Soil Enzyme Activities and Microbial Carbon Pump Promote Carbon Storage by Influencing Bacterial Communities Under Nitrogen-Rich Conditions in Tea Plantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Design
2.2. Soil Sampling and Analysis
2.3. Soil Enzymes Activities
2.4. Microbial Diversity Analysis
2.5. Calculations of MCP Efficacy
2.6. Statistical Analysis
3. Results
3.1. Response of Soil Physicochemical Properties to Fertilization
3.2. Response of Microbial Communities to Fertilization
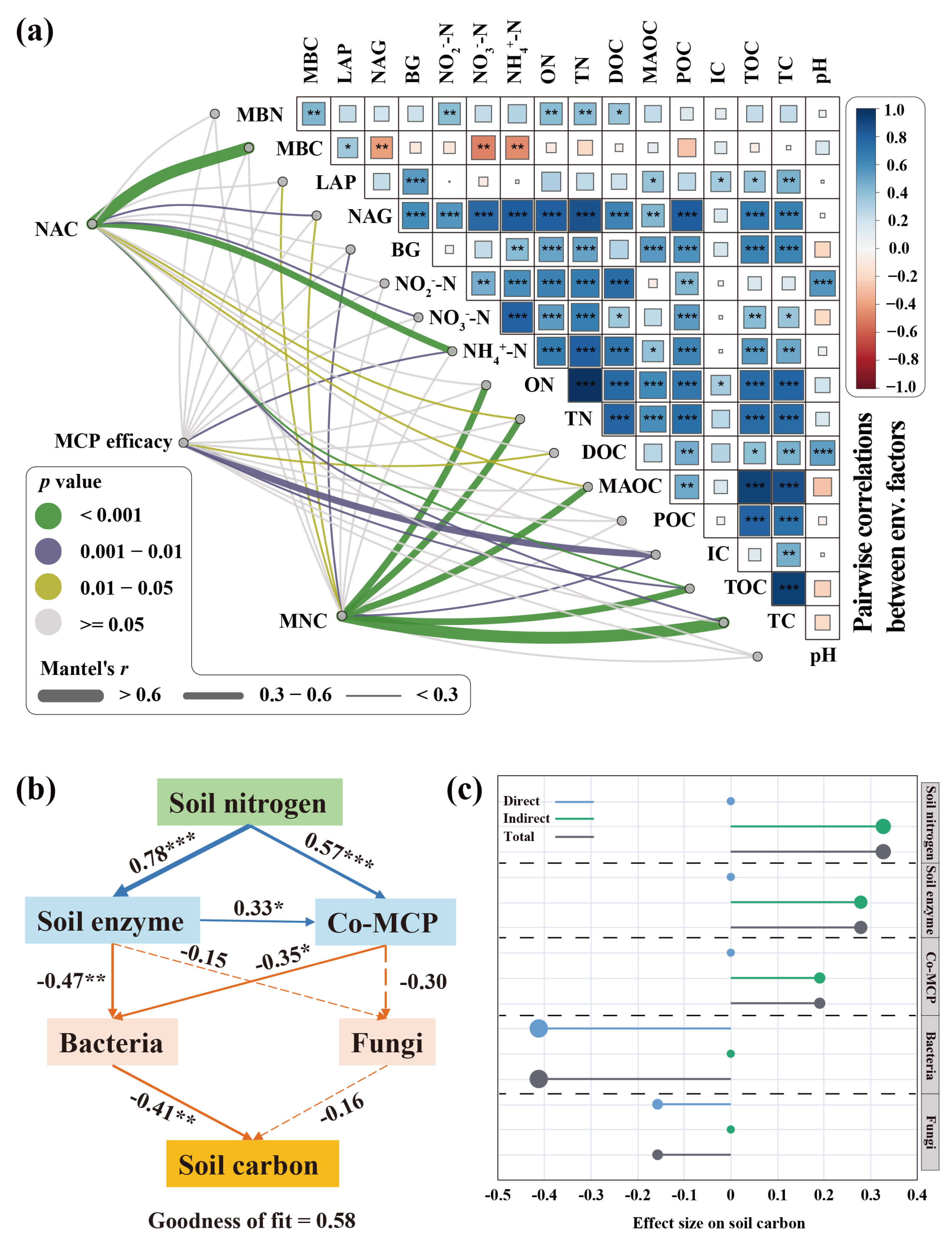
3.3. Relationship Between Microbial Community and Soil Physicochemical Properties
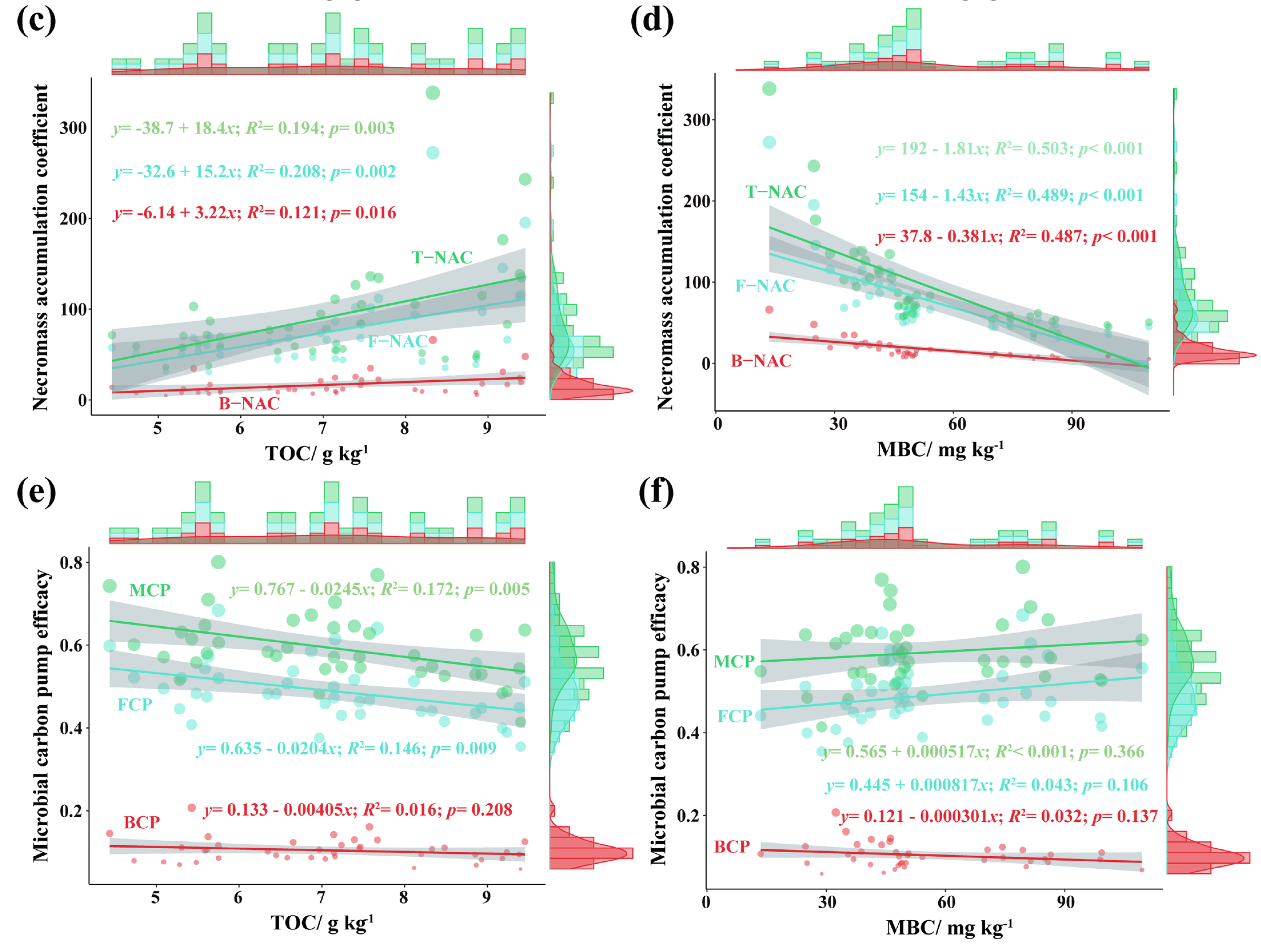
3.4. Responses of Microbial Necromass to Fertilization
3.5. Carbon–Nitrogen Coupling Processes Under Nitrogen-Rich Conditions
4. Discussion
4.1. The Impact of Soil Enzymes on C-N Coupling
4.2. Response of MCP Efficacy to Nitrogen Addition
4.3. Impact of Priming Effect on Soil Carbon Due to Fertilizer Mixing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCP | bacterial carbon pump |
| BG | β-1,4-glucosidase |
| B-NAC | bacterial necromass accumulation coefficient |
| BNC | bacterial necromass carbon |
| CK | no fertilization |
| C-N | carbon–nitrogen |
| DOC | dissolved organic carbon |
| FB | combined application of chemical fertilizer and biochar |
| FCP | fungal carbon pump |
| FF | chemical fertilizer |
| FM | combined application of chemical and microbial fertilizer |
| F-NAC | fungal necromass accumulation coefficient |
| FNC | fungal necromass carbon |
| FO | combined application of chemical and organic fertilizer |
| IC | inorganic carbon |
| LAP | leucine aminopeptidase |
| MAOC | mineral-associated organic carbon |
| MBC | microbial biomass carbon |
| MBN | microbial biomass nitrogen |
| MCP | microbial carbon pump |
| MNC | total microbial necromass carbon |
| NAG | β-1,4-N-acetylglucosaminidase |
| NAC | microbial necromass accumulation coefficient |
| NH4+-N | ammonium nitrogen |
| NO2−-N | nitrite nitrogen |
| NO3−-N | nitrate nitrogen |
| ON | organic nitrogen |
| POC | particulate organic carbon |
| SOC | soil organic carbon |
| TC | total carbon |
| TN | total nitrogen |
| T-NAC | total microbial necromass accumulation coefficient |
| TOC | total organic carbon |
References
- Yan, P.; Shen, C.; Fan, L.; Li, X.; Zhang, L.; Zhang, L.; Han, W. Tea planting affects soil acidification and nitrogen and phosphorus distribution in soil. Agric. Ecosyst. Environ. 2018, 254, 20–25. [Google Scholar] [CrossRef]
- FAO. International Tea Market: Market Situation, Prospects and Emerging Issues. 2022. Available online: https://openknowledge.fao.org (accessed on 13 January 2025).
- Yang, X.; Ni, K.; Shi, Y.; Yi, X.; Ji, L.; Wei, S.; Jiang, Y.; Zhang, Y.; Cai, Y.; Ma, Q.; et al. Metagenomics reveals N-induced changes in carbon-degrading genes and microbial communities of tea (Camellia sinensis L.) plantation soil under long-term fertilization. Sci. Total Environ. 2023, 856, 159231. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yao, Z.; Pan, Z.; Wang, R.; Yan, G.; Liu, C.; Su, Y.; Zheng, X.; Butterbach-Bahl, K. Tea-planted soils as global hotspots for N2O emissions from croplands. Environ. Res. Lett. 2020, 15, 104018. [Google Scholar] [CrossRef]
- Vitousek, P.; Howarth, R. Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry 1991, 13, 87–115. [Google Scholar] [CrossRef]
- Jiang, N.; Huang, W.; Chen, Z.; Zhang, Y.; Jiang, D.; Wu, C.; Yao, Z.; Chen, L. More labile carbon inputs lessen the positive effects of nitrogen enrichment on soil carbon storage in a temperate grassland. Plant Soil 2023, 505, 225–237. [Google Scholar] [CrossRef]
- Niu, S.; Song, L.; Wang, J.; Luo, Y.; Yu, G. Dynamic carbon-nitrogen coupling under global change. Sci. China Life Sci. 2023, 66, 771–782. [Google Scholar] [CrossRef]
- Ni, H.; Liu, C.; Sun, B.; Liang, Y. Response of global farmland soil organic carbon to nitrogen application over time depends on soil type. Geoderma 2022, 406, 115542. [Google Scholar] [CrossRef]
- Wang, X.; Feng, J.; Ao, G.; Qin, W.; Han, M.; Shen, Y.; Liu, M.; Chen, Y.; Zhu, B. Globally nitrogen addition alters soil microbial community structure, but has minor effects on soil microbial diversity and richness. Soil. Biol. Biochem. 2023, 179, 108982. [Google Scholar] [CrossRef]
- Feng, X.; Qin, S.; Zhang, D.; Chen, P.; Hu, J.; Wang, G.; Liu, Y.; Wei, B.; Li, Q.; Yang, Y.; et al. Nitrogen input enhances microbial carbon use efficiency by altering plant-microbe-mineral interactions. Glob. Chang. Biol. 2022, 28, 4845–4860. [Google Scholar] [CrossRef]
- Tian, J.; Dungait, J.A.J.; Hou, R.; Deng, Y.; Hartley, I.P.; Yang, Y.; Kuzyakov, Y.; Zhang, F.; Cotrufo, M.F.; Zhou, J. Microbially mediated mechanisms underlie soil carbon accrual by conservation agriculture under decade-long warming. Nat. Commun. 2024, 15, 377. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Jackson, R.D.; DeLucia, E.H.; Tiedje, J.M.; Liang, C. The soil microbial carbon pump: From conceptual insights to empirical assessments. Glob. Chang. Biol. 2020, 26, 6032–6039. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Canarini, A.; Zhang, W.; Lang, M.; Chen, Y.; Cui, Z.; Kuzyakov, Y.; Richter, A.; Chen, X.; Zhang, F.; et al. Microbial life-history strategies mediate microbial carbon pump efficacy in response to N management depending on stoichiometry of microbial demand. Glob. Chang. Biol. 2024, 30, e17311. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.D.; Martiny, J.B. Resistance, resilience, and redundancy in microbial communities. Proc. Natl. Acad. Sci. USA 2008, 105, 11512–11519. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Q.; Hu, X.; Fang, X.-M.; Wang, S.; Chen, F.-S. Divergent responses of soil microbial community to long-term nitrogen and phosphorus additions in a subtropical Chinese fir plantation. CATENA 2024, 242, 108132. [Google Scholar] [CrossRef]
- Mori, T. Does ecoenzymatic stoichiometry really determine microbial nutrient limitations? Soil. Biol. Biochem. 2020, 146, 107816. [Google Scholar] [CrossRef]
- Zhang, X.; Dippold, M.A.; Kuzyakov, Y.; Razavi, B.S. Spatial pattern of enzyme activities depends on root exudate composition. Soil. Biol. Biochem. 2019, 133, 83–93. [Google Scholar] [CrossRef]
- Craine, J.M.; Jackson, R.D. Plant nitrogen and phosphorus limitation in 98 North American grassland soils. Plant Soil 2009, 334, 73–84. [Google Scholar] [CrossRef]
- Henneron, L.; Kardol, P.; Wardle, D.A.; Cros, C.; Fontaine, S. Rhizosphere control of soil nitrogen cycling: A key component of plant economic strategies. New Phytol. 2020, 228, 1269–1282. [Google Scholar] [CrossRef]
- Moreau, D.; Bardgett, R.D.; Finlay, R.D.; Jones, D.L.; Philippot, L.; Power, S. A plant perspective on nitrogen cycling in the rhizosphere. Funct. Ecol. 2019, 33, 540–552. [Google Scholar] [CrossRef]
- Qiao, C.; Xu, B.; Han, Y.; Wang, J.; Wang, X.; Liu, L.; Liu, W.; Wan, S.; Tan, H.; Liu, Y.; et al. Synthetic nitrogen fertilizers alter the soil chemistry, production and quality of tea. A meta-analysis. Agron. Sustain. Dev. 2018, 38, 10. [Google Scholar] [CrossRef]
- Bonheure, D.; Willson, K. Mineral Nutrition and Fertilizers; Willson, K.C., Clifford, M.N., Eds.; Springer: Dordrecht, The Netherlands, 1992; pp. 269–329. [Google Scholar] [CrossRef]
- Wanyoko, J.; Owuor, P. Effects of plant densities and nitrogen fertilizer rates on the yield of mature seedling Kenyan tea. Tea 1995, 16, 14–20. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19960304943 (accessed on 13 January 2025).
- Ma, L.; Yang, X.; Shi, Y.; Yi, X.; Ji, L.; Cheng, Y.; Ni, K.; Ruan, J. Response of tea yield, quality and soil bacterial characteristics to long-term nitrogen fertilization in an eleven-year field experiment. Appl. Soil Ecol. 2021, 166, 103976. [Google Scholar] [CrossRef]
- Ye, J.; Wang, Y.; Wang, Y.; Hong, L.; Jia, X.; Kang, J.; Lin, S.; Wu, Z.; Wang, H. Improvement of soil acidification in tea plantations by long-term use of organic fertilizers and its effect on tea yield and quality. Front. Plant Sci. 2022, 13, 1055900. [Google Scholar] [CrossRef]
- Han, Z.; Lin, H.; Xu, P.; Li, Z.; Wang, J.; Zou, J. Impact of organic fertilizer substitution and biochar amendment on net greenhouse gas budget in a tea plantation. Agric. Ecosyst. Environ. 2022, 326, 107779. [Google Scholar] [CrossRef]
- Xie, S.; Yang, F.; Feng, H.; Yu, Z.; Liu, C.; Wei, C.; Liang, T. Organic fertilizer reduced carbon and nitrogen in runoff and buffered soil acidification in tea plantations: Evidence in nutrient contents and isotope fractionations. Sci. Total Environ. 2021, 762, 143059. [Google Scholar] [CrossRef]
- Giannitsopoulos, M.L.; Burgess, P.J.; Sakrabani, R.; Holden, A.; Saini, H.; Kirui, C. Modelling the effects of soil organic content and pH on the yield responses of tea to nitrogen fertilizer. Agric. Syst. 2023, 212, 103754. [Google Scholar] [CrossRef]
- Hu, J.; Huang, C.; Zhou, S.; Liu, X.; Dijkstra, F.A. Nitrogen addition increases microbial necromass in croplands and bacterial necromass in forests: A global meta-analysis. Soil Biol. Biochem. 2022, 165, 108500. [Google Scholar] [CrossRef]
- Fang, X.; Zhou, G.; Qu, C.; Huang, W.; Zhang, D.; Li, Y.; Yi, Z.; Liu, J. Translocating subtropical forest soils to a warmer region alters microbial communities and increases the decomposition of mineral-associated organic carbon. Soil. Biol. Biochem. 2020, 142, 107707. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Bell, C.W.; Fricks, B.E.; Rocca, J.D.; Steinweg, J.M.; McMahon, S.K.; Wallenstein, M.D. High-throughput fluorometric measurement of potential soil extracellular enzyme activities. J. Vis. Exp. 2013, e50961. [Google Scholar] [CrossRef]
- Liang, C.; Amelung, W.; Lehmann, J.; Kastner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Chang. Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef] [PubMed]
- Jiao, N.; Luo, T.; Chen, Q.; Zhao, Z.; Xiao, X.; Liu, J.; Jian, Z.; Xie, S.; Thomas, H.; Herndl, G.J.; et al. The microbial carbon pump and climate change. Nat. Rev. Microbiol. 2024, 22, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Yao, Y.; Wang, Y.; Su, X.; Ma, L.; Chen, X.; Li, Z. Microbial traits dictate soil necromass accumulation coefficient: A global synthesis. Glob. Ecol. Biogeogr. 2023, 33, 151–161. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, J.; Ao, Y.; Han, J.; Guo, Z.; Liu, X.; Zhang, J.; Mu, C.; Le Roux, X. Responses of soil N2O emissions and their abiotic and biotic drivers to altered rainfall regimes and co-occurring wet N deposition in a semi-arid grassland. Glob. Chang. Biol. 2021, 27, 4894–4908. [Google Scholar] [CrossRef]
- Li, J.; Sang, C.; Yang, J.; Qu, L.; Xia, Z.; Sun, H.; Jiang, P.; Wang, X.; He, H.; Wang, C. Stoichiometric imbalance and microbial community regulate microbial elements use efficiencies under nitrogen addition. Soil Biol. Biochem. 2021, 156, 108207. [Google Scholar] [CrossRef]
- Philippot, L.; Griffiths, B.S.; Langenheder, S. Microbial Community Resilience across Ecosystems and Multiple Disturbances. Microbiol. Mol. Biol. Rev. 2021, 85, e00026-20. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, X.; Jing, X.; Zhu, B. A meta-analysis of soil extracellular enzyme activities in response to global change. Soil Biol. Biochem. 2018, 123, 21–32. [Google Scholar] [CrossRef]
- Stock, S.C.; Köster, M.; Dippold, M.A.; Nájera, F.; Matus, F.; Merino, C.; Boy, J.; Spielvogel, S.; Gorbushina, A.; Kuzyakov, Y. Environmental drivers and stoichiometric constraints on enzyme activities in soils from rhizosphere to continental scale. Geoderma 2019, 337, 973–982. [Google Scholar] [CrossRef]
- Louca, S.; Polz, M.F.; Mazel, F.; Albright, M.B.N.; Huber, J.A.; O’Connor, M.I.; Ackermann, M.; Hahn, A.S.; Srivastava, D.S.; Crowe, S.A.; et al. Function and functional redundancy in microbial systems. Nat. Ecol. Evol. 2018, 2, 936–943. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Manici, L.M.; Caputo, F.; De Sabata, D.; Fornasier, F. The enzyme patterns of Ascomycota and Basidiomycota fungi reveal their different functions in soil. Appl. Soil. Ecol. 2024, 196, 105323. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Li, H.; Yang, S.; Semenov, M.V.; Yao, F.; Ye, J.; Bu, R.; Ma, R.; Lin, J.; Kurganova, I.; Wang, X.; et al. Temperature sensitivity of SOM decomposition is linked with a K-selected microbial community. Glob. Chang. Biol. 2021, 27, 2763–2779. [Google Scholar] [CrossRef]
- Feng, X.; Wang, S. Plant influences on soil microbial carbon pump efficiency. Glob. Chang. Biol. 2023, 29, 3854–3856. [Google Scholar] [CrossRef]
- Liang, C.; Zhu, X. The soil microbial carbon pump as a new concept for terrestrial carbon sequestration. Sci. China Earth Sci. 2021, 64, 545–558. [Google Scholar] [CrossRef]
- Qu, Y.; Tang, J.; Liu, B.; Lyu, H.; Duan, Y.; Yang, Y.; Wang, S.; Li, Z. Rhizosphere enzyme activities and microorganisms drive the transformation of organic and inorganic carbon in saline-alkali soil region. Sci. Rep. 2022, 12, 1314. [Google Scholar] [CrossRef]
- Lux, S.; Baldauf-Sommerbauer, G.; Siebenhofer, M. Hydrogenation of Inorganic Metal Carbonates: A Review on Its Potential for Carbon Dioxide Utilization and Emission Reduction. ChemSusChem 2018, 11, 3357–3375. [Google Scholar] [CrossRef]
- Bailey, V.L.; Pries, C.H.; Lajtha, K. What do we know about soil carbon destabilization? Environ. Res. Lett. 2019, 14, 083004. [Google Scholar] [CrossRef]
- Raza, S.; Miao, N.; Wang, P.; Ju, X.; Chen, Z.; Zhou, J.; Kuzyakov, Y. Dramatic loss of inorganic carbon by nitrogen-induced soil acidification in Chinese croplands. Glob. Chang. Biol. 2020, 26, 3738–3751. [Google Scholar] [CrossRef]
- Bai, Y.; Cotrufo, M.F. Grassland soil carbon sequestration: Current understanding, challenges, and solutions. Science 2022, 377, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Stark, J.; Waring, B.G. Mineral reactivity determines root effects on soil organic carbon. Nat. Commun. 2023, 14, 4962. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Schimel, J.P.; Janssens, I.A.; Song, X.; Song, C.; Yu, G.; Sinsabaugh, R.L.; Tang, D.; Zhang, X.; Thornton, P.E. Global pattern and controls of soil microbial metabolic quotient. Ecol. Monogr. 2017, 87, 429–441. [Google Scholar] [CrossRef]
- Kallenbach, C.M.; Frey, S.D.; Grandy, A.S. Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat. Commun. 2016, 7, 13630. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Moorhead, D.L.; Guo, X.; Peng, S.; Wang, Y.; Zhang, X.; Fang, L. Stoichiometric models of microbial metabolic limitation in soil systems. Glob. Ecol. Biogeogr. 2021, 30, 2297–2311. [Google Scholar] [CrossRef]
- Fontaine, S.; Barot, S.; Barre, P.; Bdioui, N.; Mary, B.; Rumpel, C. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 2007, 450, 277–280. [Google Scholar] [CrossRef]
- Yan, S.; Yin, L.; Dijkstra, F.A.; Wang, P.; Cheng, W. Priming effect on soil carbon decomposition by root exudate surrogates: A meta-analysis. Soil. Biol. Biochem. 2023, 178, 108955. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Priming effects: Interactions between living and dead organic matter. Soil. Biol. Biochem. 2010, 42, 1363–1371. [Google Scholar] [CrossRef]
- Buckeridge, K.M.; Creamer, C.; Whitaker, J. Deconstructing the microbial necromass continuum to inform soil carbon sequestration. Funct. Ecol. 2022, 36, 1396–1410. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Chen, L.; Fang, K.; Wei, B.; Qin, S.; Feng, X.; Hu, T.; Ji, C.; Yang, Y. Soil carbon persistence governed by plant input and mineral protection at regional and global scales. Ecol. Lett. 2021, 24, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, F.A.; Zhu, B.; Cheng, W. Root effects on soil organic carbon: A double-edged sword. New Phytol. 2021, 230, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Wang, T.; Xu, Y.; Deng, J.; Zhao, F.; Yang, G.; Han, X.; Feng, Y.; Ren, G. Differential soil microbial community responses to the linkage of soil organic carbon fractions with respiration across land-use changes. For. Ecol. Manag. 2018, 409, 170–178. [Google Scholar] [CrossRef]
- Wang, C.; Kuzyakov, Y. Mechanisms and implications of bacterial–fungal competition for soil resources. ISME J. 2024, 18, wrae073. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, Q.; Gao, S.; Liu, X.; Yao, Z.; Wu, H.; Qi, L.; Zhang, X. Soil Enzyme Activities and Microbial Carbon Pump Promote Carbon Storage by Influencing Bacterial Communities Under Nitrogen-Rich Conditions in Tea Plantation. Agriculture 2025, 15, 238. https://doi.org/10.3390/agriculture15030238
Shu Q, Gao S, Liu X, Yao Z, Wu H, Qi L, Zhang X. Soil Enzyme Activities and Microbial Carbon Pump Promote Carbon Storage by Influencing Bacterial Communities Under Nitrogen-Rich Conditions in Tea Plantation. Agriculture. 2025; 15(3):238. https://doi.org/10.3390/agriculture15030238
Chicago/Turabian StyleShu, Qi, Shenghua Gao, Xinmiao Liu, Zengwang Yao, Hailong Wu, Lianghua Qi, and Xudong Zhang. 2025. "Soil Enzyme Activities and Microbial Carbon Pump Promote Carbon Storage by Influencing Bacterial Communities Under Nitrogen-Rich Conditions in Tea Plantation" Agriculture 15, no. 3: 238. https://doi.org/10.3390/agriculture15030238
APA StyleShu, Q., Gao, S., Liu, X., Yao, Z., Wu, H., Qi, L., & Zhang, X. (2025). Soil Enzyme Activities and Microbial Carbon Pump Promote Carbon Storage by Influencing Bacterial Communities Under Nitrogen-Rich Conditions in Tea Plantation. Agriculture, 15(3), 238. https://doi.org/10.3390/agriculture15030238





