Exogenous Application of Thidiazuron, Carbaryl, Ethephon, and Lime Sulphur Promotes Flower Abscission and Suppresses Tea Pests in the Tea Plant Camellia sinensis (L.) O. Kuntze
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experiments and Surveys
2.2. Determination of Electrical Conductivity
2.3. Determination of Photosynthetic Pigment Contents
2.4. Determination of Leaf Chlorophyll Fluorescence
2.5. Determination of Biochemical Components
2.6. Statistical Analysis
3. Results
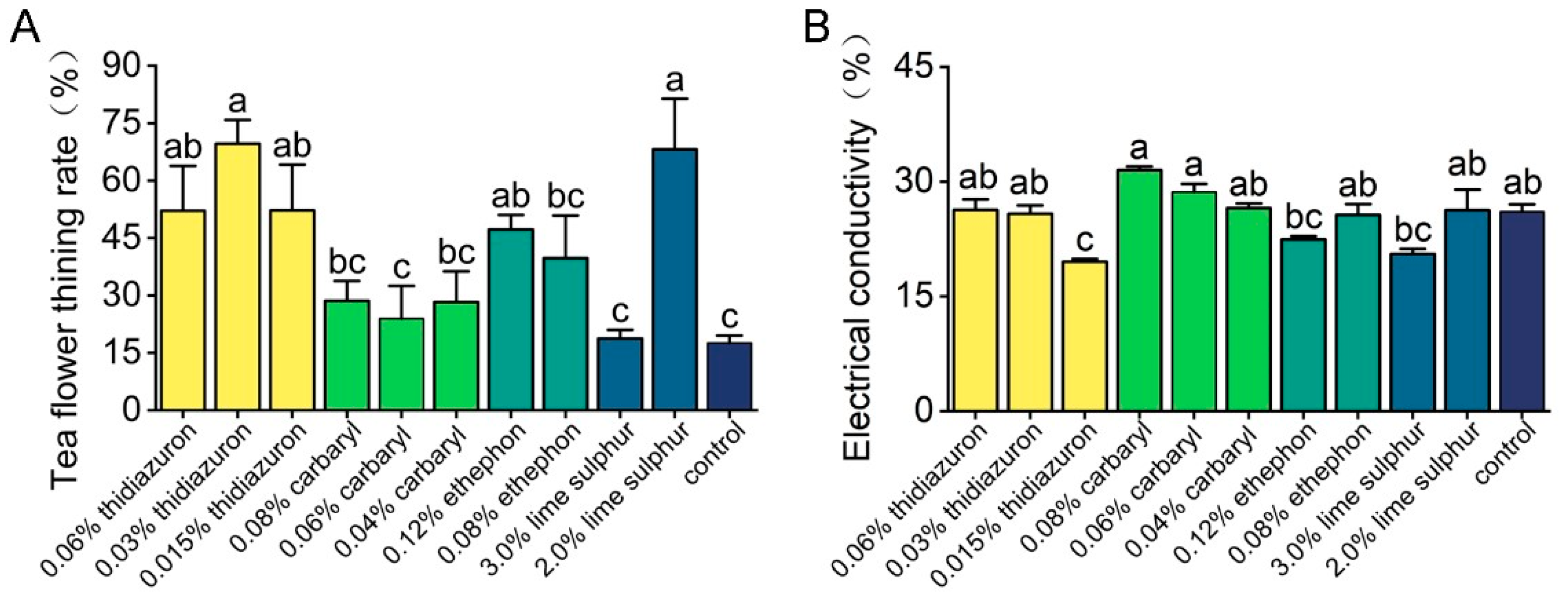
3.1. Effects of Chemicals on Tea Flower Abscission and Electrical Conductivity
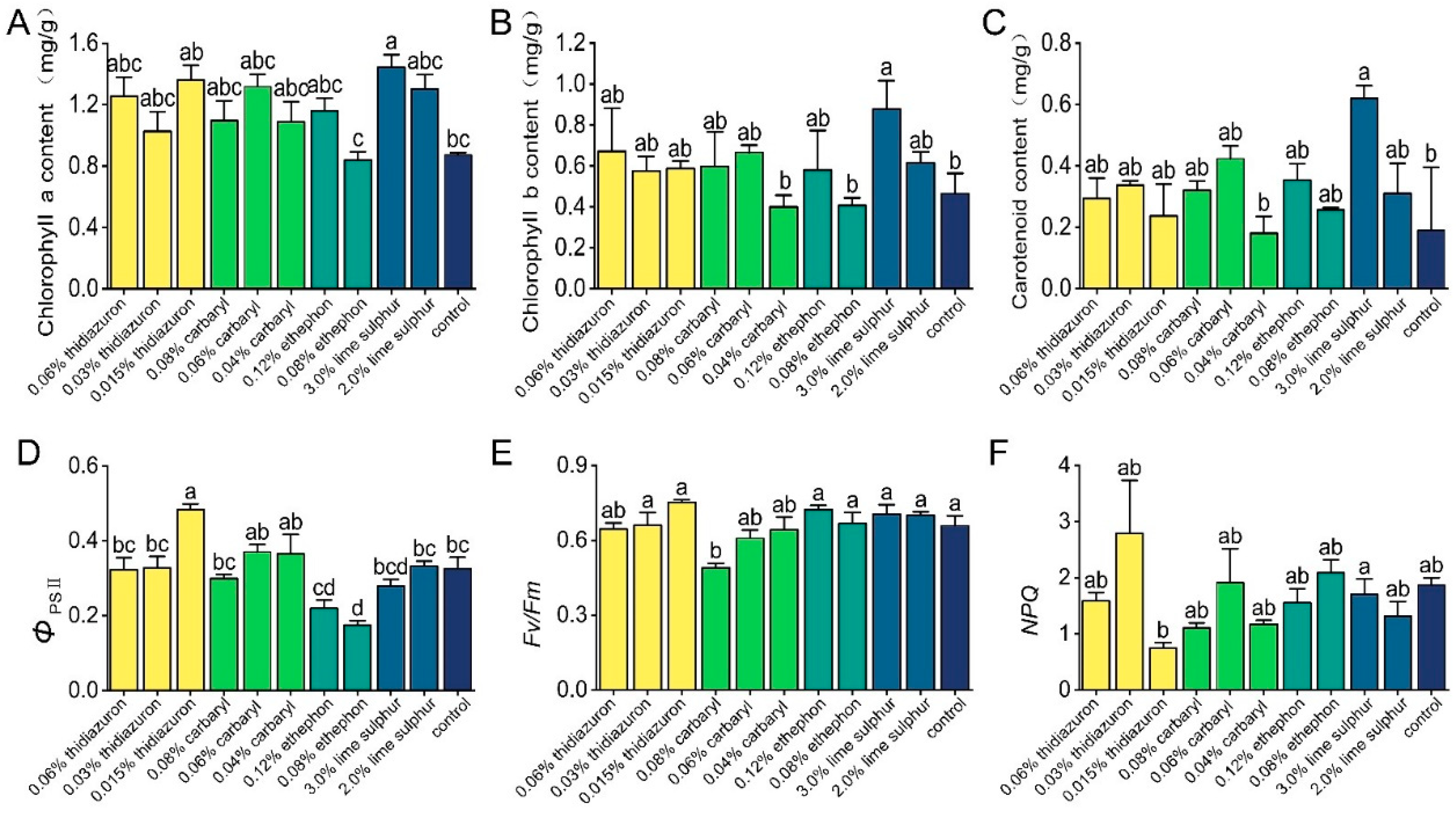
3.2. Effects of Chemicals on Pigments and Photosynthetic Characteristics
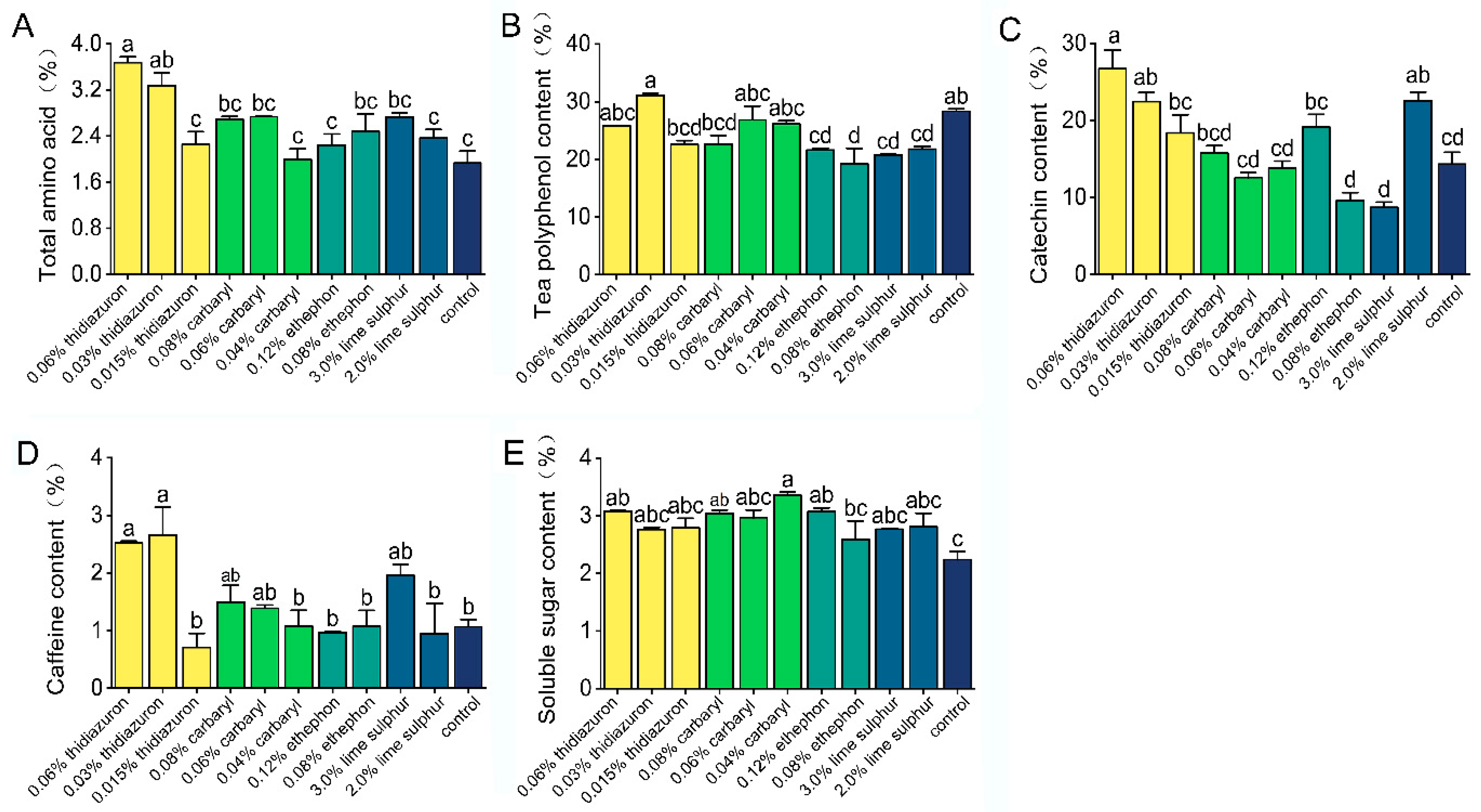
3.3. Effects of Chemicals on Biochemical Components
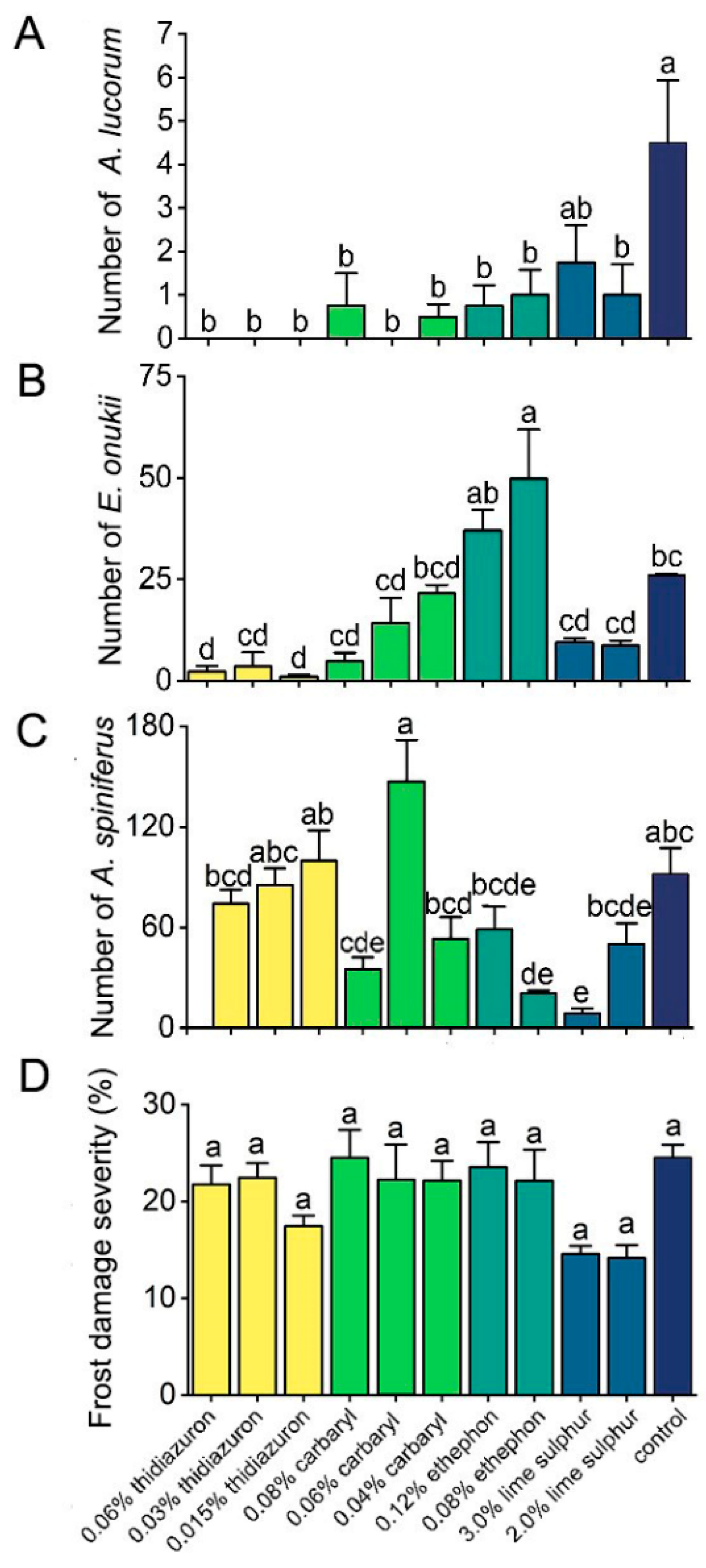
3.4. Effects of Chemicals on Insect Pests and Frost Damage on Tea Plants
3.5. Effects of Chemicals on Tea Shoots the Following Spring
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.-M.; Chen, L. Delicious and Healthy Tea: An Overview. In Global Tea Breeding: Achievements, Challenges and Perspectives; Chen, L., Apostolides, Z., Chen, Z.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–11. [Google Scholar]
- Chen, D.; Chen, G.; Sun, Y.; Zeng, X.; Ye, H. Physiological genetics, chemical composition, health benefits and toxicology of tea (Camellia sinensis L.) flower: A review. Food Res. Int. 2020, 137, 109584. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Zhang, Q.F.; Liu, M.Y.; Ma, L.F.; Shi, Y.Z.; Ruan, J.Y. Metabolomic and transcriptional analyses reveal the mechanism of C, N allocation from source leaf to flower in tea plant (Camellia sinensis. L). J. Plant Physiol. 2019, 232, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H. Analyses the way of controlling reproductive growth in tea plants. Chin. Tea 1997, 4, 6–7. [Google Scholar]
- Jiang, K.; Asami, T. Chemical regulators of plant hormones and their applications in basic research and agriculture. Biosci. Biotechnol. Biochem. 2018, 82, 1265–1300. [Google Scholar] [CrossRef] [PubMed]
- Abdelgadir, H.A.; Jäger, A.K.; Johnson, S.D.; Van Staden, J. Influence of plant growth regulators on flowering, fruiting, seed oil content, and oil quality of Jatropha curcas. S. Afr. J. Bot. 2010, 76, 440–446. [Google Scholar] [CrossRef]
- Shah, S.H.; Islam, S.; Alamri, S.; Parrey, Z.A.; Mohammad, F.; Kalaji, H.M. Plant Growth Regulators Mediated Changes in the Growth, Photosynthesis, Nutrient Acquisition and Productivity of Mustard. Agriculture 2023, 13, 570. [Google Scholar] [CrossRef]
- Agami, R.A.; Alamri, S.A.M.; Abd El-Mageed, T.A.; Abousekken, M.S.M.; Hashem, M. Salicylic acid and proline enhance water use efficiency, antioxidant defense system and tissues’ anatomy of wheat plants under field deficit irrigation stress. J. Appl. Bot. Food Qual. 2019, 92, 360–370. [Google Scholar]
- Khan, N.; Mukhtar, H. Tea and Health: Studies in Humans. Curr. Pharm. Des. 2013, 19, 6141–6147. [Google Scholar] [CrossRef]
- Tao, Q.; Zhou, Y.Y.; Guo, Q.; Liu, Y.R.; Yu, S.; Yu, C.X.; Zhang, M.C.; Li, Z.H.; Duan, L.S. A Novel Plant Growth Regulator Alleviates High-Temperature Stress in Maize. Agron. J. 2018, 110, 2350–2359. [Google Scholar] [CrossRef]
- Barchenger, D.W.; Coon, D.L.; Bosland, P.W. Efficient Breeder Seed Production Utilizing Ethephon to Promote Floral and Fruit Abscission in Ornamental Chile Peppers. Horttechnology 2016, 26, 30–35. [Google Scholar] [CrossRef]
- Gill, R.A.; Ahmar, S.; Ali, B.; Saleem, M.H.; Khan, M.U.; Zhou, W.; Liu, S. The Role of Membrane Transporters in Plant Growth and Development, and Abiotic Stress Tolerance. Int. J. Mol. Sci. 2021, 22, 12792. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, Z.; Wang, B.; Sui, S.; Li, M. Cloning of an Expansin Gene from Chimonanthus praecox Flowers and Its Expression in Flowers Treated with Ethephon or 1-Methylcyclopropene. HortScience 2012, 47, 1472–1477. [Google Scholar] [CrossRef]
- Rademacher, W. Plant Growth Regulators: Backgrounds and Uses in Plant Production. J. Plant Growth Regul. 2015, 34, 845–872. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, J. Effects of pre-bloom spraying thidiazuron and different embryo development media on seedless grape embryo rescue. N. Z. J. Crop Hortic. Sci. 2025, 53, 113–140. [Google Scholar] [CrossRef]
- Wertheim, S.J. Developments in the chemical thinning of apple and pear. Plant Growth Regul. 2000, 31, 85–100. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Zhang, X.; Wang, C.; Zhang, Z.; Sun, P. Exogenous application of ethephon regulates flower abscission, shoot growth, and secondary metabolites in Camellia sinensis. Sci. Hortic. 2022, 304, 111333. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Wu, C.; Zhao, R.; Li, M.; Cai, J.; Ma, L.; He, X.; Wu, X.; Zhenhua, Z. Effect of adjuvants on physicochemical properties of lime sulfur on flower/paraffin and application on flower thinning. Front. Plant Sci. 2023, 14, 1257672. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Seo, Y.D.; Choi, K.S. The effects of petroleum oil and lime sulfur on the mortality of Unaspis yanonensis and Aculops pelekassi in the laboratory. J. Asia-Pac. Entomol. 2010, 13, 283–288. [Google Scholar] [CrossRef]
- Robinson, T. Advances in apple culture worldwide. Rev. Bras. Frutic. 2011, 33, 37–47. [Google Scholar] [CrossRef]
- Rutkowski, K.; Łysiak, G.P. Thinning Methods to Regulate Sweet Cherry Crops—A Review. Appl. Sci. 2022, 12, 1280. [Google Scholar] [CrossRef]
- Dutta, S.K.; Gurung, G.; Yadav, A.; Laha, R.; Mishra, V.K. Factors associated with citrus fruit abscission and management strategies developed so far: A review. N. Z. J. Crop Hortic. Sci. 2023, 51, 467–488. [Google Scholar] [CrossRef]
- Ren, S.; Hu, M.; Wu, Q.; Wang, L.; Gu, H.; Chen, Z.; Ming, Z.; Li, Z. Flowering Time and Physiological Reaction of Dendrobium nobile Lindl in Response to TDZ Application. Horticulturae 2023, 9, 129. [Google Scholar] [CrossRef]
- McArtney, S.; Greene, D.; Schmidt, T.; Yuan, R. Naphthaleneacetic Acid and Ethephon Are Florigenic in the Biennial Apple Cultivars Golden Delicious and York Imperial. HortScience 2013, 48, 742–746. [Google Scholar] [CrossRef]
- Tian, Y.Y.; Chen, Z.J.; Jiang, Z.L.; Huang, X.Q.; Zhang, L.X.; Zhang, Z.Q.; Sun, P. Effects of Plant Growth Regulators on Flower Abscission and Growth of Tea Plant Camellia sinensis (L.) O. Kuntze. J. Plant Growth Regul. 2022, 41, 1161–1173. [Google Scholar] [CrossRef]
- Johnson, K.B.; Temple, T.N. Evaluation of Strategies for Fire Blight Control in Organic Pome Fruit Without Antibiotics. Plant Dis. 2013, 97, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Li, F.; Wu, Q.; Liao, B.; Yu, K.; Huo, Y.; Meng, L.; Wang, S.; Wang, B.; Du, M.; Tian, X.; et al. Thidiazuron Promotes Leaf Abscission by Regulating the Crosstalk Complexities between Ethylene, Auxin, and Cytokinin in Cotton. Int. J. Mol. Sci. 2022, 23, 2696. [Google Scholar] [CrossRef]
- Shu, H.M.; Sun, S.W.; Wang, X.J.; Chen, J.; Yang, C.Q.; Zhang, G.W.; Han, H.Y.; Li, Z.K.; Liang, T.; Liu, R.X. Thidiazuron combined with cyclanilide modulates hormone pathways and ROS systems in cotton, increasing defoliation at low temperatures. Front. Plant Sci. 2024, 15, 1333816. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Abbasi, B.H.; Zeb, A.; Xu, L.L.; Wei, Y.H. Thidiazuron: A multi-dimensional plant growth regulator. Afr. J. Biotechnol. 2011, 10, 8984–9000. [Google Scholar]
- Padmanabhan, P.; Murch, S.J.; Sullivan, J.A.; Saxena, P.K. Micropropagation of Primulina dryas (Dunn) Mich. Möller & A. Webber: High. frequency regeneration from leaf explants. Sci. Hortic. 2015, 192, 250–255. [Google Scholar]
- Yu, L.; Li, X.; Tian, H.; Liu, H.; Xiao, Y.; Liang, N.; Zhao, X.; Zhan, Y. Effects of Hormones and Epigenetic Regulation on the Callus and Adventitious Bud Induction of Fraxinus mandshurica Rupr. Forests 2020, 11, 590. [Google Scholar] [CrossRef]
- Niu, Y.; Xiang, Y. An Overview of Biomembrane Functions in Plant Responses to High-Temperature Stress. Front. Plant Sci. 2018, 9, 915. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.Y.; Gao, H.N.; Jiang, H.; Liu, C.; Li, Y.Y. MdKCS2 increased plant drought resistance by regulating wax biosynthesis. Plant Cell Rep. 2021, 40, 2357–2368. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.S.; Kwon, C. Trafficking at the host cell surface during plant immune responses. J. Plant Biol. 2012, 55, 185–190. [Google Scholar] [CrossRef]
- Rosisca, J.R.; de Oliveira, C.M.G.; Sartori, A.V.D.; Stolf-Moreira, R.; Silva, M.; Morais, H. Electrical conductivity as an indicator of damage due to low temperatures in beans leaves. Semin. Cienc. Agrar. 2019, 40, 1011–1022. [Google Scholar] [CrossRef]
- Ali, H.; Khan, M.A.; Kayani, W.K.; Khan, T.; Mashwani, Z.-u.-R.; Nazif, U.; Khan, R.S. Thidiazuron regulated growth, secondary metabolism and essential oil profiles in shoot cultures of Ajuga bracteosa. Ind. Crops Prod. 2018, 121, 418–427. [Google Scholar] [CrossRef]
- Erland, L.A.E.; Giebelhaus, R.T.; Victor, J.M.R.; Murch, S.J.; Saxena, P.K. The Morphoregulatory Role of Thidiazuron: Metabolomics-Guided Hypothesis Generation for Mechanisms of Activity. Biomolecules 2020, 10, 1253. [Google Scholar] [CrossRef] [PubMed]
- Meléndez, L.R.O.; Rodríguez, O.A.H.; Alvarez, O.C.; Mendoza, A.B.; Jurado, M.C.; Barrios, D.L.O. Does the application of growth bioregulators improve the foliar concentration of nutrients, non-structural carbohydrates and yield in pecan? Cienc. Agrotecnol. 2021, 45, e004721. [Google Scholar] [CrossRef]
- Ojeda-Barrios, D.L.; Orozco-Meléndez, L.R.; Cano-Medrano, R.; Sánchez-Chávez, E.; Parra-Quezada, R.Á.; Calderón-Jurado, M.; Jacobo-Cuellar, J.L.; Hernández-Ordoñez, E.; Cruz-Álvarez, O. Non-Structural Carbohydrates, Foliar Nutrients, Yield Components and Oxidative Metabolism in Pecan Trees in Response to Foliar Applications of Growth Regulators. Agriculture 2022, 12, 688. [Google Scholar] [CrossRef]
- Samanta, S. Potential Bioactive Components and Health Promotional Benefits of Tea (Camellia sinensis). J. Am. Nutr. Assoc. 2022, 41, 65–93. [Google Scholar] [CrossRef]
- Senanayake, S. Green tea extract: Chemistry, antioxidant properties and food applications—A review. J. Funct. Foods 2013, 5, 1529–1541. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, M.; Chen, Z. The Relative Preference of Empoasca onukii (Hemiptera: Cicadellidae) for Oviposition on Twenty-Four Tea Cultivars. J. Econ. Entomol. 2022, 115, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zhou, X.; Liao, Y.; Yang, Z. Roles of specialized metabolites in biological function and environmental adaptability of tea plant (Camellia sinensis) as a metabolite studying model. J. Adv. Res. 2021, 34, 159–171. [Google Scholar] [CrossRef]
- He, Y.; Zhang, Q.; Inostroza, A.C.; Kierszniowska, S.; Liu, L.; Li, Y.; Ruan, J. Application of metabolic fingerprinting in tea quality evaluation. Food Control 2024, 160, 110361. [Google Scholar] [CrossRef]
- Pang, X.M.; Chen, F.Y.; Liu, G.Y.; Zhang, Q.; Ye, J.H.; Lei, W.X.; Jia, X.L.; He, H.B. Comparative analysis on the quality of Wuyi Rougui (Camellia sinensis) tea with different grades. Food Sci. Technol. 2022, 42, e115321. [Google Scholar] [CrossRef]
- Yuan, A.; Liu, Z. Pollution-free organic tea garden management technology in northern China. Agric. Dev. Equip. 2013, 5, 107–108. [Google Scholar]
- Marchioretto, L.D.R.; De Rossi, A.; Amaral, L.O.d.; Ribeiro, A.M.A.d.S. Efficacy and mode of action of blossom thinners on ‘Fuji More’ apple trees. Sci. Hortic. 2019, 246, 634–642. [Google Scholar] [CrossRef]
- Shahryar, N.; Maali-Amiri, R. Metabolic acclimation of tetraploid and hexaploid wheats by cold stress-induced carbohydrate accumulation. J. Plant Physiol. 2016, 204, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Bellon, P.P.; Pietrowski, V.; Alves, L.F.A.; Rheinheimer, A.R.; Miranda, A.M.; Gazola, D. Agroecologic phytosanitary products for the control of lace bug (Vatiga manihotae) (Hemiptera: Tingidae) of Cassava. Interciencia 2014, 39, 40–45. [Google Scholar]
- Capinera, J.L. Assessment of barrier materials to protect plants from Florida leatherleaf slug (Mollusca: Gastropoda: Veronicellidae). Fla. Entomol. 2018, 101, 373–381. [Google Scholar] [CrossRef]
- Efrom, C.F.S.; Redaelli, L.R.; Meirelles, R.N.; Ourique, C.B. Selectivity of phytosanitary products used in organic farming on adult of Cryptolaemus montrouzieri (Coleoptera, Coccinellidae) under laboratory conditions. Semin. Cienc. Agrar. 2011, 32, 1429–1437. [Google Scholar] [CrossRef]
- Venzon, M.; Oliveira, R.M.; Perez, A.L.; Rodriguez-Cruz, F.A.; Martins, S. Lime sulfur toxicity to broad mite, to its host plants and to natural enemies. Pest Manag. Sci. 2013, 69, 738–743. [Google Scholar] [CrossRef]
- Ferrara, G.; Mazzeo, A.; Matarrese, A.M.S.; Pacucci, C.; Trani, A.; Fidelibus, M.W.; Gambacorta, G. Ethephon As a Potential Abscission Agent for Table Grapes: Effects on Pre-Harvest Abscission, Fruit Quality, and Residue. Front. Plant Sci. 2016, 7, 620. [Google Scholar] [CrossRef] [PubMed]
- Paul, V.; Pandey, R.; Srivastava, G.C. The fading distinctions between classical patterns of ripening in climacteric and non-climacteric fruit and the ubiquity of ethylene-An overview. J. Food Sci. Technol. 2012, 49, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Battelli, R.; Lombardi, L.; Rogers, H.J.; Picciarelli, P.; Lorenzi, R.; Ceccarelli, N. Changes in ultrastructure, protease and caspase-like activities during flower senescence in Lilium longiflorum. Plant Sci. 2011, 180, 716–725. [Google Scholar] [CrossRef]
- Zou, J.J.; Zhou, Y.; Cai, X.; Wang, C.Y. Increase in DNA fragmentation and the role of ethylene and reactive oxygen species in petal senescence of Osmanthus fragrans. Postharvest Biol. Technol. 2014, 93, 97–105. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Liu, J.F.; Yang, X.D.; Ma, R.; Liu, C.M.; Liu, Q. RNA-seq Analysis Reveals Ethylene-Mediated Reproductive Organ Development and Abscission in Soybean (Glycine max L. Merr.). Plant Mol. Biol. Rep. 2013, 31, 607–619. [Google Scholar] [CrossRef]
- Li, C.Q.; Wang, Y.; Ying, P.Y.; Ma, W.Q.; Li, J.G. Genome-wide digital transcript analysis of putative fruitlet abscission related genes regulated by ethephon in litchi. Front. Plant Sci. 2015, 6, 502. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, M.; Lun, X.; Zhang, R.; Zhang, Y.; Zhang, X.; Guan, F.; Wang, L.; Ying, Y.; Zhang, Z.; Xu, X. Exogenous Application of Thidiazuron, Carbaryl, Ethephon, and Lime Sulphur Promotes Flower Abscission and Suppresses Tea Pests in the Tea Plant Camellia sinensis (L.) O. Kuntze. Agriculture 2025, 15, 150. https://doi.org/10.3390/agriculture15020150
Jin M, Lun X, Zhang R, Zhang Y, Zhang X, Guan F, Wang L, Ying Y, Zhang Z, Xu X. Exogenous Application of Thidiazuron, Carbaryl, Ethephon, and Lime Sulphur Promotes Flower Abscission and Suppresses Tea Pests in the Tea Plant Camellia sinensis (L.) O. Kuntze. Agriculture. 2025; 15(2):150. https://doi.org/10.3390/agriculture15020150
Chicago/Turabian StyleJin, Meina, Xiaoyue Lun, Ruirui Zhang, Yu Zhang, Xiangzhi Zhang, Feiyu Guan, Liping Wang, Yiheng Ying, Zhengqun Zhang, and Xiuxiu Xu. 2025. "Exogenous Application of Thidiazuron, Carbaryl, Ethephon, and Lime Sulphur Promotes Flower Abscission and Suppresses Tea Pests in the Tea Plant Camellia sinensis (L.) O. Kuntze" Agriculture 15, no. 2: 150. https://doi.org/10.3390/agriculture15020150
APA StyleJin, M., Lun, X., Zhang, R., Zhang, Y., Zhang, X., Guan, F., Wang, L., Ying, Y., Zhang, Z., & Xu, X. (2025). Exogenous Application of Thidiazuron, Carbaryl, Ethephon, and Lime Sulphur Promotes Flower Abscission and Suppresses Tea Pests in the Tea Plant Camellia sinensis (L.) O. Kuntze. Agriculture, 15(2), 150. https://doi.org/10.3390/agriculture15020150





