Fertilizer Amount and Soil Properties Govern Differential Adsorption of Polyphosphate and Orthophosphate
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Soils and P Sources
2.2. P Adsorption Experiments
2.3. Calculations
2.3.1. P Adsorption
2.3.2. Adsorption Isotherm Equations
Langmuir Isotherm
Freundlich Isotherm
2.3.3. Statistical Analysis
3. Results
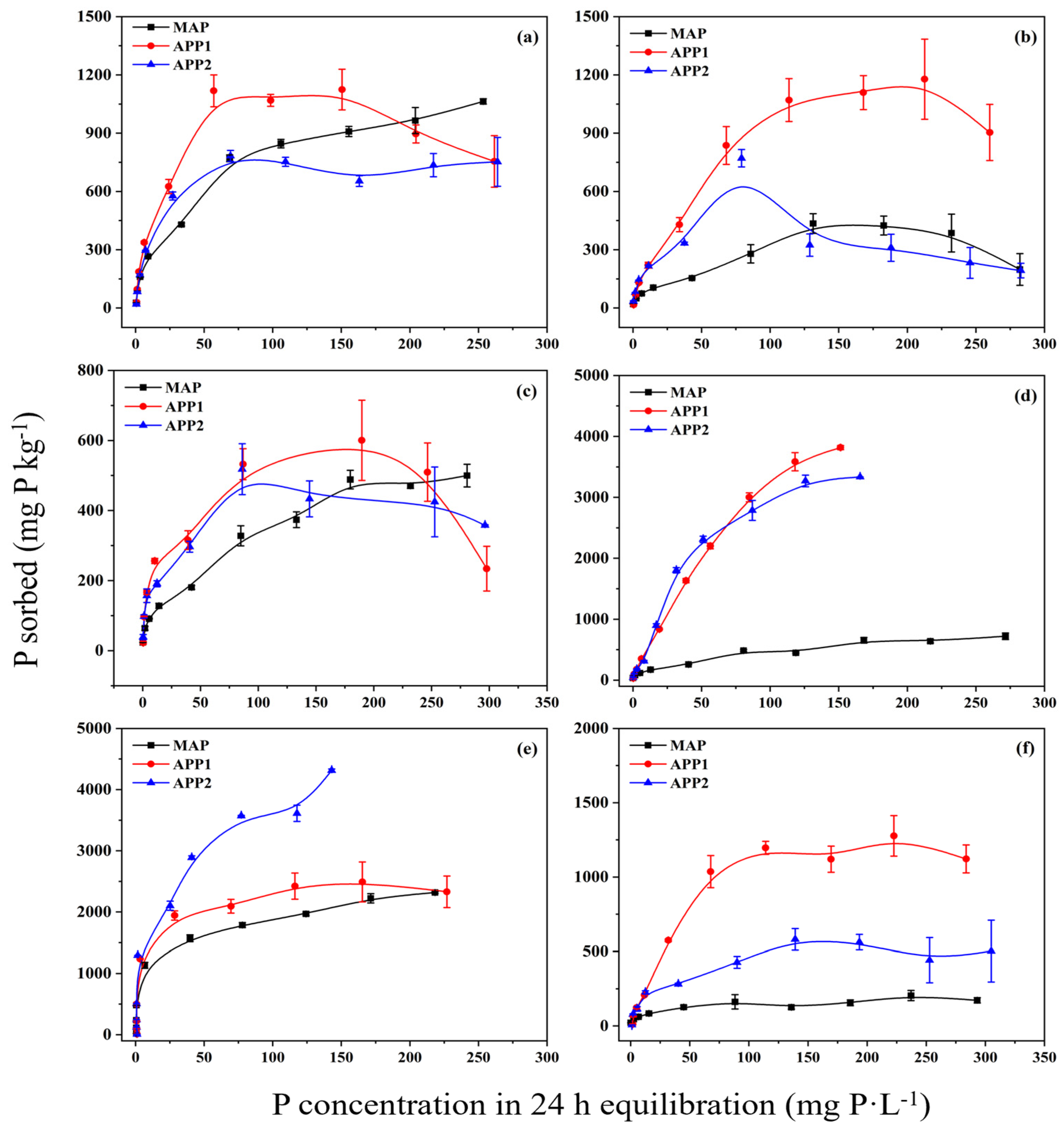
3.1. Sorption Characteristics of Total P
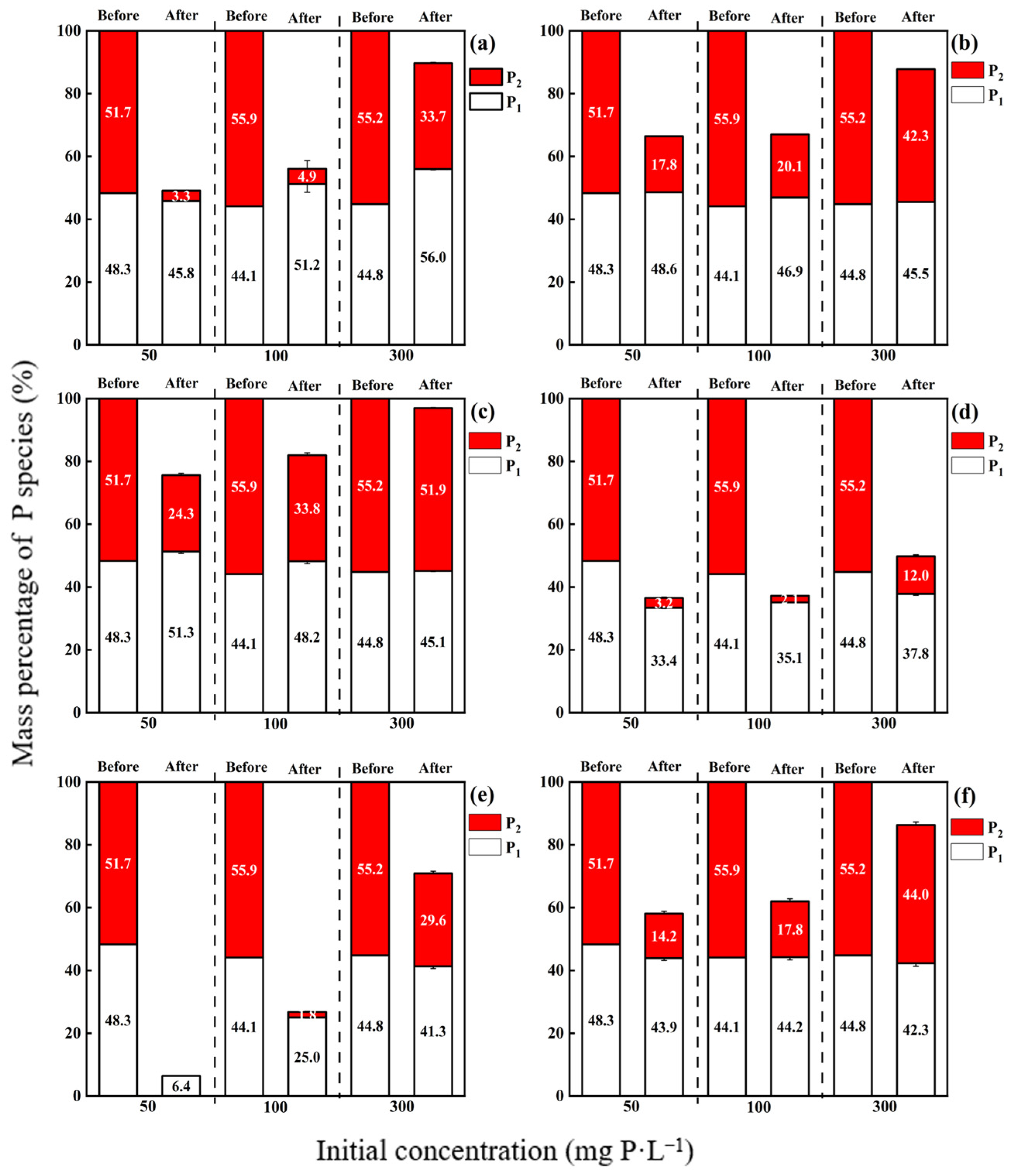
3.2. Sorption of Different P Species in APP
3.3. pH, Metal Ions and DOC in Equilibrium Solutions
3.3.1. pH
3.3.2. Metal Ions
3.3.3. DOC
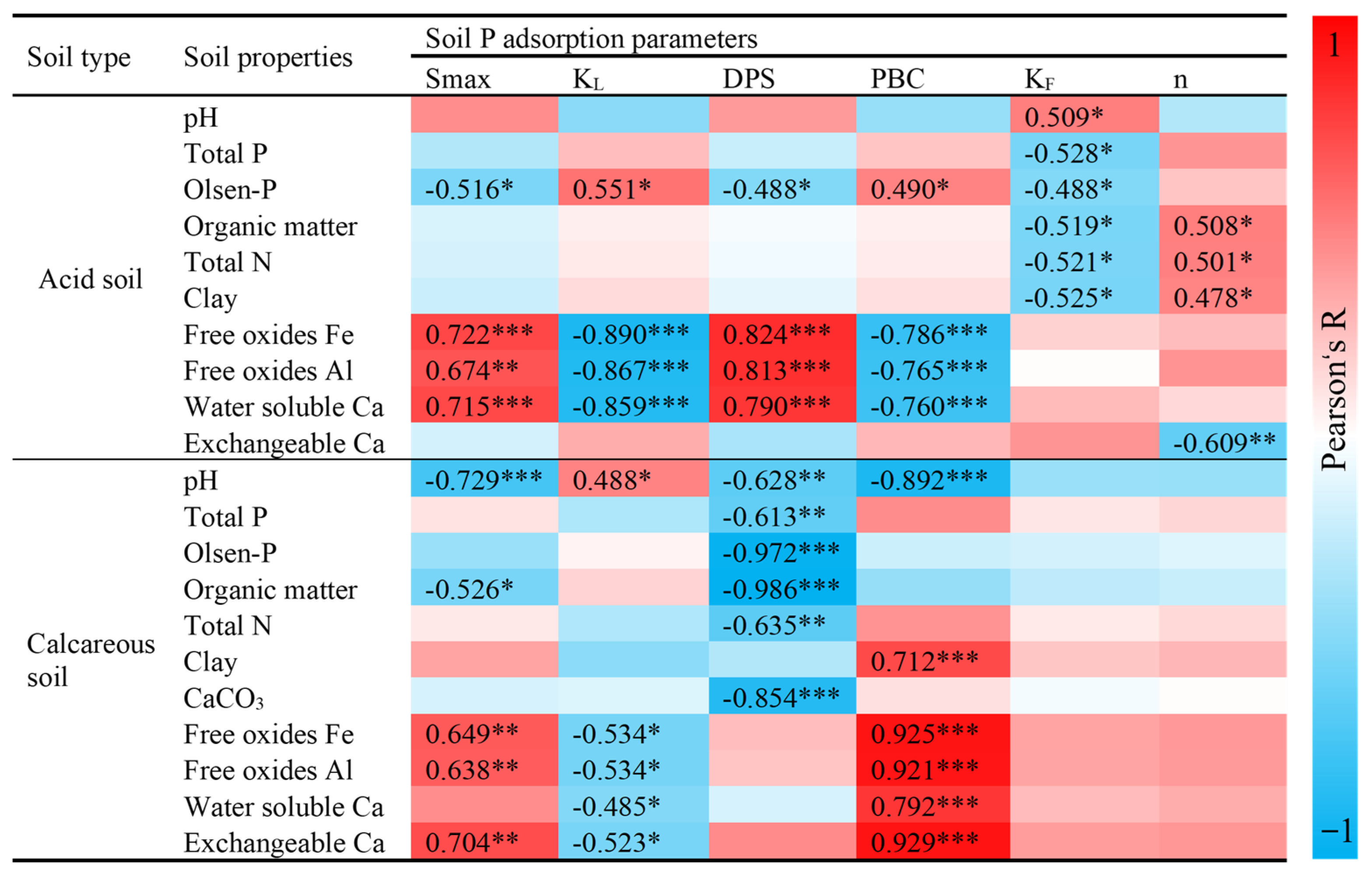
3.4. Relationship Between Soil Properties with Soil P Sorption-Desorption
4. Discussion
4.1. Trend of APP Adsorption by Soil with Increasing Concentration
4.2. Contribution of Different P Species in the APP to Total P Adsorption
4.3. Influence of Soil Properties on APP Adsorption
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuo, S.; Huang, B.; Bembenek, R. Effects of long-term phosphorus fertilization and winter cover cropping on soil phosphorus transformations in less weathered soil. Biol. Fertil. Soils 2004, 41, 116–123. [Google Scholar] [CrossRef]
- Erel, R.; Yalin, D.; Kushmaro-Bier, A.; Li, Q.; Gérard, F.; Toren, N. Soil properties and growing duration determine phosphorus phyto-availability dynamics of polyphosphate versus orthophosphate fertilizers. Plant Soil 2023, 485, 299–315. [Google Scholar] [CrossRef]
- Tao, L.; Wen, X.; Li, H.; Huang, C.; Jiang, Y.; Liu, D.; Sun, B. Influence of manure fertilization on soil phosphorous retention and clay mineral transformation: Evidence from a 16-year long-term fertilization experiment. Appl. Clay Sci. 2021, 204, 106021. [Google Scholar] [CrossRef]
- Getie, A.; Kiflu, A.; Meteke, G.; Abakumov, E. Phosphorus sorption characteristics of Luvisols and Nitisols in North Ethiopian soils. Appl. Environ. Soil Sci. 2021, 2021, 8823852. [Google Scholar] [CrossRef]
- Khan, K.S.; Naveed, M.; Qadir, M.F.; Ahmad, A.; Javed, H.H.; Ditta, A. Variation in Soil C and P Fractions Associated with Microbial Biomass. J. Soil Sci. Plant Nutr. 2023, 23, 6573–6583. [Google Scholar] [CrossRef]
- Khourchi, S.; Delaplace, P.; Bargaz, A. Polyphosphate fertilizer use efficiency strongly relies on soil physicochemical properties and root-microbial activities. Geoderma. 2023, 429, 116281. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Chu, G. Effects of short-chain polyphosphate fertilization on inorganic P transformation and mobilization of Fe, Mn and Zn in soils. Chin. J. Appl. Ecol. 2018, 29, 2970–2978. [Google Scholar]
- Chen, X.; Yang, Y.; Gong, L.; Zhang, C. Effect of ammonium polyphosphates with various degree of polymerization on growth of maize seedlings. J. Plant Nutr. Fertil. 2019, 25, 337–342. [Google Scholar]
- Mcbeath, T.M.; Armstrong, R.D.; Lombi, E.; Mclaughlin, M.J.; Holloway, R.E. Responsiveness of wheat (Triticum aestivum) to liquid and granular phosphorus fertilisers in southern Australian soils. Soil Res. 2005, 43, 203–212. [Google Scholar] [CrossRef]
- Xiong, Z.; Qiu, Y.; Guo, L.; Guo, T.; Shi, W. The availability of ammonium polyphosphate in soil and the impacting factors. J. Plant Nutr. Fertil. 2020, 26, 1473–1480. [Google Scholar]
- Hamilton, J.G.; Hilger, D.; Peak, D. Mechanisms of tripolyphosphate adsorption and hydrolysis on goethite. J. Colloid Interface Sci. 2017, 491, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Kulakovskaya, T.V.; Vagabov, V.M.; Kulaev, I.S. Inorganic polyphosphate in industry, agriculture and medicine: Modern state and outlook. Process Biochem. 2012, 47, 1–10. [Google Scholar] [CrossRef]
- Torres-Dorante, L.O.; Claassen, N.; Steingrobe, B.; Olfs, H.W. Hydrolysis rates of inorganic polyphosphates in aqueous solution as well as in soils and effects on P availability. J. Plant Nutr. Soil Sci. 2005, 168, 352–358. [Google Scholar] [CrossRef]
- Al-Kanani, T.; Mackenzie, A.F. Sorption and desorption of orthophosphate and pyrophosphate by mineral fractions of soils, goethite, and kaolinite. Can. J. Soil Sci. 1991, 71, 327–338. [Google Scholar] [CrossRef]
- Hashimoto, I.; Hughes, J.D.; Philen, O.D. Reactions of triammonium pyrophosphate with soils and soil minerals. Soil Sci. Soc. Am. J. 1969, 33, 401–405. [Google Scholar] [CrossRef]
- Savant, N.K.; Tambe, K.N. The langmuir parameters of orthophosphate and pyrophosphate sorption for ammoniated tropical soils. Commun. Soil Sci. Plant Anal. 2008, 10, 503–511. [Google Scholar] [CrossRef]
- Sutton, C.D.; Larsen, S. Pyrophosphate as a source of phosphorus for plants. Soil Sci. 1964, 97, 196–201. [Google Scholar] [CrossRef]
- Blanchar, R.W.; Hossner, L.R. Hydrolysis and sorption reactions of orthophosphate, pyrophosphate, tripolyphosphate, and trimetaphosphate anions added to an Elliot soil. Soil Sci. Soc. Am. J. 1969, 33, 141–144. [Google Scholar] [CrossRef]
- Blanchar, R.W.; Hossner, L.R. Hydrolysis and sorption of ortho-, pryo-, tripoly-, and trimetaphosphate in 32 Midwestern soils. Soil Sci. Soc. Am. J. 1969, 33, 622–625. [Google Scholar] [CrossRef]
- Xu, D. Study on the Chelation and Dissolution of Medium and Trace Elements in Water-Soluble Ammonium Polyphosphate Solution; Sichuan University: Chengdu, China, 2020. [Google Scholar]
- Khourchi, S.; Oukarroum, A.; Tika, A.; Delaplace, P.; Bargaz, A. Polyphosphate application influences morpho-physiological root traits involved in P acquisition and durum wheat growth performance. BMC Plant Biol. 2022, 22, 309. [Google Scholar] [CrossRef]
- Ji, L.; Ren, G.; Xu, D.; Fan, B.; Zhang, Z.; Yuan, T.; Yan, Z.; Wang, X. Selective adsorption of various phosphorus species coexistence in water-soluble ammonium polyphosphate on goethite: Experimental investigation and molecular dynamics simulation. Chemosphere 2022, 307, 135901. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, W.; Xie, W.; Yuan, T.; Wei, Q.; Xu, D.; Zhang, Z.; Yan, Z.; Wang, X. Selective adsorption and precipitation of pyrophosphate from water-soluble ammonium polyphosphate by calcite. J. Environ. Chem. Eng. 2022, 10, 108893. [Google Scholar] [CrossRef]
- Dari, B.; Nair, V.D.; Colee, J.; Harris, W.G.; Mylavarapu, R. Estimation of phosphorus isotherm parameters: A simple and cost-effective procedure. Front. Environ. Sci. 2015, 3, 70. [Google Scholar] [CrossRef]
- Pizzeghello, D.; Berti, A.; Nardi, S.; Morari, F. Phosphorus-related properties in the profiles of three Italian soils after long-term mineral and manure applications. Agric. Ecosyst. Environ. 2014, 189, 216–228. [Google Scholar] [CrossRef]
- Abolfazli, F.; Forghani, A.; Norouzi, M. Effects of phosphorus and organic fertilizers on phosphorus fractions in submerged soil. J. Soil Sci. Plant Nutr. 2012, 12, 349–362. [Google Scholar] [CrossRef]
- Joardar, J.C.; Sikder, S.; Hasib, S.B. Influence of Solution Concentration and Equilibrium Time on Phosphorus Sorption in Calcareous and Non-Calcareous Soils. SAARC J. Agri. 2019, 17, 117–126. [Google Scholar]
- Du, Z.; Wang, Q.; Liu, F.; Ma, H.; Ma, B.; Malhi, S.S. Movement of phosphorus in a calcareous soil as affected by humic acid. Pedosphere 2013, 23, 229–235. [Google Scholar] [CrossRef]
- Mcbeath, T.M.; Lombi, E.; Mclaughlin, M.J.; Bünemann, E.K. Pyrophosphate and orthophosphate addition to soils: Sorption, cation concentrations, and dissolved organic carbon. Soil Res. 2007, 45, 237–245. [Google Scholar] [CrossRef]
- Rahmatullah; Gill, M.A.; Wissemeier, A.H.; Steffens, D. Phosphate availability from phosphate rock as related to nitrogen form and the nitrification inhibitor DMPP. J. Plant Nutr. Soil Sci. 2006, 169, 675–678. [Google Scholar] [CrossRef]
- Mnkeni, P.N.S.; Mackenzie, A.F. Retention of ortho- and polyphosphates in some Quebec soils as affected by added organic residues and calcium carbonate. Can. J. Soil Sci. 1985, 65, 575–585. [Google Scholar] [CrossRef]
- Yuan, T.; Yan, Z.; Huang, C.; Zhang, Z.; Wang, X. Adsorption-desorption characteristics of ammonium polyphosphate in purple soils. Acta Agric. Zhejiangensis 2023, 35, 403–416. [Google Scholar]
- Olsen, S.; Cole, C.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Jo, S.; Ja, B. Total and Organic Carbon Soil Sampling and Methods of Analysis; Soil Science Society of Canada: Boca Raton, FL, USA, 2007; pp. 225–237. [Google Scholar]
- Thoma, R.; Shear, R.; Moyer, J. Comparison of conventional and automated procedures for nitrogen, phosphorus, and potassium analysis of plant material using a single digestion. Agron. J. 1967, 59, 240–243. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils. Soil Sci. 1954, 78, 154. [Google Scholar] [CrossRef]
- Mehra, O.P.; Jackson, M.L. Iron oxide removal from soils and clays by a dithionite citrate system buffered with sodium bicarbonate. Clays Clay Miner. 1960, 7, 313–317. [Google Scholar]
- Sequential spectrophotometric determination of water-soluble calcium and magnesium in soils using chlorophosphonazo I. Microchim. Acta. 1983, 81, 1–9. [CrossRef]
- Shuman, L.M.; Duncan, R.R. Soil exchangeable cations and aluminum measured by ammonium chloride, potassium chloride, and ammonium acetate. Commun. Soil Sci. Plant Anal. 1990, 21, 13–16. [Google Scholar] [CrossRef]
- Xie, W.; Wang, X.; Li, Y.; Xu, D.; Zhong, Y.; Yang, J. Simultaneous determination of various phosphates in water-soluble ammonium polyphosphate. Chromatographia 2019, 82, 1687–1695. [Google Scholar] [CrossRef]
- Graetz, D.A.; Nair, V.D. Phosphorus sorption isotherm determination. In Methods of Phosphorus Analysis for Soils, Sediments, Residuals and Water; Pierzynski, G.M., Ed.; North Carolina State University: Manhattan, NY, USA, 2000; Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=4c3a41c92a68147ee77a3fadd9f2632ac7c4ef6c (accessed on 21 January 2025).
- Pizzeghello, D.; Berti, A.; Nardi, S.; Morari, F. Phosphorus forms and P-sorption properties in three alkaline soils after long-term mineral and manure applications in north-eastern Italy. Agric. Ecosyst. Environ. 2011, 141, 58–66. [Google Scholar] [CrossRef]
- Arnold, E.; Greenberg, L.S.C.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1992. [Google Scholar]
- Yang, X.; Chen, X.; Yang, X. Effect of organic matter on phosphorus adsorption and desorption in a black soil from Northeast China. Soil Till Res. 2019, 187, 85–91. [Google Scholar] [CrossRef]
- Jalali, M.; Jalali, M. Relation between various soil phosphorus extraction methods and sorption parameters in calcareous soils with different texture. Sci. Total Environ. 2016, 566–567, 1080–1093. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, D.; Wang, X.; Yang, J.; Zhang, Z. Study on the Chelation of Ca2+/Mg2+/Zn2+ in water-soluble ammonium polyphosphate solution. Chemistry 2022, 85, 704–708. [Google Scholar]
- Xu, D.; Zhong, B.; Zhang, Z.; Xu, D.; Wang, X. Research progress of preparation and application of water-soluble ammonium polyphosphate. Chem. Ind. Eng. Prog. 2021, 40, 378–385. [Google Scholar]
- Mclaughlin, M.J.; Mcbeath, T.M.; Smernik, R.; Stacey, S.P.; Ajiboye, B.; Guppy, C. The chemical nature of P accumulation in agricultural soils—Implications for fertiliser management and design: An Australian perspective. Plant Soil 2011, 349, 69–87. [Google Scholar] [CrossRef]




| Soil Types | Red Soil | Acidic Purple Soil | Laterite Soil | Fluvo-Aquic Soil | Calcareous Purple Soil | Cinnamon Soil |
|---|---|---|---|---|---|---|
| Sampling Site | Hunan | Sichuan | Guangdong | Henan | Sichuan | Hebei |
| pH | 4.07 | 4.36 | 4.48 | 8.5 | 8.05 | 8.37 |
| Total P (g kg−1) | 2.26 | 0.51 | 0.41 | 0.86 | 0.85 | 0.54 |
| Olsen-P (mg kg−1) | 206.1 | 85.4 | 2.9 | 18.5 | 7.2 | 2.3 |
| Organic matter (g kg−1) | 26.4 | 4.5 | 8.5 | 18.7 | 8.7 | 8.0 |
| Total N (g kg−1) | 1.78 | 0.48 | 0.69 | 0.89 | 0.86 | 0.41 |
| Clay (<0.002 mm) (g kg−1) | 237 | 37 | 57 | 157 | 197 | 77 |
| CaCO3 (g kg−1) | - | - | - | 45.2 | 39.1 | 29.7 |
| Free oxides Fe (g kg−1) | 6.46 | 1.95 | 23.89 | 2.08 | 4.63 | 1.64 |
| Free oxides Al (g kg−1) | 16.03 | 4.22 | 28.29 | 1.38 | 2.54 | 1.13 |
| Water soluble Ca (g kg−1) | 0.03 | 0.03 | 0.06 | 0.12 | 0.18 | 0.05 |
| Exchangeable Ca (g kg−1) | 0.22 | 0.68 | 0.41 | 5.86 | 9.15 | 6.13 |
| P Sources | pH | Each P Species Percentage in the Total P (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | ||
| MAP | 5.4 | 100.0 | ||||||
| APP1 | 6.5 | 42.0 | 58.0 | |||||
| APP2 | 7.0 | 23.6 | 30.1 | 22.2 | 12.9 | 6.6 | 2.9 | 1.7 |
| Soils | P Sources | Langmuir Equation | Freundlich Equation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Smax (mg kg−1) | KL (L mg−1) | R2 | DPS (%) | PBC (L kg−1) | KF (L kg−1) | n | R2 | |||
| Acid soil | Red soil | MAP | 874 b | 0.057 a | 0.897 | 19.09 a | 49.49 c | 60.46 a | 0.613 b | 0.965 |
| APP1 | 1466 a | 0.045 b | 0.898 | 12.36 b | 66.11 a | 64.80 a | 0.749 a | 0.909 | ||
| APP2 | 965 b | 0.060 a | 0.983 | 17.61 a | 58.17 b | 52.61 a | 0.718 a | 0.907 | ||
| Acidic purple soil | MAP | 336 c | 0.070 a | 0.855 | 20.31 a | 23.38 b | 47.74 c | 0.394 b | 0.982 | |
| APP1 | 551 a | 0.079 a | 0.883 | 13.45 b | 43.03 a | 62.70 b | 0.494 a | 0.860 | ||
| APP2 | 474 b | 0.103 a | 0.920 | 15.27 b | 48.74 a | 85.40 a | 0.363 b | 0.935 | ||
| Laterite soil | MAP | 2016 c | 0.151 a | 0.993 | 0.14 a | 305.09 a | 315.54 b | 0.419 c | 0.823 | |
| APP1 | 2533 b | 0.111 b | 0.977 | 0.11 b | 278.48 a | 308.70 b | 0.485 b | 0.757 | ||
| APP2 | 3973 a | 0.077 c | 0.925 | 0.07 c | 304.91 a | 376.23 a | 0.547 a | 0.779 | ||
| Calcareous soil | Fluvo-aquic soil | MAP | 301 c | 0.053 a | 0.850 | 5.88 a | 15.62 c | 34.48 b | 0.431 c | 0.983 |
| APP1 | 1189 a | 0.025 c | 0.881 | 1.56 b | 29.00 b | 34.01 b | 0.752 a | 0.980 | ||
| APP2 | 841 b | 0.042 b | 0.732 | 2.16 b | 35.12 a | 59.94 a | 0.542 b | 0.975 | ||
| Calcareous purple soil | MAP | 494 c | 0.070 a | 0.829 | 1.44 a | 34.27 c | 79.48 c | 0.344 c | 0.943 | |
| APP1 | 2423 b | 0.038 b | 0.660 | 0.30 b | 92.33 a | 111.47 b | 0.683 a | 0.985 | ||
| APP2 | 3271 a | 0.026 c | 0.228 | 0.22 c | 85.10 b | 134.89 a | 0.617 b | 0.912 | ||
| Cinnamon soil | MAP | 215 c | 0.052 a | 0.913 | 1.07 a | 10.91 b | 28.25 b | 0.406 b | 0.983 | |
| APP1 | 2343 a | 0.010 b | 0.854 | 0.10 c | 23.31 a | 29.86 b | 0.826 a | 0.997 | ||
| APP2 | 484 b | 0.063 a | 0.955 | 0.48 b | 29.92 a | 59.44 a | 0.444 b | 0.959 | ||
| Soils | P Sources | Ca | Fe | Al | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 300 | 50 | 100 | 300 | 50 | 100 | 300 | |||
| Acid soil | Red soil | MAP | 5.7 a | 5.5 a | 5.1 a | 0.03 c | 0.04 c | 0.06 c | 1.19 c | 1.37 c | 1.70 c |
| APP1 | 3.8 b | 2.1 b | 1.5 c | 0.21 b | 4.96 b | 19.66 b | 2.05 b | 10.84 b | 52.71 b | ||
| APP2 | 3.4 c | 1.7 c | 1.9 b | 1.46 a | 8.85 a | 24.41 a | 6.76 a | 25.40 a | 59.13 a | ||
| Acidic purple soil | MAP | 13.8 a | 14.6 a | 15.6 b | 0.01 b | 0.02 b | 0.04 c | 0.41 b | 0.50 b | 0.61 c | |
| APP1 | 9.0 b | 10.0 c | 13.8 c | 1.98 a | 2.90 a | 7.19 b | 7.92 a | 11.17 a | 20.99 b | ||
| APP2 | 9.0 b | 12.3 b | 19.0 a | 2.55 a | 3.89 a | 11.08 a | 9.47 a | 12.38 a | 25.56 a | ||
| Laterite soil | MAP | 9.3 a | 8.5 a | 6.9 a | 0.01 b | 0.02 b | 0.06 c | 0.01 b | 0.05 b | 0.12 c | |
| APP1 | 6.7 b | 1.8 b | 1.4 c | 0.01 ab | 1.68 ab | 4.23 b | 0.00 b | 3.66 ab | 19.75 b | ||
| APP2 | 5.4 c | 1.3 b | 3.9 b | 0.03 a | 3.39 a | 12.20 a | 0.03 a | 6.18 a | 23.13 a | ||
| Calcareous soil | Fluvo-aquic soil | MAP | 48.5 a | 56.2 a | 63.1 a | 0.00 b | 0.01 c | 0.01 c | 0.00 b | 0.00 b | 0.00 c |
| APP1 | 19.1 c | 15.4 c | 13.8 c | 0.18 b | 0.45 b | 1.64 b | 0.40 ab | 1.23 a | 2.45 b | ||
| APP2 | 21.2 b | 22.9 b | 39.0 b | 0.39 a | 0.73 a | 4.00 a | 0.68 a | 1.48 a | 5.96 a | ||
| Calcareous purple soil | MAP | 95.4 a | 102.6 a | 115.0 a | 0.01 a | 0.01 b | 0.01 b | 0.00 b | 0.00 b | 0.00 b | |
| APP1 | 65.5 b | 49.0 b | 18.2 c | 0.01 a | 0.04 a | 0.42 ab | 0.05 a | 0.35 a | 3.21 a | ||
| APP2 | 66.7 b | 48.6 b | 43.6 b | 0.01 a | 0.04 a | 0.79 a | 0.05 a | 0.33 a | 3.67 a | ||
| Cinnamon soil | MAP | 49.6 a | 58.2 a | 69.7 a | 0.00 b | 0.00 c | 0.00 c | 0.00 c | 0.00 c | 0.00 c | |
| APP1 | 15.8 c | 12.3 c | 16.0 c | 0.11 a | 0.37 b | 1.27 b | 0.57 a | 1.44 b | 2.87 b | ||
| APP2 | 21.7 b | 24.3 b | 47.4 b | 0.10 a | 0.79 a | 2.51 a | 0.33 b | 2.24 a | 4.34 a | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Huang, M.; Yuan, T.; Xu, D.; Yan, Z.; Wang, X. Fertilizer Amount and Soil Properties Govern Differential Adsorption of Polyphosphate and Orthophosphate. Agriculture 2025, 15, 585. https://doi.org/10.3390/agriculture15060585
Li Y, Huang M, Yuan T, Xu D, Yan Z, Wang X. Fertilizer Amount and Soil Properties Govern Differential Adsorption of Polyphosphate and Orthophosphate. Agriculture. 2025; 15(6):585. https://doi.org/10.3390/agriculture15060585
Chicago/Turabian StyleLi, Yang, Minghui Huang, Taiyan Yuan, Dehua Xu, Zhengjuan Yan, and Xinlong Wang. 2025. "Fertilizer Amount and Soil Properties Govern Differential Adsorption of Polyphosphate and Orthophosphate" Agriculture 15, no. 6: 585. https://doi.org/10.3390/agriculture15060585
APA StyleLi, Y., Huang, M., Yuan, T., Xu, D., Yan, Z., & Wang, X. (2025). Fertilizer Amount and Soil Properties Govern Differential Adsorption of Polyphosphate and Orthophosphate. Agriculture, 15(6), 585. https://doi.org/10.3390/agriculture15060585






