of anther-specific genes in rice. J. Genet. Genom. 2019, 46, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Ke, R.; Du, M.; Yi, Y.; Chen, Y.; Wang, X.; Yao, L.; Liu, H.; Hou, X.; Xiong, L.; et al. A FLASH pipeline for arrayed CRISPR library construction and the gene function discovery of rice receptor-like kinases. Mol. Plant 2022, 15, 243–257. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, S.; Han, Y.; Liu, G.; Liu, J.; Yang, Q.; Zhang, T.; Shen, J.; Fan, X.; Zhang, C.; et al. A CRISPR/Cas9-mediated mutant library of seed-preferred genes in rice. Plant Biotechnol. J. 2024, 22, 3012–3014. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.; Alariqi, M.; Ma, Y.; Li, Y.; Liu, Z.; Zhang, R.; Jin, S.; Min, L.; Zhang, X. Efficient CRISPR/Cas9 mediated Pooled-sgRNAs assembly accelerates targeting multiple genes related to male sterility in cotton. Plant Methods 2021, 17, 16. [Google Scholar] [CrossRef]
- Sun, L.; Alariqi, M.; Wang, Y.; Wang, Q.; Xu, Z.; Zafar, M.N.; Yang, G.; Jia, R.; Hussain, A.; Chen, Y.; et al. Construction of Host Plant Insect-Resistance Mutant Library by High-Throughput CRISPR/Cas9 System and Identification of A Broad-Spectrum Insect Resistance Gene. Adv. Sci. 2023, 11, 2306157. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liang, S.; Wang, G.; Hu, T.; Fu, C.; Wang, Q.; Xu, Z.; Fan, Y.; Che, L.; Min, L.; et al. CRISPR/Cas9-mediated generation of a mutant library of cotton CDPK gene family for identifying insect-resistant genes. Plant Commun. 2024, 12, 101047. [Google Scholar] [CrossRef]
- Lorenzo, C.D.; Debray, K.; Herwegh, D.; Develtere, W.; Impens, L.; Schaumont, D.; Vandeputte, W.; Aesaert, S.; Coussens, G.; De Boe, Y.; et al. BREEDIT: A multiplex genome editing strategy to improve complex quantitative traits in maize. Plant Cell 2023, 35, 218–238. [Google Scholar] [CrossRef] [PubMed]
- Butt, H.; Eid, A.; Momin, A.A.; Bazin, J.; Crespi, M.; Arold, S.T.; Mahfouz, M.M. CRISPR directed evolution of the spliceosome for resistance to splicing inhibitors. Genome Biol. 2019, 20, 73. [Google Scholar] [CrossRef]
- Liu, X.; Qin, R.; Li, J.; Liao, S.; Shan, T.; Xu, R.; Wu, D.; Wei, P. A CRISPR-Cas9-mediated domain-specific base-editing screen enables functional assessment of ACCase variants in rice. Plant Biotechnol. J. 2020, 18, 1845–1847. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Li, S.; Ren, B.; Yan, F.; Spetz, C.; Li, X.; Zhou, X.; Zhou, H. Base-Editing-Mediated Artificial Evolution of OsALS1 In Planta to Develop Novel Herbicide-Tolerant Rice Germplasms. Mol. Plant 2020, 13, 565–572. [Google Scholar] [CrossRef]
- Xu, R.; Liu, X.; Li, J.; Qin, R.; Wei, P. Identification of herbicide resistance OsACC1 mutations via in planta prime-editing-library screening in rice. Nat. Plants 2021, 7, 888–892. [Google Scholar] [CrossRef]
- Wang, X.; Pan, W.; Sun, C.; Yang, H.; Cheng, Z.; Yan, F.; Ma, G.; Shang, Y.; Zhang, R.; Gao, C.; et al. Creating large-scale genetic diversity in Arabidopsis via base editing-mediated deep artificial evolution. Genome Biol. 2024, 25, 215. [Google Scholar] [CrossRef]
- Peng, A.; Chen, S.; Lei, T.; Xu, L.; He, Y.; Wu, L.; Yao, L.; Zou, X. Engineering canker-resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene CsLOB1 promoter in citrus. Plant Biotechnol. J. 2017, 15, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Leal, D.; Lemmon, Z.H.; Man, J.; Bartlett, M.E.; Lippman, Z.B. Engineering Quantitative Trait Variation for Crop Improvement by Genome Editing. Cell 2017, 171, 470–480. [Google Scholar] [CrossRef]
- Wang, X.; Aguirre, L.; Rodríguez-Leal, D.; Hendelman, A.; Benoit, M.; Lippman, Z.B. Dissecting cis-regulatory control of quantitative trait variation in a plant stem cell circuit. Nat. Plants 2021, 7, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Hendelman, A.; Zebell, S.; Rodriguez-Leal, D.; Dukler, N.; Robitaille, G.; Wu, X.; Kostyun, J.; Tal, L.; Wang, P.; Bartlett, M.E.; et al. Conserved pleiotropy of an ancient plant homeobox gene uncovered by cis-regulatory dissection. Cell 2021, 184, 1724–1739. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gallagher, J.; Arevalo, E.D.; Chen, R.; Skopelitis, T.; Wu, Q.; Bartlett, M.; Jackson, D. Enhancing grain-yield-related traits by CRISPR–Cas9 promoter editing of maize CLE genes. Nat. Plants 2021, 7, 287–294. [Google Scholar] [CrossRef]
- Huang, L.; Li, Q.; Zhang, C.; Chu, R.; Gu, Z.; Tan, H.; Zhao, D.; Fan, X.; Liu, Q. Creating novel Wx alleles with fine-tuned amylose levels and improved grain quality in rice by promoter editing using CRISPR/Cas9 system. Plant Biotechnol. J. 2020, 18, 2164–2166. [Google Scholar] [CrossRef]
- Zhou, S.; Cai, L.; Wu, H.; Wang, B.; Gu, B.; Cui, S.; Huang, X.; Xu, Z.; Hao, B.; Hou, H.; et al. Fine-tuning rice heading date through multiplex editing of the regulatory regions of key genes by CRISPR-Cas9. Plant Biotechnol. J. 2023, 22, 751–758. [Google Scholar] [CrossRef]
- Song, X.; Meng, X.; Guo, H.; Cheng, Q.; Jing, Y.; Chen, M.; Liu, G.; Wang, B.; Wang, Y.; Li, J.; et al. Targeting a gene regulatory element enhances rice grain yield by decoupling panicle number and size. Nat. Biotechnol. 2022, 40, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Avsec, Ž.; Weilert, M.; Shrikumar, A.; Krueger, S.; Alexandari, A.; Dalal, K.; Fropf, R.; McAnany, C.; Gagneur, J.; Kundaje, A.; et al. Base-resolution models of transcription-factor binding reveal soft motif syntax. Nat. Genet. 2021, 53, 354–366. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, Y. Beyond knockouts: Fine-tuning regulation of gene expression in plants with CRISPR-Cas-based promoter editing. New Phytol. 2023, 239, 868–874. [Google Scholar] [CrossRef]
- Cui, Y.; Cao, Q.; Li, Y.; He, M.; Liu, X.; Cubas, P. Advances in cis-element- and natural variation-mediated transcriptional regulation and applications in gene editing of major crops. J. Exp. Bot. 2023, 74, 5441–5457. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, G.; Zhao, Y.; Zhang, R.; Tang, X.; Li, L.; Jia, X.; Guo, Y.; Wu, Y.; Han, Y.; et al. An efficient CRISPR–Cas12a promoter editing system for crop improvement. Nat. Plants 2023, 9, 588–604. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Lu, J. Function and Evolution of Upstream ORFs in Eukaryotes. Trends Biochem. Sci. 2019, 44, 782–794. [Google Scholar] [CrossRef]
- Zhang, H.; Si, X.; Ji, X.; Fan, R.; Liu, J.; Chen, K.; Wang, D.; Gao, C. Genome editing of upstream open reading frames enables translational control in plants. Nat. Biotechnol. 2018, 36, 894–898. [Google Scholar] [CrossRef]
- Si, X.; Zhang, H.; Wang, Y.; Chen, K.; Gao, C. Manipulating gene translation in plants by CRISPR–Cas9-mediated genome editing of upstream open reading frames. Nat. Protoc. 2020, 15, 338–363. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Chen, K.; Zhu, H.; Zhang, R.; Zhang, H.; Li, B.; Gao, C. Fine-tuning sugar content in strawberry. Genome Biol. 2020, 21, 230. [Google Scholar] [CrossRef] [PubMed]
- Deslous, P.; Bournonville, C.; Decros, G.; Okabe, Y.; Mauxion, J.-P.; Jorly, J.; Gadin, S.; Brès, C.; Mori, K.; Ferrand, C.; et al. Overproduction of ascorbic acid impairs pollen fertility in tomato. J. Exp. Bot. 2021, 72, 3091–3107. [Google Scholar] [CrossRef] [PubMed]
- Patel-Tupper, D.; Kelikian, A.; Leipertz, A.; Maryn, N.; Tjahjadi, M.; Karavolias, N.G.; Cho, M.-J.; Niyogi, K.K. Multiplexed CRISPR—Cas9 mutagenesis of rice PSBS1 noncoding sequences for transgene—Free overexpression. Sci. Adv. 2024, 10, eadm7452. [Google Scholar] [CrossRef] [PubMed]
- Hyun, Y.; Kim, J.; Cho, S.W.; Choi, Y.; Kim, J.-S.; Coupland, G. Site-directed mutagenesis in Arabidopsis thaliana using dividing tissue-targeted RGEN of the CRISPR/Cas system to generate heritable null alleles. Planta 2014, 241, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Wei, S.; Wu, Y.; Hu, R.; Li, H.; Yang, W.; Xie, Q. High-Efficiency Genome Editing in Arabidopsis Using YAO Promoter-Driven CRISPR/Cas9 System. Mol. Plant 2015, 8, 1820–1823. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Zhang, Z.; Feng, Z.; Wei, P.; Zhang, H.; Botella, J.R.; Zhu, J.K. Development of germ-line-specific CRISPR-Cas9 systems to improve the production of heritable gene modifications in Arabidopsis. Plant Biotechnol. J. 2015, 14, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Miki, D.; Zhang, W.; Zeng, W.; Feng, Z.; Zhu, J.-K. CRISPR/Cas9-mediated gene targeting in Arabidopsis using sequential transformation. Nat. Commun. 2018, 9, 1967. [Google Scholar] [CrossRef]
- Wolter, F.; Klemm, J.; Puchta, H. Efficient in planta gene targeting in Arabidopsis using egg cell-specific expression of the Cas9 nuclease of Staphylococcus aureus. Plant J. 2018, 94, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Wang, M.; Huang, L.; Wu, F.; Tao, J.; Mo, B.; Yu, Y. A high-efficient and naked-eye visible CRISPR/Cas9 system in Arabidopsis. Planta 2023, 257, 30. [Google Scholar] [CrossRef] [PubMed]
- Decaestecker, W.; Buono, R.A.; Pfeiffer, M.L.; Vangheluwe, N.; Jourquin, J.; Karimi, M.; Van Isterdael, G.; Beeckman, T.; Nowack, M.K.; Jacobs, T.B. CRISPR-TSKO: A Technique for Efficient Mutagenesis in Specific Cell Types, Tissues, or Organs in Arabidopsis. Plant Cell 2019, 31, 2868–2887. [Google Scholar] [CrossRef]
- Feder, A.; Jensen, S.; Wang, A.; Courtney, L.; Middleton, L.; Van Eck, J.; Liu, Y.; Giovannoni, J.J. Tomato fruit as a model for tissue-specific gene silencing in crop plants. Hortic. Res. 2020, 7, 142. [Google Scholar] [CrossRef]
- Lei, J.; Dai, P.; Li, J.; Yang, M.; Li, X.; Zhang, W.; Zhou, G.; Guo, W.; Liu, X. Tissue-Specific CRISPR/Cas9 System of Cotton Pollen with GhPLIMP2b and GhMYB24 Promoters. J. Plant Biol. 2020, 64, 13–21. [Google Scholar] [CrossRef]
- He, Y.; Zhu, M.; Wang, L.; Wu, J.; Wang, Q.; Wang, R.; Zhao, Y. Programmed Self-Elimination of the CRISPR/Cas9 Construct Greatly Accelerates the Isolation of Edited and Transgene-Free Rice Plants. Mol. Plant 2018, 11, 1210–1213. [Google Scholar] [CrossRef]
- Gao, X.; Chen, J.; Dai, X.; Zhang, D.; Zhao, Y. An Effective Strategy for Reliably Isolating Heritable and Cas9-Free Arabidopsis Mutants Generated by CRISPR/Cas9-Mediated Genome Editing. Plant Physiol. 2016, 171, 1794–1800. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H. A novel CRISPR/Cas9 system for efficiently generating Cas9-free multiplex mutants in Arabidopsis. aBIOTECH 2019, 1, 6–14. [Google Scholar] [CrossRef]
- Deng, Y.; Duan, A.; Li, T.; Wang, H.; Xiong, A. A betaxanthin-based visible and fluorescent reporter for monitoring plant transformation. Crop J. 2023, 11, 666–671. [Google Scholar] [CrossRef]
- He, Y.; Zhang, T.; Sun, H.; Zhan, H.; Zhao, Y. A reporter for noninvasively monitoring gene expression and plant transformation. Hortic. Res. 2020, 7, 152. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kang, M.; Ji, Q.; Grosic, S.; Wang, K. New T-DNA binary vectors with NptII selection and RUBY reporter for efficient maize transformation and targeted mutagenesis. Plant Physiol. 2023, 192, 2598–2603. [Google Scholar] [CrossRef]
- Xu, J.; Yin, Y.; Jian, L.; Han, B.; Chen, Z.; Yan, J.; Liu, X. Seeing is believing: A visualization toolbox to enhance selection efficiency in maize genome editing. Plant Biotechnol. J. 2021, 19, 872–874. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhu, J.; Qi, X.; Cheng, B.; Liu, C.; Xie, C. Establishment of an efficient seed fluorescence reporter-assisted CRISPR/Cas9 gene editing in maize. J. Integr. Plant Biol. 2021, 63, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zeng, J.; Yuan, C.; Guo, Y.; Yu, H.; Li, Y.; Huang, C. Cas9-PF, an early flowering and visual selection marker system, enhances the frequency of editing event occurrence and expedites the isolation of genome-edited and transgene-free plants. Plant Biotechnol. J. 2019, 17, 1191–1193. [Google Scholar] [CrossRef]
- Kong, X.; Pan, W.; Sun, N.; Zhang, T.; Liu, L.; Zhang, H. GLABRA2-based selection efficiently enriches Cas9-generated nonchimeric mutants in the T1 generation. Plant Physiol. 2021, 187, 758–768. [Google Scholar] [CrossRef]
- Kelliher, T.; Starr, D.; Su, X.; Tang, G.; Chen, Z.; Carter, J.; Wittich, P.E.; Dong, S.; Green, J.; Burch, E.; et al. One-step genome editing of elite crop germplasm during haploid induction. Nat. Biotechnol. 2019, 37, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhu, L.; Zhao, B.; Zhao, Y.; Xie, Y.; Zheng, Z.; Li, Y.; Sun, J.; Wang, H. Development of a Haploid-Inducer Mediated Genome Editing System for Accelerating Maize Breeding. Mol. Plant 2019, 12, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Iwase, A.; Mita, K.; Nonaka, S.; Ikeuchi, M.; Koizuka, C.; Ohnuma, M.; Ezura, H.; Imamura, J.; Sugimoto, K. WIND1-based acquisition of regeneration competency in Arabidopsis and rapeseed. J. Plant Res. 2015, 128, 389–397. [Google Scholar] [CrossRef]
- Lowe, K.; Wu, E.; Wang, N.; Hoerster, G.; Hastings, C.; Cho, M.-J.; Scelonge, C.; Lenderts, B.; Chamberlin, M.; Cushatt, J.; et al. Morphogenic Regulators Baby boom and Wuschel Improve Monocot Transformation. Plant Cell 2016, 28, 1998–2015. [Google Scholar] [CrossRef]
- Chen, J.; Tomes, S.; Gleave, A.P.; Hall, W.; Luo, Z.; Xu, J.; Yao, J.-L. Significant improvement of apple (Malus domestica Borkh.) transgenic plant production by pre-transformation with a Baby boom transcription factor. Hortic. Res. 2022, 9, uhab014. [Google Scholar] [CrossRef]
- Zhang, T.-Q.; Lian, H.; Zhou, C.-M.; Xu, L.; Jiao, Y.; Wang, J.-W. A Two-Step Model for de Novo Activation of WUSCHEL during Plant Shoot Regeneration. Plant Cell 2017, 29, 1073–1087. [Google Scholar] [CrossRef]
- Omidbakhshfard, M.A.; Proost, S.; Fujikura, U.; Mueller-Roeber, B. Growth-Regulating Factors (GRFs): A Small Transcription Factor Family with Important Functions in Plant Biology. Mol. Plant 2015, 8, 998–1010. [Google Scholar] [CrossRef] [PubMed]
- Liebsch, D.; Palatnik, J.F. MicroRNA miR396, GRF transcription factors and GIF co-regulators: A conserved plant growth regulatory module with potential for breeding and biotechnology. Curr. Opin. Plant Biol. 2020, 53, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Martin-Ortigosa, S.; Finer, J.; Orchard, N.; Gunadi, A.; Batts, L.A.; Thakare, D.; Rush, B.; Schmitz, O.; Stuiver, M.; et al. Overexpression of the Transcription Factor GROWTH-REGULATING FACTOR5 Improves Transformation of Dicot and Monocot Species. Front. Plant Sci. 2020, 11, 572319. [Google Scholar] [CrossRef]
- Debernardi, J.M.; Tricoli, D.M.; Ercoli, M.F.; Hayta, S.; Ronald, P.; Palatnik, J.F.; Dubcovsky, J. A GRF–GIF chimeric protein improves the regeneration efficiency of transgenic plants. Nat. Biotechnol. 2020, 38, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Lian, Z.; Nguyen, C.D.; Liu, L.; Wang, G.; Chen, J.; Wang, S.; Yi, G.; Wilson, S.; Ozias-Akins, P.; Gong, H.; et al. Application of developmental regulators to improve in planta or in vitro transformation in plants. Plant Biotechnol. J. 2022, 20, 1622–1635. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, H.; Peng, J.; Yao, W.J.; Wang, Y.P.; Zhang, F.L.; Wang, S.R.; Zhao, Y.; Zhao, X.Y.; Zhang, X.S.; et al. Enhancing wheat regeneration and genetic transformation through overexpression of TaLAX1. Plant Commun. 2024, 5, 100738. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bie, X.M.; Lin, X.; Li, M.; Wang, H.; Zhang, X.; Yang, Y.; Zhang, C.; Zhang, X.S.; Xiao, J. Uncovering the transcriptional regulatory network involved in boosting wheat regeneration and transformation. Nat. Plants 2023, 9, 908–925. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, Z.; Wang, G.; Cong, Y.; Yu, L.; Jia, R.; Qin, Y.; Zhang, G.; Li, B.; Yuan, D.; et al. Single-cell resolution analysis reveals the preparation for reprogramming the fate of stem cell niche in cotton lateral meristem. Genome Biol. 2023, 24, 194. [Google Scholar]
- Zhang, Q.; Zhang, Y.; Lu, M.-H.; Chai, Y.-P.; Jiang, Y.-Y.; Zhou, Y.; Wang, X.-C.; Chen, Q.-J. A Novel Ternary Vector System United with Morphogenic Genes Enhances CRISPR/Cas Delivery in Maize. Plant Physiol. 2019, 181, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Jang, S.Y.; Jie, E.Y.; Choi, S.H.; Park, O.-S.; Bae, S.H.; Kim, H.-S.; Kim, S.W.; Hwang, G.-S.; Seo, P.J. Adenosine monophosphate enhances callus regeneration competence for de novo plant organogenesis. Mol. Plant 2023, 16, 1867–1870. [Google Scholar] [CrossRef] [PubMed]
- Che, P.; Anand, A.; Wu, E.; Sander, J.D.; Simon, M.K.; Zhu, W.; Sigmund, A.L.; Zastrow-Hayes, G.; Miller, M.; Liu, D.; et al. Developing a flexible, high-efficiency Agrobacterium-mediated sorghum transformation system with broad application. Plant Biotechnol. J. 2018, 16, 1388–1395. [Google Scholar] [CrossRef]
- Maher, M.F.; Nasti, R.A.; Vollbrecht, M.; Starker, C.G.; Clark, M.D.; Voytas, D.F. Plant gene editing through de novo induction of meristems. Nat. Biotechnol. 2019, 38, 84–89. [Google Scholar] [CrossRef]
- Ali, Z.; Abul-faraj, A.; Li, L.; Ghosh, N.; Piatek, M.; Mahjoub, A.; Aouida, M.; Piatek, A.; Baltes, N.J.; Voytas, D.F.; et al. Efficient Virus-Mediated Genome Editing in Plants Using the CRISPR/Cas9 System. Mol. Plant 2015, 8, 1288–1291. [Google Scholar] [CrossRef] [PubMed]
- Nagalakshmi, U.; Meier, N.; Liu, J.-Y.; Voytas, D.F.; Dinesh-Kumar, S.P. High-efficiency multiplex biallelic heritable editing in Arabidopsis using an RNA virus. Plant Physiol. 2022, 189, 1241–1245. [Google Scholar] [CrossRef]
- Liu, D.; Xuan, S.; Prichard, L.E.; Donahue, L.I.; Pan, C.; Nagalakshmi, U.; Ellison, E.E.; Starker, C.G.; Dinesh-Kumar, S.P.; Qi, Y.; et al. Heritable base-editing in Arabidopsis using RNA viral vectors. Plant Physiol. 2022, 189, 1920–1924. [Google Scholar] [CrossRef] [PubMed]
- Ellison, E.E.; Nagalakshmi, U.; Gamo, M.E.; Huang, P.-J.; Dinesh-Kumar, S.; Voytas, D.F. Multiplexed heritable gene editing using RNA viruses and mobile single guide RNAs. Nat. Plants 2020, 6, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Hu, J.; Sun, Y.; Li, B.; Zhang, D.; Li, W.; Liu, J.; Li, D.; Gao, C.; Zhang, Y.; et al. Highly efficient heritable genome editing in wheat using an RNA virus and bypassing tissue culture. Mol. Plant 2021, 14, 1787–1798. [Google Scholar] [CrossRef]
- Gao, Q.; Xu, W.Y.; Yan, T.; Fang, X.D.; Cao, Q.; Zhang, Z.J.; Ding, Z.H.; Wang, Y.; Wang, X.B. Rescue of a plant cytorhabdovirus as versatile expression platforms for planthopper and cereal genomic studies. New Phytol. 2019, 223, 2120–2133. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, X.; Liu, H.; Li, Z. Highly efficient DNA-free plant genome editing using virally delivered CRISPR–Cas9. Nat. Plants 2020, 6, 773–779. [Google Scholar] [CrossRef]
- Yang, L.; Machin, F.; Wang, S.; Saplaoura, E.; Kragler, F. Heritable transgene-free genome editing in plants by grafting of wild-type shoots to transgenic donor rootstocks. Nat. Biotechnol. 2023, 41, 958–967. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, K.; Li, T.; Zhang, Y.; Wang, Y.; Zhao, Q.; Liu, J.; Zhang, H.; Liu, C.; Ran, Y.; et al. Efficient DNA-free genome editing of bread wheat using CRISPR/Cas9 ribonucleoprotein complexes. Nat. Commun. 2017, 8, 14261. [Google Scholar] [CrossRef] [PubMed]
- Malnoy, M.; Viola, R.; Jung, M.-H.; Koo, O.-J.; Kim, S.; Kim, J.-S.; Velasco, R.; Nagamangala Kanchiswamy, C. DNA-Free Genetically Edited Grapevine and Apple Protoplast Using CRISPR/Cas9 Ribonucleoproteins. Front. Plant Sci. 2016, 7, 1904. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Chen, K.; Yan, Y.; Zhang, Y.; Gao, C. Genotyping genome-edited mutations in plants using CRISPR ribonucleoprotein complexes. Plant Biotechnol. J. 2018, 16, 2053–2062. [Google Scholar] [CrossRef] [PubMed]
- Sant’Ana, R.R.A.; Caprestano, C.A.; Nodari, R.O.; Agapito-Tenfen, S.Z. PEG-Delivered CRISPR-Cas9 Ribonucleoproteins System for Gene-Editing Screening of Maize Protoplasts. Genes 2020, 11, 1029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Iaffaldano, B.; Qi, Y. CRISPR ribonucleoprotein-mediated genetic engineering in plants. Plant Commun. 2021, 2, 100168. [Google Scholar] [CrossRef]
- Banakar, R.; Schubert, M.; Collingwood, M.; Vakulskas, C.; Eggenberger, A.L.; Wang, K. Comparison of CRISPR-Cas9/Cas12a Ribonucleoprotein Complexes for Genome Editing Efficiency in the Rice Phytoene Desaturase (OsPDS) Gene. Rice 2020, 13, 4. [Google Scholar] [CrossRef]
- Liu, W.; Rudis, M.R.; Cheplick, M.H.; Millwood, R.J.; Yang, J.-P.; Ondzighi-Assoume, C.A.; Montgomery, G.A.; Burris, K.P.; Mazarei, M.; Chesnut, J.D.; et al. Lipofection-mediated genome editing using DNA-free delivery of the Cas9/gRNA ribonucleoprotein into plant cells. Plant Cell Rep. 2019, 39, 245–257. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, J.; Choi, S.A.; Kim, Y.-S.; Koo, O.; Choi, S.H.; Ahn, W.S.; Jie, E.Y.; Kim, S.W. Efficient genome editing using CRISPR–Cas9 RNP delivery into cabbage protoplasts via electro-transfection. Plant Biotechnol. Rep. 2020, 14, 695–702. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Meng, D.; Zhong, Y.; Chen, C.; Dong, X.; Xu, X.; Chen, B.; Li, W.; Li, L.; et al. A 4-bp Insertion at ZmPLA1 Encoding a Putative Phospholipase A Generates Haploid Induction in Maize. Mol. Plant 2017, 10, 520–522. [Google Scholar] [CrossRef] [PubMed]
- Kelliher, T.; Starr, D.; Richbourg, L.; Chintamanani, S.; Delzer, B.; Nuccio, M.L.; Green, J.; Chen, Z.; McCuiston, J.; Wang, W.; et al. MATRILINEAL, a sperm-specific phospholipase, triggers maize haploid induction. Nature 2017, 542, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Gilles, L.M.; Khaled, A.; Laffaire, J.B.; Chaignon, S.; Gendrot, G.; Laplaige, J.; Bergès, H.; Beydon, G.; Bayle, V.; Barret, P.; et al. Loss of pollen-specific phospholipase NOT LIKE DAD triggers gynogenesis in maize. EMBO J. 2017, 36, 707–717. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, Y.; Liu, C.; Liu, Y.; Wang, Y.; Liang, D.; Liu, J.; Sahoo, G.; Kelliher, T. OsMATL mutation induces haploid seed formation in indica rice. Nat. Plants 2018, 4, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhong, Y.; Qi, X.; Chen, M.; Liu, Z.; Chen, C.; Tian, X.; Li, J.; Jiao, Y.; Wang, D.; et al. Extension of the in vivo haploid induction system from diploid maize to hexaploid wheat. Plant Biotechnol. J. 2019, 18, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Liu, C.; Qi, X.; Jiao, Y.; Wang, D.; Wang, Y.; Liu, Z.; Chen, C.; Chen, B.; Tian, X.; et al. Mutation of ZmDMP enhances haploid induction in maize. Nat. Plants 2019, 5, 575–580. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, B.; Li, M.; Wang, D.; Jiao, Y.; Qi, X.; Wang, M.; Liu, Z.; Chen, C.; Wang, Y.; et al. A DMP-triggered in vivo maternal haploid induction system in the dicotyledonous Arabidopsis. Nat. Plants 2020, 6, 466–472. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Yue, Y.; Zhao, H.; Fei, X.; E, L.; Liu, C.; Chen, S.; Lai, J.; Song, W. Loss-of-function alleles of ZmPLD3 cause haploid induction in maize. Nat. Plants 2021, 7, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Impens, L.; Lorenzo, C.D.; Vandeputte, W.; Wytynck, P.; Debray, K.; Haeghebaert, J.; Herwegh, D.; Jacobs, T.B.; Ruttink, T.; Nelissen, H.; et al. Combining multiplex gene editing and doubled haploid technology in maize. New Phytol. 2023, 239, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Kuppu, S.; Ron, M.; Marimuthu, M.P.A.; Li, G.; Huddleson, A.; Siddeek, M.H.; Terry, J.; Buchner, R.; Shabek, N.; Comai, L.; et al. A variety of changes, including CRISPR/Cas9-mediated deletions, in CENH3 lead to haploid induction on outcrossing. Plant Biotechnol. J. 2020, 18, 2068–2080. [Google Scholar] [CrossRef]
- Lv, J.; Yu, K.; Wei, J.; Gui, H.; Liu, C.; Liang, D.; Wang, Y.; Zhou, H.; Carlin, R.; Rich, R.; et al. Generation of paternal haploids in wheat by genome editing of the centromeric histone CENH3. Nat. Biotechnol. 2020, 38, 1397–1401. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, M.; Albertsen, M.C.; Young, J.K.; Cigan, A.M. Concurrent modifications in the three homeologs of Ms45 gene with CRISPR-Cas9 lead to rapid generation of male sterile bread wheat (Triticum aestivum L.). Plant Mol. Biol. 2018, 97, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Okada, A.; Arndell, T.; Borisjuk, N.; Sharma, N.; Watson-Haigh, N.S.; Tucker, E.J.; Baumann, U.; Langridge, P.; Whitford, R. CRISPR/Cas9-mediated knockout of Ms1 enables the rapid generation of male-sterile hexaploid wheat lines for use in hybrid seed production. Plant Biotechnol. J. 2019, 17, 1905–1913. [Google Scholar] [CrossRef]
- Du, M.; Zhou, K.; Liu, Y.; Deng, L.; Zhang, X.; Lin, L.; Zhou, M.; Zhao, W.; Wen, C.; Xing, J.; et al. A biotechnology-based male-sterility system for hybrid seed production in tomato. Plant J. 2020, 102, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhou, Y.; Kishchenko, O.; Stepanenko, A.; Jatayev, S.; Zhang, D.; Borisjuk, N. Gene editing to facilitate hybrid crop production. Biotechnol. Adv. 2021, 46, 107676. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Peng, Z.; Tang, D.; Yang, Z.; Li, D.; Xu, Y.; Zhang, C.; Huang, S. Generation of self-compatible diploid potato by knockout of S-RNase. Nat. Plants 2018, 4, 651–654. [Google Scholar] [CrossRef]
- Qin, X.; Li, W.; Liu, Y.; Tan, M.; Ganal, M.; Chetelat, R.T. A farnesyl pyrophosphate synthase gene expressed in pollen functions in S-RNase-independent unilateral incompatibility. Plant J. 2018, 93, 417–430. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Y.; Li, B.; Liu, Z.; Khan, F.; Zhang, T.; Zhou, G.; Tu, J.; Shen, J.; Yi, B.; et al. Functional Analysis of M-Locus Protein Kinase Revealed a Novel Regulatory Mechanism of Self-Incompatibility in Brassica napus L. Int. J. Mol. Sci. 2019, 20, 3303. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhu, C.; Zheng, M.; Liu, M.; Zhang, D.; Liu, B.; Li, Q.; Si, J.; Ren, X.; Song, H. CRISPR/Cas9-mediated multiple gene editing in Brassica oleracea var. capitata using the endogenous tRNA-processing system. Hortic. Res. 2019, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, A.; Wani, S.H.; Saleem, F.; Yu, M.; Zhou, M.; Shabala, S.; Foyer, C. Rewilding crops for climate resilience: Economic analysis and de novo domestication strategies. J. Exp. Bot. 2021, 72, 6123–6139. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.-Y.; Fan, L. Orphan Crops and their Wild Relatives in the Genomic Era. Mol. Plant 2021, 14, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, S.; Han, B.; Li, J. The integrated genomics of crop domestication and breeding. Cell 2022, 185, 2828–2839. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H.; Li, J. De novo domestication: Retrace the history of agriculture to design future crops. Curr. Opin. Biotechnol. 2023, 81, 102946. [Google Scholar] [CrossRef]
- Curtin, S.; Qi, Y.; Peres, L.E.P.; Fernie, A.R.; Zsögön, A. Pathways to de novo domestication of crop wild relatives. Plant Physiol. 2022, 188, 1746–1756. [Google Scholar] [CrossRef]
- Li, T.; Yang, X.; Yu, Y.; Si, X.; Zhai, X.; Zhang, H.; Dong, W.; Gao, C.; Xu, C. Domestication of wild tomato is accelerated by genome editing. Nat. Biotechnol. 2018, 36, 1160–1163. [Google Scholar] [CrossRef]
- Zsögön, A.; Čermák, T.; Naves, E.R.; Notini, M.M.; Edel, K.H.; Weinl, S.; Freschi, L.; Voytas, D.F.; Kudla, J.; Peres, L.E.P. De novo domestication of wild tomato using genome editing. Nat. Biotechnol. 2018, 36, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, T.; Meng, X.; Du, H.; Zhang, J.; Liu, G.; Chen, M.; Jing, Y.; Kou, L.; Li, X.; et al. A route to de novo domestication of wild allotetraploid rice. Cell 2021, 184, 1156–1170. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, Z.H.; Reem, N.T.; Dalrymple, J.; Soyk, S.; Swartwood, K.E.; Rodriguez-Leal, D.; Van Eck, J.; Lippman, Z.B. Rapid improvement of domestication traits in an orphan crop by genome editing. Nat. Plants 2018, 4, 766–770. [Google Scholar] [CrossRef] [PubMed]
| Tools | Websites | References |
|---|---|---|
| CRISPR-P 2.0 | http://cbi.hzau.edu.cn/CRISPR2, accessed on 24 December 2024/ | [159] |
| CRISPR-Local | http://crispr.hzau.edu.cn/CRISPR-Local/, accessed on 24 December 2024 | [160] |
| CRISPR-GE | http://skl.scau.edu.cn/, accessed on 24 December 2024 | [161] |
| Cas designer | http://rgenome.net/cas-designer/, accessed on 24 December 2024 | [162] |
| CRISPOR | http://crispor.org, accessed on 24 December 2024 | [163] |
| TIDE | http://tide.nki.nl, accessed on 24 December 2024 | [4] |
| ICE | https://ice.synthego.com, accessed on 24 December 2024 | [4] |
| Hi-TOM2 | http://www.hi-tom.net/hitom2/, accessed on 24 December 2024 | [165] |
| CRISPResso2 | https://github.com/pinellolab/CRISPResso2, accessed on 24 December 2024 | [166] |
| CRISPR-GRANT | https://github.com/fuhuancheng/CRISPR-GRANT, accessed on 24 December 2024 | [167] |
| CrisprStitch | https://zhangtaolab.org/software/crisprstitch, accessed on 24 December 2024 | [168] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, W.; Wang, Z.; Liu, L. The Continuous Improvement of the Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)–CRISPR-Associated Protein System Has Led to Its Highly Efficient Application in Plants. Agriculture 2025, 15, 29. https://doi.org/10.3390/agriculture15010029
Tan W, Wang Z, Liu L. The Continuous Improvement of the Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)–CRISPR-Associated Protein System Has Led to Its Highly Efficient Application in Plants. Agriculture. 2025; 15(1):29. https://doi.org/10.3390/agriculture15010029
Chicago/Turabian StyleTan, Wanqing, Zhiyuan Wang, and Liezhao Liu. 2025. "The Continuous Improvement of the Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)–CRISPR-Associated Protein System Has Led to Its Highly Efficient Application in Plants" Agriculture 15, no. 1: 29. https://doi.org/10.3390/agriculture15010029
APA StyleTan, W., Wang, Z., & Liu, L. (2025). The Continuous Improvement of the Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)–CRISPR-Associated Protein System Has Led to Its Highly Efficient Application in Plants. Agriculture, 15(1), 29. https://doi.org/10.3390/agriculture15010029







