Effects of Energy Intake on Nutrient Digestibility, Nitrogen Metabolism, Energy Utilization, Serum Biochemical Indices, and Rumen Microbiota in Lanzhou Fat-Tailed Sheep
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design, Diet, and Feeding Management
2.2. Feed, Fecal and Urine Collection
2.3. Measurements
Nutrient content in feces)/Nutrient content in feed × 100%
− Urinary energy (UE) − CH4-E
2.4. Determination of Serum Biochemical Indices
2.5. Rumen Fluid Collection and Rumen Microbial Analysis
2.6. Statistical Analysis
3. Results
3.1. Growth Performance and Nutrient Apparent Digestibility
3.2. Nitrogen Metabolism
3.3. Energy Utilization
3.4. Serum Biochemical Indices
3.5. Rumen Microbial Composition
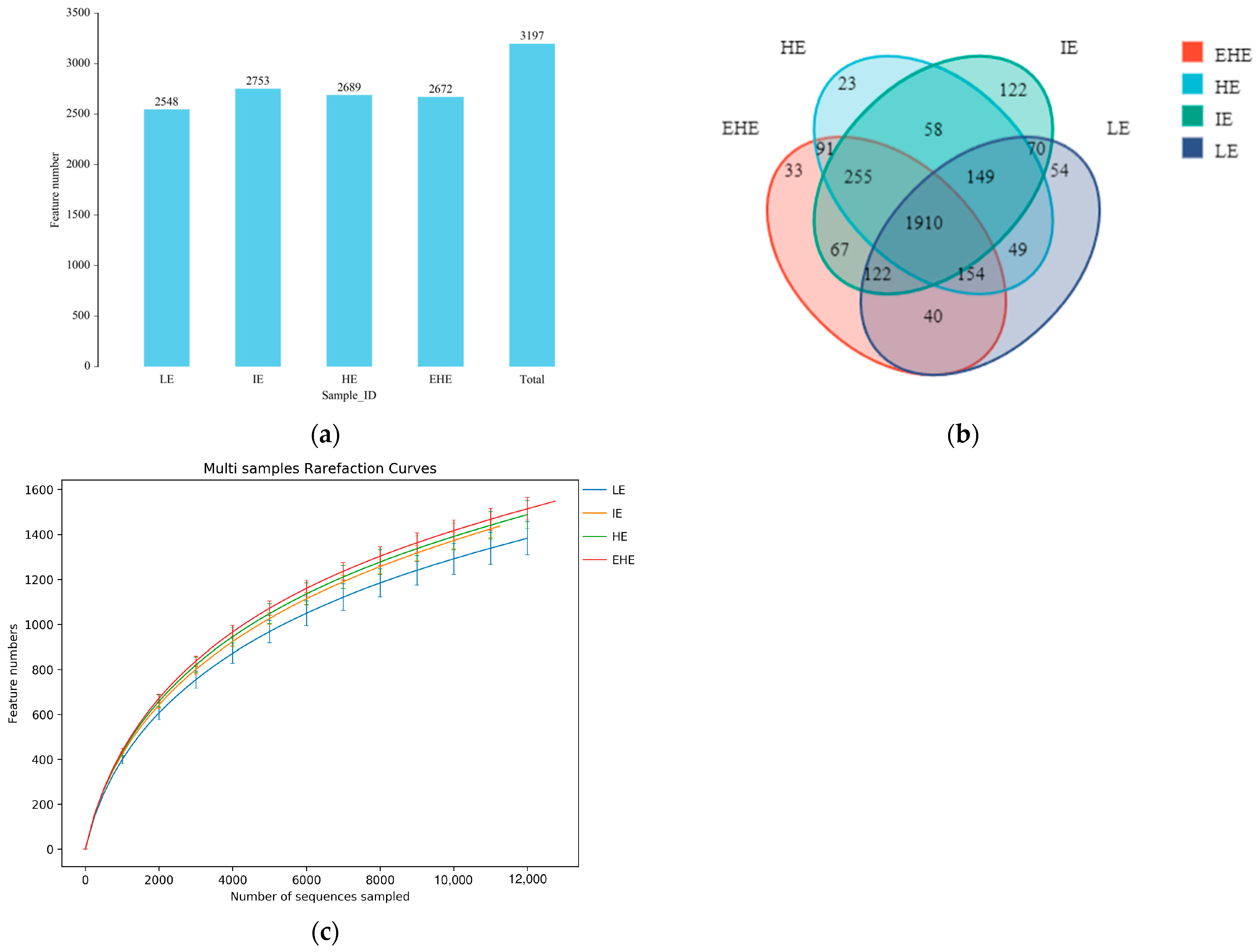
3.5.1. Sample Sequencing Results
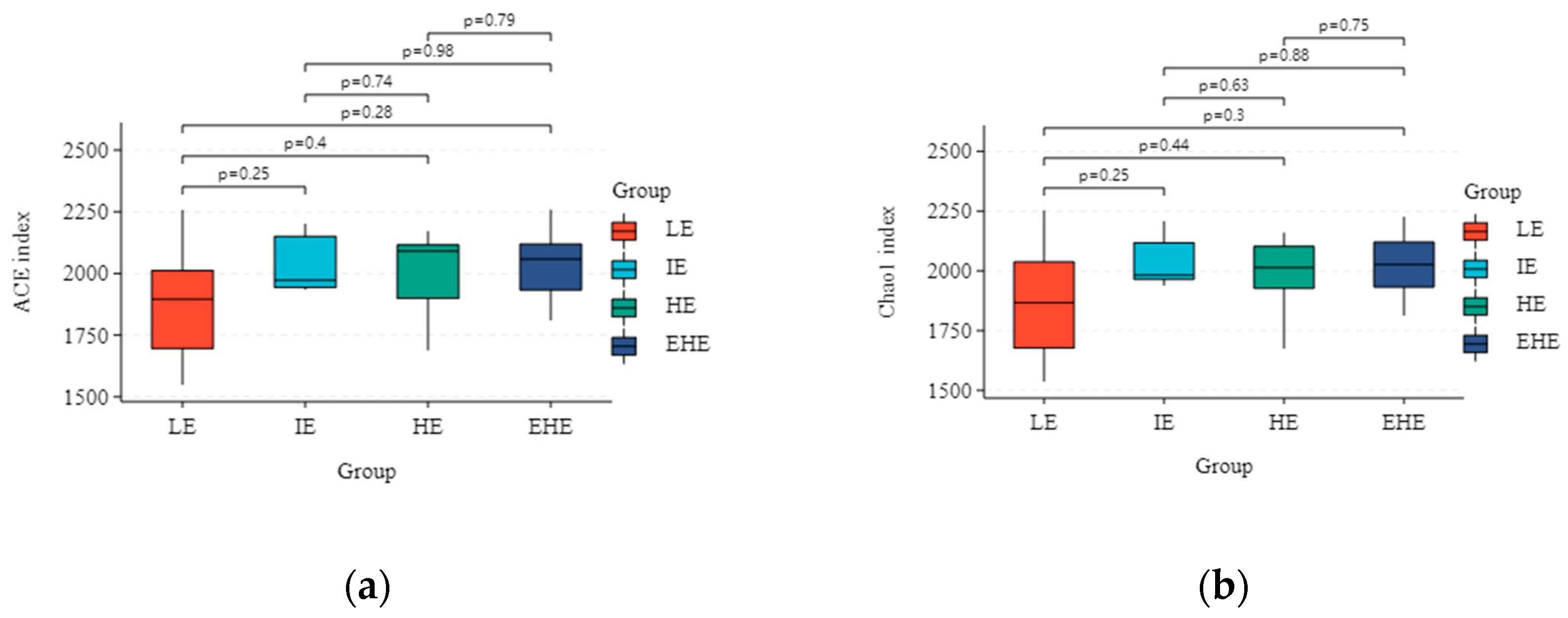
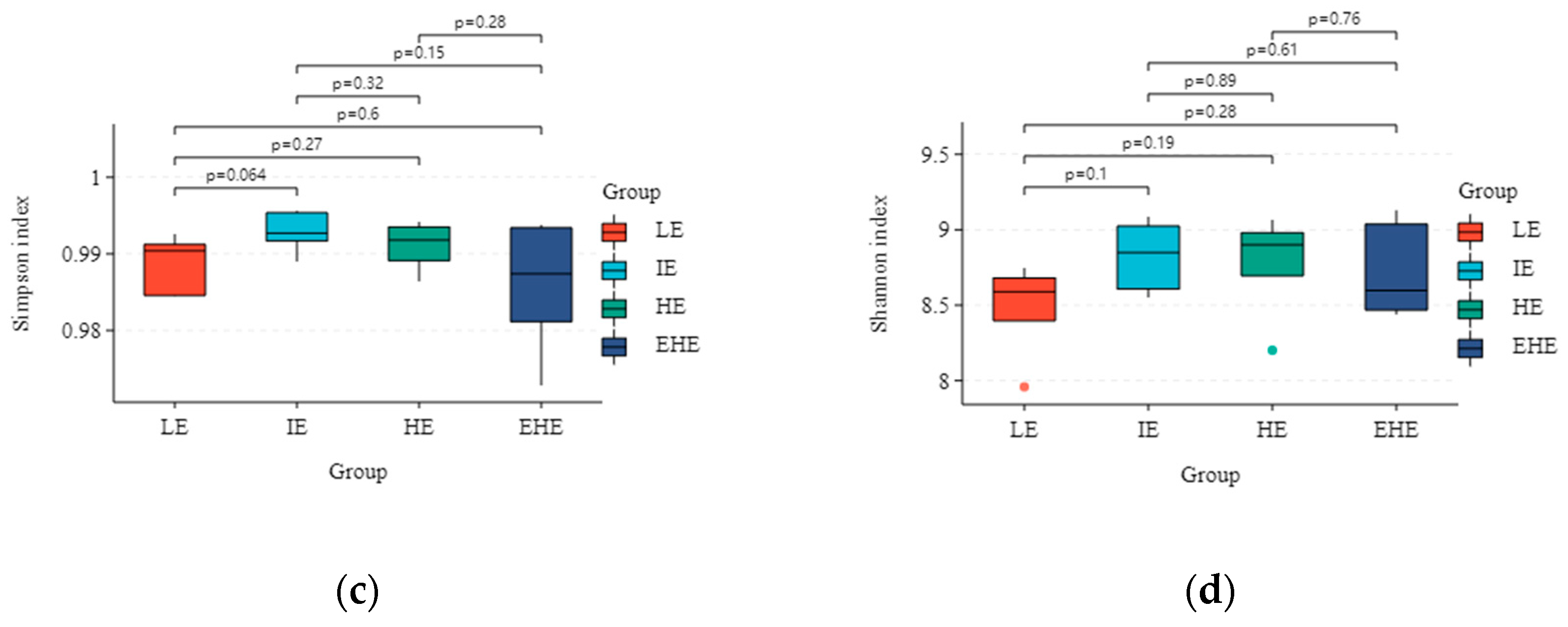
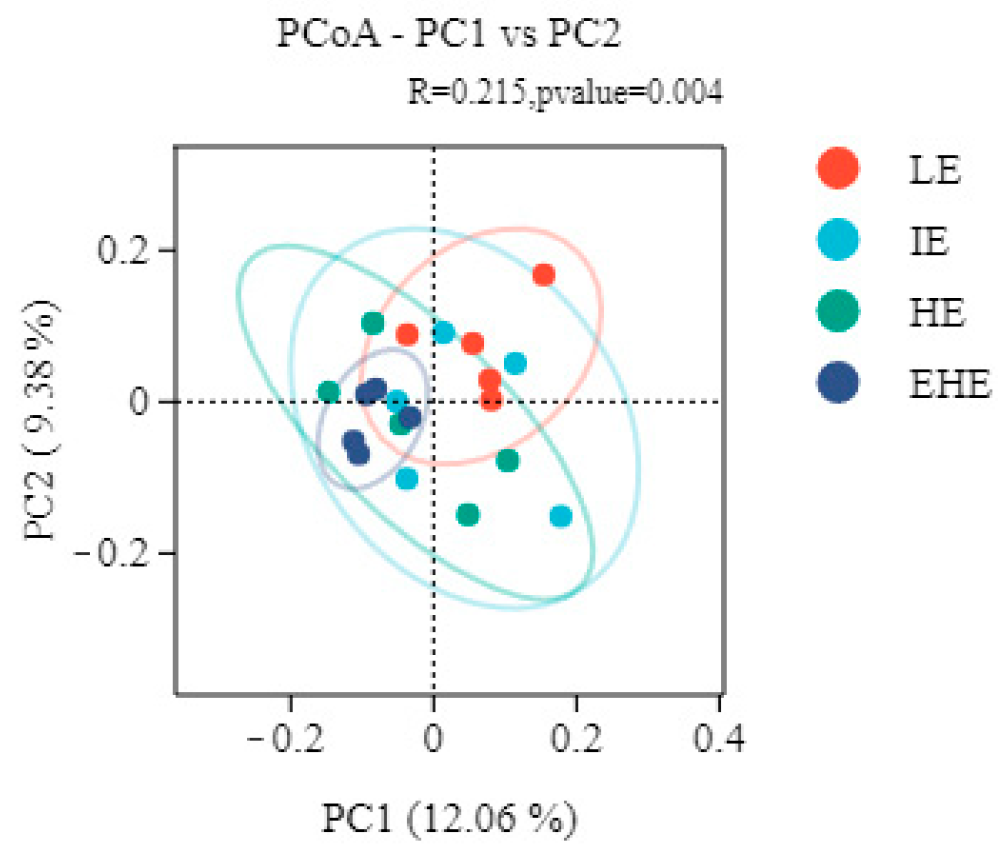
3.5.2. Alpha Diversity and Beta Diversity
3.5.3. Taxonomy of Rumen Bacterial Composition
4. Discussion
4.1. Effect of Dietary Energy Level on Growth Performance and Nutrient Apparent Digestibility
4.2. Effect of Dietary Energy Level on Nitrogen Metabolism
4.3. Effect of Dietary Energy Level on Energy Utilization
4.4. Effect of Dietary Energy Level on Serum Biochemical Indicators
4.5. Effect of Dietary Energy Level on Rumen Microbial Composition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H.F.; Wei, T.; Zhang, J.S.; Xu, H.W.; Gao, D.D.; Cao, X.; Cai, Y.; Yang, J.T.; Wang, R.; Guo, P.H. A suitable prediction model of body weight and trait measurements of Lanzhou fat-tailed sheep. Pratacult. Sci. 2023, 40, 1648–1655. [Google Scholar]
- Bai, J.; Zhao, E.L.; Li, M.F.; Xin, J.P.; Xu, L.J.; Qu, M.R.; Yi, Z.H.; Yang, S.T.; Yang, J.J.; Li, Y.J. Effects of Dietary Energy Level on Growth Performance, Nutrient Digestionand Energy Metabolism of Jinjiang Steers in Early Stage of Fattening. Chin. J. Anim. Nutr. 2019, 31, 692–698. [Google Scholar]
- Wang, Q.; Wang, Y.; Hussain, T.; Dai, C.; Li, J.; Huang, P.; Li, Y.; Ding, X.; Huang, J.; Ji, F.; et al. Effects of dietary energy level on growth performance, blood parameters and meat quality in fattening male Hu lambs. J. Anim. Physiol. Anim. Nutr. 2020, 104, 418–430. [Google Scholar]
- Luo, S.-F.; Wang, Y.-C.; Wang, X.; Dai, C.-P.; Wang, Q.-Y. Dietary energy and protein levels on lactation performance and progeny growth of Hu sheep. J. Appl. Anim. Res. 2022, 50, 526–533. [Google Scholar]
- Zhou, J.; Wang, W.; Jing, X.; Degen, A.; Guo, Y.; Kang, J.; Shang, Z.; Yu, Z.; Qiu, Q.; Guo, X.; et al. Effect of dietary energy on digestibilities, rumen fermentation, urinary purine derivatives and serum metabolites in Tibetan and small-tailed Han sheep. J. Anim. Physiol. Anim. Nutr. 2019, 103, 977–987. [Google Scholar]
- Liu, H.; Zhou, J.; Degen, A.; Liu, H.; Cao, X.; Hao, L.; Shang, Z.; Ran, T.; Long, R. A comparison of average daily gain, apparent digestibilities, energy balance, rumen fermentation parameters, and serum metabolites between yaks (Bos grunniens) and Qaidam cattle (Bos taurus) consuming diets differing in energy level. Anim. Nutr. 2023, 12, 77–86. [Google Scholar]
- Zhu, Y.; Sun, G.; Luosang-dunzhu; Li, X.; Luosang-zhaxi; Suolang-zhaxi; Suolang; Ciyang; Cidan-yangji; Basang-wangdui; et al. High energy level diet improves the growth performance and rumen fermentation of yaks in cold weather. Front. Vet. Sci. 2023, 10, 1212422. [Google Scholar] [CrossRef]
- Mohd Azmi, A.F.; Hafandi, A.; Mohd Nor, N.; Goh, Y.; Saad, M.; Abu Bakar, M.Z.; Abdullah, P.; Jayanegara, A.; Abu Hassim, H. Effects of Concentrate and Bypass Fat Supplementations on Growth Performance, Blood Profile, and Rearing Cost of Feedlot Buffaloes. Animals 2021, 11, 2105. [Google Scholar] [CrossRef]
- Kang, J.; Wang, W.; Guo, Y.; Jing, X.; Zhong, C.; Guo, W.; Long, R.; Zhou, J. Effects of different dietary energy levels on apparent digestibility, nitrogen metabolism and growth performance of Tibetan sheep under low nitrogen conditions. Acta Pratacult. Sin. 2018, 27, 166–174. [Google Scholar]
- Huws, S.A.; Creevey, C.J.; Oyama, L.B.; Mizrahi, I.; Denman, S.E.; Popova, M.; Muñoz-Tamayo, R.; Forano, E.; Waters, S.M.; Hess, M.; et al. Addressing Global Ruminant Agricultural Challenges Through Understanding the Rumen Microbiome: Past, Present, and Future. Front. Microbiol. 2018, 9, 2161. [Google Scholar]
- Wang, Q.; Wang, Y.; Wang, X.; Dai, C.; Tang, W.; Li, J.; Huang, P.; Li, Y.; Ding, X.; Huang, J.; et al. Effects of dietary energy levels on rumen fermentation, microbiota, and gastrointestinal morphology in growing ewes. Food Sci. Nutr. 2020, 8, 6621–6632. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, G.; Zhang, W.; Zhou, J.; Cong, H.; Yang, G.; Liu, G. Rumen microbiota helps Tibetan sheep obtain energy more efficiently to survive in the extreme environment of the Qinghai–Tibet Plateau. Front. Microbiol. 2024, 15, 1431063. [Google Scholar]
- Ge, T.; Yang, C.; Li, B.; Huang, X.; Zhao, L.; Zhang, X.; Tian, L.; Zhang, E. High-energy diet modify rumen microbial composition and microbial energy metabolism pattern in fattening sheep. BMC Vet. Res. 2023, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.A.; Yang, C.; Zhang, J.; Kalwar, Q.; Liang, Z.; Li, C.; Du, M.; Yan, P.; Long, R.; Han, J.; et al. Effects of Dietary Energy Levels on Rumen Fermentation, Microbial Diversity, and Feed Efficiency of Yaks (Bos grunniens). Front. Microbiol. 2020, 11, 625. [Google Scholar]
- Cui, K.; Qi, M.; Wang, S.; Diao, Q.; Zhang, N. Dietary energy and protein levels influenced the growth performance, ruminal morphology and fermentation and microbial diversity of lambs. Sci. Rep. 2019, 9, 16612. [Google Scholar]
- Song, S.Z.; Zhu, C.Y.; Liu, L.S.; Gong, X.Y.; Luo, R.R. The effect of tail-docking on adipocyte structure and lipid metabolism-related gene expression in Lanzhou fat-tailed sheep. Acta Pratacult. Sin. 2024, 33, 94–104. [Google Scholar]
- Chen, K.; Shui, Y.; Deng, M.; Guo, Y.; Sun, B.; Liu, G.; Liu, D.; Li, Y. Effects of different dietary energy levels on growth performance, meat quality and nutritional composition, rumen fermentation parameters, and rumen microbiota of fattening Angus steers. Front. Microbiol. 2024, 15, 1378073. [Google Scholar]
- NY/T816-2021; Nutrient Requirements of Meat-Type Sheep and Goat. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2021.
- An, X.; Zhang, L.; Luo, J.; Zhao, S.; Jiao, T. Effects of Oat Hay Content in Diets on Nutrient Metabolism and the Rumen Microflora in Sheep. Animals 2020, 10, 2341. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Lu, M.; Zhao, S.; Li, W.; Quan, G.; Xue, B. Effects of Dietary Energy Levels on Growth Performance, Nutrient Digestibility, Rumen Barrier and Microflora in Sheep. Animals 2024, 14, 2525. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Dai, C.; Li, J.; Huang, P.; Li, Y.; Ding, X.; Huang, J.; Hussain, T.; Yang, H. Effects of dietary energy on growth performance, carcass characteristics, serum biochemical index, and meat quality of female Hu lambs. Anim. Nutr. 2020, 6, 499–506. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, J.; Ahmad, A.A.; Bao, P.; Guo, X.; Long, R.; Ding, X.; Yan, P. Dietary Energy Levels Affect Growth Performance through Growth Hormone and Insulin-Like Growth Factor 1 in Yak (Bos grunniens). Animals 2019, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Sileshi, G.; Mitiku, E.; Mengistu, U.; Adugna, T.; Fekede, F. Effects of dietary energy and protein levels on nutrient intake, digestibility, and body weight change in Hararghe highland and Afar sheep breeds of Ethiopia. J. Adv. Vet. Anim. Res. 2021, 8, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.P.; Zhou, J.W.; Wang, W.J.; Degen, A.A.; Guo, Y.M.; Kang, J.P.; Xu, W.X.; Liu, P.P.; Yang, C.; Shi, F.Y.; et al. Tibetan sheep are better able to cope with low energy intake than Small-tailed Han sheep due to lower maintenance energy requirements and higher nutrient digestibilities. Anim. Feed Sci. Technol. 2019, 254, 114200. [Google Scholar] [CrossRef]
- Huo, L.M.; Cao, Y.F.; Gao, Y.X.; Li, Y.; Li, Q.F.; Li, J.G. The Effect of Dietary Energy Levels on Growth Performance and Rumen Fermentation in Chinese Holstein Heifers. Acta Vet. Zootech. Sin. 2019, 50, 332–342. [Google Scholar]
- Schroeder, G.F.; Titgemeyer, E.C.; Awawdeh, M.S.; Smith, J.S.; Gnad, D.P. Effects of energy source on methionine utilization by growing steers1. J. Anim. Sci. 2006, 84, 1505–1511. [Google Scholar] [CrossRef]
- Hanlon, M.; Moorby, J.; McConochie, H.; Foskolos, A. Effects of addition of nutritionally improved straw in dairy cow diets at 2 starch levels. J. Dairy Sci. 2020, 103, 10233–10244. [Google Scholar] [CrossRef]
- Liu, J.W.; Zhang, X.L.; Li, X.; Wang, L.; Qin, L.H.; Ban, Z.B.; Wu, J.; Zhang, G.L.; Wan, F.C. Effects of Dietary Energy Levels on Metabolism and Serum Parameters of Steppe Red Cattle. Sci. Agric. Sin. 2020, 53, 2502–2511. [Google Scholar]
- Su, H.; Akins, M.S.; Esser, N.M.; Ogden, R.; Coblentz, W.K.; Kalscheur, K.F.; Hatfield, R. Effects of feeding alfalfa stemlage or wheat straw for dietary energy dilution on nutrient intake and digestibility, growth performance, and feeding behavior of Holstein dairy heifers. J. Dairy Sci. 2017, 100, 7106–7115. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Li, Z.; Li, Y.; Lai, C.; Piao, X.; Milgen, J.; Wang, F. Metabolizable energy requirement for maintenance estimated by regression analysis between body weight gain and metabolizable energy intake in growing pigs. Asian-Australas. J. Anim. Sci. 2019, 32, 1397. [Google Scholar] [CrossRef]
- Hu, H.W.; Cui, H.T.; Zhao, Y.J. Study on Energy Requirement of Huanghuai Goat Ram in Growing Period. China Herbiv. Sci. 2024, 44, 52–58. [Google Scholar]
- Subepang, S.; Suzuki, T.; Phonbumrung, T.; Sommart, K. Enteric methane emissions, energy partitioning, and energetic efficiency of zebu beef cattle fed total mixed ration silage. Asian-Australas. J. Anim. Sci. 2018, 32, 548–555. [Google Scholar] [PubMed]
- Gao, H.; Yu, Y.; Yin, Y.; Zhao, Q.; Wu, H.; Li, H.; Chen, L.; Lv, Y.; Zhang, Y.; Leng, J. Study on Energy Requirement of Yunshang Black Goat Growing and Fattening Rams. Chin. J. Anim. Nutr. 2023, 35, 1740–1748. [Google Scholar]
- Bao, K.; Wang, X.; Wang, K.; Li, G.; Liu, H. Energy and Protein Requirements for the Maintenance of Growing Male Sika Deer (Cervus nippon). Front. Vet. Sci. 2021, 8, 745426. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wang, Z.; Yan, T.; Chang, S.; Hou, F. Modulation of feed digestibility, nitrogen metabolism, energy utilisation and serum biochemical indices by dietary Ligularia virgaurea supplementation in Tibetan sheep. Animal 2023, 17, 100910. [Google Scholar]
- Ren, J.G.; Guan, P.; Huang, J.Z.; Sheng, Y.Y.; Bao, T.Z.; Ma, Z.P.; Qian, W.X. Effects of Dietary Energy Level on Growth-fattening Performance and Serum Biochemical Indices of Angus Bulls. China Anim. Husb. Vet. Med. 2023, 50, 966–976. [Google Scholar]
- Zhang, Q.X.; Gao, A.Q.; Shi, X.X.; Niu, Z.Y.; Li, G.; Xing, C.; Zhu, Y.X.; Zhang, R.H. Effect of Different Linseed Oil Forms Supplementation on Growth Performance, Slaughter Performance and Serum Biochemical Indexes of Mutton Sheep. China Anim. Husb. Vet. Med. 2018, 45, 415–421. [Google Scholar]
- Ley, R.E.; Lozupone, C.A.; Hamady, M.; Knight, R.; Gordon, J.I. Worlds within worlds: Evolution of the vertebrate gut microbiota. Nat. Rev. Microbiol. 2008, 6, 776–788. [Google Scholar]
- Cheng, Z.; Liu, J.; Yu, Y.; Liu, W.; Li, X.; Li, F.; Zang, C.; Yang, K. Niacin alters ruminal microbial composition and metabolites in sheep fed a high-concentrate diet. Front. Vet. Sci. 2025, 12, 1510617. [Google Scholar]
- Evans, N.J.; Brown, J.M.; Murray, R.D.; Getty, B.; Birtles, R.J.; Hart, C.A.; Carter, S.D. Characterization of Novel Bovine Gastrointestinal Tract Treponema Isolates and Comparison with Bovine Digital Dermatitis Treponemes. Appl. Environ. Microbiol. 2011, 77, 138–147. [Google Scholar] [CrossRef]
- Lv, X.; Cui, K.; Qi, M.; Wang, S.; Diao, Q.; Zhang, N. Ruminal Microbiota and Fermentation in Response to Dietary Protein and Energy Levels in Weaned Lambs. Animals 2020, 10, 109. [Google Scholar] [CrossRef]
- Thomas, F.; Hehemann, J.-H.; Rebuffet, E.; Czjzek, M.; Michel, G. Environmental and Gut Bacteroidetes: The Food Connection. Front. Microbiol. 2011, 2, 93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zheng, N.; Shen, W.; Zhao, S.; Wang, J. Synchrony Degree of Dietary Energy and Nitrogen Release Influences Microbial Community, Fermentation, and Protein Synthesis in a Rumen Simulation System. Microorganisms 2020, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- Gharechahi, J.; Vahidi, M.F.; Bahram, M.; Jianlin, H.; Salekdeh, G.H. Metagenomic analysis reveals a dynamic microbiome with diversified adaptive functions to utilize high lignocellulosic forages in the cattle rumen. ISME J. 2021, 15, 1108–1120. [Google Scholar] [PubMed]
- Lan, W.; Yang, C. Ruminal methane production: Associated microorganisms and the potential of applying hydrogen-utilizing bacteria for mitigation. Sci. Total Environ. 2019, 654, 1270–1283. [Google Scholar]
- Kotarski, S.F.; Waniska, R.D.; Thurn, K.K. Starch hydrolysis by the ruminal microflora. J. Nutr. 1992, 122, 178–190. [Google Scholar]
- Mao, S.Y.; Zhang, R.Y.; Wang, D.S.; Zhu, W.Y. Impact of subacute ruminal acidosis (SARA) adaptation on rumen microbiota in dairy cattle using pyrosequencing. Anaerobe 2013, 24, 12–19. [Google Scholar]
- An, D.; Dong, X.; Dong, Z. Prokaryote diversity in the rumen of yak (Bos grunniens) and Jinnan cattle (Bos taurus) estimated by 16S rDNA homology analyses. Anaerobe 2005, 11, 207–215. [Google Scholar]
| Item | Treatments | |||
|---|---|---|---|---|
| LE | IE | HE | EHE | |
| Ingredients, % | ||||
| Wheat straw | 59.00 | 52.00 | 47.00 | 42.00 |
| Alfalfa | 11.00 | 18.00 | 20.00 | 20.00 |
| Corn | 7.00 | 13.60 | 22.50 | 28.00 |
| Soybean meal | 8.00 | 6.00 | 5.50 | 4.50 |
| Wheat bran | 13.17 | 8.57 | 3.17 | 3.67 |
| Sodium bicarbonate | 0.07 | 0.07 | 0.07 | 0.07 |
| Salt | 0.76 | 0.76 | 0.76 | 0.76 |
| Premix | 1.00 | 1.00 | 1.00 | 1.00 |
| Total | 100 | 100 | 100 | 100 |
| Nutrition level | ||||
| ME MJ/d | 6.77 | 7.22 | 7.72 | 8.20 |
| Starch, % | 13.50 | 22.64 | 33.63 | 36.39 |
| CP | 10.52 | 10.53 | 10.52 | 10.52 |
| EE, % | 2.28 | 2.27 | 2.32 | 2.47 |
| ADF, % | 41.85 | 40.00 | 40.34 | 39.58 |
| NDF, % | 61.00 | 62.34 | 59.50 | 58.34 |
| Ca, % | 0.51 | 0.64 | 0.68 | 0.67 |
| P, % | 0.29 | 0.27 | 0.23 | 0.25 |
| Items | Treatments | p-Value | |||
|---|---|---|---|---|---|
| LE | IE | HE | EHE | ||
| IBW, kg | 27.22 ± 2.30 | 27.34 ± 1.06 | 26.70 ± 1.30 | 27.26 ± 1.58 | 0.993 |
| FBW, kg | 27.61 ± 2.28 | 27.78 ± 1.18 | 27.54 ± 1.48 | 28.38 ± 1.52 | 0.984 |
| ADG, g/d | 9.12 ± 10.04 c | 18.52 ± 4.57 bc | 42.12 ± 7.42 ab | 47.0 ± 11.14 a | 0.019 |
| DMI, kg/d | 1.26 ± 0.01 d | 1.31 ± 0.00 c | 1.34 ± 0.00 b | 1.36 ± 0.00 a | <0.001 |
| Apparent digestibility, % | |||||
| DM | 48.03 ± 0.73 c | 50.50 ± 0.59 b | 52.61 ± 0.51 a | 53.00 ± 0.87 a | <0.001 |
| CP | 57.05 ± 1.04 c | 59.81 ± 1.18b c | 62.17 ± 1.31 b | 65.95 ± 0.77 a | <0.001 |
| ADF | 53.75 ± 0.76 | 53.95 ± 0.77 | 53.04 ± 0.49 | 51.94 ± 0.98 | 0.248 |
| NDF | 55.90 ± 0.67 | 56.27 ± 0.58 | 54.69 ± 0.53 | 54.24 ± 0.88 | 0.114 |
| Item | Treatments | p-Value | |||
|---|---|---|---|---|---|
| LE | IE | HE | EHE | ||
| NI (g/d) | 10.83 ± 0.07 a | 10.56 ± 0.03 b | 10.18 ± 0.03 c | 10.10 ± 0.01 c | <0.001 |
| FN output (g/d) | 5.75 ± 0.08 a | 5.27 ± 0.06 b | 4.91 ± 0.06 c | 4.75 ± 0.09 c | <0.001 |
| UN output (g/d) | 1.09 ± 0.04 | 1.07 ± 0.03 | 1.03 ± 0.03 | 1.04 ± 0.03 | 0.454 |
| MN output (g/d) | 6.83 ± 0.09 a | 6.33 ± 0.06 b | 5.93 ± 0.07 c | 5.79 ± 0.10 c | <0.001 |
| RN (g/d) | 3.99 ± 0.08 b | 4.22 ± 0.06 a | 4.25 ± 0.06 a | 4.32 ± 0.10 a | 0.020 |
| Efficiency, % | |||||
| FN/NI | 53.10 ± 0.67 a | 49.91 ± 0.60 b | 48.17 ± 0.51 bc | 47.00 ± 0.90 c | <0.001 |
| UN/NI | 10.07 ± 0.36 | 10.11 ± 0.27 | 10.07 ± 0.26 | 10.27 ± 0.27 | 0.960 |
| MN/NI | 63.16 ± 0.71 a | 60.02 ± 0.58 b | 58.24 ± 0.59 ab | 57.27 ± 0.96 b | <0.001 |
| RN/NI | 36.83 ± 0.71 a | 39.98 ± 0.58 ab | 41.76 ± 0.59 b | 42.74 ± 0.96 c | <0.001 |
| Item | Treatments | p-Value | |||
|---|---|---|---|---|---|
| LE | IE | HE | EHE | ||
| GE intake, MJ/d | 14.59 ± 0.10 d | 15.26 ± 0.04 c | 16.01 ± 0.05 b | 16.74 ± 0.02 a | <0.001 |
| FE output, MJ/d | 6.79 ± 0.11 a | 6.85 ± 0.09 a | 6.28 ± 0.08 b | 5.96 ± 1.11 c | <0.001 |
| UE output, MJ/d | 0.31 ± 0.01 | 0.31 ± 0.02 | 0.30 ± 0.02 | 0.35 ± 0.01 | 0.154 |
| CH4-E, MJ/d | 0.90 ± 0.01 d | 0.93 ± 0.00 c | 0.97 ± 0.00 b | 1.01 ± 0.00 a | <0.001 |
| DE, MJ/d | 7.80 ± 0.11 d | 8.40 ± 0.09 c | 9.73 ± 0.07 b | 10.79 ± 0.11 a | <0.001 |
| ME, MJ/d | 6.59 ± 0.11 d | 7.16 ± 0.09 c | 8.46 ± 0.07 b | 9.43 ± 0.11 a | <0.001 |
| Efficiency | |||||
| DE/GE intake, % | 53.46 ± 0.69 c | 55.08 ± 0.58 c | 60.77 ± 0.46 b | 64.42 ± 0.64 a | <0.001 |
| ME/GE intake, % | 45.18 ± 0.70 d | 46.92 ± 0.57 c | 52.82 ± 0.45 b | 56.35 ± 0.64 a | <0.001 |
| ME/DE | 84.42 ± 0.28 c | 85.15 ± 0.23 b | 86.90 ± 0.17 a | 87.43 ± 0.17 a | <0.001 |
| FE/GE intake, % | 46.54 ± 0.69 c | 44.92 ± 0.58 b | 39.23 ± 0.46 a | 35.58 ± 0.64 a | <0.001 |
| UE/GE intake, % | 2.11 ± 0.09 | 2.04 ± 0.11 | 1.89 ± 0.09 | 2.06 ± 0.08 | 0.357 |
| CH4-E/GE intake, % | 6.17 ± 0.01 a | 6.11 ± 0.00 b | 6.06 ± 0.00 c | 6.02 ± 0.00 d | <0.001 |
| Item | Treatments | p-Value | |||
|---|---|---|---|---|---|
| LE | IE | HE | EHE | ||
| TP (g/L) | 68.46 ± 1.05 | 69.04 ± 2.97 | 69.88 ± 0.96 | 70.14 ± 2.30 | 0.929 |
| ALB (g/L) | 26.02 ± 0.38 | 26.84 ± 0.63 | 25.00 ± 1.13 | 25.36 ± 1.02 | 0.481 |
| GLB (g/L) | 42.20 ± 2.53 | 42.44 ± 1.09 | 43.32 ± 0.84 | 45.14 ± 1.31 | 0.559 |
| ALB/GLB ratio | 0.38 ± 0.01 | 0.39 ± 0.01 | 0.36 ± 0.01 | 0.36 ± 0.01 | 0.445 |
| UREA (mmol/L) | 7.77 ± 0.39 | 7.08 ± 0.44 | 7.06 ± 0.36 | 7.35 ± 0.66 | 0.704 |
| GLU (mmol/L) | 1.63 ± 0.20 | 1.68 ± 0.14 | 1.72 ± 0.05 | 1.90 ± 0.55 | 0.923 |
| TG (mmol/L) | 0.20 ± 0.01 | 0.26 ± 0.03 | 0.21 ± 0.03 | 0.24 ± 0.03 | 0.320 |
| TC (mmol/L) | 1.64 ± 0.15 | 2.03 ± 0.12 | 1.90 ± 0.22 | 1.99 ± 0.14 | 0.373 |
| Item | Treatments | p-Value | |||
|---|---|---|---|---|---|
| LE | IE | HE | EHE | ||
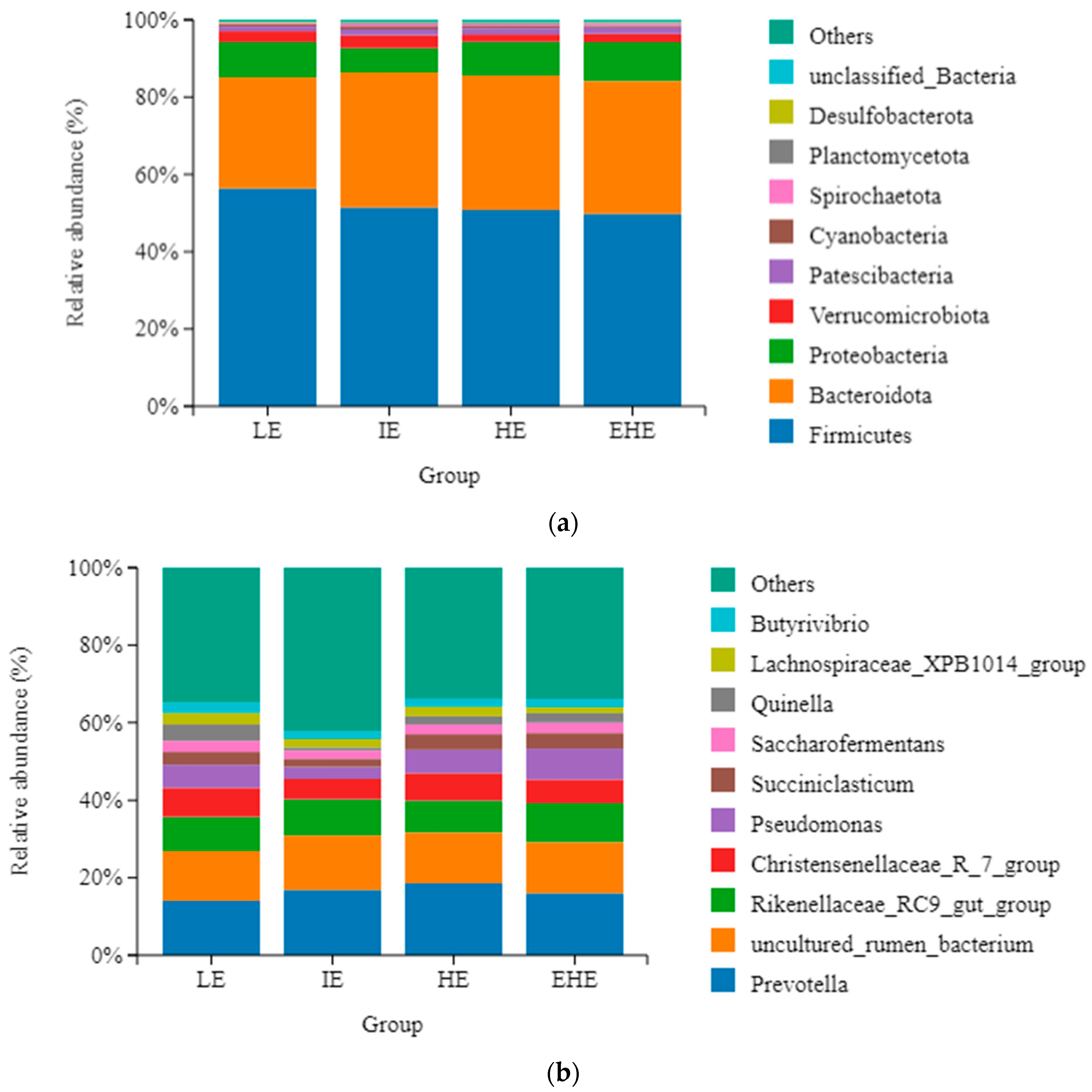
| Firmicutes | 56.26 ± 3.11 | 51.48 ± 1.82 | 50.89 ± 3.15 | 49.82 ± 1.52 | 0.312 |
| Bacteroidota | 28.79 ± 1.75 | 34.99 ± 1.70 | 34.70 ± 2.67 | 34.44 ± 2.06 | 0.149 |
| Proteobacteria | 9.14 ± 1.51 | 6.21 ± 1.62 | 8.72 ± 0.89 | 9.95 ± 2.14 | 0.415 |
| Verrucomicrobiota | 2.86 ± 0.48 ab | 3.37 ± 1.11 a | 1.94 ± 0.12 c | 2.17 ± 0.26 bc | 0.010 |
| Patescibacteria | 1.22 ± 0.14 | 1.45 ± 0.36 | 1.57 ± 0.21 | 1.67 ± 0.25 | 0.635 |
| Cyanobacteria | 0.68 ± 0.08 | 0.77 ± 0.17 | 0.56 ± 0.14 | 0.51 ± 0.09 | 0.460 |
| Spirochaetota | 0.31 ± 0.10 | 0.59 ± 0.14 | 0.47 ± 0.05 | 0.44 ± 0.08 | 0.305 |
| Planctomycetota | 0.00 ± 0.00 | 0.56 ± 0.23 | 0.46 ± 0.19 | 0.39 ± 0.26 | 0.251 |
| Desulfobacterota | 0.24 ± 0.06 a | 0.12 ± 0.02 b | 0.14 ± 0.04 ab | 0.15 ± 0.02 ab | 0.034 |
| unclassified_Bacteria | 0.10 ± 0.02 | 0.12 ± 0.02 | 0.20 ± 0.07 | 0.15 ± 0.03 | 0.349 |
| Item | Treatments | p-Value | |||
|---|---|---|---|---|---|
| LE | IE | HE | EHE | ||
| Prevotella | 14.08 ± 0.99 | 16.97 ± 1.58 | 18.56 ± 2.52 | 16.06 ± 1.80 | 0.390 |
| uncultured_rumen_bacterium | 12.83 ± 0.93 | 14.04 ± 1.01 | 13.04 ± 1.23 | 13.26 ± 0.67 | 0.832 |
| Rikenellaceae_RC9_gut_group | 8.88 ± 0.87 | 9.28 ± 1.74 | 8.23 ± 0.56 | 10.06 ± 0.35 | 0.651 |
| Christensenellaceae_R_7_group | 7.54 ± 2.01 | 5.21 ± 0.82 | 7.03 ± 0.81 | 6.03 ± 0.46 | 0.528 |
| Pseudomonas | 5.89 ± 1.63 | 3.01 ± 1.10 | 5.98 ± 0.81 | 7.90 ± 2.38 | 0.231 |
| Succiniclasticum | 3.34 ± 0.48 ab | 2.18 ± 0.41 b | 4.07 ± 0.73 a | 4.05 ± 0.54 a | 0.027 |
| Saccharofermentans | 2.83 ± 0.22 | 2.11 ± 0.41 | 2.52 ± 0.33 | 2.66 ± 0.34 | 0.485 |
| Lachnospiraceae_XPB1014_group | 3.05 ± 0.46 | 2.23 ± 0.48 | 2.64 ± 1.19 | 1.58 ± 0.35 | 0.514 |
| Quinella | 0.42 ± 1.82 | 0.73 ± 0.53 | 2.15 ± 1.14 | 2.32 ± 2.12 | 0.472 |
| Butyrivibrio | 5.59 ± 0.48 | 2.00 ± 0.35 | 1.98 ± 0.26 | 2.13 ± 0.40 | 0.648 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, W.; Zheng, J.; Jiao, N.; Ma, C.; Li, H.; Zhang, J.; Yang, J.; Xu, H.; Cai, Y.; Gao, D.; et al. Effects of Energy Intake on Nutrient Digestibility, Nitrogen Metabolism, Energy Utilization, Serum Biochemical Indices, and Rumen Microbiota in Lanzhou Fat-Tailed Sheep. Agriculture 2025, 15, 698. https://doi.org/10.3390/agriculture15070698
Feng W, Zheng J, Jiao N, Ma C, Li H, Zhang J, Yang J, Xu H, Cai Y, Gao D, et al. Effects of Energy Intake on Nutrient Digestibility, Nitrogen Metabolism, Energy Utilization, Serum Biochemical Indices, and Rumen Microbiota in Lanzhou Fat-Tailed Sheep. Agriculture. 2025; 15(7):698. https://doi.org/10.3390/agriculture15070698
Chicago/Turabian StyleFeng, Wangmei, Juanshan Zheng, Na Jiao, Chi Ma, Honghe Li, Junsong Zhang, Jutian Yang, Hongwei Xu, Yong Cai, Dandan Gao, and et al. 2025. "Effects of Energy Intake on Nutrient Digestibility, Nitrogen Metabolism, Energy Utilization, Serum Biochemical Indices, and Rumen Microbiota in Lanzhou Fat-Tailed Sheep" Agriculture 15, no. 7: 698. https://doi.org/10.3390/agriculture15070698
APA StyleFeng, W., Zheng, J., Jiao, N., Ma, C., Li, H., Zhang, J., Yang, J., Xu, H., Cai, Y., Gao, D., Cao, X., Feng, X., & Guo, P. (2025). Effects of Energy Intake on Nutrient Digestibility, Nitrogen Metabolism, Energy Utilization, Serum Biochemical Indices, and Rumen Microbiota in Lanzhou Fat-Tailed Sheep. Agriculture, 15(7), 698. https://doi.org/10.3390/agriculture15070698







