Dynamic Modeling of Convective Drying of Pineapple Peels: Bioactive, Physical, and Thermal Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Processing
2.1.1. Drying
2.1.2. Drying Kinetics
2.1.3. Effective Diffusivity
2.1.4. Thermodynamic Properties
2.1.5. Preparation of Pineapple Peel Flour
2.1.6. Characterization of Fresh and Flour Pineapple Peels
2.2. Ascorbic Acid
2.2.1. Total Phenolic Compounds
2.2.2. Total Tannins and Flavonoids
2.2.3. Total Carotenoids
2.3. Physical Characterization of Flours
2.3.1. Absolute, Apparent, and Compact Density
2.3.2. Hausner Ratio and Carr Index
2.3.3. Porosity and Angle of Repose
2.4. Thermal Analyses
2.4.1. Differential Scanning Calorimetry and Thermogravimetry
2.4.2. Fourier Transform Infrared Spectroscopy (FT-IR)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Drying Kinetics of Pineapple Peels
3.2. Mathematical Modeling
3.3. Effective Diffusivity
3.4. Thermodynamic Properties
3.5. Bioactive Compounds
3.6. Physical Properties
3.7. Thermal Properties and Spectrometry
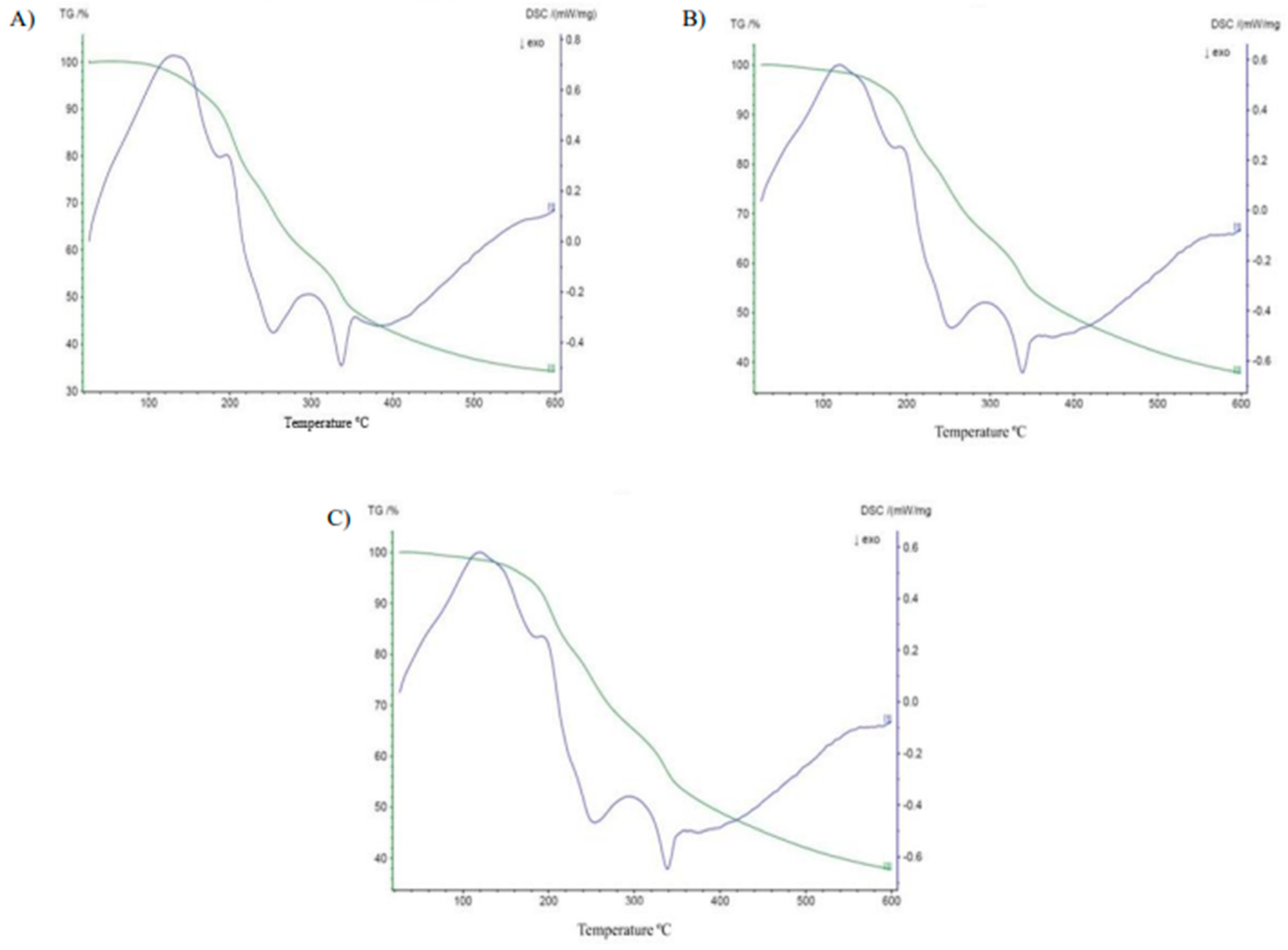
3.7.1. Differential Scanning Calorimetry and Thermogravimetry
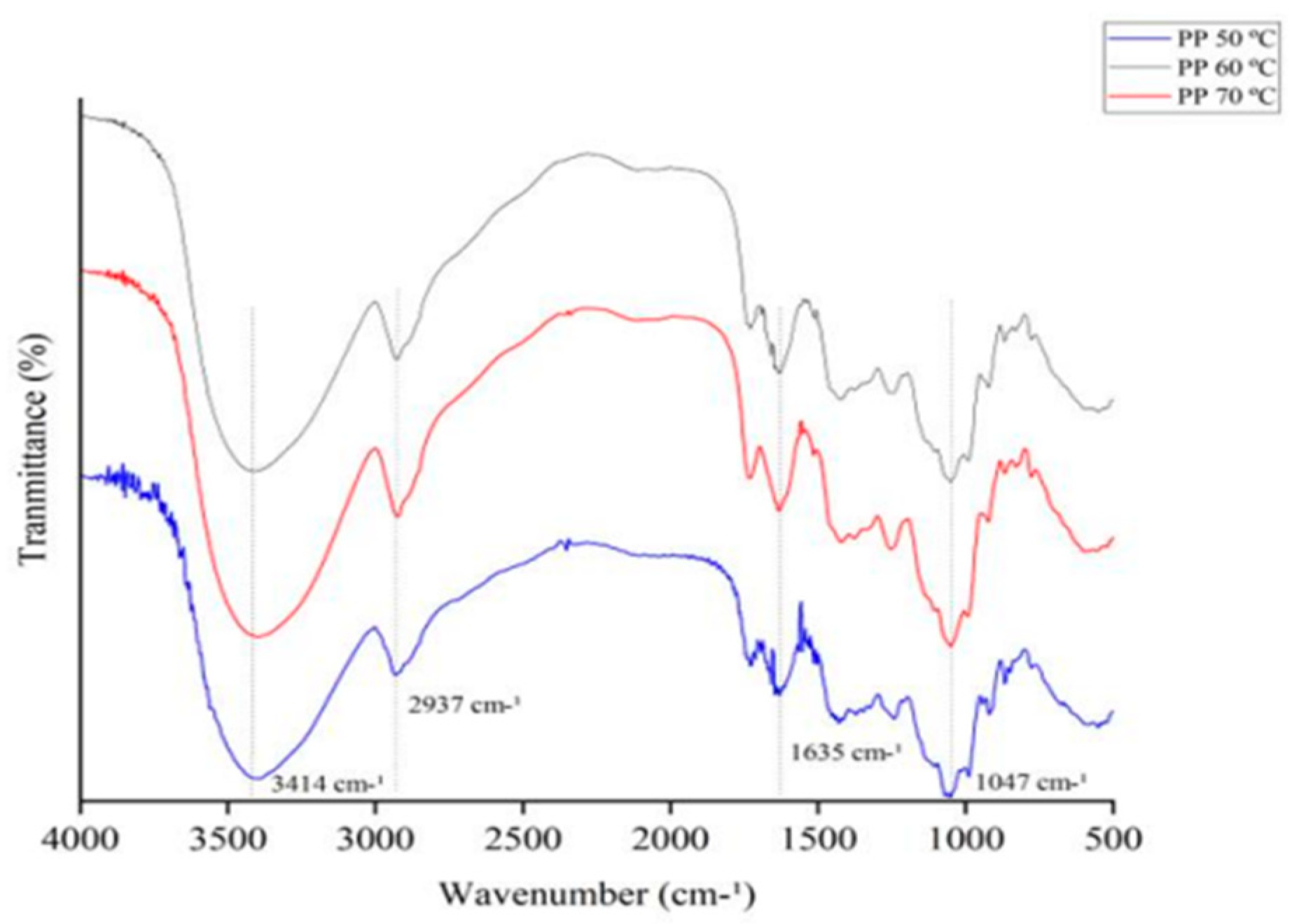
3.7.2. Fourier Transform Infrared Spectroscopy (FT-IR)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rinaldo, D. Carbohydrate and bioactive compounds composition of starchy tropical fruits and tubers, in relation to pre and postharvest conditions: A review. J. Food Sci. 2020, 85, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, H.; Qin, Q.; Wang, J.; Nie, Y.; Wen, L.; Tang, Y.; Qu, M. The investigation of peripheral inflammatory and oxidative stress biomarkers in dementia with Lewy Bodies, compared with Alzheimer’s Disease, and mild cognitive impairment. Neuroscience 2025, 568, 209–218. [Google Scholar] [CrossRef]
- FAO. Análisis del Mercado de las Principales Frutas Tropicales: Panorama General de Febrero de 2020; The Food and Agriculture Organization United Nations: Rome, Italy, 2020; Volume 6. [Google Scholar]
- Pereira, L.F.A.; Firmo, W.C.A.; Coutinho, D.F. A importância do reaproveitamento de resíduos da indústria alimentícia: O caso do processamento de frutas. Res. Soc. Dev. 2022, 11, e38111234089. [Google Scholar] [CrossRef]
- Suri, S.; Singh, A.; Nema, P.K. Infrared drying of Kinnow (Citrus reticulata) peel waste: Kinetics and quality characterization. Biomass Convers. Biorefinery 2024, 14, 7579–7590. [Google Scholar] [CrossRef]
- Bas-Bellver, C.; Barrera, C.; Betoret, N.; Seguí, L. Impact of disruption and drying conditions on physicochemical, functional and antioxidant properties of powdered ingredients obtained from Brassica vegetable by-products. Foods 2022, 11, 3663. [Google Scholar] [CrossRef]
- Abbas, S.; Shanbhag, T.; Kothare, A. Applications of bromelain from pineapple waste towards acne. Saudi J. Biol. Sci. 2021, 28, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Misran, E.; Bani, O.; Situmeang, E.M.; Purba, A.S. Banana stem based activated carbon as a low-cost adsorbent for methylene blue removal: Isotherm, kinetics, and reusability. Alex. Eng. J. 2022, 61, 1946–1955. [Google Scholar] [CrossRef]
- Vega-Galvez, A.; Pasten, A.; Uribe, E.; Mejias, N.; Araya, M.; Vidal, R.L.; Valenzuela-Barra, G.; Delporte, C. Comprehensive Assessment of Anti-Inflammatory, Antiproliferative and Neuroprotective Properties of Cauliflower after Dehydration by Different Drying Methods. Foods 2024, 13, 3162. [Google Scholar] [CrossRef]
- Diógenes, A.M.G.; Figueirêdo, R.M.F.; Queiroz, A.J.M.; Ferreira, J.P.L.; Silva, W.P.; Gomes, J.P.; Santos, F.S.; Castro, D.S.; Oliveira, M.N.; Santos, D.C.; et al. Mathematical Models to Describe the Foam Mat Drying Process of Cumbeba Pulp (Tacinga inamoena) and Product Quality. Foods 2022, 11, 1751. [Google Scholar] [CrossRef]
- Kumar, S.N.; Rajabathar, J.R.; Muthusamy, K.; Kavitha, N.P. Estimation of effective diffusivity, thermodynamic parameter and drying kinetics exploration in coffee berries drying. React. Kinet. Mech. Catal. 2023, 136, 1371–1384. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I.; Mujumdar, A. A comprehensive review of recent advances in renewable-based drying technologies for a sustainable future. Dry. Echnol. 2022, 40, 1029–1050. [Google Scholar] [CrossRef]
- BRASIL. Agência Nacional de Vigilância Sanitária (Anvisa). Resolução RDC nº 275, de 21 de Outubro de 2002. Dispõe Sobre o Regulamento Técnico de Procedimentos Operacionais Padronizados Aplicados aos Estabelecimentos Produtores/Industrializadores de Alimentos e a Lista de Verificação das Boas Práticas de Fabricação em Alimentos. Diário Oficial da União: Seção 1, Brasília, DF, 23 out. 2002. Available online: https://www.gov.br/servidor/pt-br/siass/centrais_conteudo/manuais/resolucao-rdc-anvisa-n-275-de-21-de-outubro-de-2002.pdf/view (accessed on 20 January 2025).
- IAL-Instituto Adolfo Lutz. Normas Analíticas do Instituto Adolfo Lutz: Métodos Químicos e Físicos Para Análise de Alimentos, 1st ed.; IAL: São Paulo, Brasil, 2008; 1020p. [Google Scholar]
- Lewis, W.K. The Rate of Drying of Solid Materials. J. Ind. Eng. Chem. 1921, 13, 427–432. [Google Scholar] [CrossRef]
- Page, G.E. Factors Influencing the Maximum Rates of Air Drying Shelled Corn in Thin Layers. Master’s Thesis, Purdue University, West Lafayette, IN, USA, 1949. [Google Scholar]
- Henderson, S.M. Progress in Developing the Thin Layer Drying Equation. Trans. Am. Soc. Agric. Eng. 1974, 17, 1167–1168. [Google Scholar] [CrossRef]
- Karathanos, V.T. Determination of water content of dried fruits by drying kinetics. J. Food Eng. 1999, 39, 337–344. [Google Scholar] [CrossRef]
- Thompson, T.L.; Peart, P.M.; Foster, G.H. Mathematical simulation of corn during drying—A new model. Trans. Am. Soc. Agric. Eng. 1968, 11, 582–586. [Google Scholar] [CrossRef]
- Henderson, S.M.; Pabis, S. Grain Drying Theory I. Temperature Effects on Drying Coefficient. J. Agric. Eng. Res. 1961, 6, 169–174. [Google Scholar]
- Midilli, A.; Kucuk, H.; Yapar, Z.A. A new model for single-layer drying. Dry. Technol. 2002, 20, 1503–1513. [Google Scholar] [CrossRef]
- Sharaf-Eldeen, Y.I.; Blaisdell, J.L.; Hamdy, M.Y. A Model for Ear Corn Drying. Trans. Am. Soc. Agric. Eng. 1980, 23, 1261–1265. [Google Scholar] [CrossRef]
- Corrêa Filho, L.C.; Andrade, E.T.; Martinazzo, A.P.; D’andrea, E.M.; Sousa, F.A.; Figueira, V.G. Drying kinetics, shrinkage and analysis of liquid diffusion of figs. Rev. Bras. Eng. Agrícola Ambient. 2015, 19, 797–802. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 43rd ed.; Association Official Analytical Chemistry: Washington, DC, USA, 2009; 1018p, Available online: https://www.scirp.org/reference/referencespapers?referenceid=429305 (accessed on 20 January 2025).
- Waterhouse, A.L.; Laurie, V.F. Oxidation of Wine Phenolics: A Critical Evaluation and Hypotheses. Am. J. Enol. Vitic. 2006, 57, 306–313. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Swain, T. Changes in tannins in ripening fruits. Phytochemistry 1963, 2, 371–383. [Google Scholar] [CrossRef]
- Francis, F.J. Analysis of anthocyanins. In Anthocyanins as Food Colors; Markakis, P., Ed.; Academic Press: New York, NY, USA, 1982; pp. 181–207. [Google Scholar]
- Higby, W.K. A Simplified Method for Determination of Some Aspects of the Carotenoid Distribution in Natural and Carotene-Fortified Orange Juice. J. Food Sci. 1962, 27, 42–49. [Google Scholar] [CrossRef]
- Pragati, S.; Genitha, I.; Ravish, K. Comparative study of ripe and unripe banana flour during storage. J. Food P. Technol. 2014, 5, 1–6. [Google Scholar]
- Politi, F.A.S. Estudos Farmacognósticos e Avaliação de Atividades Biológicas de Extratos Obtidos das Cascas Pulverizadas de Endopleurauchi (Huber) Cuatrec. (Humiriaceae). Master’s Thesis, Universidade Estadual Paulista, Araraquara, Brasil, 2009. [Google Scholar]
- Tonon, R.V. Secagem por Atomização do Suco de Açaí: Influência das Variáveis de Processo, Qualidade e Estabilidade do Produto. Ph.D. Thesis, Universidade Estadual de Campinas, Campinas, Brasil, 2009. [Google Scholar]
- Hausner, H.H. Friction conditions in a mass of metal powder. Int. J. Powder Met. 1967, 3, 7–13. [Google Scholar]
- Carr, R. Evaluating Flow Properties of Solids. Chem. Eng. 1965, 72, 69–72. [Google Scholar]
- Syamaladevi, D.P.; Spudich, J.A.; Sowdhamini, R. Structural and Functional Insights on the Myosin Superfamily. Bioinform. Biol. Insights 2012, 6, BBI-S8451. [Google Scholar] [CrossRef]
- Bhandari, B.R.; Datta, N.; D’arcy, B.R.; Rintoul, G.B. Co-crystallization of Honey with Sucrose. Lebensm. Wiss. Technol. 1998, 31, 138–142. [Google Scholar] [CrossRef]
- Xiang, S.; Yang, J.; Chen, Y.; Zhong, M.E.; Xiang, Z.; Zhou, Z. Enhancing the slow-release performance of urea by biochar polyurethanes co-coating. J. Coat. Technol. Res. 2024, 1–9. [Google Scholar] [CrossRef]
- Silva, F.A.S.; Azevedo, C.A.V. The Assistat Software Version 7.7 and Its Use in the Analysis of Experimental Data. Afr. J. Agric. Res. 2016, 11, 3733–3740. [Google Scholar]
- Aral, S.; Beşe, A.V. Convective drying of hawthorn fruit (Crataegus spp.): Effect of experimental parameters on drying kinetics, color, shrinkage, and rehydration capacity. Food Chem. 2016, 210, 577–584. [Google Scholar] [CrossRef]
- Dhara, J.; Saha, S.; Saha, M.; Chakraborty, R. Study on drying kinetics, antioxidant activity, total bioactive compounds, physicochemical properties and microstructural characteristics of dehydrated star fruits (Averrhoa carambola) by different drying methods. Sustain. Food Technol. 2023, 1, 590–602. [Google Scholar] [CrossRef]
- Alibas, I.; Zia, M.P.; Yilmaz, A.; Asik, B.B. Drying kinetics and quality characteristics of green apple peel (Mallus communis L. var. “Granny Smith”) used in herbal tea production. J. Food Process. Preserv. 2020, 44, e14332. [Google Scholar] [CrossRef]
- Ambawat, S.; Sharma, A.; Saini, R.K. Mathematical modeling of thin layer drying kinetics and moisture diffusivity study of pretreated Moringa oleifera leaves using fluidized bed dryer. Processes 2022, 10, 2464. [Google Scholar] [CrossRef]
- Zeng, Z.; Han, C.; Wang, Q.; Yuan, H.; Zhang, X.; Li, B. Analysis of drying characteristic, effective moisture diffusivity and energy, exergy and environment performance indicators during thin layer drying of tea in a convective-hot air dryer. Front. Sustain. Food Syst. 2024, 8, 1371696. [Google Scholar] [CrossRef]
- Cavalcanti-Mata, M.E.; Duarte, M.E.; Tolentino, M.; Mendes, F.A.; Batista, L.; De Lima, J.M.; Lúcio, A.; Nascimento, A.P.; Almeida, R.D.; Lisboa, H.M. Drying Kinetics of Industrial Pineapple Waste: Effective Diffusivity and Thermodynamic Properties Resulting from New Mathematical Models Derived from the Fick Equation. Processes 2024, 12, 1198. [Google Scholar] [CrossRef]
- Reis, C.G.D.; Figueirêdo, R.M.F.D.; Queiroz, A.J.D.M.; Paiva, Y.F.; Amadeu, L.T.S.; Santos, F.S.D.; Ferreira, J.P.D.L.; Lima, T.L.B.D.; Andrade, F.S.; Gomes, J.P.; et al. Pineapple peel flours: Drying kinetics, thermodynamic properties, and physicochemical characterization. Processes 2023, 11, 3161. [Google Scholar] [CrossRef]
- Kaveh, M.; Jahanbakhshi, A.; Abbaspour-Gilandeh, Y.; Taghinezhad, E.; Moghimi, M.B.F. The effect of ultrasound pre-treatment on quality, drying, and thermodynamic attributes of almond kernel under convective dryer using ANNs and ANFIS network. J. Food Process Eng. 2018, 41, e12868. [Google Scholar] [CrossRef]
- Deng, L.Z.; Mujumdar, A.S.; Yang, W.X.; Zhang, Q.; Zheng, Z.A.; Wu, M.; Xiao, H.W. Hot air impingement drying kinetics and quality attributes of orange peel. J. Food Process. Preserv. 2020, 44, e14294. [Google Scholar] [CrossRef]
- Lehmad, M.; El Hachimi, Y.; Lhomme, P.; Mghazli, S.; Abdenouri, N. Comprehensive Analysis of Adsorption–Desorption Isotherms, Drying Kinetics, and Nutritional Quality of Black Soldier Fly (Hermetia illucens) Larvae. Food Biophys. 2024, 19, 938–954. [Google Scholar] [CrossRef]
- Zogzas, N.P.; Maroulis, Z.B.; Marinos-Kouris, D. Moisture Diffusivity Data Compilation in Foodstuffs. Dry Technol. 1996, 14, 2225–2253. [Google Scholar] [CrossRef]
- De Jesus Junqueira, J.R.; Do Carmo, J.R.; Miyagusku, L.; Balbinoti, T.C.V.; de Carvalho Rafael Salgado Junqueira, M.; de Lucena, R.F.P. Infrared Drying of Bocaiuva (Acrocomia aculeata) Slices: Drying Kinetics, Energy Consumption, and Quality Characteristics. Food Biophys. 2024, 19, 885–894. [Google Scholar] [CrossRef]
- Akhoundzadeh Yamchi, A.; Hosainpour, A.; Hassanpour, A.; Rezvanivand Fanaei, A. Developing ultrasound-assisted infrared drying technology for bitter melon (Momordica charantia). J. Food Process Eng. 2024, 47, e14516. [Google Scholar] [CrossRef]
- El-Mesery, H.S.; Qenawy, M.; Hu, Z.; Alshaer, W.G. Evaluation of infrared drying for okra: Mathematical modelling, moisture diffusivity, energy activity and quality attributes. Case Stud. Therm. Eng. 2023, 50, 103451. [Google Scholar] [CrossRef]
- Moura, H.V.; De Figueirêdo, R.M.F.; Queiroz, A.J.M.; Silva, E.T.V.; Esmero, J.A.D.; Lisbôa, J.F. Mathematical modeling and thermodynamic properties of the drying kinetics of trapiá residues. J. Food Process Eng. 2021, 44, e13768. [Google Scholar] [CrossRef]
- Fabela-Morón, M.F. Bioactive compounds, sensory attributes, and flavor perceptions involved in taste-active molecules in fruits and vegetables. Front. Nutr. 2024, 11, 1427857. [Google Scholar] [CrossRef]
- Hoyos-Martínez, P.L.; Merle, J.; Labidi, J.; Bouhtoury, F.C. Tannins extraction: A key point for their valorization and cleaner production. J. Clean. Prod. 2019, 206, 1138–1155. [Google Scholar] [CrossRef]
- Deng, H.; Cai, Z.; Xiang, S.; Guo, Q.; Huang, W.; Liang, G. Karyotype Analysis of Diploid and Spontaneously Occurring Tetraploid Blood Orange [Citrus sinensis (L.) Osbeck] Using Multicolor FISH With Repetitive DNA Sequences as Probes. Front. Plant Sci. 2019, 10, 331. [Google Scholar] [CrossRef]
- Gondim, C.D.M.; De Figueirêdo, R.M.F.; Queiroz, A.J.D.M.; Esmero, J.A.D.; Moura, H.V.; Moreira, I.D.S.J. Thermodynamic properties and bioactive compounds of mandacaru dried waste. Food Process. Preserv. 2022, 46, e17230. [Google Scholar] [CrossRef]
- Albuquerque, J.C.; De Figueirêdo, R.M.F.; De Melo Queiroz, A.J.; dos Santos, F.S.; Santos, N.C.; de Oliveira Carvalho, R.; Gregório, M.G.; Moura, H.V.; de Macedo Albuquerque Junior, N.; Amadeu, L.T.S.; et al. Processing of Maranhão mango peels by convective drying and freeze-drying: Kinetic study, functional and thermal properties. Food Meas. 2024, 18, 6295–6309. [Google Scholar] [CrossRef]
- Kaushik, A.; Saxena, D.C.; Singh, S. Functional, Thermal, Pasting, and Antioxidant Properties of Flour from Indian Browntop Millet (Brachiaria ramosa) Cultivars. Food Biophys. 2024, 19, 637–652. [Google Scholar] [CrossRef]
- Seerangurayar, T.; Manickavasagan, A.; Al-Ismaili, A.M.; Al-Mulla, Y.A. Effect of carrier agents on flowability and microstructural properties of foam-mat freeze dried date powder. J. Food Eng. 2017, 215, 33–43. [Google Scholar] [CrossRef]
- Santhalakshmy, S.; Bosco, S.J.D.; Francis, S.; Sabeena, M. Effect of inlet temperature on physicochemical properties of spray-dried jamun fruit juice powder. Powder Technol. 2015, 274, 37–43. [Google Scholar] [CrossRef]
- Gomez-Caturla, J.; Ivorra-Martinez, J.; Fenollar, O.; Balart, R.; Garcia-Garcia, D.; Dominici, F.; Torre, L. Development of starch-rich thermoplastic polymers based on mango kernel flour and different plasticizers. Int. J. Biol. Macromol. 2024, 264, 130773. [Google Scholar] [CrossRef] [PubMed]
- Peres, C.B.; de Morais, L.C.; Resende, P.M.R. Carbon adsorption on waste biomass of passion fruit peel: A promising machine learning model for CO2 capture. J. CO2 Util. 2024, 80, 102680. [Google Scholar] [CrossRef]
- de Melo, A.M.; Barbi, R.C.T.; Costa, B.P.; Ikeda, M.; Carpiné, D.; Ribani, R.H. Valorization of the agro-industrial by-products of bacupari (Garcinia brasiliensis (Mart.)) through production of flour with bioactive properties. Food Biosci. 2022, 45, 101343. [Google Scholar] [CrossRef]
- Barbi, R.C.T.; Hornung, P.S.; Ávila, S.; Alves, F.E.D.S.B.; Beta, T.; Ribani, R.H. Ripe and unripe inajá (Maximilia maripa) fruit: A new high source of added value bioactive compounds. Food Chem. 2020, 331, 127333. [Google Scholar] [CrossRef]
- Sakulnarmrat, K.; Sittiwong, W.; Konczak, I. Encapsulation of mangosteen pericarp anthocyanin-rich extract by spray drying. Int. J. Food Sci. Technol. 2022, 57, 1237–1247. [Google Scholar] [CrossRef]
- Damto, T.; Birhanu, T.; Zewdu, A. Physicochemical and antioxidant characterization of commercially available honey sample from Addis Ababa market, Ethiopia. Heliyon 2023, 9. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, M.; Jiang, Q.; Mujumdar, A.S. Intelligent system/equipment for quality deterioration detection of fresh food: Recent advances and application. Foods 2024, 13, 1662. [Google Scholar] [CrossRef]
- Kaushal, R.; Kaur, B.; Panesar, P.S.; Anal, A.K.; Chu-Ky, S. Valorization of pineapple rind for bromelain extraction using microwave assisted technique: Optimization, purification, and structural characterization. J. Food Sci. Technol. 2024, 61, 551–562. [Google Scholar] [CrossRef]
- Yanuhar, U.; Suryanto, H.; Aminnudin, A.; Wijaya, H.W.; Maulana, J.; Caesar, N.R.; Irawan, Y.S.; Binoj, J.S. Utilization of Pineapple Peel Waste/ZnO Nanoparticles Reinforcement for Cellulose-Based Nanocomposite Membrane and Its Characteristics. J. Polym. Environ. 2024, 32, 3749–3764. [Google Scholar] [CrossRef]

| Model | Equation | References |
|---|---|---|
| Newton | [15] | |
| Page | [16] | |
| Henderson and Pabis | [17] | |
| Henderson and Pabis modified | [18] | |
| Thompson | [19] | |
| Two terms | [20] | |
| Midilli | [21] | |
| Diffusion approximation | [22] | |
| Two-Term exponential | [23] |
| Model | Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Newton | k | R2 | MSD | χ2 (×10−1) | |||||
| 50 | 0.0076 | 0.9960 | 0.0309 | 0.0098 | |||||
| 60 | 0.0147 | 0.9993 | 0.0132 | 0.0018 | |||||
| 70 | 0.0202 | 0.9997 | 0.0084 | 0.0007 | |||||
| Page | k | n | R2 | DQM | χ2 (×10−1) | ||||
| 50 | 0.0181 | 0.8155 | 0.9998 | 0.0074 | 0.0006 | ||||
| 60 | 0.0193 | 0.9327 | 0.9997 | 0.0084 | 0.0008 | ||||
| 70 | 0.0193 | 1.0116 | 0.9997 | 0.0082 | 0.0007 | ||||
| Henderson e Pabis | k | a | R2 | DQM | χ2 (×10−1) | ||||
| 50 | 0.0068 | 0.9421 | 0.9979 | 0.0225 | 0.0054 | ||||
| 60 | 0.9881 | 0.0144 | 0.9993 | 0.0125 | 0.0017 | ||||
| 70 | 0.0204 | 1.0089 | 0.9997 | 0.0078 | 0.0007 | ||||
| Henderson e Pabis modificado | a | k | B | k0 | C | k1 | R2 | DQM | χ2 (×10−1) |
| 50 | −0.0436 | 0.0068 | 1.0294 | 0.0068 | −0.0436 | 0.0068 | 0.9979 | 0.0225 | 0.0061 |
| 60 | 0.2461 | 0.0345 | 0.3204 | 0.0117 | 0.4470 | 0.0118 | 0.9999 | 0.0156 | 0.0034 |
| 70 | 0.0512 | 0.0404 | 0.4598 | 0.0199 | 0.5010 | 0.0199 | 0.9997 | 0.0137 | 0.0026 |
| Thompson | a | b | R2 | DQM | χ2 (×10−1) | ||||
| 50 | −10.3192 | 0.3212 | 0.9993 | 0.0129 | 0.0018 | ||||
| 60 | −19.6820 | 0.5620 | 0.9998 | 0.0069 | 0.0005 | ||||
| 70 | −1872.48 | 6.1474 | 0.9997 | 0.0084 | 0.0008 | ||||
| Two-Term | a | k0 | B | k1 | R2 | DQM | χ2 (×10−1) | ||
| 50 | 0.2338 | 0.0336 | 0.7719 | 0.00053 | 0.9999 | 0.0029 | 0.0001 | ||
| 60 | 0.2460 | 0.0345 | 0.7675 | 0.0118 | 0.9999 | 0.0056 | 0.0004 | ||
| 70 | 0.9608 | 0.0199 | 0.0511 | 0.0404 | 0.9997 | 0.0077 | 0.0007 | ||
| Midilli | a | k | N | b (×10−2) | R2 | DQM | χ2 (×10−1) | ||
| 50 | 1.0109 | 0.0202 | 0.7954 | -0.0005 | 0.9998 | 0.0066 | 0.0005 | ||
| 60 | 1.0169 | 0.0218 | 0.9085 | 0.0001 | 0.9998 | 0.0073 | 0.0006 | ||
| 70 | 1.0101 | 0.0208 | 0.9962 | 0.0000 | 0.9997 | 0.0078 | 0.0007 | ||
| Diffusion approximation | a | k | B | R2 | DQM | χ2 (×10−1) | |||
| 50 | 0.2331 | 0.0318 | 0.1675 | 0.9999 | 0.0031 | 0.0001 | |||
| 60 | 0.3053 | 0.0275 | 0.4122 | 0.9998 | 0.0066 | 0.0005 | |||
| 70 | −0.0151 | 0.6600 | 0.0312 | 0.9998 | 0.0075 | 0.0006 | |||
| Two-Term exponential | a | k | R2 | DQM | χ2 (×10−1) | ||||
| 50 | 0.1825 | 0.0320 | 0.9997 | 0.0084 | 0.0007 | ||||
| 60 | 0.0197 | 0.7226 | 0.9994 | 0.0121 | 0.0016 | ||||
| 70 | 0.0038 | 5.2396 | 0.9997 | 0.0089 | 0.0009 | ||||
| Residue | T (°C) | Def (m2/s) | R2 |
|---|---|---|---|
| Pineapple peels | 50 | 2.83 × 10−10 | 0.9974 |
| 60 | 5.64 × 10−10 | 0.9926 | |
| 70 | 7.96 × 10−10 | 0.9898 |
| Residue | D0 (m2/s) | Ea (kJ/mol) | R2 |
|---|---|---|---|
| Pineapple peels | 1.65 × 10−2 | 47.90 | 0.9705 |
| Residue | T (°C) | ΔH (kJ/mol) | ΔS (kJ/mol) | ΔG (kJ/mol) |
|---|---|---|---|---|
| Pineapple peels | 50 | 45.21 | −0.2797 | 135.60 |
| 60 | 45.13 | −0.2800 | 138.40 | |
| 70 | 45.04 | −0.2802 | 141.20 |
| Parameters | Drying Temperature (°C) | |||
|---|---|---|---|---|
| Fresh | 50 | 60 | 70 | |
| Ascorbic acid (mg/100 g d.b.) | 99.48 ± 0.74 a | 68.55 ± 1.43 bc | 61.58 ± 0.63 c | 72.29 ± 5.70 b |
| Total phenolic compound (mg GAE/100 g d.b.) | 1740.90 ± 5.02 a | 1114.86 ± 0.67 c | 1153.68 ± 0.61 b | 1147.45 ± 0.44 b |
| Total tannins (mg TAE/100 g d.b.) | 613.42 ± 0.38 d | 626.97 ± 1.69 c | 698.96 ± 0.47 b | 720.14 ± 0.49 a |
| Total flavonoids (mg/100 g d.b.) | 75.96 ± 0.06 a | 13.44 ± 0.06 d | 14.47 ± 0.02 c | 16.01 ± 0.04 b |
| Total anthocyanins (mg/100 g d.b.) | 8.10 ± 0.02 a | 3.09 ± 0.005 d | 3.52 ± 0.003 c | 6.11 ± 0.014 b |
| Total carotenoids (mg/100 g d.b.) | 0.955 ± 0.015 a | 0.253 ± 0.0004 d | 0.386 ± 0.002 b | 0.339 ± 0.003 c |
| Parâmetro | Drying Temperature (°C) | ||
|---|---|---|---|
| 50 | 60 | 70 | |
| BApparent density (g/cm3) | 0.574 ± 0.01 b | 0.580 ± 0.01 b | 0.622 ± 0.01 a |
| Compacted density (g/cm3) | 0.624 ± 0.02 c | 0.665 ± 0.01 b | 0.701 ± 0.01 a |
| Absolute density (g/cm3) | 2.43 ± 0.17 a | 2.51 ± 0.01 a | 2.57 ± 0.05 a |
| Carr Index (%) | 8.00 ± 2.00 b | 12.67 ± 1.15 a | 11.33 ± 2.31 b |
| Hausner Ratio | 1.09 ± 0.02 b | 1.15 ± 0.02 a | 1.13 ± 0.0 ab |
| Angle of repose (°) | 25.67 ± 1.45 a | 21.18 ± 1.45 b | 21.22 ± 0.38 b |
| Sample | TGA | ||
|---|---|---|---|
| Event | Δm (%) | ΔT (°C) | |
| PP 50 °C | 1° | 5.84 | 87.11–160.59 |
| 2° | 16.90 | 160.59–219.85 | |
| 3° | 17.24 | 219.85–294.95 | |
| 4° | 10.42 | 294.95–340.00 | |
| 5° | 15.12 | 340.00–584.37 | |
| PP 60 °C | 1° | 3.88 | 68.34–154.68 |
| 2° | 15.68 | 154.68–234.73 | |
| 3° | 12.19 | 234.73–279.99 | |
| 4° | 14.62 | 279.99–354.82 | |
| 5° | 15.26 | 354.82–584.42 | |
| PP 70 °C | 1° | 1.52 | 82.44–137.69 |
| 2° | 9.10 | 137.69–205.66 | |
| 3° | 12.08 | 205.66–280.02 | |
| 4° | 8.47 | 280.02–349.83 | |
| 5° | 7.56 | 349.83–584.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, R.d.O.; de Figueirêdo, R.M.F.; Queiroz, A.J.d.M.; dos Santos, F.S.; Gregório, M.G.; Amadeu, L.T.S.; Moura, H.V.; Junior, N.d.M.A.; Andrade, F.S.; Cruz, E.B.C.; et al. Dynamic Modeling of Convective Drying of Pineapple Peels: Bioactive, Physical, and Thermal Properties. Agriculture 2025, 15, 609. https://doi.org/10.3390/agriculture15060609
Carvalho RdO, de Figueirêdo RMF, Queiroz AJdM, dos Santos FS, Gregório MG, Amadeu LTS, Moura HV, Junior NdMA, Andrade FS, Cruz EBC, et al. Dynamic Modeling of Convective Drying of Pineapple Peels: Bioactive, Physical, and Thermal Properties. Agriculture. 2025; 15(6):609. https://doi.org/10.3390/agriculture15060609
Chicago/Turabian StyleCarvalho, Raniza de Oliveira, Rossana Maria Feitosa de Figueirêdo, Alexandre José de Melo Queiroz, Francislaine Suelia dos Santos, Mailson Gonçalves Gregório, Lumara Tatiely Santos Amadeu, Henrique Valentim Moura, Nailton de Macedo Albuquerque Junior, Fabrícia Santos Andrade, Emily Bezerra Coutinho Cruz, and et al. 2025. "Dynamic Modeling of Convective Drying of Pineapple Peels: Bioactive, Physical, and Thermal Properties" Agriculture 15, no. 6: 609. https://doi.org/10.3390/agriculture15060609
APA StyleCarvalho, R. d. O., de Figueirêdo, R. M. F., Queiroz, A. J. d. M., dos Santos, F. S., Gregório, M. G., Amadeu, L. T. S., Moura, H. V., Junior, N. d. M. A., Andrade, F. S., Cruz, E. B. C., Lara, E. Z., Gomes, J. P., & Madruga, M. S. (2025). Dynamic Modeling of Convective Drying of Pineapple Peels: Bioactive, Physical, and Thermal Properties. Agriculture, 15(6), 609. https://doi.org/10.3390/agriculture15060609








