Genetic Diversity of Local Wheat (Triticum aestivum L.) and Traceability in the Production of Galician Bread (Protected Geographical Indication) by Microsatellites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Wheat Flour, Sourdough and Bread
2.3. DNA Isolation and PCR Conditions
2.4. Data Analysis
3. Results
3.1. Genetic Diversity
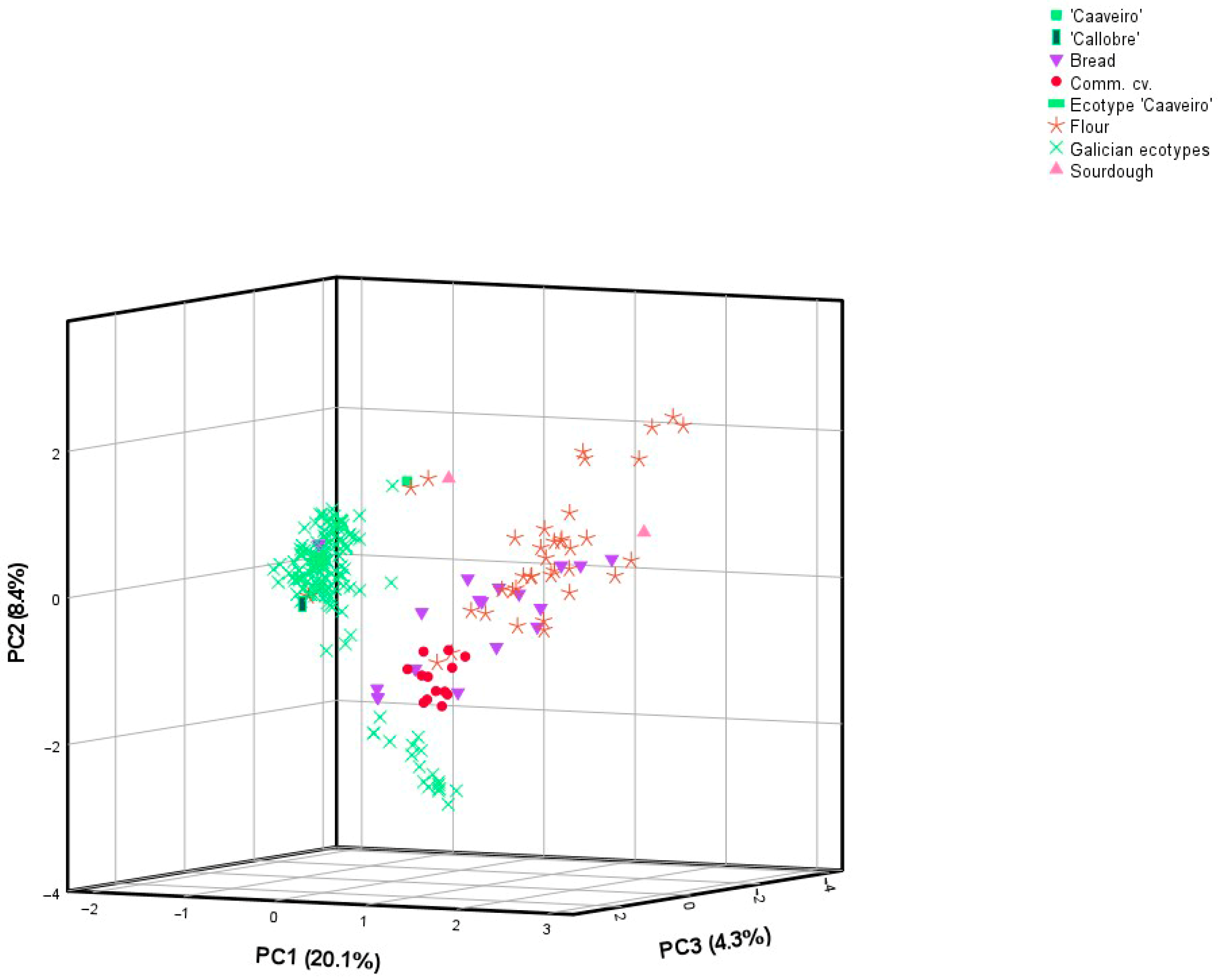
3.2. Genetic Structure, Dissimilarity of Allelic Data and Factorial Component Analysis
3.3. Traceability of Galician Wheat in Flour, Sourdough and Bread Samples
4. Discussion
4.1. Genetic Diversity
4.2. Genetic Structure, Dissimilarity of Allelic Data and Factorial Component Analysis
4.3. Traceability of Galician Wheat in Flour, Sourdough and Bread Samples
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pascual, L.; Ruiz, M.; López-Fernández, M.; Pérez-Peña, H.; Benavente, E.; Vázquez, J.F.; Sansaloni, C.; Giraldo, P. Genomic Analysis of Spanish Wheat Landraces Reveals Their Variability and Potential for Breeding. BMC Genom. 2020, 21, 122. [Google Scholar] [CrossRef] [PubMed]
- Marín-Sanz, M.; Sánchez-León, S.; León, E.; Barro, F. Comparative Characterization of the Gluten and Fructan Contents of Breads from Industrial and Artisan Bakeries: A Study of Food Products in the Spanish Market. Food Nutr. Res. 2022, 66, 8472. [Google Scholar] [CrossRef]
- Abdurezake, M.; Bekeko, Z.; Mohammed, A. Genetic Variability and Path Coefficient Analysis among Bread Wheat (Triticum Aestivum L.) Genotypes for Yield and Yield-related Traits in Bale Highlands, Southeastern Ethiopia. Agrosyst. Geosci. Environ. 2024, 7, 20515. [Google Scholar] [CrossRef]
- Hunter, M.C.; Smith, R.G.; Schipanski, M.E.; Atwood, L.W.; Mortensen, D.A. Agriculture in 2050: Recalibrating Targets for Sustainable Intensification. Bioscience 2017, 67, 386–391. [Google Scholar] [CrossRef]
- Patpour, M.; Hovmøller, M.S.; Justesen, A.F.; Newcomb, M.; Olivera, P.; Jin, Y.; Szabo, L.J.; Hodson, D.; Shahin, A.A.; Wanyera, R.; et al. Emergence of Virulence to SrTmp in the Ug99 Race Group of Wheat Stem Rust, Puccinia Graminis f. Sp. Tritici in Africa. Plant Dis. 2016, 100, 522. [Google Scholar] [CrossRef]
- Gadea, M. Los Trigos Españoles. Agric. Rev. Agropecu. 1949, 197–203. [Google Scholar]
- Gómez-Ibarlucea, C. Los Cereales de Invierno En Galicia. I: Situación Actual y Perspectivas. Agricultura 1989, 685, 720–723. [Google Scholar]
- Anuario de Estadística Agraria; MAPA (Ministerio de Agricultura, Pesca y Alimentación): Madrid, Spain, 2024.
- Ramil Rego, P. Paleothnobotánica de Yacimientos Arqueológicos Holocenos de Galicia (No Cantábrico). Munibe Antropol. Arkeol. 1993, 45, 165–174. [Google Scholar]
- Álvarez Núñez, A.; Ramil Rego, P.; Aira Rodríguez, M.J. Estudio Paleocarpológico Realizado En El Castro de Penalba (Campolameiro, Pontevedra. España). Bot. Complut. 1990, 16, 81–90. [Google Scholar]
- Téllez Molina, R.; Alonso Peña, M. Los Trigos de la Ceres Hispánica de Lagasca y Clemente; Instituto Nacional de Investigaciones Agronomicas: Madrid, Spain, 1952. [Google Scholar]
- Planellas Giralt, J. Ensayo de Una Flora Fanerogámica Gallega; Imprenta y litografía de D. Juan Rey Romero: Santiago, Spain, 1852. [Google Scholar]
- Merino Román, B. Flora Descriptiva e Ilustrada de Galicia; Merino Román, B., Ed.; Tipografía Galaica: Santiago, Spain, 1909; Volume 3. [Google Scholar]
- Gadea, M. Trigos Españoles; Instituto Nacional de Investigaciones Agronómicas: Madrid, Spain, 1954. [Google Scholar]
- Sánchez-Monge, E. Catálogo Genético de Trigos Españoles; Ministerio de Agricultura: Madrid, Spain, 1957. [Google Scholar]
- Sahuquillo, E.; Vila, M.I.F. Trigos de Cultivo Tradicional En Galicia: Caracterización Botánica e Agronómica; Xunta de Galicia: Santiago de Compostela, Spain, 1991. [Google Scholar]
- Zamora, L.U. Estudio Agromorfológico y Caracterización de Las Gluteninas de Alto Peso Molecular de Los Ecotipos Autóctonos Gallegos de Triticum aestivum l. 1. Ph.D. Thesis, Universidad de Santiago de Compostela, Galicia, Spain, 2021. [Google Scholar]
- Urquijo, L. ¿Cómo Recuperar Los Ecotipos Autóctonos? In Respostas Ás Preguntas Sobre o Pan e o Cereal do País, Serie Recursos Alimentarios 1; Romero Rodriguez, M., Pereira Lorenzo, S., Eds.; Monografías do Ibader: Lugo, Spain, 2018; pp. 33–38. [Google Scholar]
- European Commission Regulation 2019/2182 of 16 December 2019 Entering a Name in the Register of Protected Designations of Origin and Protected Geographical Indications [Pan Galego (PGI)]. Off. J. Eur. Union 2019, 62, L330.
- Perry, D.J.; Lee, S.J. Droplet Digital PCR for Verification of Interspersed Refuge in Midge Tolerant Wheat Varietal Blends. Can. J. Plant Sci. 2017, 97, 257–265. Available online: https://cdnsciencepub.com/doi/full/10.1139/cjps-2016-0223#.WlMAnv5Dsb0 (accessed on 10 December 2024).
- Morcia, C.; Bergami, R.; Scaramagli, S.; Ghizzoni, R.; Carnevali, P.; Terzi, V. A Chip Digital PCR Assay for Quantification of Common Wheat Contamination in Pasta Production Chain. Foods 2020, 9, 911. [Google Scholar] [CrossRef] [PubMed]
- Pasqualone, A.; Montemurro, C.; Grinn-Gofron, A.; Sonnante, G.; Blanco, A. Detection of Soft Wheat in Semolina and Durum Wheat Bread by Analysis of DNA Microsatellites. J. Agric. Food Chem. 2007, 55, 3312–3318. [Google Scholar] [CrossRef]
- Morcia, C.; Terzi, V.; Ghizzoni, R.; Vaiuso, C.; Delogu, C.; Andreani, L.; Venturini, A.; Carnevali, P.; Pompa, P.P.; Tumino, G. Digital PCR for Genotype Quantification: A Case Study in a Pasta Production Chain. Biology 2021, 10, 419. [Google Scholar] [CrossRef]
- Sonnante, G.; Montemurro, C.; Morgese, A.; Sabetta, W.; Blanco, A.; Pasqualone, A. DNA Microsatellite Region for a Reliable Quantification of Soft Wheat Adulteration in Durum Wheat-Based Foodstuffs by Real-Time PCR. J. Agric. Food Chem. 2009, 57, 10199–10204. [Google Scholar] [CrossRef]
- Carloni, E.; Amagliani, G.; Omiccioli, E.; Ceppetelli, V.; Del Mastro, M.; Rotundo, L.; Brandi, G.; Magnani, M. Validation and Application of a Quantitative Real-Time PCR Assay to Detect Common Wheat Adulteration of Durum Wheat for Pasta Production. Food Chem. 2017, 224, 86–91. [Google Scholar] [CrossRef]
- Fanelli, V.; Dellino, M.; Taranto, F.; De Giovanni, C.; Sabetta, W.; De Vita, P.; Montemurro, C. Varietal Identification in Pasta through an SSR-based Approach: A Case Study. J. Sci. Food Agric. 2023, 103, 5521–5528. [Google Scholar] [CrossRef]
- Su, W.; Xu, H.; Sun, L.; Lu, C.; Wu, R. Genetic Diversity Analysis of Volunteer Wheat Based on SSR Markers. J. Genet. 2023, 102, 54. [Google Scholar] [CrossRef]
- Ahmed, H.G.M.D.; Kashif, M.; Rashid, M.A.R.; Sajjad, M.; Zeng, Y. Genome Wide Diversity in Bread Wheat Evaluated by SSR Markers. Int. J. Agric. Biol. 2020, 24, 263–272. [Google Scholar] [CrossRef]
- Dagnaw, T.; Mulugeta, B.; Haileselassie, T.; Geleta, M.; Ortiz, R.; Tesfaye, K. Genetic Diversity of Durum Wheat (Triticum Turgidum, L. Ssp. Durum, Desf) Germplasm as Revealed by Morphological and SSR Markers. Genes 2023, 14, 1155. [Google Scholar] [CrossRef]
- Christov, N.K.; Tsonev, S.; Dragov, R.; Taneva, K.; Bozhanova, V.; Todorovska, E.G. Genetic Diversity and Population Structure of Modern Bulgarian and Foreign Durum Wheat Based on Microsatellite and Agronomic Data. Biotechnol. Biotechnol. Equip. 2022, 36, 637–652. [Google Scholar] [CrossRef]
- Devi, S.; Singh, V.; Yashveer, S.; Dalal, M.S.; Paras; Chawla, R.; Akbarzai, D.K.; Chaurasia, H. Molecular Characterization of Bread Wheat (Triticum aestivum) Genotypes Using SSR Markers. Indian J. Agric. Sci. 2023, 93, 948–953. [Google Scholar] [CrossRef]
- Jabari, M.; Golparvar, A.; Sorkhilalehloo, B.; Shams, M. Investigation of Genetic Diversity of Iranian Wild Relatives of Bread Wheat Using ISSR and SSR Markers. J. Genet. Eng. Biotechnol. 2023, 21, 73. [Google Scholar] [CrossRef] [PubMed]
- Ismail, R.M.; Fathallah, F.B.; El-Shafei, A.; Shoaib, R.M.; Mahfouz, S.A.; EL-Demardash, I.S. Enhancing Wheat Genetic Resources through Diallel Crosses for Production of New Lines. Egypt. J. Agron. 2023, 45, 139–155. [Google Scholar] [CrossRef]
- Kara, K.; Rached-Kanouni, M.; Mnasri, S.; Khammar, H.; Ben Naceur, M. Genetic Variability Assessment in Bread Wheat (Triticum Aestivum) Grown in Algeria Using Microsatellites SSR Markers. Biodiversitas 2020, 21, 6. [Google Scholar] [CrossRef]
- Tahir, O.; Bangash, S.A.K.; Ibrahim, M.; Shahab, S.; Khattak, S.H.; Ud Din, I.; Khan, M.N.; Hafeez, A.; Wahab, S.; Ali, B.; et al. Evaluation of Agronomic Performance and Genetic Diversity Analysis Using Simple Sequence Repeats Markers in Selected Wheat Lines. Sustainability 2022, 15, 293. [Google Scholar] [CrossRef]
- Tsonev, S.; Christov, N.K.; Mihova, G.; Dimitrova, A.; Todorovska, E.G. Genetic Diversity and Population Structure of Bread Wheat Varieties Grown in Bulgaria Based on Microsatellite and Phenotypic Analyses. Biotechnol. Biotechnol. Equip. 2021, 35, 1520–1533. [Google Scholar] [CrossRef]
- Khodadadi, E.; Farokhzadeh, S. Assessing Genetic Diversity of Indigenous Bread and Durum Wheat Cultivars in Iran Using Inter Simple Sequence Repeat (ISSR) Markers. Genet. Resour. Crop Evol. 2024, 71, 3135–3150. [Google Scholar] [CrossRef]
- Ramos-Cabrer, A.M.; Fernández-Canto, N.; Almeida-García, F.; Gorostidi, A.; Lombardero-Fernández, M.; Romero-Rodríguez, M.Á.; Pereira-Lorenzo, S. Traceability of the Local Cultivar ‘Caaveiro’ in Flour Mixtures Used to Produce Galician Bread by Simple Sequence Repeats and Droplet Digital Polymerase Chain Reaction Technology. Int. J. Food. Sci. Technol. 2022, 57, 7085–7098. [Google Scholar] [CrossRef]
- Fernández-Canto, M.N.; García-Gómez, M.B.; Boado-Crego, S.; Vázquez-Odériz, M.L.; Muñoz-Ferreiro, M.N.; Lombardero-Fernández, M.; Pereira-Lorenzo, S.; Romero-Rodríguez, M.Á. Element Content in Different Wheat Flours and Bread Varieties. Foods 2022, 11, 3176. [Google Scholar] [CrossRef]
- Fernández-Canto, N.; Romero-Rodríguez, M.Á.; Ramos-Cabrer, A.M.; Pereira-Lorenzo, S.; Lombardero-Fernández, M. Polarized Light Microscopy Guarantees the Use of Autochthonous Wheat in the Production of Flour for the Protected Geographical Indication ‘Galician Bread’. Food Control 2023, 147, 109597. [Google Scholar] [CrossRef]
- Fdez-Vidal, X.R.; Fernández-Canto, N.; Romero-Rodríguez, M.Á.; Ramos-Cabrer, A.M.; Pereira-Lorenzo, S.; Lombardero-Fernández, M. Neural Networks Allow the Automatic Verification of the Type of Flour, Analysing the Starch Granule Morphology, to Ensure the Protected Geographical Indication ‘Galician Bread’. Food Control 2024, 158, 110198. [Google Scholar] [CrossRef]
- Villa, T.C.C.; Maxted, N.; Scholten, M.; Ford-Lloyd, B. Defining and Identifying Crop Landraces. Plant Genet. Resour. 2005, 3, 373–384. [Google Scholar] [CrossRef]
- Ruiz, M.; Giraldo, P.; Royo, C.; Villegas, D.; Aranzana, M.J.; Carrillo, J.M. Diversity and Genetic Structure of a Collection of Spanish Durum Wheat Landraces. Crop Sci. 2012, 52, 2262–2275. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research—An Update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of Population Structure Using Multilocus Genotype Data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Rosenberg, N.A.; Donnelly, P. Association Mapping in Structured Populations. Am. J. Hum. Genet. 2000, 67, 170–181. [Google Scholar] [CrossRef]
- Pereira-Lorenzo, S.; Ramos-Cabrer, A.M.; Barreneche, T.; Mattioni, C.; Villani, F.; Díaz-Hernández, B.; Martín, L.M.; Robles-Loma, A.; Cáceres, Y.; Martín, A. Instant Domestication Process of European Chestnut Cultivars. Ann. Appl. Biol. 2019, 174, 74–85. [Google Scholar] [CrossRef]
- Porras-Hurtado, L.; Ruiz, Y.; Santos, C.; Phillips, C.; Carracedo, Á.; Lareu, M.V. An Overview of STRUCTURE: Applications, Parameter Settings, and Supporting Software. Front. Genet. 2013, 4, 98. [Google Scholar] [CrossRef]
- Pereira-Lorenzo, S.; Urrestarazu, J.; Ramos-Cabrer, A.M.; Miranda, C.; Pina, A.; Dapena, E.; Moreno, M.A.; Errea, P.; Llamero, N.; Díaz-Hernández, M.B.; et al. Analysis of the Genetic Diversity and Structure of the Spanish Apple Genetic Resources Suggests the Existence of an Iberian Genepool. Ann. Appl. Biol. 2017, 171, 424–440. [Google Scholar] [CrossRef]
- Alessandri, S.; Cabrer, A.M.R.; Martìn, M.A.; Mattioni, C.; Pereira-Lorenzo, S.; Dondini, L. Genetic Characterization of Italian and Spanish Wild and Domesticated Chestnut Trees. Sci. Hortic. 2022, 295, 110882. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the Number of Clusters of Individuals Using the Software STRUCTURE: A Simulation Study. Mol. Ecol 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Smouse, P.E.; Quattro, J.M. Analysis of Molecular Variance Inferred from Metric Distances among DNA Haplotypes: Application to Human Mitochondrial DNA Restriction Data. Genetics 1992, 131, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Michalakis, Y.; Excoffier, L. A Generic Estimation of Population Subdivision Using Distances Between Alleles With Special Reference for Microsatellite Loci. Genetics 1996, 142, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Lorenzo, S.; Ramos-Cabrer, A.M.; Barreneche, T.; Mattioni, C.; Villani, F.; Díaz-Hernández, M.B.; Martín, L.M.; Martín, Á. Database of European Chestnut Cultivars and Definition of a Core Collection Using Simple Sequence Repeats. Tree Genet. Genomes 2017, 13, 114. [Google Scholar] [CrossRef]
- Perrier, X.; Flori, A.; Bonnot, F. Data Analysis Methods. In Genetic Diversity of Cultivated Tropical Plants; Hamon, P., Seguin, M., Perrier, X., Glaszmann, J.C., Eds.; Science Publishers: Enfield, NH, USA; Montpellier, France, 2003; pp. 43–76. [Google Scholar]
- Perrier, X.; Jacquemoud-Collet, J.P. DARwin Software. In Genetic Diversity of Cultivated Tropical Plants; Hamon, P., Seguin, M., Perrier, X., Glaszmann, J.C., Eds.; Science Publishers: Enfield, NH, USA; Montpellier, France, 2003; pp. 43–76. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, version 28.0; IBM Corporation: Armonk, NY, USA, 2021.
- Goodman, L.A. The Analysis of Multidimensional Contingency Tables When Some Variables Are Posterior to Others: A Modified Path Analysis Approach. Biometrika 1973, 60, 179–192. [Google Scholar] [CrossRef]
- Llauradó, M.; Moreno- González, J. Classification of Northern Spanish Populations of Maize by Methods of Numerical Taxonomy. I. Morphological Traits. Maydica 1993, 38, 15–21. [Google Scholar]
- Pereira-Lorenzo, S.; Fernández-López, J.; Moreno-González, J. Variability and Grouping of Northwestern Spanish Chestnut Cultivars. I. Morphological Traits. J. Am. Soc. Hortic. Sci. 1996, 121, 183–189. [Google Scholar] [CrossRef]
- Earl, D.A.; VonHoldt, B.M. Structure Harvester: A Website and Program for Visualizing Structure Output and Implementing the Evanno Method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Zhang, P.; Dreisigacker, S.; Buerkert, A.; Alkhanjari, S.; Melchinger, A.E.; Warburton, M.L. Genetic Diversity and Relationships of Wheat Landraces from Oman Investigated with SSR Markers. Genet. Resour. Crop Evol. 2006, 53, 1351–1360. [Google Scholar] [CrossRef]
- Ballesta, P.; Maldonado, C.; Mora-Poblete, F.; Mieres-Castro, D.; del Pozo, A.; Lobos, G.A. Spectral-Based Classification of Genetically Differentiated Groups in Spring Wheat Grown under Contrasting Environments. Plants 2023, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Mondini, L.; Farina, A.; Porceddu, E.; Pagnotta, M.A. Analysis of Durum Wheat Germplasm Adapted to Different Climatic Conditions. Ann. Appl. Biol. 2010, 156, 211–219. [Google Scholar] [CrossRef]
- Alipour, H.; Bihamta, M.R.; Mohammadi, V.; Peyghambari, S.A.; Bai, G.; Zhang, G. Genotyping-by-Sequencing (GBS) Revealed Molecular Genetic Diversity of Iranian Wheat Landraces and Cultivars. Front. Plant Sci 2017, 8, 1293. [Google Scholar] [CrossRef] [PubMed]
- Amallah, L.; Taghouti, M.; Rhrib, K.; Gaboun, F.; Arahou, M.; Hassikou, R.; Diria, G. Validation of Simple Sequence Repeats Associated with Quality Traits in Durum Wheat. J. Crop Sci. Biotechnol 2016, 19, 137–150. [Google Scholar] [CrossRef]
- Arora, A.; Kundu, S.; Dilbaghi, N.; Sharma, I.; Tiwari, R. Population Structure and Genetic Diversity among Indian Wheat Varieties Using Microsatellite (SSR) Markers. Aust. J. Crop Sci. 2014, 8, 1281–1289. [Google Scholar]
- Zeven, A.C. Landraces: A Review of Definitions and Classifications. Euphytica 1998, 104, 127–139. [Google Scholar] [CrossRef]
- Brown, A.H.D. Isozymes, Plant Population Genetic Structure and Genetic Conservation. Theor. Appl. Genet. 1978, 52, 145–157. [Google Scholar] [CrossRef]
- Zeven, A.C. Traditional Maintenance Breeding of Landraces: 1. Data by Crop. Euphytica 2000, 116, 65–85. [Google Scholar] [CrossRef]
- Miyahara, T.; Miyake, N.; Sawahuji, K.; Kitta, K.; Nakamura, K.; Kondo, K.; Ozeki, Y. Wheat DNA Fragmentation of Commercial Processed Foods II. Mater. Methods. 2016, 23, 141–148. [Google Scholar]




| SSR (Locus) | Allelic Rank [43] | No. Alleles in Previous Works in Spain [38] | Alleles in Previous Works in Spain [38] | Common Alleles (Present Study) | Exclusive Alleles (Present Study) | Exclusive and Previously Unreported Alleles in Flours, Sourdoughs, and Breads |
|---|---|---|---|---|---|---|
| Xgwm0148 | 138–164 | 8 | 89, 138, 140, 142, 144, 146, 160, 164 | 142, 144, 146 | 89 2, 138 1, 140 1, 160 1, 164 1 | 140 1, 160 1, 162 3,4, 164 1, 166 1,3,4 |
| Xgwm0155 | 139–147 | 6 | 139, 141, 143, 145, 147, 149 | 141, 143, 145, 147 | 139 2, 149 1 | 124 1,3,4, 128 1,3,4 |
| Xgwm0156 | 283–317 | 11 | 283, 287, 289, 291, 293, 296, 300, 312, 315, 317, 319 | 287, 289, 291, 312 | 283 1, 293 2, 296 2, 300 1, 319 2 | 283 1, 300 1, 315 1,3, 317 1,3, 321 1,3,4 |
| Xgwm0186 | 117–137 | 8 | 100, 117, 119, 121, 123, 127, 129, 137 | 119, 121 | 100 1, 117 2, 123 2, 127 1, 129 1, 137 1 | 100 1, 117 2, 123 2, 127 1, 129 1, 137 1 |
| Xgwm0234 | 198–245 | 12 | 198, 201, 224, 226, 228, 230, 234, 236, 238, 240, 242, 244 | 201, 226, 228, 234, 236, 238, 240, 242, 244 | 198 1, 224 2, 230 2 | 198 1 |
| Xgwm0312 | 184–250 | 27 | 184, 192, 194, 196, 208, 210, 212, 214, 218, 220, 222, 224, 226, 228, 230, 232, 235, 237, 239, 241, 243, 245, 248, 250, 252, 256, 258 | 184, 220, 222, 230, 235 | 192 1, 194 1, 196 1, 208 1, 210 2, 212 2, 218 1, 224 2, 226 2, 228 2, 230, 232 2, 237 2, 239 2, 241 2, 243 2, 245 2, 248 2, 250 2, 252 2, 256 2, 258 2 | 192 1, 194 1, 196 1, 208 1, 214 3, 218 1, 228 2, 241 2, 243 2, 250 2, 252 2 |
| Xgwm0332 (A/B) | 190–245 | 22 | 190, 193, 195, 200, 204, 208, 211, 213, 217, 219, 221, 223, 225, 227, 229, 231, 233, 236, 241, 245, 250, 256 | A (190, 193, 195)/B (200, 211, 221, 236) | B (204 2, 208 1, 213 2, 217 2, 219 2, 223 2, 225 2, 227 2, 229 2, 231 2, 233 2, 241 1, 250 1, 256 2) | B (206 3,4, 208 1, 217 2, 229 2, 233 2, 241 1, 250 1) |
| Xgwm0570 | 96–159 | 13 | 96, 106, 136, 138, 141, 143, 145, 148, 151, 153, 155, 157, 159 | 136, 143, 145, 148, 151, 153 | 106 1, 138 1, 141 2, 155 2, 157 2, 159 2 | 106 1, 136, 138 1, 155 2, 159 2 |
| Xgwm0577 | 128–219 | 24 | 128, 130, 134, 136, 138, 140, 142, 147, 150, 152, 155, 157, 159, 162, 176, 203, 205, 207, 209, 211, 213, 215, 217, 219 | 140, 150, 152, 155, 157, 159, 162, 203, 205 | 128 1, 130 1, 136 2, 138 2, 142, 176 2, 207 2, 209 2, 211 2, 213 2, 215 1, 219 1 | 128 1, 130 1, 134 1,3, 136 2, 142, 147 1,3, 207 2 |
| Xgwm0060 | 116–138 | 10 | 116, 118, 122, 126, 128, 130, 132, 134, 136, 138 | 128, 134, 136, 138 | 118 1, 122 1, 126 1, 130 2, 132 2 | 116 1,3, 118 1, 120 3,4, 126 1, 132 2 |
| Xgwm0088 | 141–151 | 6 | 141, 143, 145, 147, 149, 151 | 143, 145 | 141 2, 147 1, 149 1, 151 1 | 147 1, 149 1, 151 1 |
| Xgwm0513 (A/B) | 148–213 | 17 | 148, 150, 154, 160, 162, 174, 184, 188, 189, 194, 200, 203, 205, 207, 209, 211, 213 | A (148, 150, 154)/B (211, 213) | A (160 1, 162 1)/B (188 1, 189 1, 200 2, 203 1, 205 1, 207 2, 209 2) | A (160 1, 162 1, 174 3/B (184 1,3, 188 1, 194 1,3, 203 1, 205 1) |
| Xgwm0389 | 114–151 | 15 | 114, 116, 118, 120, 122, 124, 126, 128, 131, 133, 135, 137, 139, 143, 151 | 116, 118, 120, 122, 133, 135 | 114 2124 2, 128 2, 131 2, 137 1, 139 1, 143 1 | 126 1,3, 139 1, 143 1, 151 1,3 |
| BARC155 | 180–206 | 9 | 180, 184, 186, 188, 194, 198, 201, 203, 206 | 184, 201, 203 | 180 1, 186 1, 188 1, 194 2, 198 2, 206 2 | 188 1 |
| BARC80 | 101–133 | 6 | 101, 103, 106, 109, 114, 133 | 106, 109 | 101 2, 103 2, 114 1 | 114 1, 118 3,4 |
| WMC468 | 129–157 | 12 | 129, 131, 133, 135, 140, 144, 146, 148, 151, 153, 155, 157 | 133, 135, 151, 155, 157 | 129 1, 140 2, 144 2, 146 1, 148 1, 153 2 | 129 1, 131 3, 146 1, 153 2 |
| Xgwm0002 (A/B) | 116–277 | 11 | 116, 118, 120, 127, 220, 222, 224, 231, 255, 257, 277 | A (116, 118, 120, 127)/B (220, 222, 224, 257) | B (231 1, 255 1) | B (231 1, 277 3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urquijo-Zamora, L.; Pereira-Lorenzo, S.; Romero-Rodríguez, Á.; Lombardero-Fernández, M.; Ramos-Cabrer, A.M.; Fernández-Otero, C.I. Genetic Diversity of Local Wheat (Triticum aestivum L.) and Traceability in the Production of Galician Bread (Protected Geographical Indication) by Microsatellites. Agriculture 2025, 15, 51. https://doi.org/10.3390/agriculture15010051
Urquijo-Zamora L, Pereira-Lorenzo S, Romero-Rodríguez Á, Lombardero-Fernández M, Ramos-Cabrer AM, Fernández-Otero CI. Genetic Diversity of Local Wheat (Triticum aestivum L.) and Traceability in the Production of Galician Bread (Protected Geographical Indication) by Microsatellites. Agriculture. 2025; 15(1):51. https://doi.org/10.3390/agriculture15010051
Chicago/Turabian StyleUrquijo-Zamora, Luís, Santiago Pereira-Lorenzo, Ángeles Romero-Rodríguez, Matilde Lombardero-Fernández, Ana María Ramos-Cabrer, and Cristina Isabel Fernández-Otero. 2025. "Genetic Diversity of Local Wheat (Triticum aestivum L.) and Traceability in the Production of Galician Bread (Protected Geographical Indication) by Microsatellites" Agriculture 15, no. 1: 51. https://doi.org/10.3390/agriculture15010051
APA StyleUrquijo-Zamora, L., Pereira-Lorenzo, S., Romero-Rodríguez, Á., Lombardero-Fernández, M., Ramos-Cabrer, A. M., & Fernández-Otero, C. I. (2025). Genetic Diversity of Local Wheat (Triticum aestivum L.) and Traceability in the Production of Galician Bread (Protected Geographical Indication) by Microsatellites. Agriculture, 15(1), 51. https://doi.org/10.3390/agriculture15010051







