Assessment of Physiological Traits of Fragaria vesca Genotypes Under Water Deficit Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
- Decis Mega 50 EW, targeting aphids (Aphidoidea) at a dosage of 0.25 L/ha.
- Mospilan 20 SP, targeting thrips (Thysanoptera, aphids (Aphidoidea), and greenhouse whitefly (Trialeurodes vaporariorum) at a concentration of 4 g per 10 L of water, applied at 3 L per 100 m2.
2.3. Measurements
- the relative leaf water content index (RWC) by the method of Yamasaki and Dillenburg [39];
- the gas exchange parameters, i.e., assimilation intensity CO2 net (Pn), transpiration intensity (E), stomatal conductivity H2O (gs) and substomatal CO2 concentration (ci), were measured using a TPS-2 portable gas analyzer with a PLC-4 (PP Systems, Amesbury, MA, USA); the photosynthetic water utilization factor (WUE) was determined on the basis of the Pn/E quotient;
- the fluorescence parameters of chlorophyll “a” were determined using a Handy PEA spectrofluorometer (Hansatech Ltd., Kings Lynn, UK), based on the standard procedure of the apparatus (3 × 650 nm LED, maximum actinic light intensity 3000 μmol m−2 s−1). The measurement was carried out in each variant on 15 randomly selected, fully grown bean leaves (the repetition was the measurement on one leaf), in a place previously darkened for 20 min, using factory-made clips (the irradiation area was 4 mm). The following parameters were measured: initial (zero) fluorescence, index of excitation energy loss in power antennas (F0), maximum fluorescence, after reduction of acceptors in PS II and after darkroom adaptation (FM), variable fluorescence, determined after dark adaptation (the parameter depends on maximum quantum efficiency of PS II) (FV = FM − F0), maximum potential efficiency of the photochemical reaction in PS II, determined after dark adaptation, after reduction of acceptors in PS II (FV/FM), chlorophyll fluorescence growth time from the beginning of the measurement to reaching maximum (TFM), and the area above the fluorescence induction curve of chlorophyll “a” between points F0 and FM proportional to the size of the pool of reduced plastoquinone electron acceptors in PSII (AM (Area)).
2.4. Meteorological Data
2.5. Statistical Analysis
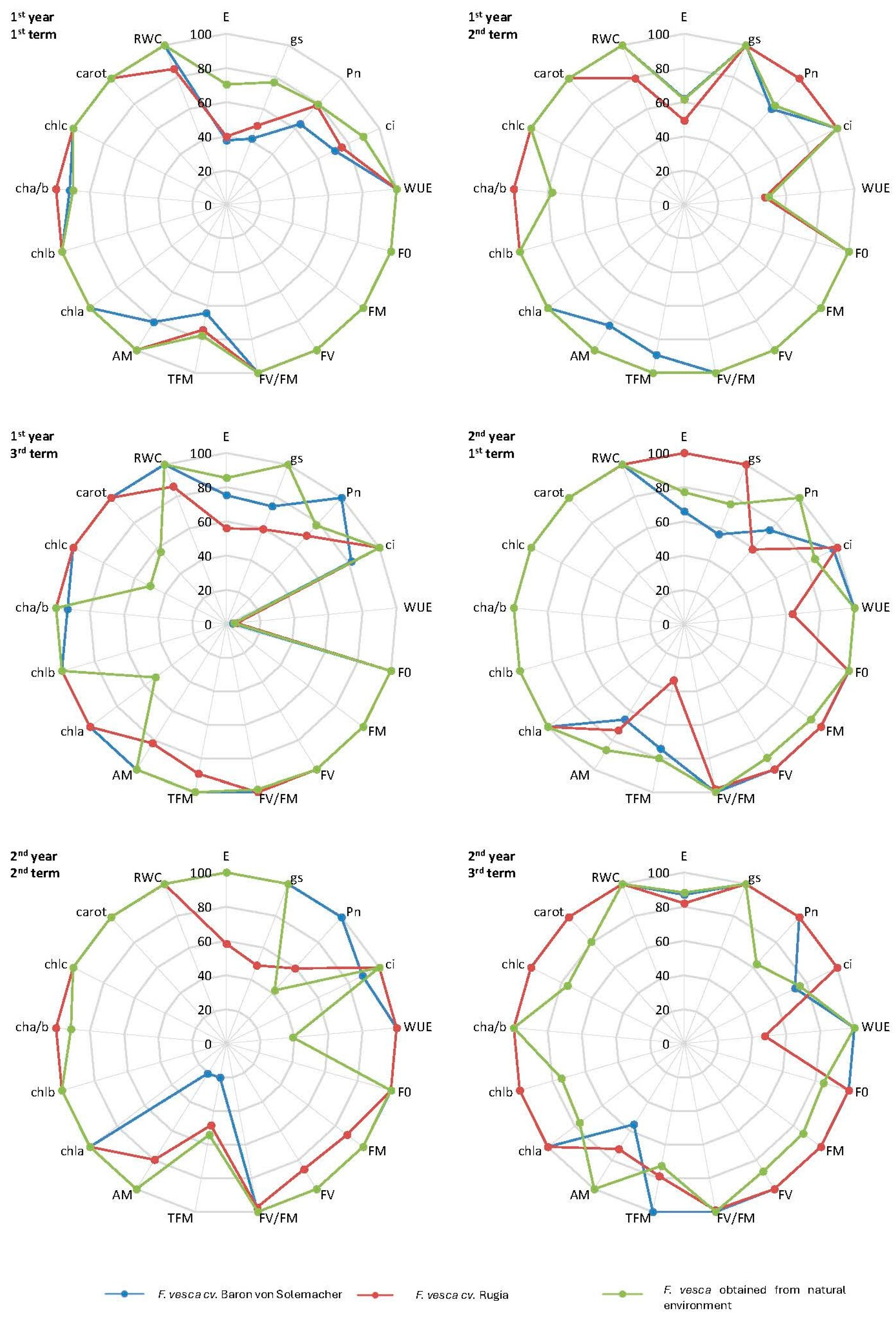
2.6. Radar Charts
- SDef—parameter value in conditions of deficit soil moisture;
- SCtrl—parameter value in conditions of control soil moisture.
- DDef—parameter value in conditions of deficit soil moisture;
- DCtrl—parameter value in conditions of control soil moisture.
3. Results
3.1. Evaluation of Differences in Physiological Traits, Chemical Composition, and Yield Among Genotypes
3.1.1. Gas Exchange Parameters
3.1.2. Chlorophyll “a” Fluorescence Parameters
3.1.3. Content of Photosynthetic Pigments
3.1.4. Relative Water Content in Leaves (RWC)
3.1.5. Fruit Yield and Weight of a Single Wild Strawberry Fruit
3.1.6. Fresh and Dry Mass of the Root System
3.1.7. Chemical Composition
3.2. Evaluation of Differences in Physiological Traits, Chemical Composition, and Yield Among Genotypes in Various Soil Moisture Conditions
3.2.1. Gas Exchange Parameters
3.2.2. Chlorophyll “a” Fluorescence Parameters
3.2.3. Content of Photosynthetic Pigments
3.2.4. Relative Water Content in Leaves (RWC)
3.2.5. Fruit Yield and Weight of a Single Wild Strawberry Fruit
3.2.6. Fresh and Dry Mass of the Root System
3.2.7. Chemical Composition
3.2.8. Radar Charts
4. Discussion
5. Conclusions
- This study enabled a comparative analysis of the physiological responses of Fragaria vesca plants, including two cultivated varieties (‘Rugia’ and ‘Baron von Solemacher’) and a wild strawberry population obtained from a natural habitat. The key findings are as follows:
- F. vesca genotypes exhibit differences in physiological traits. Wild plants display distinct physiological patterns compared to cultivated varieties, including higher chlorophyll fluorescence values and greater assimilation pigment content.
- Water deficiency in the substrate significantly impacts the physiological characteristics of F. vesca plants. Drought conditions lead to reductions in gas exchange parameters, water use efficiency, chlorophyll “a” fluorescence parameters, relative water content in leaves, and the mass of fruits. Conversely, drought increases the content of elements such as K, Ca, Mg, Na, Cu, Zn, and Mn in various plant organs.
- F. vesca genotypes differ in their physiological responses to water deficit in the substrate. Plants from the natural environment demonstrate lower sensitivity, in terms of physiological responses, to water deficit compared to cultivated varieties.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamdan, M.; Shafar, J. Effect of water irrigation techniques on growth and yield of rock melon (Cucumis melo Linn cv. Glamour). Adv. Plant Sci. Technol. 2017, 24, 80–84. [Google Scholar]
- Ghaderi, N.; Normohammadi, S.; Javadi, T. Morpho-physiological responses of strawberry (Fragaria × ananassa) to exogenous salicylic acid application under drought stress. J. Agric. Sci. Technol. 2015, 17, 167–178. [Google Scholar]
- Klamkowski, K.; Treder, W.; Wójcik, K. Effects of long-term water stress on leaf gas exchange, growth and yield of three strawberry cultivars. J. Agric. Sci. Technol. 2015, 17, 167–178. [Google Scholar]
- Naumann, G.; Alfieri, L.; Wyser, K.; Mentaschi, L.; Betts, R.A.; Carrao, H.; Spinoni, J.; Vogt, J.; Feyen, L. Global Changes in Drought Conditions Under Different Levels of Warming. Geophys. Res. Lett. 2018, 45, 3285–3296. [Google Scholar] [CrossRef]
- Shao, H.-B.; Bu, L.-Y.; Jaleel, C.; Zhao, C.-X. Water-deficit stress-induced anatomical changes in higher plants. Comptes Rendus Biol. 2008, 331, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Doroszewski, A.; Jadczyszyn, J.; Kozyra, J.; Pudełko, R.; Stuczyński, T.; Mizak, K.; Łopatka, A.; Koza, P.; Górski, T.; Wróblewska, E. Podstawy systemu monitoringu suszy rolniczej (Fundamentals of the agricultural drought monitoring system). Water Environ. Rural Areas 2015, 2, 77–91. [Google Scholar]
- Boczoń, A.; Wróbel, M. Wpływ suszy na pobór wody przez sosnę zwyczajną (Pinus sylvestris L.) o różnej pozycji w drzewostanie (The influence of drought on the water uptake by Scots pines (Pinus sylvestris L.) at different positions in the tree stand). For. Res. Work 2015, 76, 370–376. [Google Scholar]
- Treder, W.; Wójcik, K.; Tryngiel-Grać, A.; Krzewińska, D.; Klamkowski, K. Rozwój nawodnień roślin sadowniczych w świetle badań ankietowych (Development of irrigation of orchard plants reflected by survey investigations). Infrastruct. Ecol. Rural Areas 2011, 5, 61–69. [Google Scholar]
- Dietz, K.; Zörb, C.; Geilfus, C. Drought and crop yield. Plan Biol. 2021, 23, 881–893. [Google Scholar] [CrossRef]
- Liu, F.; Savic, S.; Jensen, C.; Shahnazari, A.; Jacobsen, S.; Stikic, R.; Andersen, M. Water relations and yield of lysimeter-grown strawberries under limited irrigation. Sci. Hortic. 2007, 111, 128–132. [Google Scholar] [CrossRef]
- Żebrowska, J.; Dyduch, M. Quantitative assessment of the morphogenetic efficiency in strawberry Fragaria × ananassa Duch. Folia Hortic. 2009, 21, 53–64. [Google Scholar] [CrossRef]
- Statistics Poland, Agriculture Department. Crop Production in 2016; Statistical Publishing Establishment: Warsaw, Poland, 2017.
- Statistics Poland, Agriculture Department. Production of Agricultural and Horticultural Crops in 2020; Statistical Publishing Establishment: Warsaw, Poland, 2021.
- Alsheikh, M.; Suso, H.; Robson, M.; Battey, N.; Wetten, A. Appropriate choice of antibiotic and Agrobacterium strain improves transformation of antibiotic-sensitive Fragaria vesca and F. v. semperflorens. Plant Cell Rep. 2002, 20, 1173–1180. [Google Scholar] [CrossRef]
- Caulet, R.-P.; Gradinariu, G.; Iurea, D.; Morariu, A. Influence of furostanol glycosides treatments on strawberry (Fragaria × ananassa Duch.) growth and photosynthetic characteristics under drought conditions. Sci. Hort. 2014, 169, 179–188. [Google Scholar] [CrossRef]
- Klamkowski, K.; Treder, K. wpływ deficytu wody na wymianę gazową liści, wzrost i plonowanie dwóch odmian truskawki uprawianych pod osłonami (Effect of water deficit on gas exchange parameters, growth and yield of two strawberry cultivars grown under greenhouse conditions). Infrastruct. Ecol. Rural Areas 2011, 5, 105–113. [Google Scholar]
- Sun, C.; Li, X.; Hu, Y.; Zhao, P.; Xu, T.; Sun, J.; Gao, X. Proline, sugars, and antioxidant enzymes respond to drought stress in the leaves of strawberry plants. Hortic. Sci. Technol. 2015, 33, 625–632. [Google Scholar] [CrossRef]
- Yin, B.; Wang, Y.; Liu, P.; Hu, J.; Zhen, W. Effects of vesicular-arbuscular mycorrhiza on the protective system in strawberry leaves under drought stress. Front. Agric. China 2010, 4, 165–169. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Nulit, R.; Sakimin, S.Z. Influence of drought stress on growth, biochemical changes and leaf gas exchange of strawberry (Fragaria × ananassa Duch.) in Indonesia. AIMS Agric. Food. 2022, 7, 37–60. [Google Scholar]
- Jiang, L.; Song, R.; Wang, X.; Wang, J.; Wu, C. Transcriptomic and Metabolomic Analyses Provide New Insights into the Response of Strawberry (Fragaria × ananassa Duch.) to Drought Stress. Horticulturae 2024, 10, 734. [Google Scholar] [CrossRef]
- Mikiciuk, G.; Mikiciuk, M.; Hawrot-Paw, M. Influence of superabsorbent polymers on the chemical composition of strawberry (Fragaria × ananassa Duch.) and biological activity in the soil. Folia Hortic. 2015, 27, 63–69. [Google Scholar] [CrossRef]
- Krishna, K.V.; Manasa, N.; Krishna, N.V. Drought Resistance in Wheat (Triticum aestivum L.). J. Adv. Biol. Biotechnol. 2024, 27, 409–415. [Google Scholar] [CrossRef]
- Sun, F.; Chen, Q.; Chen, Q.; Jiang, M.; Qu, Y. Yield-based drought tolerance index evaluates the drought tolerance of cotton germplasm lines in the interaction of genotype-by-environment. PeerJ 2023, 11, e14367. [Google Scholar] [CrossRef] [PubMed]
- Bartczak, M.; Lisiecka, J.; Knaflewski, M. Correlation between selected parameters of planting material and strawberry yield. Folia Hortic. 2010, 22, 9–12. [Google Scholar] [CrossRef]
- Błaszczyk, J. Influence of harvest date and storage conditions on the content of chlorophyll pigments in pear peels. Folia Hortic. 2012, 24, 91–95. [Google Scholar] [CrossRef]
- Ghaderi, N.; Siosemardeh, A. Response to drought stress of two strawberry cultivars (cv. Kurdistan and Selva). Hortic. Environ. Biotechnol. 2011, 52, 6–12. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Fahadi Hoveizeh, N.; Kadkhodaei, S.; Vaculík, M. Physiological and biochemical responses of commercial strawberry cultivars under optimal and drought stress conditions. Plants 2023, 12, 496. [Google Scholar] [CrossRef]
- Docimo, T.; Paesano, A.; D’Agostino, N.; D’Amelia, V.; Garramone, R.; Carputo, D.; Aversano, R. Exploring CDF gene family in wild potato under salinity stress unveils promising candidates for developing climate-resilient crops. Sci. Rep. 2024, 14, 24619. [Google Scholar] [CrossRef] [PubMed]
- Najafipour, B.; Mirlohi, A.; Majidi, M.M.; Saeidi, G.; Abtahi, M. Wild barley genomic resources for drought adaptability and quality improvement. J. Cereal Sci. 2023, 114, 103802. [Google Scholar] [CrossRef]
- Żurawicz, E. Truskawka i Poziomka (Strawberry and Wild Strawberry); Państwowe Wydawnictwo Rolnicze i Leśne: Warsaw, Poland, 2005. [Google Scholar]
- Kaya, C.; Şenbayram, M.; Akram, N.A.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Sulfur-enriched leonardite and humic acid soil amendments enhance tolerance to drought and phosphorus deficiency stress in maize (Zea mays L.). Sci. Rep. 2020, 10, 6432. [Google Scholar] [CrossRef]
- Wang, X.; Fu, J.; Min, Z.; Zou, D.; Liu, H.; Wang, J.; Zheng, H.; Jia, L.; Yang, L.; Xin, W.; et al. Response of rice with overlapping growth stages to water stress by assimilates accumulation and transport and starch synthesis of superior and inferior grains. Int. J. Mol. Sci. 2022, 23, 11157. [Google Scholar] [CrossRef] [PubMed]
- Frost, C.J.; Nyamdari, B.; Tsai, C.J.; Harding, S.A. The tonoplast-localized sucrose transporter in Populus (PtaSUT4) regulates whole-plant water relations, responses to water stress, and photosynthesis. PLoS ONE 2012, 7, e44467. [Google Scholar] [CrossRef]
- Küçükyumuk, C.; Yıldız, H.; Meriç, M.K. The response of Braeburn apple to regulated deficit irrigation. J. Agric. Sci. 2020, 26, 154–163. [Google Scholar] [CrossRef]
- Jones, H.G. Monitoring plant and soil water status: Established and novel methods revisited and their relevance to studies of drought tolerance. J. Exp. Bot. 2007, 58, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I.; Allen, M.B.; Whatley, F.R. Photosynthesis by isolated chloroplasts. IV. General concept and comparison of three photochemical reactions. Biochim. Biophys. Acta 1956, 20, 449–461. [Google Scholar] [CrossRef]
- Lichtenthaler, H.; Wellburn, A. Determinations of total carotenoids and chlorophyll a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1966, 11, 591–592. [Google Scholar] [CrossRef]
- Hager, A.; Mayer-Berthenrath, T. Die Isolierung und quantative Bestimung der Carotenoide und Chlorophyll von Blatern, Algen und isolierten Chloroplasten mit Hilfe Dunnschichtchromatographischer Methoden. Planta 1966, 69, 198–217. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, S.; Rebello Dillenburg, I. Measurements of leaf relative water content in Araucaria angustifolia. Rev. Bras. Fisiol. Veg. 1999, 11, 69–75. [Google Scholar]
- Cameron, J.S.; Hartley, C.A. Gas exchange characteristics of Fragaria chiloensis genotypes. Hort. Sci. 1990, 25, 327–329. [Google Scholar] [CrossRef]
- Gao, S.; Yan, Q.; Chen, L.; Song, Y.; Li, J.; Fu, C.; Dong, M. Effects of ploidy level and haplotype on variation of photosynthetic traits: Novel evidence from two Fragaria species. PLoS ONE 2017, 12, e0179899. [Google Scholar] [CrossRef]
- Lapshin, V.I.; Yakovenko, V.V.; Shcheglov, S.N.; Podorojny, V.N. A methodical approach for evaluating the variability of productivity and fruit quality in the genetic collections of strawberry (Fragaria × ananassa Duch.). Vavilovskij Žurnal Genet. I Sel. 2019, 23, 675–682. [Google Scholar] [CrossRef]
- Kiprono, D.; Thagun, C.; Kodama, Y. Light-dependent chloroplast relocation in wild strawberry (Fragaria vesca). Plant Signal. Behav. 2024, 19, 2342744. [Google Scholar] [CrossRef]
- Na, Y.W.; Jeong, H.J.; Lee, S.Y.; Choi, H.G.; Kim, S.H.; Rho, I.R. Chlorophyll fluorescence as a diagnostic tool for abiotic stress tolerance in wild and cultivated strawberry species. Hortic. Environ. Biotechnol. 2014, 55, 280–286. [Google Scholar] [CrossRef]
- Malambane, G.; Madumane, K.; Sewelo, L.T.; Batlang, U. Drought stress tolerance mechanisms and their potential common indicators to salinity, insights from the wild watermelon (Citrullus lanatus): A review. Front. Plant Sci. 2023, 13, 1074395. [Google Scholar] [CrossRef]
- Skender, A.; Ajdinović, T.; Bećirspahić, D. The comparison of phenotypic characteristics of current varieties and wild species of Fragaria. Genetika 2015, 47, 45–52. [Google Scholar] [CrossRef]
- Ulrich, D.; Olbricht, K. Diversity of metabolite patterns and sensory characters in wild and cultivated strawberries 1. J. Berry Res. 2014, 4, 11–17. [Google Scholar] [CrossRef]
- Wei, Y.; Kong, Y.; Li, H.; Yao, A.; Han, J.; Zhang, W.; Li, X.; Han, D. Genome-Wide Characterization and Expression Profiling of the AP2/ERF Gene Family in Fragaria vesca L. Int. J. Mol. Sci. 2024, 25, 7614. [Google Scholar] [CrossRef] [PubMed]
- Brasileiro, A.; Morgante, C.; Araujo, A.; Leal-Bertioli, S.; Silva, A.; Martins, A.; Vinson, C.; Santos, C.; Bonfim, O.; Togawa, R.; et al. Transcriptome profiling of wild Arachis from water-limited environments uncovers drought tolerance candidate genes. Plant Mol. Biol. Rep. 2015, 33, 1876–1892. [Google Scholar] [CrossRef]
- Schulze, J.; Erhardt, A.; Stoll, P. Reduced clonal reproduction indicates low potential for establishment of hybrids between wild and cultivated strawberries (Fragaria vesca × F. × ananassa). Ecol. Res. 2013, 28, 43–52. [Google Scholar] [CrossRef]
- Rugienius, R.; Bendokas, V.; Siksnianas, T.; Stanys, V.; Sasnauskas, A.; Kazanaviciute, V. Characteristics of Fragaria vesca yield parameters and anthocyanin accumulation under water deficit stress. Plants 2021, 10, 557. [Google Scholar] [CrossRef]
- Rokosa, M.; Mikiciuk, M. Assessment of physiological and morphological traits of plants of the genus Fragaria under conditions of water deficit-a study review. Acta Sci. Pol. Hortorum Cultus 2020, 19, 21–39. [Google Scholar] [CrossRef]
- Vinskienė, J.; Bendokas, V.; Stanys, V.; Sasnauskas, A.; Rugienius, R. The effect of osmotic stress, lighting spectrum and temperature on growth and gene expression related to anthocyanin biosynthetic pathway in wild strawberry (L.). Folia Hortic. 2023, 35, 419–431. [Google Scholar] [CrossRef]
- Nezhadahmadi, A.; Faruq, G.; Rashid, K. The impact of drought stress on morphological and physiological parameters of three strawberry varieties in different growing conditions. Pak. J. Agric. Sci. 2015, 52, 79–92. [Google Scholar]
- Al-Yasi, H.; Attia, H.; Alamer, K.; Hassan, F.; Ali, E.; Elshazly, S.; Siddique, K.; Hessini, K. Impact of drought on growth, photosynthesis, osmotic adjustment, and cell wall elasticity in Damask rose. Plant Physiol. Biochem. 2020, 150, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Perin, E.; Messias, R.; Galli, V.; Borowski, J.; Souza, E.; Avila, L.; Bamberg, A.; Rombaldi, C. Mineral content and antioxidant compounds in strawberry fruit submitted to drough stress. Food Sci. Technol. Int. 2019, 39, 245–254. [Google Scholar] [CrossRef]
- Keutgen, A.J.; Pawelzik, E. Quality and nutritional value of strawberry fruit under long term salt stress. Food Chem. 2008, 107, 1413–1420. [Google Scholar] [CrossRef]
- Buchanan, B.; Gruissem, W.; Jones, R. Photosynthesis. In Biochemistry and Molecular Biology of Plants; Wiley Blackwell: Hoboken, NJ, USA; American Society of Plants Biologists: Rockville, MD, USA, 2000; pp. 568–629. ISBN 978-0-470-71421-8. [Google Scholar]
- Martinez, J.P.; Ledent, J.F.; Bajji, M.; Kinet, J.M.; Lutts, S. Effect of water stress on growth, Na+ and K+ accumulation and water use efficiency in relation to osmotic adjustment in two populations of Atriplex halimus L. Plant Growth Regul. 2003, 41, 63–73. [Google Scholar] [CrossRef]
- Yurela, I. Copper in plants. Braz. J. Plant Physiol. 2005, 17, 145–156. [Google Scholar] [CrossRef]
- Gupta, B.; Shrestha, J. Abiotic stress adaptation and tolerance mechanisms in crop plants. Front. Plant Sci. 2023, 14, 1278895. [Google Scholar] [CrossRef]
- Munné-Bosch, S. Vitamin E function in stress sensing and signaling in plants. Dev. Cell 2019, 48, 290–292. [Google Scholar] [CrossRef] [PubMed]
- Lata, C.; Prasad, M. Role of DREBs in regulation of abiotic stress responses in plants. J. Exp. Bot. 2011, 62, 4731–4748. [Google Scholar] [CrossRef]
- Craine, J.M.; Nippert, J.B.; Elmore, A.J.; Skibbe, A.M.; Hutchinson, S.L.; Brunsell, N.A. Timing of climate variability and grassland productivity. Proc. Natl. Acad. Sci. USA 2012, 09, 3401–3405. [Google Scholar] [CrossRef]
- Maqbool, A.; Bakhsh, A.; Aksoy, E. Effects of natural variations on biofortification. In Wild Germplasm for Genetic Improvement in Crop Plants; Academic Press: Cambridge, MA, USA, 2021; pp. 115–138. [Google Scholar]
- Dawson, W.; Bòdis, J.; Bucharova, A.; Catford, J.; Duncan, R.; Fraser, L.; Groenteman, R.; Kelly, R.; Moore, J.; Pärtel, M.; et al. Root traits vary as much as leaf traits and have consistent phenotypic plasticity among 14 populations of a globally widespread herb. Funct. Ecol. 2024, 4, 926–941. [Google Scholar] [CrossRef]
- Bowsher, A.W.; Miller, B.J.; Donovan, L.A. Evolutionary divergences in root system morphology, allocation, and nitrogen uptake in species from high-versus low-fertility soils. Funct. Plant Biol. 2015, 43, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Jacques, C.; Salon, C.; Barnard, R.L.; Vernoud, V.; Prudent, M. Drought stress memory at the plant cycle level: A review. Plants 2021, 10, 1873. [Google Scholar] [CrossRef] [PubMed]
- Omondi, E.O.; Lin, C.Y.; Huang, S.M.; Liao, C.A.; Lin, Y.P.; Oliva, R.; van Zonneveld, M. Landscape genomics reveals genetic signals of environmental adaptation of African wild eggplants. Ecol. Evol. 2024, 14, e11662. [Google Scholar] [CrossRef]
| pH | Fine Soil Fractions [%] | S0 [g·cm−3] | Content of P | Content of K | Content of Mg | Salinity [g·NaCl·dm−3] | Wtv | |
|---|---|---|---|---|---|---|---|---|
| H2O | KCl | [mg · 100 g−1] | ||||||
| 6.70 | 5.98 | 21.00 | 1.25 | 5.87 | 12.66 | 5.98 | 0.45 | 37.10 |
| Years | Months | Average/Sum | ||||||
|---|---|---|---|---|---|---|---|---|
| IV | V | VI | VII | VIII | IX | X | ||
| Mean air temperature (°C) | ||||||||
| 2017 | 7.4 | 14.1 | 17.2 | 17.7 | 18.2 | 13.6 | 11.2 | 14.2 |
| Deviation | −1.8 | 0.5 | 0.4 | −1.2 | −0.3 | −0.7 | 1.7 | −0.2 |
| 2018 | 12.3 | 16.6 | 18.5 | 20.0 | 20.0 | 15.4 | 10.3 | 16.2 |
| Deviation | 3.1 | 3.0 | 1.7 | 1.1 | 1.5 | 1.1 | 0.8 | 1.8 |
| 2019 | 10.1 | 12.1 | 21.5 | 18.8 | 20.1 | 14.5 | 10.7 | 15.4 |
| Deviation | 0.9 | −1.5 | 4.7 | −0.1 | 1.6 | 0.2 | 1.2 | 1.0 |
| 1991–2020 | 9.2 | 13.6 | 16.8 | 18.9 | 18.5 | 14.3 | 9.5 | 14.4 |
| Sunshine duration (h) | ||||||||
| 2017 | 143.2 | 240.3 | 215.5 | 197.1 | 229.1 | 130.6 | 96.4 | 11,252.2 |
| % of the norm | 71.9 | 98.3 | 89.0 | 80.1 | 99.5 | 81.6 | 96.8 | 88.1 |
| 2018 | 257.6 | 349.8 | 253.6 | 301.0 | 269.2 | 204.0 | 170.36 | 1805.8 |
| % of the norm | 129.3 | 143.1 | 104.7 | 122.3 | 116.9 | 127.5 | 171.3 | 127.0 |
| 2019 | 285.7 | 198.2 | 345.5 | 222.3 | 239.1 | 168.5 | - | 1459.3 |
| % of the norm | 143.4 | 81.1 | 142.7 | 90.3 | 103.8 | 105.3 | x | 102.6 |
| 1991–2020 | 199.3 | 244.5 | 242.2 | 246.2 | 230.3 | 160.0 | 99.6 | 1422.1 |
| Total precipitation (mm) | ||||||||
| 2017 | 42.2 | 90.8 | 132.7 | 192.5 | 43.8 | 30.4 | 93.9 | 626.3 |
| % of the norm | 135.3 | 162.7 | 224.5 | 252.6 | 72.6 | 63.7 | 215.9 | 167.5 |
| 2018 | 26.8 | 22.5 | 15.0 | 92.8 | 21.4 | 16.3 | 20.2 | 215.0 |
| % of the norm | 85.9 | 40.3 | 25.4 | 121.8 | 35.5 | 34.2 | 46.4 | 57.5 |
| 2019 | 10.7 | 68.7 | 70.8 | 23.5 | 41.8 | 63.2 | 46.1 | 324.8 |
| % of the norm | 34.3 | 123.1 | 119.8 | 30.8 | 69.3 | 132.5 | 106.0 | 86.9 |
| 1991–2020 | 31.2 | 55.8 | 59.1 | 76.2 | 60.3 | 47.1 | 43.5 | 373.8 |
| Relative air humidity (%) | ||||||||
| 2017 | 73.8 | 70.9 | 74.5 | 79.4 | 76.5 | 82.1 | 85.6 | 77.5 |
| Deviation | 3.0 | −0.6 | 1.6 | 5.0 | 0.6 | 0.9 | 0.1 | 1.5 |
| 2018 | 69.3 | 63.7 | 66.4 | 71.5 | 67.8 | 74.2 | 79.1 | 70.3 |
| Deviation | −1.5 | −7.8 | −6.5 | −2.9 | −8.1 | −7.0 | −6.4 | −5.7 |
| 2019 | 60.0 | 71.2 | 65.5 | 70.0 | 69.7 | 78.2 | 84.3 | 71.3 |
| Deviation | −10.8 | −0.3 | −7.4 | −4.4 | −6.2 | −3.0 | −1.2 | −4.8 |
| 1991–2020 | 70.8 | 71.5 | 72.9 | 74.4 | 75.9 | 81.2 | 85.5 | 76.0 |
| Parameters | Genotypes | Measurement Terms | |||||
|---|---|---|---|---|---|---|---|
| 1st Year | 2nd Year | ||||||
| July | August | September | July | August | September | ||
| E [mmol H2O∙m−2∙s−1] | B | 1.105 a* | 0.502 b | 0.967 a | 0.707 a | 0.841 ab | 0.573 b |
| R | 1.017 b | 0.645 a | 1.036 a | 0.571 b | 0.992 a | 0.525 c | |
| N | 1.120 a | 0.653 a | 0.726 b | 0.687 a | 0.737 b | 0.907 a | |
| gs [mol H2O∙m−2∙s−1] | B | 0.094 a | 0.137 a | 0.061 ab | 0.064 a | 0.043 b | 0.063 b |
| R | 0.087 a | 0.107 b | 0.064 a | 0.048 b | 0.053 a | 0.049 b | |
| N | 0.086 a | 0.150 a | 0.050 b | 0.060 a | 0.038 b | 0.120 a | |
| Pn [mmol H2O∙m−2∙s−1] | B | 6.747 a | 2.200 b | 4.727 a | 3.760 a | 1.520 b | 2.513 b |
| R | 6.847 a | 1.780 b | 3.740 ab | 3.487 a | 3.873 a | 4.107 a | |
| N | 7.307 a | 3.253 a | 3.427 b | 3.760 a | 4.260 a | 4.893 a | |
| ci [μmol CO2∙mol−1] | B | 311.00 a | 421.33 ab | 315.00 a | 298.20 a | 355.73 a | 498.47 a |
| R | 299.93 a | 426.20 a | 302.53 a | 280.87 a | 289.40 b | 371.47 b | |
| N | 279.80 b | 411.73 b | 304.53 a | 298.87 a | 244.33 c | 449.80 a | |
| WUE | B | 6.201 a | 3.484 b | 5.167 a | 5.409 a | 1.922 c | 4.656 b |
| R | 6.747 a | 3.812 b | 3.799 a | 6.253 a | 4.028 b | 5.432 b | |
| N | 6.519 a | 5.168 a | 5.173 a | 5.328 a | 6.533 a | 20.713 a | |
| Parameters | Genotypes | Measurement Terms | |||||
|---|---|---|---|---|---|---|---|
| 1st Year | 2nd Year | ||||||
| July | August | September | July | August | September | ||
| F0 | B | 600.786 a* | 616.857 a | 587.429 a | 259.143 a | 261.857 a | 266.857 a |
| R | 594.375 a | 612.857 a | 598.250 a | 268.625 a | 273.125 a | 278.188 a | |
| N | 600.429 a | 594.000 a | 582.929 a | 258.571 a | 229.857 b | 250.643 a | |
| FM | B | 2804.571 a | 2523.143 b | 2917.929 ab | 1246.571 b | 1222.714 b | 1270.500 a |
| R | 2795.000 a | 2680.688 ab | 2821.563 b | 1346.688 a | 1398.500 a | 1323.500 a | |
| N | 2998.429 a | 2782.643 a | 3067.071 a | 1333.143 a | 992.571 c | 1258.643 a | |
| FV | B | 2203.786 a | 1906.286 b | 2330.500 ab | 987.429 b | 960.857 b | 1003.643 a |
| R | 2200.625 a | 2068.250 ab | 2223.313 b | 1078.063 a | 1125.375 a | 954.313 a | |
| N | 2398.000 a | 2188.643 a | 2484.143 a | 1074.571 a | 762.714 c | 1008.000 a | |
| FV/FM | B | 0.782 a | 0.754 b | 0.796 ab | 0.792 a | 0.785 b | 0.780 ab |
| R | 0.784 a | 0.771 ab | 0.785 b | 0.799 a | 0.804 a | 0.772 b | |
| N | 0.793 a | 0.779 a | 0.809 a | 0.805 a | 0.743 ab | 0.801 a | |
| TFM | B | 373.571 b | 240.714 b | 305.000 ab | 578.571 b | 277.857 a | 535.000 a |
| R | 404.375 b | 236.875 b | 279.375 b | 493.751 c | 324.375 a | 518.750 a | |
| N | 507.143 a | 273.571 a | 339.286 a | 671.429 a | 317.144 a | 560.000 a | |
| AM | B | 52,530.786 b | 31,023.786 b | 45,809.500 b | 28,356.286 b | 16,458.857 b | 22,092.714 b |
| R | 54,069.125 b | 35,609.250 ab | 42,670.813 b | 30,417.688 b | 23,104.000 a | 23,176.813 b | |
| N | 68,608.929 a | 39,444.500 a | 57,824.214 a | 40,694.786 a | 17,188.714 b | 32,145.643 a | |
| Parameters | Genotypes | Measurement Terms | |||||
|---|---|---|---|---|---|---|---|
| 1st Year | 2nd Year | ||||||
| July | August | September | July | August | September | ||
| Chlorophyll “a” | B | 1.711 ab* | 0.851 b | 1.427 b | 1.327 b | 1.952 a | 1.857 b |
| R | 1.360 b | 1.090 b | 1.042 c | 1.355 b | 1.528 b | 1.641 b | |
| N | 1.995 a | 1.420 a | 1.777 a | 2.116 a | 1.780 ab | 2.201 a | |
| Chlorophyll “b” | B | 0.622 a | 0.565 a | 0.531 b | 0.564 a | 0.627 a | 0.627 ab |
| R | 0.523 a | 0.562 a | 0.523 b | 0.654 a | 0.486 b | 0.591 b | |
| N | 0.702 a | 0.700 a | 0.855 a | 0.846 a | 0.508 ab | 0.721 a | |
| Chlorophyll “a” to chlorophyll “b” ratio | B | 2.747 a | 1.528 b | 2.684 a | 2.385 a | 3.113 b | 2.963 a |
| R | 2.632 a | 1.932 a | 2.074 a | 2.208 a | 3.154 b | 2.780 b | |
| N | 2.839 a | 2.028 a | 2.107 a | 2.523 a | 3.504 a | 3.056 a | |
| Total chlorophyll | B | 2.333 ab | 1.415 b | 1.958 b | 2.333 ab | 1.415 b | 1.958 b |
| R | 1.882 b | 1.652 b | 1.565 c | 1.882 b | 1.652 b | 1.565 c | |
| N | 2.697 a | 2.119 a | 2.631 a | 2.697 a | 2.119 a | 2.631 a | |
| Carotenoids | B | 0.959 ab | 0.672 b | 0.866 b | 0.683 b | 0.966 a | 0.933 b |
| R | 0.728 b | 0.748 b | 0.639 c | 0.696 b | 0.902 ab | 0.825 b | |
| N | 1.179 a | 1.009 a | 1.170 a | 1.050 a | 0.772 b | 1.120 a | |
| Genotypes | Measurement Terms | |||||
|---|---|---|---|---|---|---|
| 1st Year | 2nd Year | |||||
| July | August | September | July | August | September | |
| B | 54.445 b* | 54.765 b | 56.308 ab | 45.247 a | 42.064 a | 46.258 b |
| R | 62.644 a | 55.159 b | 61.177 a | 39.875 a | 40.545 ab | 58.035 a |
| N | 58.285 ab | 65.073 a | 54.813 b | 37.150 a | 30.509 b | 52.958 ab |
| Parameters | Genotypes | 1st Year | 2nd Year |
|---|---|---|---|
| Fresh Fruit Mass Yield | B | 23.79 a* | 33.11 a |
| R | 22.20 a | 23.27 b | |
| N | - | - | |
| Single Fruit Mass | B | 0.65 a | 0.55 a |
| R | 0.59 a | 0.54 a | |
| N | - | - |
| Genotypes | Fresh Mass of the Root System | Dry Mass of the Root System |
|---|---|---|
| B | 11.81 a* | 6.83 a |
| R | 13.30 a | 6.83 a |
| N | 17.80 a | 10.47 a |
| Plant Organ | Genotypes | K [g‧kg−1] | Ca [g‧kg−1] | Mg [g‧kg−1] | Na [g‧kg−1] | Cu [mg‧kg−1] | Zn [mg‧kg−1] | Mn [mg‧kg−1] | Mo [mg‧kg−1] | Fe [mg‧kg−1] |
|---|---|---|---|---|---|---|---|---|---|---|
| Leaves | B | 97.6 a* | 66.9 a | 13.5 b | 4.8 a | 37.3 a | 172.5 ab | 201.8 a | 11.7 a | 1.9 a |
| R | 106.9 a | 69.7 a | 15.7 a | 2.8 a | 52.2 a | 236.4 a | 205.4 a | 6.6 b | 1.8 a | |
| N | 96.5 a | 51.9 b | 17.2 a | 2.7 a | 45.6 a | 146.8 b | 139.2 b | 12.8 a | 0.7 b | |
| Crowns | B | 82.9 a | 115.6 b | 3.9 b | 28.0 ab | 87.9 ab | 155.2 b | 31.4 b | 17.6 a | 0.2 a |
| R | 92.2 a | 91.4 b | 3.4 b | 37.4 a | 117.1 a | 141.3 b | 30.8 b | 16.4 a | 0.2 a | |
| N | 50.4 b | 159.7 a | 5.4 a | 10.0 b | 74.4 b | 212.9 a | 61.8 a | 17.3 a | 0.2 a | |
| Roots | B | 25.9 a | 80.7 a | 13.4 a | 6.1 a | 92.5 b | 853.5 ab | 373.3 b | 12.4 a | 6.3 b |
| R | 24.5 a | 63.4 a | 13.4 a | 5.9 a | 111.4 a | 677.8 b | 388.0 b | 9.4 a | 6.4 b | |
| N | 12.9 b | 60.2 a | 9.8 b | 5.9 a | 71.8 c | 883.8 a | 940.8 a | 14.6 a | 13.7 a | |
| Average | B | 69.9 a | 89.9 a | 9.8 a | 32.6 a | 73.7 ab | 375.4 a | 189.0 a | 14.2 a | 2.6 a |
| R | 74.5 a | 74.8 a | 10.8 a | 15.4 a | 93.6 a | 351.8 a | 208.1 a | 10.8 b | 2.8 a | |
| N | 53.3 a | 90.6 a | 10.8 a | 6.2 a | 63.9 b | 414.5 a | 380.6 a | 14.9 a | 4.9 a |
| Parameters | Genotypes | SML | Measurement Terms | |||||
|---|---|---|---|---|---|---|---|---|
| 1st Year | 2nd year | |||||||
| July | August | September | July | August | September | |||
| E [mmol H2O∙m−2∙s−1] | B | Ctrl | 1.105 ab | 1.041 a | 0.967 a | 0.707 a | 0.841 bc | 0.573 c |
| Def | 0.417 d | 0.645 a | 0.727 b | 0.465 c | 1.097 a | 0.499 cd | ||
| R | Ctrl | 1.017 b | 1.021 a | 1.036 a | 0.571 b | 0.992 ab | 0.252 e | |
| Def | 0.405 d | 0.502 a | 0.579 b | 0.565 b | 0.578 d | 0.431 d | ||
| N | Ctrl | 1.120 a | 1.059 a | 0.726 b | 0.687 a | 0.735 cd | 0.907 a | |
| Def | 0.789 c | 0.653 a | 0.619 b | 0.530 bc | 0.733 cd | 0.801 b | ||
| Average | Ctrl | 1.081 A | 1.040 A | 0.910 A | 0.655 A | 0.856 A | 0.577 A | |
| Def | 0.537 B | 0.600 B | 0.642 B | 0.520 B | 0.803 A | 0.574 A | ||
| gs [mol H2O∙m−2∙s−1] | B | Ctrl | 0.094 a | 0.137 a | 0.061 a | 0.064 a | 0.043 bc | 0.063 a |
| Def | 0.039 c | 0.254 a | 0.045 a | 0.036 c | 0.050 ab | 0.082 a | ||
| R | Ctrl | 0.087 a | 0.107 a | 0.064 a | 0.048 b | 0.053 a | 0.055 a | |
| Def | 0.043 c | 0.182 a | 0.038 a | 0.046 b | 0.026 e | 0.049 a | ||
| N | Ctrl | 0.086 a | 0.150 a | 0.050 a | 0.060 a | 0.038 cd | 0.113 a | |
| Def | 0.066 b | 0.305 a | 0.042 a | 0.045 b | 0.032 de | 0.120 a | ||
| Average | Ctrl | 0.089 A | 0.131 B | 0.058 A | 0.058 A | 0.045 A | 0.083 A | |
| Def | 0.049 B | 0.247 A | 0.042 B | 0.042 B | 0.036 B | 0.077 A | ||
| Pn [mmol H2O∙m−2∙s−1] | B | Ctrl | 6.747 a | 2.200 a | 4.727 a | 3.760 a | 1.520 d | 2.513 c |
| Def | 4.313 a | 1.667 a | 3.873 a | 2.787 b | 3.133 bc | 4.513 b | ||
| R | Ctrl | 6.847 a | 1.780 a | 3.740 a | 3.487 a | 3.873 ab | 4.107 bc | |
| Def | 5.380 a | 1.773 a | 2.607 a | 2.060 c | 2.300 cd | 3.667 bc | ||
| N | Ctrl | 7.307 a | 3.253 a | 3.427 a | 3.533 a | 4.260 a | 7.780 a | |
| Def | 5.800 a | 2.553 a | 2.673 a | 3.840 a | 1.787 d | 4.893 b | ||
| Average | Ctrl | 6.697 A | 2.411 A | 3.964 A | 3.593 A | 3.218 A | 5.320 A | |
| Def | 5.164 B | 1.998 A | 3.051 B | 2.896 B | 2.407 B | 3.838 B | ||
| ci [μmol CO2∙mol−1] | B | Ctrl | 311.000 a | 421.333 a | 315.000 a | 298.200 ab | 355.733 a | 498.467 a |
| Def | 220.667 c | 441.600 a | 257.133 a | 290.800 c | 316.133 ab | 361.333 a | ||
| R | Ctrl | 299.933 ab | 426.200 a | 302.533 a | 280.867 bc | 289.400 bc | 371.467 a | |
| Def | 225.933 c | 434.200 a | 282.533 a | 320.133 a | 300.533 b | 323.800 a | ||
| N | Ctrl | 279.800 b | 411.733 a | 304.533 a | 298.867 ab | 244.333 c | 449.800 a | |
| Def | 250.000 c | 434.733 a | 286.467 a | 255.400 c | 319.000 ab | 339.800 a | ||
| Average | Ctrl | 296.911 A | 419.756 B | 307.356 A | 292.644 A | 296.489 A | 439.911 A | |
| Def | 232.200 B | 436.844 A | 275.378 B | 278.778 A | 311.889 A | 341.644 B | ||
| WUE | B | Ctrl | 6.201 c | 3.484 a | 5.167 a | 5.409 bc | 1.922 c | 4.656 c |
| Def | 11.071 b | 1.668 a | 0.196 a | 6.593 b | 2.938 bc | 9.285 bc | ||
| R | Ctrl | 6.747 c | 3.812 a | 3.799 a | 6.253 ab | 4.028 b | 20.713 a | |
| Def | 13.763 a | 1.806 a | 0.231 a | 3.979 c | 3.760 b | 9.805 b | ||
| N | Ctrl | 6.519 c | 5.168 a | 5.173 a | 5.328 bc | 6.533 a | 5.432 bc | |
| Def | 7.561 c | 2.569 a | 0.243 a | 7.685 a | 2.554 bc | 9.967 b | ||
| Average | Ctrl | 6.489 B | 4.155 A | 4.713 A | 5.663 A | 4.161 A | 10.267 A | |
| Def | 10.798 A | 2.014 B | 0.223 B | 6.089 A | 3.084 B | 9.686 A | ||
| Parameters | Genotypes | SML | Measurement Terms | |||||
|---|---|---|---|---|---|---|---|---|
| 1st Year | 2nd Year | |||||||
| July | August | September | July | August | September | |||
| F0 | B | Ctrl | 600.786 a | 616.857 a | 587.429 a | 259.143 a | 261.857 a | 266.857 ab |
| Def | 598.333 a | 620.000 a | 585.933 a | 252.200 a | 266.733 a | 274.867 ab | ||
| R | Ctrl | 594.375 a | 610.143 a | 598.250 a | 268.625 a | 273.125 a | 278.188 a | |
| Def | 608.929 a | 612.438 a | 594.000 a | 258.214 a | 267.500 a | 271.857 ab | ||
| N | Ctrl | 600.429 a | 594.000 a | 582.929 a | 258.571 a | 229.857 a | 250.643 b | |
| Def | 603.867 a | 593.200 a | 593.600 a | 247.200 a | 223.067 a | 211.933 c | ||
| Average | Ctrl | 598.341 A | 607.977 A | 589.932 A | 262.409 A | 255.773 A | 265.818 A | |
| Def | 603.591 A | 907.727 A | 591.114 A | 252.409 B | 252.091 A | 252.455 A | ||
| FM | B | Ctrl | 2804.571 a | 2523.143 a | 2917.929 a | 1246.571 c | 1222.714 b | 1270.500 a |
| Def | 2936.867 a | 2729.667 a | 2959.667 a | 1272.400 bc | 1305.333 ab | 1179.800 a | ||
| R | Ctrl | 2701.214 a | 2680.688 a | 2821.563 a | 1346.688 a | 1398.500 a | 1232.500 a | |
| Def | 2795.000 a | 2733.000 a | 2998.643 a | 1201.357 c | 1237.857 b | 1162.857 a | ||
| N | Ctrl | 2998.429 a | 2782.643 a | 3067.071 a | 1333.143 ab | 992.571 c | 1258.643 a | |
| Def | 3005.067 a | 2928.533 a | 2922.600 a | 1237.867 c | 887.333 d | 1097.000 a | ||
| Average | Ctrl | 2862.773 A | 2663.000 B | 2930.341 A | 1310.523 A | 1213.409 A | 1525.909 A | |
| Def | 2885.136 A | 2798.523 A | 2959.432 A | 1238.023 B | 1141.364 B | 1146.182 B | ||
| FV | B | Ctrl | 2203.786 a | 1906.286 a | 2330.500 a | 987.429 cb | 960.857 b | 1003.643 a |
| Def | 2338.533 a | 2109.667 a | 2373.733 a | 1020.200 ab | 1038.600 ab | 904.933 a | ||
| R | Ctrl | 2200.625 a | 2068.250 a | 2223.313 a | 1078.063 a | 1125.375 a | 954.313 a | |
| Def | 2092.286 a | 2122.857 a | 2404.643 a | 943.143 c | 970.357 b | 891.000 a | ||
| N | Ctrl | 2398.000 a | 2188.643 a | 2484.143 a | 1074.571 a | 762.714 c | 1008.000 a | |
| Def | 2401.200 a | 2335.333 a | 2329.000 a | 990.667 bc | 664.267 d | 885.067 a | ||
| Average | Ctrl | 2264.432 A | 2055.023 B | 2340.409 A | 1048.114 A | 957.636 A | 987.091 A | |
| Def | 2281.545 A | 2190.795 A | 2368.318 A | 985.614 B | 889.273 B | 893.727 B | ||
| FV/FM | B | Ctrl | 0.782 a | 0.754 a | 0.796 a | 0.792 ab | 0.785 a | 0.780 a |
| Def | 0.796 a | 0.769 a | 0.800 a | 0.801 a | 0.795 a | 0.765 a | ||
| R | Ctrl | 0.784 a | 0.771 a | 0.785 a | 0.799 a | 0.804 a | 0.772 a | |
| Def | 0.765 a | 0.775 a | 0.800 a | 0.784 b | 0.783 a | 0.764 a | ||
| N | Ctrl | 0.793 a | 0.779 a | 0.809 a | 0.805 a | 0.743 a | 0.801 a | |
| Def | 0.797 a | 0.796 a | 0.796 a | 0.800 a | 0.737 a | 0.805 a | ||
| Average | Ctrl | 0.786 A | 0.768 A | 0.796 A | 0.799 A | 0.779 A | 0.783 A | |
| Def | 0.787 A | 0.780 A | 0.798 A | 0.795 A | 0.772 A | 0.778 A | ||
| TFM | B | Ctrl | 373.571 a | 240.714 a | 305.000 a | 578.571 d | 277.857 a | 535.000 a |
| Def | 506.667 a | 266.667 a | 341.333 a | 726.667 b | 500.000 a | 611.333 a | ||
| R | Ctrl | 404.375 a | 236.875 a | 279.375 a | 493.751 e | 324.375 a | 518.750 a | |
| Def | 507.143 a | 251.429 a | 309.286 a | 821.429 a | 491.429 a | 628.571 a | ||
| N | Ctrl | 507.143 a | 273.571 a | 339.286 a | 671.429 c | 317.144 a | 560.000 a | |
| Def | 620.000 a | 276.000 a | 356.667 a | 806.667 a | 462.000 a | 713.333 a | ||
| Average | Ctrl | 427.273 B | 249.773 B | 306.591 A | 577.273 B | 307.273 B | 537.045 B | |
| Def | 545.455 A | 265.000 A | 336.364 A | 784.091 A | 484.318 A | 651.591 A | ||
| AM | B | Ctrl | 52,530.79 a | 31,023.79 a | 45,809.50 bc | 28,356.29 a | 16,458.86 c | 22,092.71 a |
| Def | 62,576.73 a | 36,214.73 a | 52,129.73 ab | 38,018.53 a | 29,509.93 a | 31,889.93 a | ||
| R | Ctrl | 54,069.13 a | 35,609.25 a | 42,670.81 c | 30,417.69 a | 23,104.00 b | 23,176.81 a | |
| Def | 52,151.14 a | 34,992.36 a | 50,287.57 abc | 38,595.50 a | 27,784.21 a | 29,535.43 a | ||
| N | Ctrl | 68,608.93 a | 39,444.50 a | 57,824.21 a | 40,694.79 a | 17,188.72 c | 32,145.64 a | |
| Def | 73,210.20 a | 43,184.27 a | 50,782.27 abc | 46,048.60 a | 19,009.80 c | 36,060.13 a | ||
| Average | Ctrl | 58,205.96 A | 35,370.55 A | 48,491.02 A | 33,031.77 B | 19,107.50 B | 25,685.59 B | |
| Def | 62,884.55 A | 38,201.77 A | 51,084.02 A | 40,939.64 A | 25,381.25 A | 32,562.43 A | ||
| Parameters | Genotypes | SML | Measurement Terms | |||||
|---|---|---|---|---|---|---|---|---|
| 1st Year | 2nd Year | |||||||
| July | August | September | July | August | September | |||
| Chlorophyll “a” | B | Ctrl | 1.711 a | 0.851 d | 1.427 b | 1.327 a | 1.952 a | 1.857 bc |
| Def | 1.727 a | 1.409 b | 1.386 b | 1.852 a | 2.372 a | 1.717 bc | ||
| R | Ctrl | 1.360 a | 1.090 c | 1.042 c | 1.355 a | 1.528 a | 1.641 bc | |
| Def | 1.629 a | 1.274 bc | 1.460 b | 1.553 a | 2.143 a | 2.009 ab | ||
| N | Ctrl | 1.995 a | 1.420 b | 1.777 a | 2.116 a | 1.780 a | 2.201 a | |
| Def | 2.194 a | 1.629 a | 0.923 d | 2.250 a | 2.034 a | 1.685 bc | ||
| Average | Ctrl | 1.689 A | 1.120 B | 1.415 A | 1.599 B | 1.754 B | 1.900 A | |
| Def | 1.850 A | 1.437 A | 1.256 B | 1.885 A | 2.183 A | 1.804 A | ||
| Chlorophyll “b” | B | Ctrl | 0.622 a | 0.565 c | 0.531 b | 0.564 a | 0.627 a | 0.627 abc |
| Def | 0.685 a | 0.709 b | 0.553 b | 0.705 a | 0.768 a | 0.627 abc | ||
| R | Ctrl | 0.523 a | 0.562 c | 0.523 b | 0.606 a | 0.486 a | 0.591 bc | |
| Def | 0.741 a | 0.652 bc | 0.601 b | 0.654 a | 0.695 a | 0.693 ab | ||
| N | Ctrl | 0.702 a | 0.700 b | 0.387 c | 0.846 a | 0.508 a | 0.721 a | |
| Def | 0.860 a | 1.037 a | 0.855 a | 0.824 a | 0.637 a | 0.537 c | ||
| Average | Ctrl | 0.616 B | 0.609 B | 0.637 A | 0.688 A | 0.541 B | 0.646 A | |
| Def | 0.762 A | 0.800 A | 0.514 B | 0.712 A | 0.700 A | 0.619 A | ||
| Chlorophyll “a” to chlorophyll “b” ratio | B | Ctrl | 2.747 a | 1.528 b | 2.684 a | 2.385 a | 3.113 bc | 2.963 ab |
| Def | 2.525 a | 1.986 a | 2.506 a | 2.607 a | 3.091 bc | 2.730 c | ||
| R | Ctrl | 2.632 a | 1.932 a | 2.074 a | 2.208 a | 3.154 bc | 2.780 c | |
| Def | 2.190 a | 1.950 a | 2.429 a | 2.565 a | 3.083 c | 2.898 bc | ||
| N | Ctrl | 2.839 a | 2.028 a | 2.107 a | 2.523 a | 3.504 a | 3.056 ab | |
| Def | 2.557 a | 1.573 b | 2.386 a | 2.732 a | 3.196 b | 3.136 a | ||
| Average | Ctrl | 2.739 A | 1.829 A | 2.288 A | 2.372 A | 3.257 A | 2.933 A | |
| Def | 2.424 B | 1.836 A | 2.440 A | 2.635 A | 3.123 B | 2.921 A | ||
| Total chlorophyll | B | Ctrl | 2.333 a | 1.415 a | 1.958 b | 1.890 a | 2.579 a | 2.483 abc |
| Def | 2.411 a | 2.117 a | 1.938 b | 2.556 a | 3.139 a | 2.343 abc | ||
| R | Ctrl | 1.882 a | 1.652 a | 1.565 c | 2.008 a | 2.014 a | 2.231 c | |
| Def | 2.369 a | 1.926 a | 2.061 b | 2.159 a | 2.837 a | 2.701 ab | ||
| N | Ctrl | 2.697 a | 2.119 a | 2.631 a | 2.962 a | 2.288 a | 2.921 a | |
| Def | 3.053 a | 2.666 a | 1.309 d | 3.073 a | 2.670 a | 2.222 c | ||
| Average | Ctrl | 2.304 A | 1.729 B | 2.051 A | 2.286 A | 2.294 B | 2.545 A | |
| Def | 2.611 A | 2.236 A | 1.769 B | 2.596 A | 2.882 A | 2.422 A | ||
| Carotenoids | B | Ctrl | 0.959 a | 0.672 a | 0.866 b | 0.683 a | 0.966 a | 0.933 bc |
| Def | 1.023 a | 0.983 a | 0.920 b | 0.917 a | 1.155 a | 0.912 bc | ||
| R | Ctrl | 0.728 a | 0.748 a | 0.639 c | 0.696 a | 0.772 a | 0.825 c | |
| Def | 0.992 a | 0.866 a | 0.846 b | 0.808 a | 1.058 a | 1.047 ab | ||
| N | Ctrl | 1.179 a | 1.009 a | 1.170 a | 1.050 a | 0.902 a | 1.120 a | |
| Def | 1.403 a | 1.204 a | 0.667 c | 1.108 a | 1.047 a | 0.902 bc | ||
| Average | Ctrl | 0.955 B | 0.810 B | 0.891 A | 0.810 B | 0.880 B | 0.959 A | |
| Def | 1.139 A | 1.018 A | 0.811 B | 0.944 A | 1.087 A | 0.954 A | ||
| Genotypes | SML | Measurement Terms | |||||
|---|---|---|---|---|---|---|---|
| 1st Year | 2nd Year | ||||||
| July | August | September | July | August | September | ||
| B | Ctrl | 54.445 bc | 54.765 a | 56.308 a | 45.245 a | 42.064 a | 46.258 a |
| Def | 57.062 bc | 55.160 a | 63.152 a | 45.247 a | 29.790 a | 42.738 a | |
| R | Ctrl | 62.644 a | 55.159 a | 61.177 a | 39.875 a | 40.545 a | 58.035 a |
| Def | 53.366 c | 43.781 a | 52.717 a | 38.022 a | 31.226 a | 50.866 a | |
| N | Ctrl | 58.285 b | 65.073 a | 54.813 a | 37.150 a | 30.509 a | 52.958 a |
| Def | 56.126 bc | 60.660 a | 56.865 a | 35.471 a | 18.812 a | 47.108 a | |
| Average | Ctrl | 58.458 A | 58.332 A | 57.432 A | 40.757 A | 37.706 A | 52.417 A |
| Def | 55.518 B | 53.200 B | 57.578 A | 37.285 A | 26.609 B | 46.904 A | |
| Parameters | Genotypes | SML | 1st Year | 2nd Year |
|---|---|---|---|---|
| Fresh Fruit Mass Yield | B | Ctrl | 23.79 a | 33.11 a |
| Def | 5.29 a | 14.83 a | ||
| R | Ctrl | 22.20 a | 23.27 a | |
| Def | 5.64 a | 14.62 a | ||
| N | Ctrl | - | - | |
| Def | - | - | ||
| Average | Ctrl | 23.00 A | 29.19 A | |
| Def | 5.46 B | 14.72 B | ||
| Single Fruit Mass | B | Ctrl | 0.65 a | 0.55 a |
| Def | 0.36 a | 0.38 a | ||
| R | Ctrl | 0.59 a | 0.54 a | |
| Def | 0.39 a | 0.38 a | ||
| N | Ctrl | - | - | |
| Def | - | - | ||
| Average | Ctrl | 0.62 A | 0.55 A | |
| Def | 0.37 B | 0.38 B |
| Genotypes | SML | Fresh Mass of the Root System | Dry Mass of the Root System |
|---|---|---|---|
| B | Ctrl | 11.81 a | 6.83 a |
| Def | 6.74 a | 4.40 a | |
| R | Ctrl | 12.30 a | 6.53 a |
| Def | 8.26 a | 4.40 a | |
| N | Ctrl | 17.80 a | 10.47 a |
| Def | 23.80 a | 15.31 a | |
| Average | Ctrl | 13.64 a | 7.66 a |
| Def | 16.61 a | 10.46 a |
| Plant Organ | Genotypes | SML | K [g‧kg−1] | Ca [g‧kg−1] | Mg [g‧kg−1] | Na [g‧kg−1] | Cu [mg‧kg−1] | Zn [mg‧kg−1] | Mn [mg‧kg−1] | Mo [mg‧kg−1] | Fe [mg‧kg−1] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaves | B | Ctrl | 97.6 a | 66.9 ab | 13.5 a | 64.8 a | 37.3 a | 172.5 bc | 201.8 ab | 11.7 a | 1.9 b |
| Def | 100.4 a | 70.2 a | 16.6 a | 4.9 a | 53.4 a | 225.0 ab | 241.8 a | 12.8 a | 2.6 a | ||
| R | Ctrl | 106.9 a | 69.7 a | 15.7 a | 2.8 a | 52.2 a | 236.4 ab | 205.4 ab | 6.6 a | 1.8 b | |
| Def | 118.0 a | 68.4 a | 17.0 a | 5.5 a | 56.0 a | 228.0 ab | 186.4 b | 6.7 a | 1.2 bc | ||
| N | Ctrl | 96.5 a | 51.9 bc | 17.2 a | 2.7 a | 45.6 a | 146.8 c | 139.2 c | 12.8 a | 0.7 c | |
| Def | 108.7 a | 42.2 c | 18.2 a | 4.6 a | 46.0 a | 288.2 a | 225.8 ab | 12.4 a | 1.8 b | ||
| Average | Ctrl | 100.5 B | 62.5 A | 15.6 B | 20.5 A | 45.6 A | 186.1 B | 180.7 B | 10.3 A | 1.4 A | |
| Def | 109.0 A | 60.6 A | 17.3 A | 5.0 A | 51.8 A | 247.1 A | 218.0 A | 10.6 A | 1.8 A | ||
| Crowns | B | Ctrl | 82.9 a | 115.6 bc | 3.9 a | 28.0 a | 87.9 a | 155.2 a | 31.4 a | 17.6 a | 0.2 a |
| Def | 92.2 a | 129.7 b | 4.2 a | 49.8 a | 84.1 a | 160.8 a | 49.8 a | 14.6 a | 0.5 a | ||
| R | Ctrl | 92.2 a | 91.4 c | 3.4 a | 37.4 a | 117.1 a | 141.3 a | 30.8 a | 16.4 a | 0.2 a | |
| Def | 104.3 a | 120.3 bc | 3.9 a | 56.6 a | 85.6 a | 164.1 a | 34.5 a | 13.9 a | 0.3 a | ||
| N | Ctrl | 50.4 a | 159.7 a | 5.4 a | 10.0 a | 74.4 a | 212.9 a | 61.8 a | 17.3 a | 0.2 a | |
| Def | 54.9 a | 130.2 b | 5.1 a | 20.8 a | 64.0 a | 245.6 a | 77.2 a | 18.6 a | 0.3 a | ||
| Average | Ctrl | 75.2 A | 122.2 A | 4.3 A | 25.1 B | 93.1 A | 169.8 A | 41.3 A | 17.1 A | 0.2 B | |
| Def | 83.8 A | 126.7 A | 4.4 A | 42.4 A | 77.9 A | 190.2 A | 53.8 A | 15.7 A | 0.3 A | ||
| Roots | B | Ctrl | 25.9 a | 80.7 a | 13.4 a | 6.1 a | 92.5 bc | 853.5 bc | 373.3 a | 12.4 a | 6.3 a |
| Def | 26.9 a | 88.3 a | 12.1 a | 8.6 a | 121.2 a | 844.4 b | 422.2 a | 19.0 a | 7.5 a | ||
| R | Ctrl | 24.5 a | 63.4 a | 13.4 a | 5.9 a | 111.4 a | 677.8 c | 388.0 a | 9.4 a | 6.4 a | |
| Def | 29.0 a | 103.3 a | 13.1 a | 8.9 a | 107.6 ab | 913.2 b | 309.0 a | 14.0 a | 7.3 a | ||
| N | Ctrl | 12.9 a | 60.2 a | 9.8 b | 5.9 a | 71.8 d | 883.8 b | 940.8 a | 14.6 a | 13.7 a | |
| Def | 20.9 a | 77.6 a | 12.9 a | 9.6 a | 93.0 c | 1232.3 a | 926.8 a | 12.4 a | 12.6 a | ||
| Average | Ctrl | 20.7 A | 67.2 B | 12.1 A | 6.0 B | 91.9 B | 801.6 B | 581.2 A | 12.1 A | 9.0 A | |
| Def | 25.9 A | 90.6 A | 12.7 A | 9.0 A | 108.3 A | 979.8 A | 525.9 A | 15.3 A | 8.9 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rokosa, M.; Mikiciuk, M.; Ptak, P. Assessment of Physiological Traits of Fragaria vesca Genotypes Under Water Deficit Conditions. Agriculture 2025, 15, 70. https://doi.org/10.3390/agriculture15010070
Rokosa M, Mikiciuk M, Ptak P. Assessment of Physiological Traits of Fragaria vesca Genotypes Under Water Deficit Conditions. Agriculture. 2025; 15(1):70. https://doi.org/10.3390/agriculture15010070
Chicago/Turabian StyleRokosa, Marta, Małgorzata Mikiciuk, and Piotr Ptak. 2025. "Assessment of Physiological Traits of Fragaria vesca Genotypes Under Water Deficit Conditions" Agriculture 15, no. 1: 70. https://doi.org/10.3390/agriculture15010070
APA StyleRokosa, M., Mikiciuk, M., & Ptak, P. (2025). Assessment of Physiological Traits of Fragaria vesca Genotypes Under Water Deficit Conditions. Agriculture, 15(1), 70. https://doi.org/10.3390/agriculture15010070






