The Influence of Substrate Composition on Nutritional Content and Biological Activity of Some Pleurotus Mushrooms Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Biologic Material
2.2. Mushroom Cultivation
2.3. Sample Preparation
2.4. Crude Protein and Crude Fiber Content Determination
2.5. Total Phenolic Content Analysis
2.6. Total Flavonoid Content Evaluation
2.7. Phenolic Compounds by High-Performance Liquid Chromatography Analysis
2.8. Antimicrobial Activity
- N = CFU-colony forming units or number of viable cells per mL;
- m = arithmetic means of colonies measured using three plates that were inoculated with the same dilution;
- c = dilution inverse factor from which inoculation was performed;
- 100 = result ratio to 1 mL.
2.9. Antioxidant Activity
2.10. Statistical Data Analysis
3. Results
3.1. Crude Protein and Crude Fiber Content
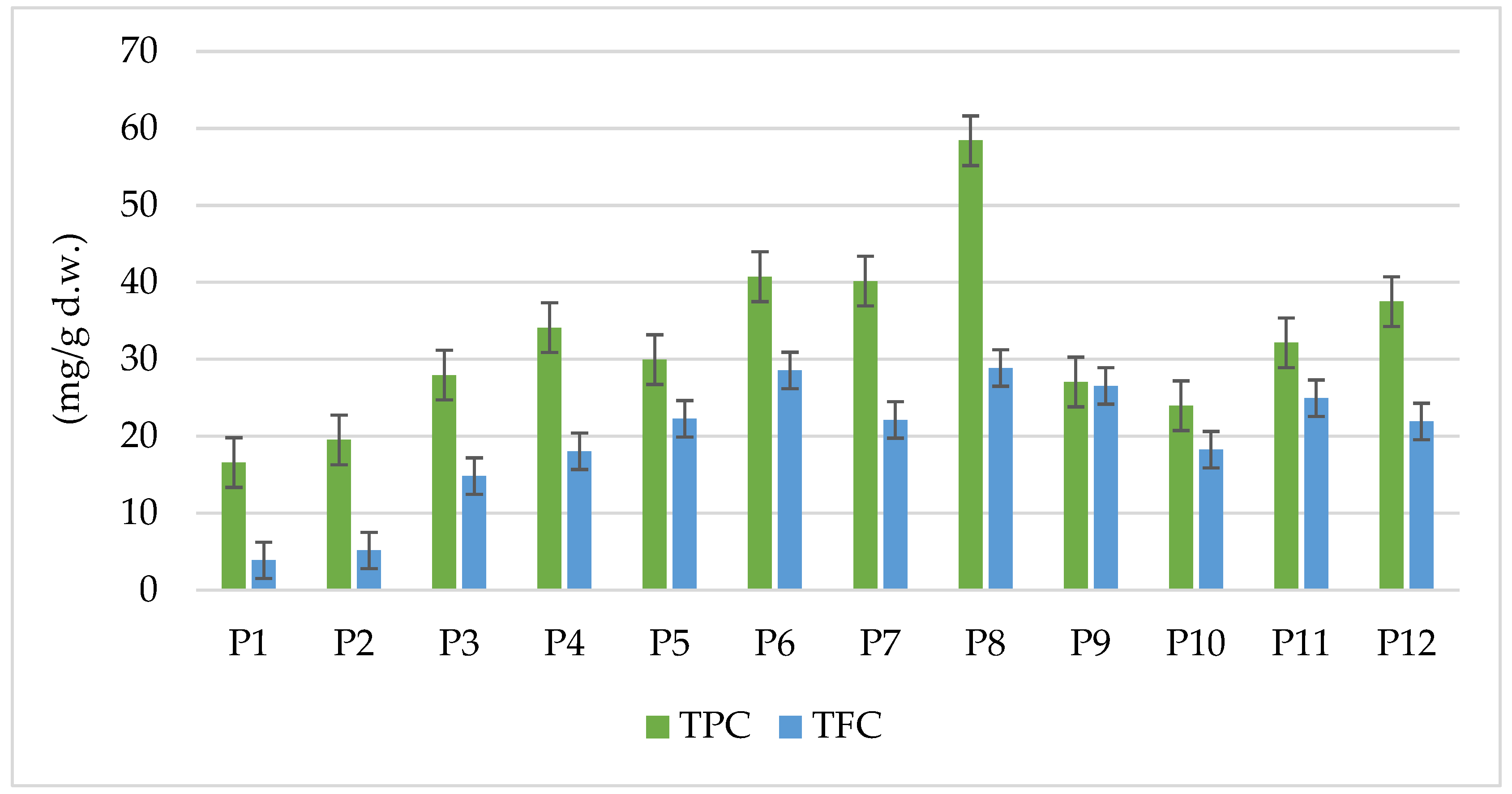
3.2. Phenolic and Polyphenol Compounds Content
3.3. Antimicrobial Activity
3.4. Antioxidant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSG | Brewery-spent grains |
| EMMs | Edible and medicinal mushrooms |
| N | Nitrogen |
| CPC | Crude protein content |
| CFC | Crude fiber content |
| TPC | Total polyphenols content |
| TFC | Total flavones content |
| TSA | Tryptone soy agar |
| ATTC | American Type Culture Collection |
| MIC | Minimum inhibitory concentration |
| MBC | Minimum bactericidal concentration |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| IC50 | Half maximal inhibitory concentration |
| RSA | Radical scavenging activity |
References
- Ferdes, M.; Dincă, M.N.; Ionescu, M.; Ștefan, E.M.; Ionescu, A.D.; Munteanu, M.G. Submerged Cultivation of Some Species of Edible Basidiomycetes for The Simultaneous Biosynthesis of Biomass and Laccase Enzyme. Sci. Bull. Ser. F. Biotechnol. 2024, 28, 44–49. [Google Scholar]
- Chang, S.T. The world mushroom industry: Trends and technological development. Int. J. Med. Mushrooms 2006, 8, 297–314. [Google Scholar]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 376387. [Google Scholar]
- Doroški, A.; Klaus, A.; Režek-Jambrak, A.; Djekic, I. Food waste originated material as an alternative substrate used for the cultivation of oyster mushroom (Pleurotus ostreatus): A review. Sustainability 2022, 14, 12509. [Google Scholar] [CrossRef]
- Barros, L.; Cruz, T.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C. Wild and commercial mushrooms as source of nutrients and nutraceuticals. Food Chem. Toxicol. 2008, 46, 2742–2747. [Google Scholar]
- Cohen, N.; Cohen, J.; Asatiani, M.D.; Varshney, V.K.; Yu, H.T.; Yang, Y.C.; Wasser, S.P. Chemical composition and nutritional and medicinal value of fruit bodies and submerged cultured mycelia of culinary-medicinal higher Basidiomycetes mushrooms. Int. J. Med. Mushrooms 2014, 16, 273–291. [Google Scholar] [CrossRef]
- Chang, R. Functional properties of edible mushrooms. Nutr. Rev. 1996, 54, S91–S93. [Google Scholar]
- Wong, J.H.; Ng, T.B.; Cheung, R.C.F.; Ye, X.J.; Wang, H.X.; Lam, S.K.; Lin, P.; Chan, Y.S.; Fang, E.F.; Ngai, P.H.K. Proteins with antifungal properties and other medicinal applications from plants and mushrooms. Appl. Microbiol. Biotechnol. 2010, 87, 1221–1235. [Google Scholar] [CrossRef]
- Dufossé, L.; Fouillaud, M.; Caro, Y. Fungi and fungal metabolites for the improvement of human and animal nutrition and health. J. Fungi 2021, 7, 274. [Google Scholar] [CrossRef]
- Alam, N.; Yoon, K.N.; Lee, J.S.; Lee, M.W.; Lee, T.S. Pleurotus nebrodensis ameliorates atherogenic lipid and histological function in hypercholesterolemic rats. Int. J. Pharmacol. 2011, 7, 455–462. [Google Scholar]
- Wasser, S. Medicinal mushroom science: Current perspectives, advances, evidences, and challenges. Biomed. J. 2014, 37, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Chilanti, G.; da Rosa, L.O.; Poleto, L.; Branco, C.S.; Camassola, M.; Fontana, R.C.; Dillon, A.J. Effect of different substrates on Pleurotus spp. cultivation in Brazil-Ergothioneine and lovastatin. J. Food Compos. Anal. 2022, 107, 104367. [Google Scholar] [CrossRef]
- Gąsecka, M.; Mleczek, M.; Siwulski, M.; Niedzielski, P. Phenolic composition and antioxidant properties of Pleurotus ostreatus and Pleurotus eryngii enriched with selenium and zinc. Eur. Food Res. Technol. 2015, 242, 723–732. [Google Scholar] [CrossRef]
- Golak-Siwulska, I.; Kałużewicz, A.; Spiżewski, T.; Siwulski, M.; Sobieralski, K. Bioactive compounds and medicinal properties of Oyster mushrooms (Pleurotus sp.). Folia Hortic. 2018, 30, 191–201. [Google Scholar] [CrossRef]
- Selvamani, S.; El-Enshasy, H.A.; Dailin, D.J.; Malek, R.A.; Hanapi, S.Z.; Ambehabati, K.K.; Moloi, N. Antioxidant compounds of the edible mushroom Pleurotus ostreatus. Int. J. Biotechnol. Wellness Ind. 2018, 7, 1–14. [Google Scholar] [CrossRef]
- Vamanu, E.; Ene, M.; Pelinescu, D.; Sarbu, I.; Vamanu, A.; Nita, S. Determination of antioxidant and antimicrobial properties of alcoholic extract from Pleurotus ostreatus M2191 mycelium obtained in the presence of various nitrogen sources. Rev. Chim. 2011, 62, 1189–1194. [Google Scholar]
- Okafor, D.C.; Onuegbu, N.C.; Odimegwu, N.E.; Ibeabuchi, J.C.; Njoku, N.E.; Agunwa, I.M.; Ofoedu, C.E.; Njoku, C.C. Antioxidant and antimicrobial activities of oyster mushroom. Am. J. Food Sci. Technol. 2017, 5, 64–69. [Google Scholar]
- Ferreira, I.C.; Barros, L.; Abreu, R. Antioxidants in wild mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Vunduk, J.; Petrović, P.; Van Griensven, L. Antioxidants of edible mushrooms. Molecules 2015, 20, 19489–19525. [Google Scholar] [CrossRef]
- Popa, A.; Israel-Roming, F. Methods for identifying lignin degrading microorganisms. Sci. Bull. Ser. F. Biotechnol. 2023, 27, 68–77. [Google Scholar]
- Popa, A. Biodegradation of lignocellulosic substrates with improved fungal strains. Sci. Bull. Ser. F. Biotechnol. 2024, 28, 81–86. [Google Scholar]
- Hřebečková, T.; Wiesnerová, L.; Hanč, A. Change in agrochemical and biochemical parameters during the laboratory vermicomposting of spent mushroom substrate after cultivation of Pleurotus ostreatus. Sci. Total. Environ. 2020, 739, 140085. [Google Scholar]
- El-Ramady, H.; Abdalla, N.; Fawzy, Z.; Badgar, K.; Llanaj, X.; Törős, G.; Prokisch, J. Green biotechnology of oyster mushroom (Pleurotus ostreatus L.): A sustainable strategy for myco-remediation and bio-fermentation. Sustainability 2022, 14, 3667. [Google Scholar] [CrossRef]
- Rusu, I.C.; Zăgrean, A.V.; Israel-Roming, F. Fruiting yield of some Pleurotus strains. Curr. Trends Nat. Sci. 2024, 13, 186–197. [Google Scholar]
- Uzun, Y.; Gen, H.; Tun, Y.; Demirel, K. Determination of protein and nitrogen fractions of wild edible mushrooms. Asian J. Chem. 2009, 21, 2769–2776. [Google Scholar]
- Oprea, O.B.; Popa, M.E.; Apostol, L.; Gaceu, L. Research on the potential use of grape seed flour in the bakery industry. Foods 2022, 11, 1589. [Google Scholar] [CrossRef]
- Arlet, A.C.I.; Tociu, M.; Balanuca, B.; Israel-Roming, F. Extraction and evaluation of total phenolics content from red corn bran. Sci. Bull. Ser. F. Biotechnol. 2023, 27, 60–67. [Google Scholar]
- Mulțescu, M.; Susman, I.E.; Culețu, A.; Zamfir, M.; Sanmartin, A.M.; Israel-Roming, F. The influence of starter cultures of lactic acid bacteria on fermentation of white cabbage. AgroLife Sci. J. 2024, 13, 300–309. [Google Scholar]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar]
- Giurescu, I.; Șesan, T.E.; Badju, S.; Lupu, C.; Oancea, F. Preparation of compost from sea buckthorn branches by using a multipurpose Trichoderma strain. Sci. Bull. Ser. F. Biotechnol. 2023, 27, 97–104. [Google Scholar]
- Pascariu, O.E.; Dias, L.G.; Israel-Roming, F. Optimization of Extraction Method of Bioactive Compounds from Elderberries (Sambucus nigra L.) and Testing Extract Stability. Horticulturae 2024, 10, 743. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [PubMed]
- Irimescu, L.S.; Olivares, C.G.; Preda, C.I.; Diguță, C.F.; Gabriela, L.; Bălan, D.; Matei, F. Characterisation of the antimicrobial and antioxidant profile of Phalaenopsis orchid wastes. AgroLife Sci. J. 2021, 10, 101–108. [Google Scholar]
- Frîncu, M.; Dumitrache, C.; Petre, A.C.; Andrei, M.; Teodorescu, R.I.; Bărbulescu, D.I.; Matei, F. Physico-chemical characterization of some sources of grape marc from Pietroasa vineyard. AgroLife Sci. J. 2023, 12, 81–86. [Google Scholar]
- Cueva, M.B.R.; Hernández, A.; Niño-Ruiz, Z. Influence of C/N ratio on productivity and the protein contents of Pleurotus ostreatus grown in differents residue mixtures. Rev. Fac. Cienc. Agrar. 2017, 49, 331–344. [Google Scholar]
- Irshad, A.; Tahir, A.; Sharif, S.; Khalid, A.; Ali, S.; Naz, A.; Ameen, A. Research Article Determination of Nutritional and Biochemical Composition of Selected Pleurotus spps. BioMed Res. Int. 2023, 1, 8150909. [Google Scholar]
- Krüzselyi, D.; Kovács, D.; Vetter, J. Chemical analysis of king oyster mushroom (Pleurotus eryngii) fruitbodies. Acta Aliment. 2016, 45, 20–27. [Google Scholar]
- Ogundare, G.R.; Itelima, J.U.; Onwuliri, F.C. Evaluation of Phytochemical, Proximate and Elemental Composition of Wild Edible Mushrooms in Nigeria. Int. J. Mod. Pharm. Res. 2024, 8, 1–7. [Google Scholar]
- Tshinyangu, K.K. Effect of grass hay substrate on nutritional value of Pleurotus ostreatus var. columbinus. Food/Nahrung 1996, 40, 79–83. [Google Scholar]
- Rezaeian, S.; Saadatmand, S.; Sattari, T.N.; Mirshamsi, A. Antioxidant potency of Iranian newly cultivated wild mushrooms of Agaricus and Pleurotus species. Biomed. Res. 2015, 26, 534–542. [Google Scholar]
- Elhusseiny, S.M.; El-Mahdy, T.S.; Awad, M.F.; Elleboudy, N.S.; Farag, M.M.; Aboshanab, K.M.; Yassien, M.A. Antiviral, cytotoxic, and antioxidant activities of three edible agaricomycetes mushrooms: Pleurotus columbinus, Pleurotus sajor-caju, and Agaricus bisporus. J. Fungi 2021, 7, 645. [Google Scholar] [CrossRef] [PubMed]
- El-Razek, A.; Amal, M.; Ibrahim, A.; Elattar, A.; Asker, D. Utilization of Agro-Wastes to Produce Oyster Mushroom (Pleurotus ostreatus) with High Antioxidant and Antimicrobial Activities. Alex. J. Food Sci. Technol. 2020, 17, 1–17. [Google Scholar]
- Palacios, I.; Lozano, M.; Moro, C.; D’Arrigo, M.; Rostagno, M.A.; Martínez, J.A.; Villares, A. Antioxidant properties of phenolic compounds occurring in edible mushrooms. Food Chem. 2011, 128, 674–678. [Google Scholar]
- Jabłońska-Ryś, E.; Sławińska, A.; Szwajgier, D. Effect of lactic acid fermentation on antioxidant properties and phenolic acid contents of oyster (Pleurotus ostreatus) and chanterelle (Cantharellus cibarius) mushrooms. Food Sci. Biotechnol. 2016, 25, 439–444. [Google Scholar]
- Kim, M.-Y.; Seguin, P.; Ahn, J.-K.; Kim, J.-J.; Chun, S.-C.; Kim, E.-H.; Seo, S.-H.; Kang, E.-Y.; Kim, S.-L.; Park, Y.-J. Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea. J. Agric. Food Chem. 2008, 56, 7265–7270. [Google Scholar]
- Angelini, P.; Pellegrino, R.M.; Tirillini, B.; Flores, G.A.; Alabed, H.B.; Ianni, F.; Ferrante, C. Metabolomic profiling and biological activities of Pleurotus columbinus Quél. Cultivated on different agri-food byproducts. Antibiotics 2021, 10, 1245. [Google Scholar] [CrossRef]
- Radzki, W.; Tutaj, K.; Skrzypczak, K.; Michalak-Majewska, M.; Gustaw, W. Ethanolic extracts of six cultivated mushrooms as a source of bioactive compounds. Appl. Sci. 2023, 14, 66. [Google Scholar] [CrossRef]
- Matkovits, A.; Fodor, M.; Jókai, Z. Analysis of Polyphenol Patterns of Pleurotus ostreatus Cultivars by UHPLC-ESI-MS/MS.; Application of FT-NIR and Chemometric Methods, Classification Options. Chemosensors, 2024; 12, 19. [Google Scholar]
- Reis, F.S.; Martins, A.; Barros, L.; Ferreira, I.C. Antioxidant properties and phenolic profile of the most widely appreciated cultivated mushrooms: A comparative study between in vivo and in vitro samples. Food Chem. Toxicol. 2012, 50, 1201–1207. [Google Scholar]
- Vamanu, E. Antioxidant properties of mushroom mycelia obtained by batch cultivation and tocopherol content affected by extraction procedures. BioMed Res. Int. 2014, 974804. [Google Scholar]
- Jayakumar, T.; Thomas, P.A.; Geraldine, P. In-vitro antioxidant activities of an ethanolic extract of the oyster mushroom, Pleurotus ostreatus. Innov. Food Sci. Emerg. Technol. 2009, 10, 228–234. [Google Scholar]
- Akyüz, M.; Onganer, A.N.; Erecevit, P.; Kirbag, S. Flavonoid contents and 2, 2-diphenyl-1-picrylhydrazyl radical scavenging activity of some edible mushrooms from Turkey: A. bisporus and Pleurotus spp. Curr. Top. Nutraceuticals Res. 2009, 10, 133. [Google Scholar]
- Ecevit, K.; Barros, A.A.; Silva, J.M.; Reis, R.L. Preventing microbial infections with natural phenolic compounds. Futur. Pharmacol. 2022, 2, 460–498. [Google Scholar] [CrossRef]
- Khalil, M.I.; Alam, N. Antibacterial activity of oyster mushroom against pathogenic bacteria. Bangladesh J. 2006, 18, 79–83. [Google Scholar]
- Akyuz, M.; Onganer, A.; Erecevıt, P.; Kırbag, S. Antimicrobial activity of some edible mushrooms in the eastern and southeast Anatolia region of Turkey. Gazi Univ. J. Sci. 2010, 23, 125–130. [Google Scholar]
- Younis, A.M.; Wu, F.S.; El Shikh, H.H. Antimicrobial activity of extracts of the oyster culinary medicinal mushroom Pleurotus ostreatus (higher basidiomycetes) and identification of a new antimicrobial compound. Int. J. Med. Mushrooms 2015, 17, 579–590. [Google Scholar] [CrossRef]
- Singh, S.K.; Khajuria, R.; Kaur, L. Biodegradation of ciprofloxacin by white rot fungus Pleurotus ostreatus. 3 Biotech 2017, 7, 1–8. [Google Scholar]
- Arbaayah, H.H.; Umi, K.Y. Antioxidant properties in the oyster mushrooms (Pleurotus spp.) and split gill mushroom (Schizophyllum commune) ethanolic extracts. Mycosphere 2013, 4, 661–673. [Google Scholar]


| Sample | Species/Strain | Substrate Variant | Morphological Part |
| P1 | Pleurotus eryngii PeM-41 | V0 | Stipes |
| P2 | Pleurotus eryngii PeM-41 | V1 | Stipes |
| P3 | Pleurotus eryngii PeM-41 | V0 | Pileus |
| P4 | Pleurotus eryngii PeM-41 | V1 | Pileus |
| P5 | Pleurotus ostreatus PoM-77 | V0 | Stipes |
| P6 | Pleurotus ostreatus PoM-77 | V1 | Stipes |
| P7 | Pleurotus ostreatus PoM-77 | V0 | Pileus |
| P8 | Pleurotus ostreatus PoM-77 | V1 | Pileus |
| P9 | Pleurotus columbinus PcM-98 | V0 | Stipes |
| P10 | Pleurotus columbinus PcM-98 | V1 | Stipes |
| P11 | Pleurotus columbinus PcM-98 | V0 | Pileus |
| P12 | Pleurotus columbinus PcM-98 | V1 | Pileus |
| Compound | Gallic Acid | Chlorogenic Acid | Caffeic Acid | Syringic Acid | p-Coumaric Acid | Rutin | Quercetin |
|---|---|---|---|---|---|---|---|
| Sample | μg/g d.w. | μg/g d.w. | μg/g d.w. | μg/g d.w. | μg/g d.w. | μg/g d.w. | μg/g d.w. |
| P1 | 113.35 ± 1.88 | 0.866 ± 0.07 | 0.10 ± 0.04 | 0.50 ± 0.04 | 0.16 ± 0.01 | 1.50 ± 0.01 | <LoD |
| P2 | 84.57 ± 0.04 | 0.56 ± 0.06 | 0.89 ± 0.05 | 0.49 ± 0.03 | 0.16 ± 0.02 | 1.60 ± 0.0 | <LoD |
| P3 | 119.62 ± 0.86 | 0.66 ± 0.12 | 1.36 ± 0.05 | 0.10 ± 0.01 | 0.97 ± 0.07 | 1.25 ± 0.22 | <LoD |
| P4 | 121.22 ± 3.11 | 0.60 ± 0.01 | 1.05 ± 0.05 | 1.47 ± 0.07 | 2.33 ± 0.09 | 1.12 ± 0.05 | <LoD |
| P5 | 140.27 ± 1.55 | 4.84 ± 0.45 | 1.83 ± 0.07 | 1.74 ± 0.32 | 1.03 ± 0.03 | 1.45 ± 0.06 | 0.15 ± 0.04 |
| P6 | 121.60 ± 8.46 | 4.88 ± 0.13 | 1.71 ± 0.04 | 1.97 ± 0.02 | 1.95 ± 0.03 | 0.53 ± 0.08 | 0.16 ± 0.01 |
| P7 | 155.32 ± 4.21 | 11.06 ± 0.28 | 3.38 ± 0.05 | 1.55 ± 0.24 | 1.73 ± 0.22 | 0.70 ± 0.02 | 0.47 ± 0.07 |
| P8 | 183.33 ± 2.18 | 9.42 ± 0.02 | 2.45 ± 0.06 | 1.52 ± 0.10 | 6.95 ± 0.01 | 0.68 ± 0.23 | 0.57 ± 0.02 |
| P9 | 126.50 ± 2.62 | 2.58 ± 0.08 | 1.52 ± 0.27 | 1.97 ± 0.01 | 0.62 ± 0.08 | 0.25 ± 0.07 | 0.47 ± 0.064 |
| P10 | 89.41 ± 0.96 | 2.06 ± 0.01 | 0.10 ± 0.01 | 0.51 ± 0.01 | 0.81 ± 0.04 | 0.03 ± 0.03 | 2.65 ± 0.11 |
| P11 | 146.90 ± 1.90 | 3.09 ± 0.06 | 2.28 ± 0.04 | 0.19 ± 0.19 | 2.13 ± 0.06 | 0.98 ± 0.08 | 1.37 ± 0.19 |
| P12 | 104.25 ± 5.38 | 2.22 ± 0.08 | 1.67 ± 0.05 | 0.16 ± 0.16 | 1.58 ± 0.01 | 0.46 ± 0.01 | 2.12 ± 0.01 |
| Sample | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pathogen | ||||||||||||
| S. aureus ATCC 6538 S. pyogenes ATCC 19615 | - - | - - | - - | - - | 4 + 13 +++ | 7 ++ 13 +++ | 6 ++ 10 +++ | 8 ++ 15 +++ | 5 ++ 14 +++ | 3 + 8 ++ | 11 +++ 12 +++ | 7 ++ 6 ++ |
| Sample | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pathogen | ||||||||||||
| S. aureus 6538 | - | - | - | - | 6.25 | 6.25 | 1.25 | 6.25 | 6.25 | 6.25 | 6.25 | 1.25 |
| S. pyogenes 19615 | - | - | - | - | 1.25 | 1.25 | 6.25 | 1.25 | 1.25 | - | 1.25 | 6.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusu, I.-C.; Pascariu, O.-E.; Popa, A.; Diguță, C.-F.; Apostol, L.; Nicolcioiu, M.-B.; Zăgrean, A.V.; Israel-Roming, F. The Influence of Substrate Composition on Nutritional Content and Biological Activity of Some Pleurotus Mushrooms Extracts. Agriculture 2025, 15, 791. https://doi.org/10.3390/agriculture15070791
Rusu I-C, Pascariu O-E, Popa A, Diguță C-F, Apostol L, Nicolcioiu M-B, Zăgrean AV, Israel-Roming F. The Influence of Substrate Composition on Nutritional Content and Biological Activity of Some Pleurotus Mushrooms Extracts. Agriculture. 2025; 15(7):791. https://doi.org/10.3390/agriculture15070791
Chicago/Turabian StyleRusu, Ionuț-Cristian, Oana-Elena Pascariu, Aglaia Popa (Burlacu), Camelia-Filofteia Diguță, Livia Apostol, Mihai-Bogdan Nicolcioiu, Alexandru Valentin Zăgrean, and Florentina Israel-Roming. 2025. "The Influence of Substrate Composition on Nutritional Content and Biological Activity of Some Pleurotus Mushrooms Extracts" Agriculture 15, no. 7: 791. https://doi.org/10.3390/agriculture15070791
APA StyleRusu, I.-C., Pascariu, O.-E., Popa, A., Diguță, C.-F., Apostol, L., Nicolcioiu, M.-B., Zăgrean, A. V., & Israel-Roming, F. (2025). The Influence of Substrate Composition on Nutritional Content and Biological Activity of Some Pleurotus Mushrooms Extracts. Agriculture, 15(7), 791. https://doi.org/10.3390/agriculture15070791







