Reducing Postharvest Losses in Organic Apples: The Role of Yeast Consortia Against Botrytis cinerea
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Isolates and Consortia
2.2. Selected Apple Pathogen Inoculum
2.3. Fruit
2.4. Wound Colonization by Yeast Isolates
2.5. Effect of Selected Yeast Isolates and Consortia on the Rate of Spore Germination of B. cinerea In Vitro
2.6. Efficacy of Yeast Isolates and Consortia in Inhibiting Grey Mould Decay of Apples at 4 °C and at 23 °C
2.7. Statistical Analysis
3. Results
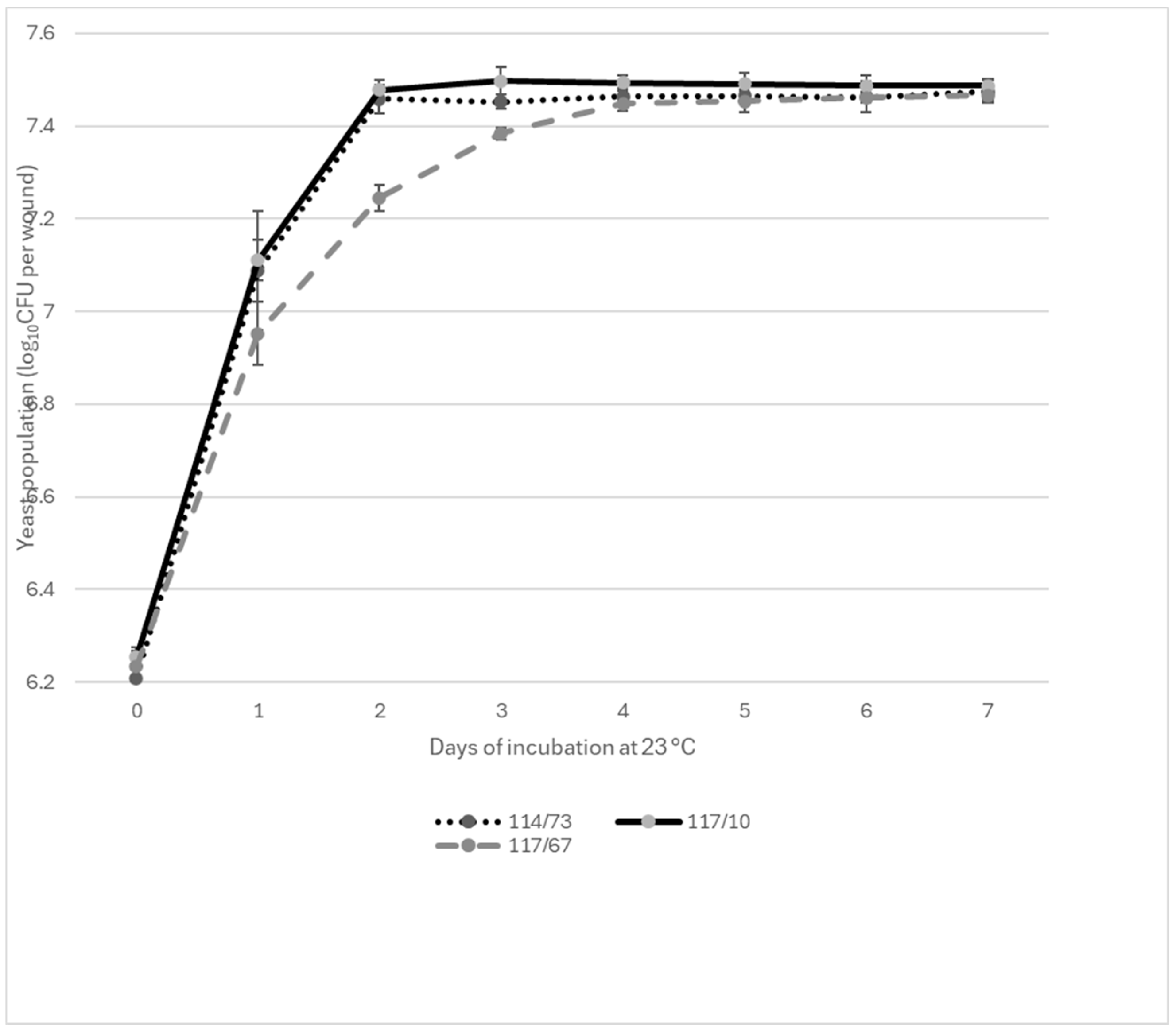
3.1. Wound Colonization by Yeast Isolates
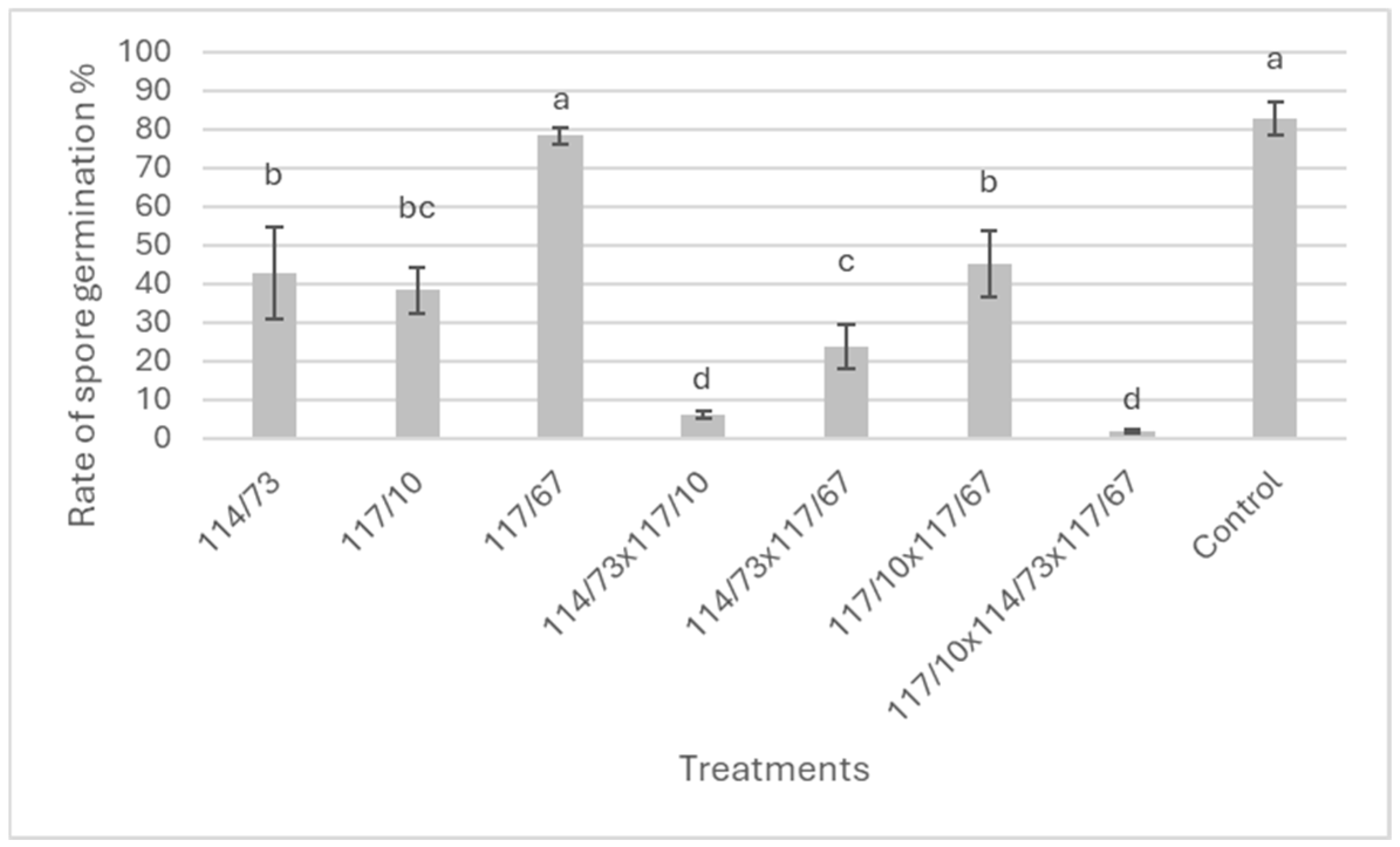
3.2. Effect of Yeast Isolates and Consortia on the Rate of Spore Germination of B. cinerea In Vitro
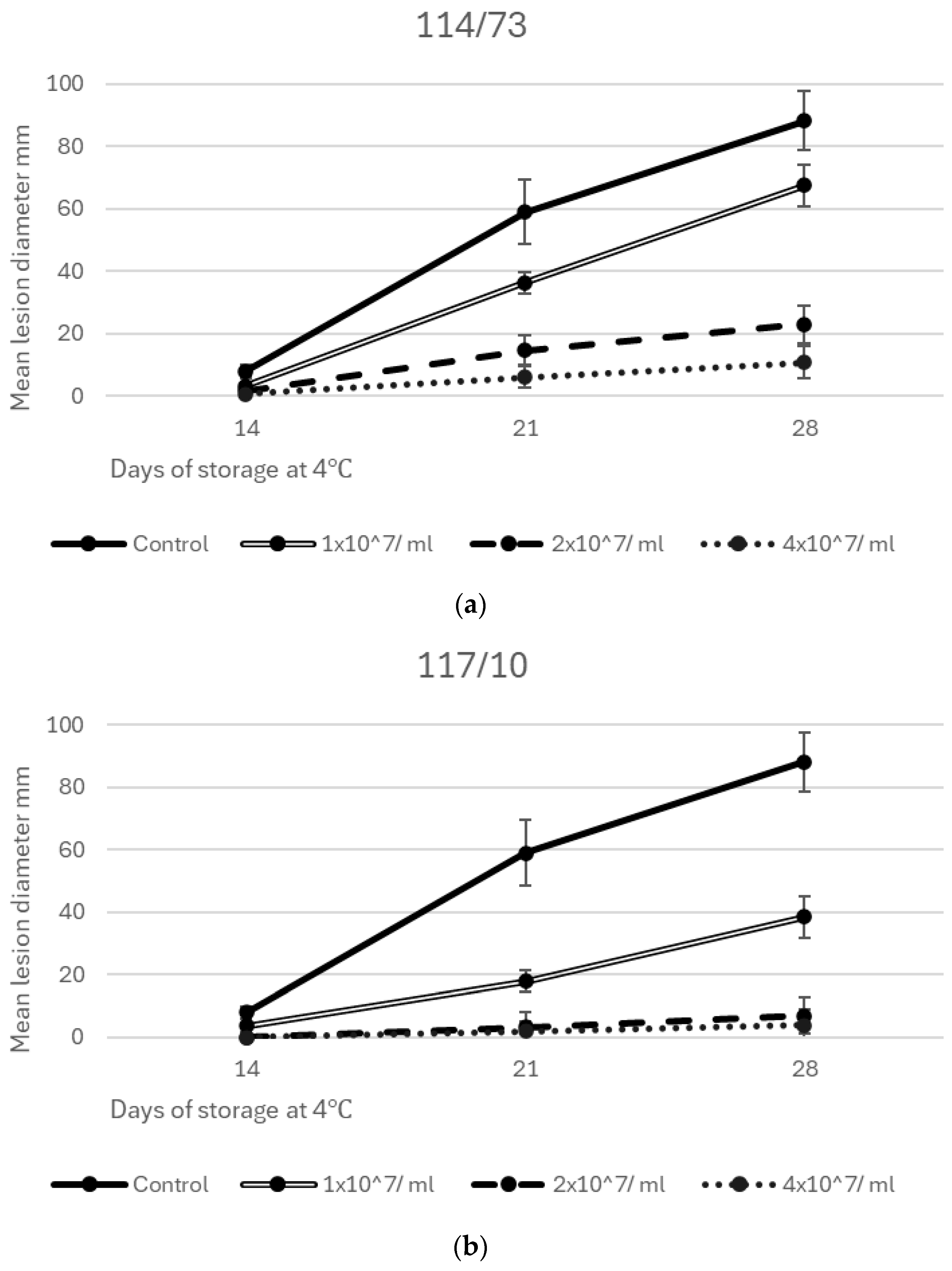
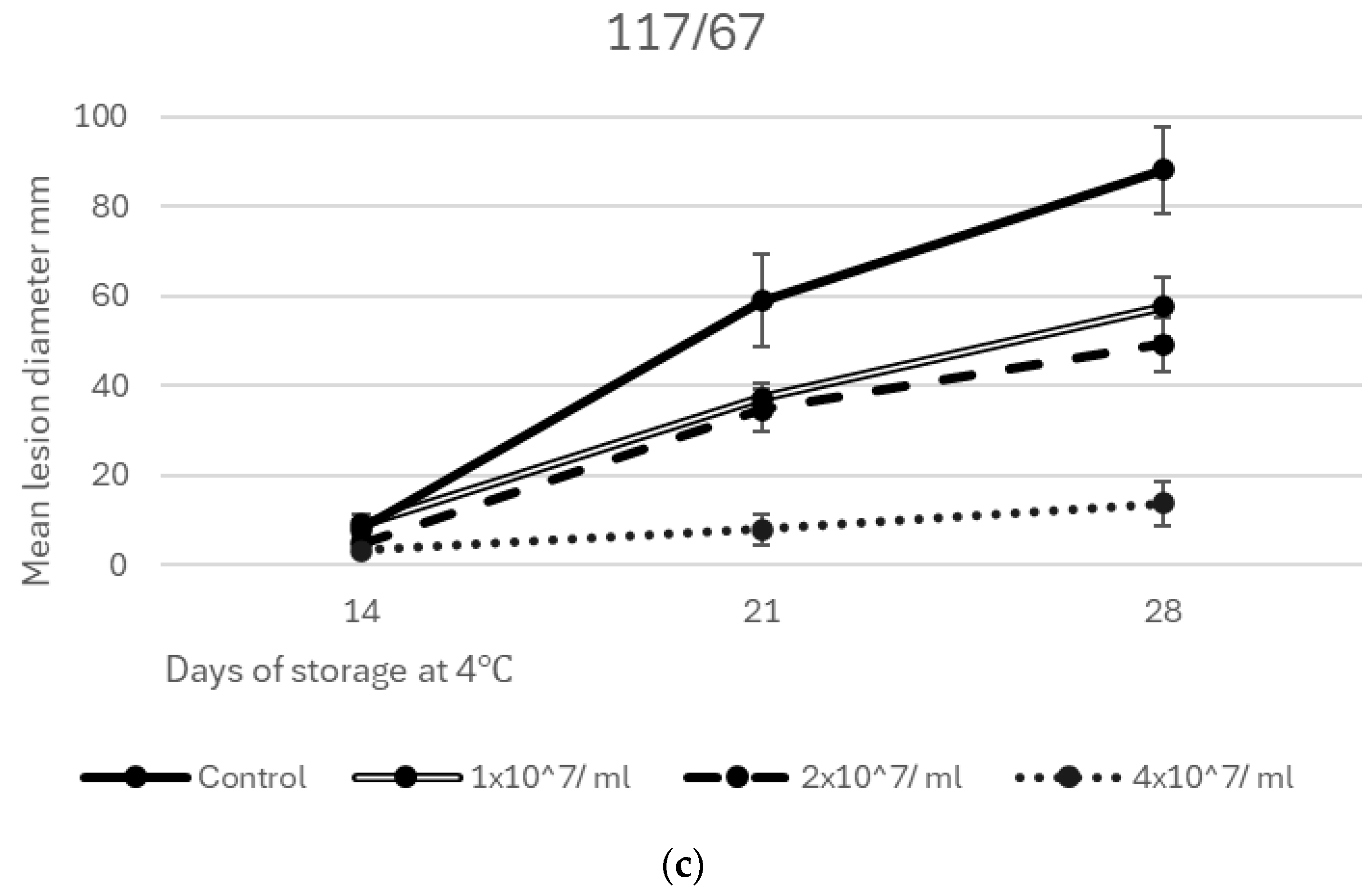
3.3. Efficacy of Yeast Isolates and Consortia in Inhibiting Grey Mould Decay of Apples at 4 °C
3.4. Efficacy of Yeast Isolates and Consortia in Inhibiting Grey Mould Decay of Apples at 23 °C
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Argenta, L.C.; de Freitas, S.T.; Mattheis, J.P.; Vieira, M.J.; Ogoshi, C. Characterization and quantification of postharvest losses of apple fruit stored under commercial conditions. HortScience 2021, 56, 608–616. [Google Scholar] [CrossRef]
- Leng, J.; Yu, L.; Dai, Y.; Leng, Y.; Wang, C.; Chen, Z.; Sui, Y. Recent advances in research on biocontrol of postharvest fungal decay in apples. Crit. Rev. Food Sci. Nutr. 2023, 63, 10607–10620. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; He, J.; Liu, H.; Zhou, H. The phenylpropanoid pathway affects apple fruit resistance to Botrytis cinerea. J. Phytopathol. 2018, 166, 206–215. [Google Scholar] [CrossRef]
- Hua, L.; Yong, C.; Zhanquan, Z.; Boqiang, L.; Guozheng, Q.; Shiping, T. Pathogenic mechanisms and control strategies of Botrytis cinerea causing post-harvest decay in fruits and vegetables. Food Qual. Saf. 2018, 2, 111–119. [Google Scholar] [CrossRef]
- Bi, K.; Liang, Y.; Mengiste, T.; Sharon, A. Killing softly: A roadmap of Botrytis cinerea pathogenicity. Trends Plant Sci. 2023, 28, 211–222. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.D.C.; Kumar, A.; Fadiji, A.E.; Babalola, O.O.; Puopolo, G.; Santoyo, G. Agroecological management of the grey mould fungus Botrytis cinerea by plant growth-promoting bacteria. Plants 2023, 12, 637. [Google Scholar] [CrossRef]
- Nakajima, M.; Akutsu, K. Virulence factors of Botrytis cinerea. J. Gen. Plant Pathol. 2014, 80, 15–23. [Google Scholar] [CrossRef]
- Di Francesco, A.; Aprea, E.; Gasperi, F.; Parenti, A.; Placì, N.; Rigosi, F.; Baraldi, E. Apple pathogens: Organic essential oils as an alternative solution. Sci. Hortic. 2022, 300, 111075. [Google Scholar] [CrossRef]
- Soppelsa, S.; Van Hemelrijck, W.; Bylemans, D.; Andreotti, C. Essential oils and chitosan applications to protect apples against postharvest diseases and to extend shelf life. Agronomy 2023, 13, 822. [Google Scholar] [CrossRef]
- Popescu, P.A.; Palade, L.M.; Nicolae, I.C.; Popa, E.E.; Miteluț, A.C.; Drăghici, M.C.; Popa, M.E. Chitosan-based edible coatings containing essential oils to preserve the shelf life and postharvest quality parameters of organic strawberries and apples during cold storage. Foods 2022, 11, 3317. [Google Scholar] [CrossRef]
- Mitra, A.; Selvam, S.P.; Shehabudheen, S.; Anitha, P.M.; Kumar, M.M. Efficiency evaluation of cinnamon essential oil loaded nanoliposomal coating for the post-harvest management of apple (Malus domestica). Int. J. Emerg. Technol. 2020, 11, 554–559. [Google Scholar]
- Lyousfi, N.; Letrib, C.; Legrifi, I.; Blenzar, A.; El Khetabi, A.; El Hamss, H.; Lahlali, R. Combination of sodium bicarbonate (SBC) with bacterial antagonists for the control of brown rot disease of fruit. J. Fungi 2022, 8, 636. [Google Scholar] [CrossRef] [PubMed]
- Serna-Escolano, V.; Gutiérrez-Pozo, M.; Dobón-Suárez, A.; Zapata, P.J.; Giménez, M.J. Effect of preharvest treatments with sodium bicarbonate and potassium silicate in Navel and Valencia oranges to control fungal decay and maintain quality traits during cold storage. Agronomy 2023, 13, 2925. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, C.; Guan, X.; Lian, S.; Li, B.; Wang, C. Biocontrol efficiency of Meyerozyma guilliermondii Y-1 against apple postharvest decay caused by Botryosphaeria dothidea and the possible mechanisms of action. Int. J. Food Microbiol. 2021, 338, 108957. [Google Scholar] [CrossRef]
- Sun, C.; Huang, Y.; Lian, S.; Saleem, M.; Li, B.; Wang, C. Improving the biocontrol efficacy of Meyerozyma guilliermondii Y-1 with melatonin against postharvest gray mold in apple fruit. Postharvest Biol. Technol. 2021, 171, 111351. [Google Scholar] [CrossRef]
- Kowalska, J.; Krzymińska, J.; Tyburski, J. Yeasts as a potential biological agent in plant disease protection and yield improvement—A short review. Agriculture 2022, 12, 1404. [Google Scholar] [CrossRef]
- Hassan, Z.U.; Al Thani, R.; Atia, F.A.; Alsafran, M.; Migheli, Q.; Jaoua, S. Application of yeasts and yeast derivatives for the biological control of toxigenic fungi and their toxic metabolites. Environ. Technol. Innov. 2021, 22, 101447. [Google Scholar] [CrossRef]
- Canonico, L.; Agarbati, A.; Galli, E.; Comitini, F.; Ciani, M. Metschnikowia pulcherrima as biocontrol agent and wine aroma enhancer in combination with a native Saccharomyces cerevisiae. LWT 2023, 181, 114758. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, R.; Xiong, B. Management of postharvest diseases of kiwifruit with a combination of the biocontrol yeast Candida oleophila and an oligogalacturonide. Biol. Control 2021, 156, 104549. [Google Scholar] [CrossRef]
- Li, X.; Yu, L.; An, F.; Bai, H.; Wisniewski, M.; Wang, Z. Caffeic acid increases the fitness of Candida oleophila to the microenvironment of kiwifruit and its biocontrol performance against postharvest decay fungi. Postharvest Biol. Technol. 2023, 196, 112177. [Google Scholar] [CrossRef]
- Ayogu, P.; Martins, V.; Gerós, H. Grape berry native yeast microbiota: Advancing trends in the development of sustainable vineyard pathogen biocontrol strategies. OENO One 2024, 58, 1. [Google Scholar] [CrossRef]
- Godana, E.A.; Yang, Q.; Wang, K.; Zhang, H.; Zhang, X.; Zhao, L.; Legrand, N.N.G. Bio-control activity of Pichia anomala supplemented with chitosan against Penicillium expansum in postharvest grapes and its possible inhibition mechanism. LWT 2020, 124, 109188. [Google Scholar] [CrossRef]
- Elkhairy, B.M.; Salama, N.M.; Desouki, A.M.; Abdelrazek, A.B.; Soliman, K.A.; Ibrahim, S.A.; Khalil, H.B. Towards unlocking the biocontrol potential of Pichia kudriavzevii for plant fungal diseases: In vitro and in vivo assessments with candidate secreted protein prediction. BMC Microbiol. 2023, 23, 356. [Google Scholar] [CrossRef]
- Fernandez-San Millan, A.; Fernandez-Irigoyen, J.; Santamaria, E.; Larraya, L.; Ancin, M.; Farran, I.; Veramendi, J. Successful biocontrol of Pichia spp. strains against Botrytis cinerea infection in apple fruit: Unraveling protection mechanisms from proteomic insights. LWT 2024, 201, 116253. [Google Scholar] [CrossRef]
- Cignola, R.; Zucchinali, S.; Firrao, G.; Di Francesco, A. Aspects of the biocontrol activity of Aureobasidium spp. strain against Penicillium expansum of apple. Ann. Appl. Biol. 2024, 184, 307–313. [Google Scholar] [CrossRef]
- Remolif, G.; Schiavon, G.; Garello, M.; Spadaro, D. Efficacy of postharvest application of Aureobasidium pullulans to control white haze on apples and effect on the fruit mycobiome. Horticulturae 2024, 10, 927. [Google Scholar] [CrossRef]
- Biasi, A.; Zhimo, V.Y.; Kumar, A.; Abdelfattah, A.; Salim, S.; Feygenberg, O.; Droby, S. Changes in the fungal community assembly of apple fruit following postharvest application of the yeast biocontrol agent Metschnikowia fructicola. Horticulturae 2021, 7, 360. [Google Scholar] [CrossRef]
- Fernandez-San Millan, A.; Gamir, J.; Farran, I.; Larraya, L.; Veramendi, J. Identification of new antifungal metabolites produced by the yeast Metschnikowia pulcherrima involved in the biocontrol of postharvest plant pathogenic fungi. Postharvest Biol. Technol. 2022, 192, 111995. [Google Scholar]
- Acar, E.G.; Dikmetas, D.N.; Devecioglu, D.; Ozer, E.M.; Sarikece, H.; Karbancioglu-Guler, F. Antagonistic activities of Metschnikowia pulcherrima isolates against Penicillium expansum on Amasya apples. Curr. Microbiol. 2024, 81, 180. [Google Scholar] [CrossRef]
- Moënne-Loccoz, Y.; Mavingui, P.; Combes, C.; Normand, P.; Steinberg, C. Microorganisms and Biotic Interactions. In Environmental Microbiology: Fundamentals and Applications: Microbial Ecology; Springer: Dordrecht, The Netherlands, 2015; pp. 395–444. [Google Scholar]
- Etesami, H.; Beattie, G.A. Plant-Microbe Interactions in Adaptation of Agricultural Crops to Abiotic Stress Conditions. In Probiotics and Plant Health; Springer: Berlin/Heidelberg, Germany, 2017; pp. 163–200. [Google Scholar]
- Negi, R.; Sharma, B.; Jan, T.; Kaur, T.; Chowdhury, S.; Kapoor, M.; Yadav, A.N. Microbial consortia: Promising tool as plant bioinoculants for agricultural sustainability. Curr. Microbiol. 2024, 81, 222. [Google Scholar] [CrossRef]
- Remlein-Starosta, D.; Krzymińska, J.; Kowalska, J.; Bocianowski, J. Evaluation of yeast-like fungi to protect Virginia mallow (Sida hermaphrodita) against Sclerotinia sclerotiorum. Can. J. Plant Sci. 2016, 96, 243–251. [Google Scholar] [CrossRef]
- Dwivedi, M.; Singh, P.; Pandey, A.K. Botrytis fruit rot management: What have we achieved so far? Food Microbiol. 2024, 122, 104564. [Google Scholar] [CrossRef] [PubMed]
- Elmer, P.A.; Michailides, T.J. Epidemiology of Botrytis cinerea in Orchard and Vine Crops. In Botrytis: Biology, Pathology and Control; Springer: Dordrecht, The Netherlands, 2007; pp. 243–272. [Google Scholar]
- Tuyet, B. Preharvest Conditions Affecting Apple Quality, Antioxidant Responses, and Susceptibility to Infection by Grey Mould (Botrytis cinerea). Ph.D. Thesis, University of Gothenburg, Gothenburg, Sweden, 6 December 2020. [Google Scholar]
- Głos, H.; Bryk, H.; Michalecka, M.; Puławska, J. The recent occurrence of biotic postharvest diseases of apples in Poland. Agronomy 2022, 12, 399. [Google Scholar] [CrossRef]
- Shewa, A.G.; Gobena, D.A.; Ali, M.K. Review on postharvest quality and handling of apple. Int. J. Agric. Sci. Food Technol. 2022, 8, 28–32. [Google Scholar]
- Gomomo, Z.; Fanadzo, M.; Mewa-Ngongang, M.; Hoff, J.; Van der Rijst, M.; Okudoh, V.; du Plessis, H.W. Control of mould spoilage on apples using yeasts as biological control agents. Pol. J. Food Nutr. Sci. 2022, 72, 119–128. [Google Scholar] [CrossRef]
- Mbili, N.C.; Yobo, K.S.; Laing, M.D. Isolation and in vivo Screening of Yeast Antagonists for the Control of Botrytis cinerea and Penicillium expansum of Pome Fruit. In V International Symposium on Postharvest Pathology: From Consumer to Laboratory-Sustainable Approaches to Managing Postharvest; ISHS: Liège, Belgium, 2019. [Google Scholar]
- Zhao, L.; Wang, Y.; Dhanasekaran, S.; Guo, Z.; Chen, S.; Zhang, X.; Zhang, H. Efficacy of Wickerhamomyces anomalus yeast in the biocontrol of blue mold decay in apples and investigation of the mechanisms involved. BioControl 2021, 66, 547–558. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, L.; Chen, Y.; Dhanasekaran, S.; Chen, X.; Zhang, X.; Yang, X.; Wu, M.; Song, Y.; Zhang, H. Study on the control effect and physiological mechanism of Wickerhamomyces anomalus on primary postharvest diseases of peach fruit. Int. J. Food Microbiol. 2024, 413, 110575. [Google Scholar] [CrossRef]
- Zhao, Q.; Shi, Y.; Ngea, G.L.N.; Zhang, X.; Yang, Q.; Zhang, Q.; Xu, X.; Zhang, H. Changes of the microbial community in kiwifruit during storage after postharvest application of Wickerhamomyces anomalus. Food Chem. 2023, 404, 134593. [Google Scholar] [CrossRef]
- Raynaldo, F.A.; Ackah, M.; Ngea, G.L.N.; Rehman, S.A.; Yang, Q.; Wang, K.; Zhang, X.; Zhang, H. The potentiality of Wickerhamomyces anomalus against postharvest black spot disease in cherry tomatoes and insights into the defense mechanisms involved. Postharvest Biol. Technol. 2024, 209, 112699. [Google Scholar] [CrossRef]
- Raynaldo, F.A.; Dhanasekaran, S.; Ngea, G.L.N.; Yang, Q.; Zhang, X.; Zhang, H. Investigating the biocontrol potentiality of Wickerhamomyces anomalus against postharvest gray mold decay in cherry tomatoes. Sci. Hortic. 2021, 285, 110137. [Google Scholar] [CrossRef]
- Kheireddine, A.; Palmieri, D.; Vitullo, D.; Barberio, A.; Zouaoui, M.; De Curtis, F.; Sadfi-Zouaoui, N.; Lima, G. Characterization of new yeast isolates collected from different fruits in Tunisia and biocontrol activity against Penicillium expansum on apples. J. Plant Pathol. 2021, 103, 1169–1184. [Google Scholar] [CrossRef]
- Wei, Y.; Mao, S.; Tu, K. Effect of preharvest spraying Cryptococcus laurentii on postharvest decay and quality of strawberry. Biol. Control 2014, 73, 68–74. [Google Scholar] [CrossRef]
- Agarbati, A.; Canonico, L.; Pecci, T.; Romanazzi, G.; Ciani, M.; Comitini, F. Biocontrol of non-Saccharomyces yeasts in vineyards against the grey mould disease agent Botrytis cinerea. Microorganisms 2022, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Kristjuhan, A.; Kristjuhan, K.; Tamm, T. Richness of yeast community associated with apple fruits in Estonia. Heliyon 2024, 10, e27885. [Google Scholar] [CrossRef] [PubMed]
- Al Riachy, R.; Strub, C.; Durand, N.; Chochois, V.; Lopez-Lauri, F.; Fontana, A.; Schorr-Galindo, S. The influence of long-term storage on the epiphytic microbiome of postharvest apples and on Penicillium expansum occurrence and patulin accumulation. Toxins 2024, 16, 102. [Google Scholar] [CrossRef]
- Stanevičienė, R.; Lukša, J.; Strazdaitė-Žielienė, Ž.; Ravoitytė, B.; Losinska-Sičiūnienė, R.; Mozūraitis, R.; Servienė, E. Mycobiota in the carposphere of sour and sweet cherries and antagonistic features of potential biocontrol yeasts. Microorganisms 2021, 9, 1423. [Google Scholar] [CrossRef]
- Du, X.; Li, S.; Luo, A.; Yin, X.; Fan, K.; Mou, L.; Li, J. Evaluating the fungal pathogens’ inhibition efficiency of composite film combined with antagonistic yeasts and sodium alginate on peach. Coatings 2023, 13, 417. [Google Scholar] [CrossRef]
- Zhimo, V.Y.; Biasi, A.; Kumar, A.; Feygenberg, O.; Salim, S.; Vero, S.; Wisniewski, M.; Droby, S. Yeasts and bacterial consortia from kefir grains are effective biocontrol agents of postharvest diseases of fruits. Microorganisms 2020, 8, 428. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Xu, J.; Xin, F.; Jiang, L. Applications of synthetic microbial consortia in biological control of mycotoxins and fungi. Curr. Opin. Food Sci. 2023, 53, 101074. [Google Scholar] [CrossRef]
- Cáceres, H.; Barriga-Sánchez, M.; Bendezú, L.; Huamán, E.; Becerra-Canales, B.; Almanza, A.; Ortiz-Campos, J.; Barra-Bucarei, L. Antagonist activity of yeasts and lactic acid bacteria against phytopathogenic strains of economic importance in agriculture. Chilean J. Agric. Res. 2024, 84, 663–673. [Google Scholar] [CrossRef]
- Czajkowski, R.; Maciag, T.; Krzyzanowska, D.M.; Jafra, S. Biological Control Based on Microbial Consortia–From Theory to Commercial Products. In How Research Can Stimulate the Development of Commercial Biological Control Against Plant Diseases; Springer Nature: London, UK, 2020; pp. 183–202. [Google Scholar]
- Minchev, Z.; Kostenko, O.; Soler, R.; Pozo, M.J. Microbial consortia for effective biocontrol of root and foliar diseases in tomato. Front. Plant Sci. 2021, 12, 756368. [Google Scholar] [CrossRef] [PubMed]
- Edward-Rajanayagam, R.M.A.; Narváez-Zapata, J.A.; Ramírez-González, M.D.S.; de la Cruz-Arguijo, E.A.; López-Meyer, M.; Larralde-Corona, C.P. Yeast mixtures for postharvest biocontrol of diverse fungal rots on Citrus limon var Eureka. Horticulturae 2023, 9, 573. [Google Scholar] [CrossRef]
- Guetsky, R.; Shtienberg, D.; Elad, Y.; Fischer, E.; Dinoor, A. Improving biological control by combining biocontrol agents each with several mechanisms of disease suppression. Phytopathology 2002, 92, 976–985. [Google Scholar] [CrossRef]
- El Ghaouth, A.; Wilson, C.L.; Wisniewski, M. Control of postharvest decay of apple fruit with Candida saitoana and induction of defense responses. Phytopathology 2003, 93, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Chen, L.; Sui, Y.; Chen, C.; Zhang, W.; Zhou, J.; Dong, W.; Jiang, M.; Xin, F.; Ochsenreither, K. Biotechnological potential and applications of microbial consortia. Biotechnol. Adv. 2020, 40, 107500. [Google Scholar] [CrossRef]
- Negi, R.; Sharma, B.; Parastesh, F.; Kaur, S.; Khan, S.S.; Kour, D.; Singh, S.; Rai, A.K.; Rustagi, S.; Yadav, N.; et al. Microbial consortia mediated regulation of plant defense: A promising tool for sustaining crop protection. Physiol. Mol. Plant Pathol. 2024, 134, 102393. [Google Scholar] [CrossRef]
- Herrera-Balandrano, D.D.; Wang, S.Y.; Wang, C.X.; Shi, X.C.; Liu, F.Q.; Laborda, P. Antagonistic mechanisms of yeasts Meyerozyma guilliermondii and M. caribbica for the control of plant pathogens: A review. Biol. Control 2023, 186, 105333. [Google Scholar]
- Bhan, C.; Asrey, R.; Singh, D.; Meena, N.K.; Vinod, B.R.; Menaka, M. Bioefficacy of bacteria and yeast bioagents on disease suppression and quality retention of stored Kinnow mandarin fruits. Food Biosci. 2023, 53, 102743. [Google Scholar] [CrossRef]
- Nasahi, C.; Yusuf, A.R.; Hartati, S.; Kurniadie, D.; Subakti-Putri, S.N. Yeast potential in controlling Aspergillus sp. causing fruit rot disease in Dekopon oranges (Citrus reticulata ‘Shiranui’). Res. Crops 2023, 24, 407–415. [Google Scholar] [CrossRef]
- Podgórska-Kryszczuk, I. Biological control of Aspergillus flavus by the yeast Aureobasidium pullulans in vitro and on tomato fruit. Plants 2023, 12, 236. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, K.; Liu, J.; Zhao, Z.; Guo, B.; Wang, X.; Xiang, W.; Zhao, J. Identification and evaluation of an endophytic antagonistic yeast for the control of gray mold (Botrytis cinerea) in apple and mechanisms of action. Food Microbiol. 2024, 123, 104583. [Google Scholar] [CrossRef] [PubMed]
- Oztekin, S.; Dikmetas, D.N.; Devecioglu, D.; Acar, E.G.; Karbancioglu-Guler, F. Recent insights into the use of antagonistic yeasts for sustainable biomanagement of postharvest pathogenic and mycotoxigenic fungi in fruits with their prevention strategies against mycotoxins. J. Agric. Food Chem. 2023, 71, 9923–9950. [Google Scholar] [CrossRef] [PubMed]
- Grzegorczyk, M.; Szalewicz, A.; Żarowska, B.; Połomska, X.; Wątorek, W.; Wojtatowicz, M. Drobnoustroje w biologicznej ochronie roślin przed chorobami grzybowymi. Acta Sci. Pol. Biotechnol. 2015, 14, 19–42. [Google Scholar]
- Ali, A.; Ölmez, F.; Zeshan, M.A.; Mubeen, M.; Iftikhar, Y.; Sajid, A.; Solanki, M.K. Yeast-based solutions in controlling plant pathogens. Biocatal. Agric. Biotechnol. 2024, 58, 103199. [Google Scholar] [CrossRef]
- Bitas, V.; Kim, H.S.; Bennett, J.W.; Kang, S. Sniffing on microbes: Diverse roles of microbial volatile organic compounds in plant health. Mol. Plant-Microbe Interact. 2013, 26, 835–843. [Google Scholar] [CrossRef]
- Agirman, B.; Erten, H. Biocontrol ability and action mechanisms of Aureobasidium pullulans GE17 and Meyerozyma guilliermondii KL3 against Penicillium digitatum DSM2750 and Penicillium expansum DSM62841 causing postharvest diseases. Yeast 2020, 37, 437–448. [Google Scholar] [CrossRef]
- Yang, T.; Wang, C.; Li, C.; Sun, R.; Yang, M. Antagonistic effects of volatile organic compounds of Saccharomyces cerevisiae NJ-1 on the growth and toxicity of Aspergillus flavus. Biol. Control 2023, 177, 105093. [Google Scholar] [CrossRef]
- Zou, X.; Wei, Y.; Zhu, J.; Sun, J.; Shao, X. Volatile organic compounds of Scheffersomyces spartinae W9 have antifungal effect against Botrytis cinerea on strawberry fruit. Foods 2023, 12, 3619. [Google Scholar] [CrossRef]
- Moir, A.; Corfe, B.M.; Behravan, J. Spore germination. Cell Mol. Life Sci. 2002, 59, 403–409. [Google Scholar] [CrossRef]

| Yeast Isolate | Control | |||
|---|---|---|---|---|
| Yeast Concentration | 114/73 | 117/10 | 117/67 | |
| 1 × 107 CFU/mL | 67.50 ± 6.58 b* | 38.40 ± 6.41 de | 57.60 ± 4.46 bc | 88.10 ± 9.52 a |
| 2 × 107 CFU/mL | 23.00 ± 5.95 ef | 6.90 ± 1.22 g | 49.20 ± 7.82 cd | |
| 4 × 107 CFU/mL | 10.80 ± 4.98 fg | 3.90 ± 3.40 g | 13.70 ± 2.72 fg | |
| Combination | Day | ||
|---|---|---|---|
| 14 | 21 | 28 | |
| 114/73 × 117/10 | 0.00 ± 00 b | 1.60 ± 0.58 c | 1.90 ± 0.92 c |
| 114/73 × 117/67 | 1.20 ± 0.68 b | 14.50 ± 2.76 b | 22.00 ± 3.59 b |
| 117/10 × 117/67 | 1.00 ± 0.89 b | 11.40 ± 3.61 bc | 20.30 ± 2.36 b |
| 117/10 × 114/73 × 117/67 | 0.00 ± 00 b | 0.50 ± 0.45 c | 1.20 ± 0.51 c |
| Control | 7.90 ± 2.15 a | 58.90 ± 11.85 a | 88.10 ± 9.52 a |
| Combination | Day | ||
|---|---|---|---|
| 7 | 10 | 14 | |
| 114/73 | 20.00 ± 3.61 c | 31.17 ± 6.54 bc | 61.17 ± 4.37 b |
| 117/10 | 21.83 ± 6.15 c | 32.33 ± 11.07 bc | 48.17 ± 21.22 bcd |
| 117/67 | 37.17 ± 6.64 b | 51.00 ± 7.33 b | 71.17 ± 5.78 ab |
| 114/73 × 117/10 | 20.17 ± 2.13 c | 23.67 ± 0.27 bc | 28.67 ± 3.28 cd |
| 114/73 × 117/67 | 36.67 ± 1.16 b | 46.00 ± 4.23 b | 52.83 ± 2.79 bc |
| 117/10 × 117/67 | 31.67 ± 5.33 b | 47.67 ± 7.01 b | 55.67 ± 2.37 b |
| 117/10 × 114/73 × 117/67 | 17.50 ± 4.99 c | 20.67 ± 4.81 c | 23.50 ± 4.89 d |
| Control | 55.83 ± 5.47 a | 81.67 ± 5.33 a | 94.00 ± 3.61 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzymińska, J.; Kowalska, J. Reducing Postharvest Losses in Organic Apples: The Role of Yeast Consortia Against Botrytis cinerea. Agriculture 2025, 15, 602. https://doi.org/10.3390/agriculture15060602
Krzymińska J, Kowalska J. Reducing Postharvest Losses in Organic Apples: The Role of Yeast Consortia Against Botrytis cinerea. Agriculture. 2025; 15(6):602. https://doi.org/10.3390/agriculture15060602
Chicago/Turabian StyleKrzymińska, Joanna, and Jolanta Kowalska. 2025. "Reducing Postharvest Losses in Organic Apples: The Role of Yeast Consortia Against Botrytis cinerea" Agriculture 15, no. 6: 602. https://doi.org/10.3390/agriculture15060602
APA StyleKrzymińska, J., & Kowalska, J. (2025). Reducing Postharvest Losses in Organic Apples: The Role of Yeast Consortia Against Botrytis cinerea. Agriculture, 15(6), 602. https://doi.org/10.3390/agriculture15060602







