Evaluation of the Potential of Pyrimidine Nucleoside Antibiotics Against Alternaria spp. Resistant to QoIs Fungicides: Insights for the Management of Ginseng Alternaria Leaf and Stem Blight Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungicides
2.2. Isolates
2.3. Sensitivity of Alternaria spp. Isolates to PNA
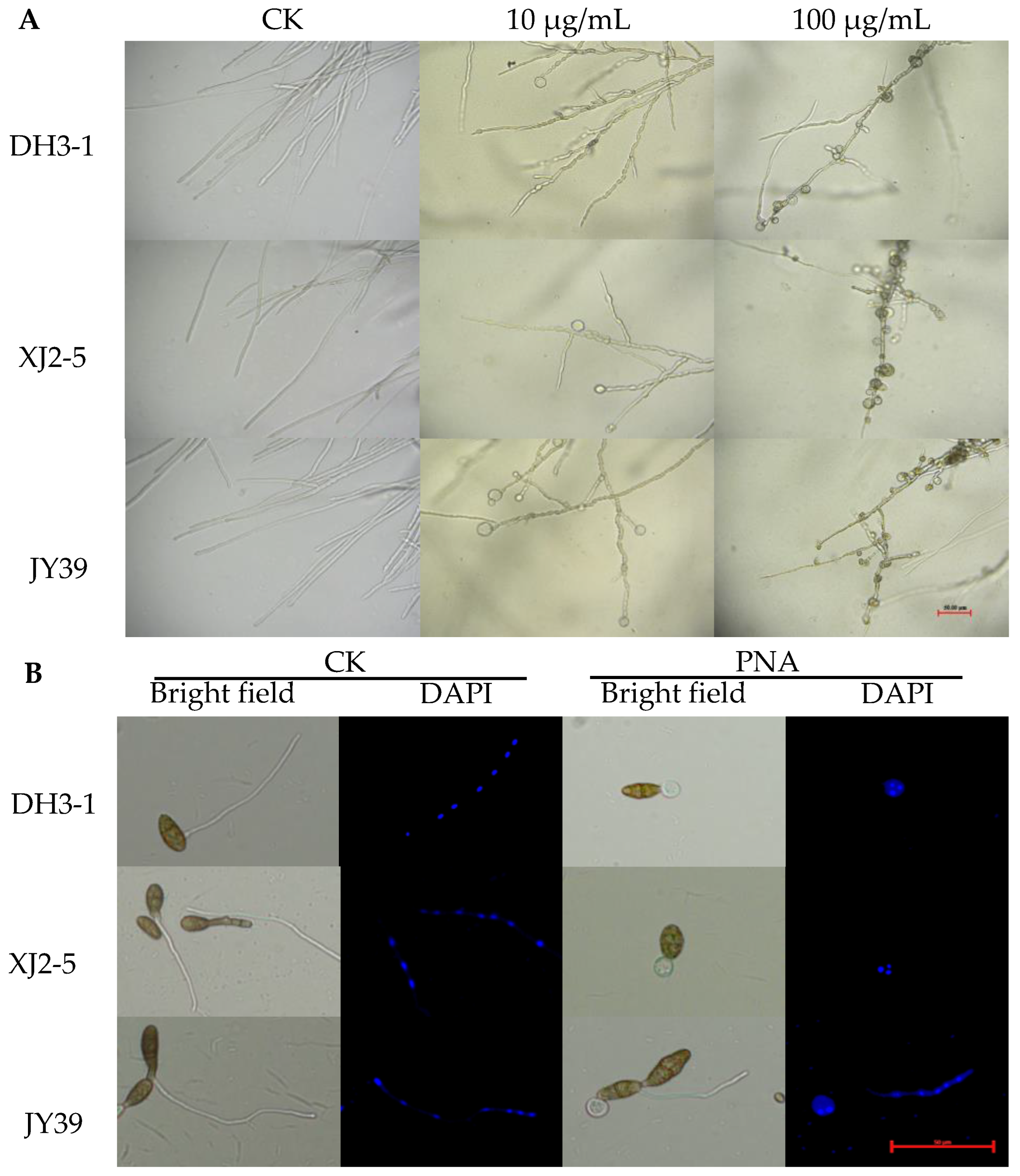
2.4. Effects of PNA on the Morphology of Mycelia and Conidia Germ Tubes of A. alternata
2.5. Test for Biochemical Activity of PNA on A. alternata
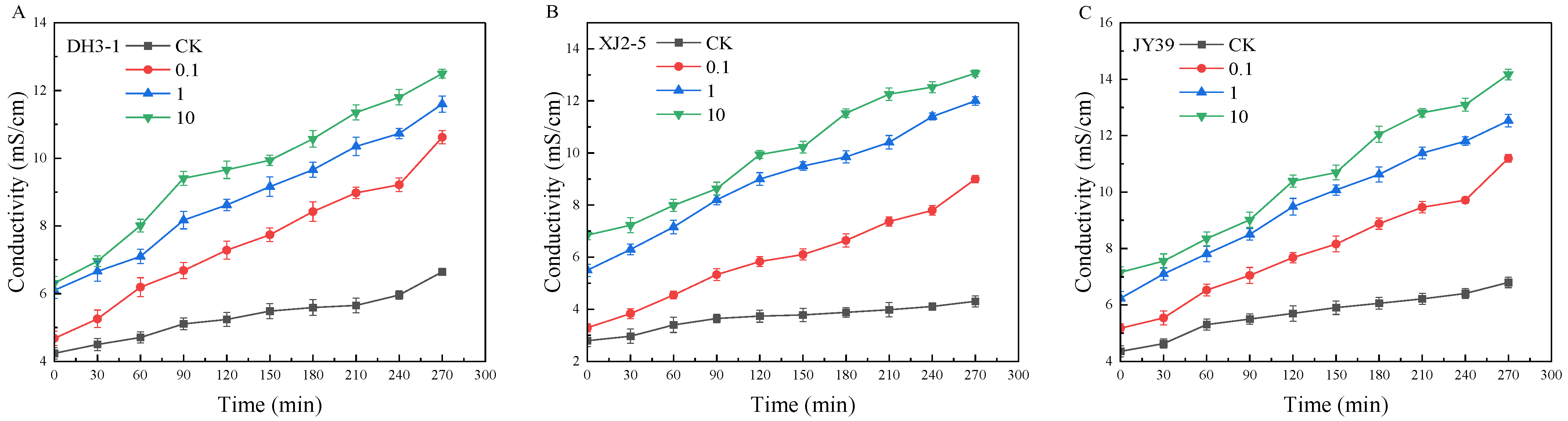
2.5.1. Determination of Cell Membrane Permeability
2.5.2. Determination of the Ergosterol Content
2.5.3. Determination of the DNA and Protein Content
2.6. Protective and Curative Efficacy
2.7. Test for Field Efficacy of PNA Against GALSB Disease
2.8. Cross-Resistance Tests Between PNA and Other Fungicides
2.9. Statistical Analysis
3. Results
3.1. Baseline Sensitivity of Alternaria spp. to PNA
3.2. Effects of PNA on the Morphology of Mycelia and Conidia Germination
3.3. Biochemical Activity of PNA on A. alternata
3.3.1. Cell Membrane Permeability of A. alternata Isolates
3.3.2. Ergosterol Content of A. alternata Isolates
3.3.3. DNA and Protein Content of A. alternata Isolates
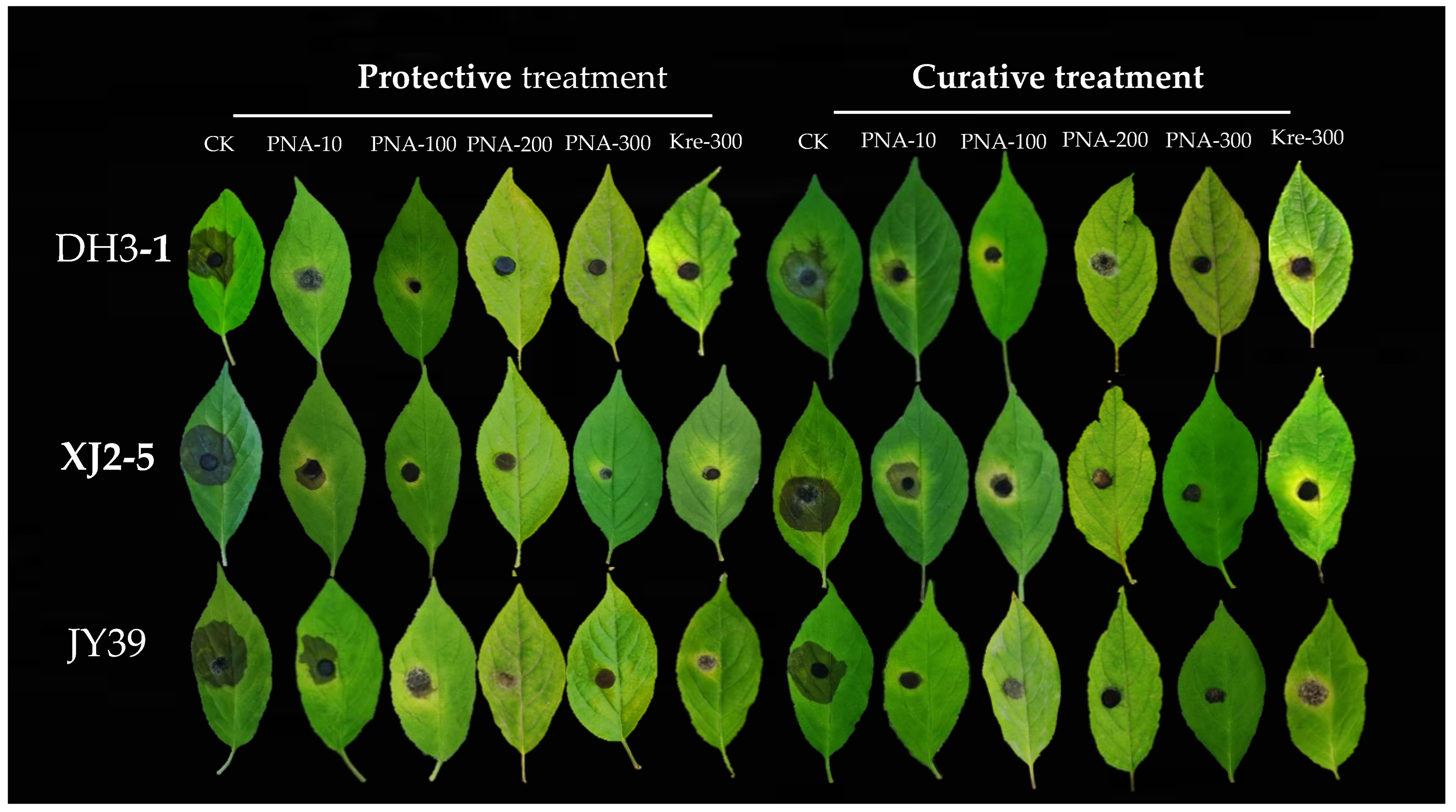
3.4. In Vitro Efficacy of PNA for Controlling GALSB Disease
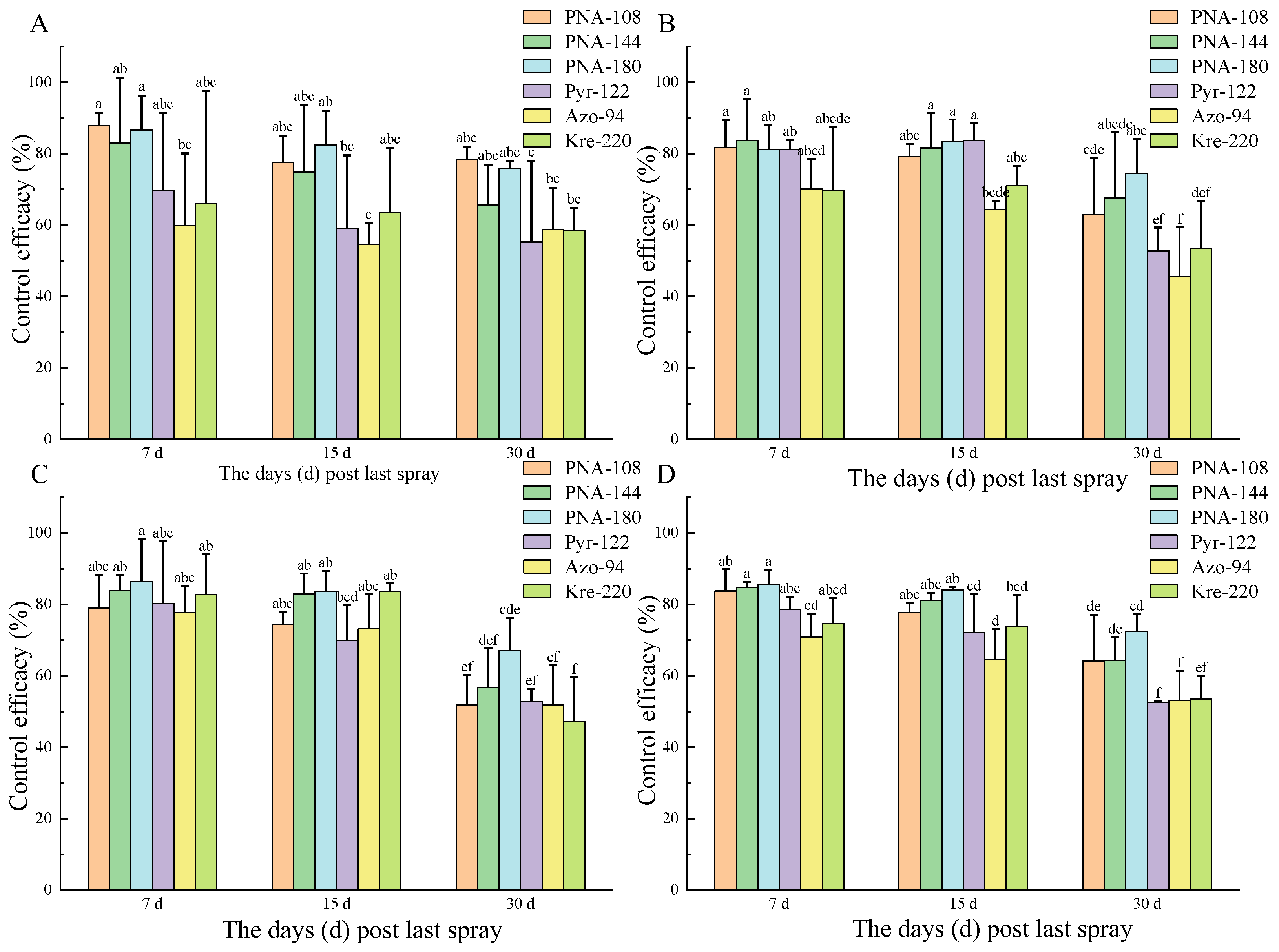
3.5. Field Efficacy of PNA Against GALSB Disease
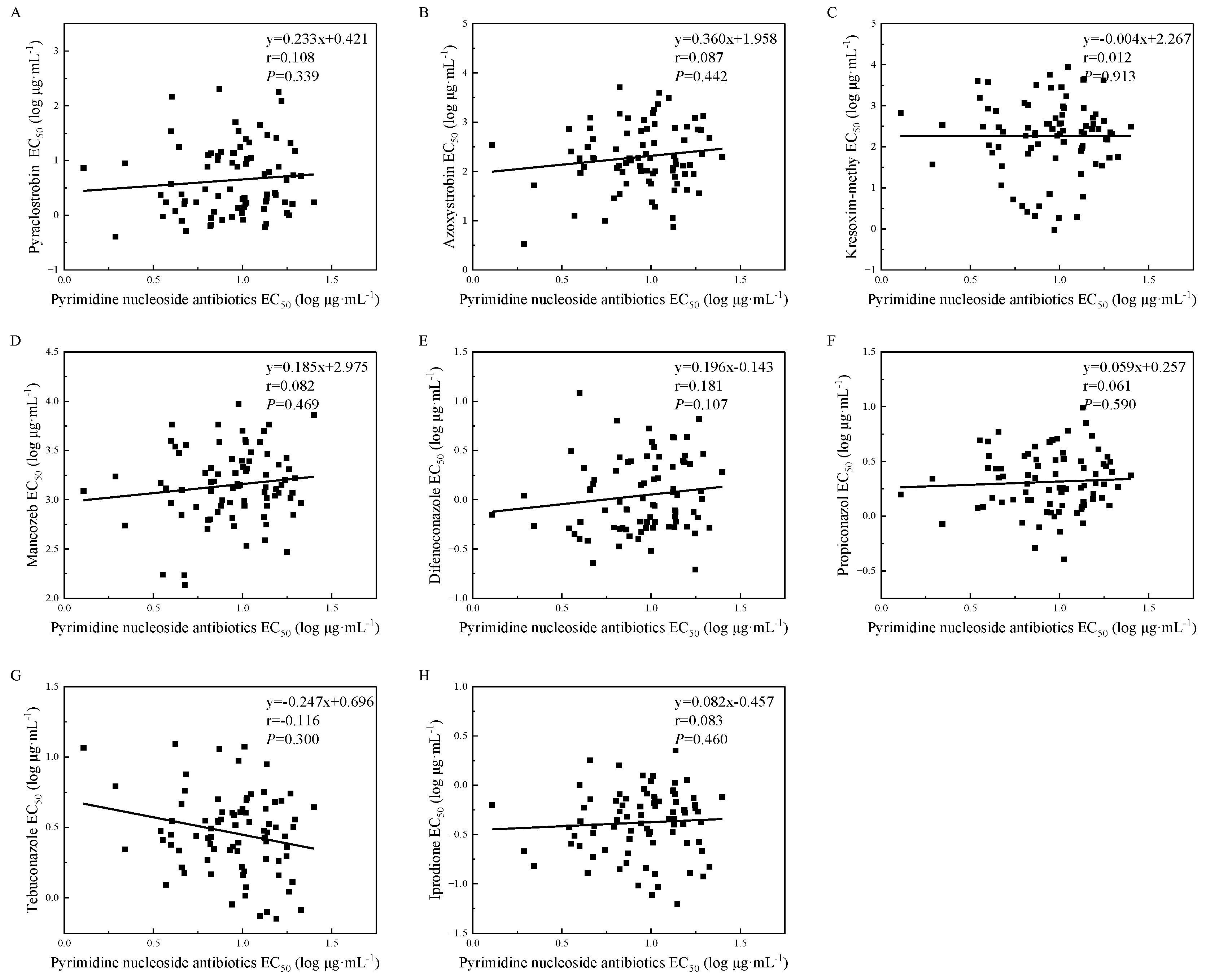
3.6. Cross-Resistance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PNA | Pyrimidine nucleoside antibiotics |
| GALSB | Ginseng Alternaria leaf and stem blight |
| TA | Technical grade |
| AS | Aqueous solution |
| SC | Suspension concentrates |
| WP | Wettable powder |
| DHE | Dehydroergosterol |
| OD | Optical density |
| CE | Control efficacy |
References
- Deng, J.X.; Paul, N.C.; Park, M.S.; Yu, S.H. Molecular characterization, morphology, and pathogenicity of Alternaria panax from araliaceous plants in Korea. Mycol. Progress. 2013, 12, 383–396. [Google Scholar] [CrossRef]
- Gao, J.; Yang, M.; Xie, Z.; Lu, B.; Tom, H.; Liu, L. Morphological and molecular identification and pathogenicity of Alternaria spp. associated with ginseng in Jilin province, China. Can. J. Plant Pathol. 2021, 43, 537–550. [Google Scholar] [CrossRef]
- Wei, S.; Sun, Y.; Xi, G.; Zhang, H.; Xiao, M.; Yin, R. Development of a single-tube nested PCR-lateral flow biosensor assay for rapid and accurate detection of Alternaria panax Whetz. PLoS ONE 2018, 13, e0206462. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Wang, W.; Liu, Z. Genome resource of American ginseng black spot pathogen Alternaria panax. Plant Dis. 2022, 106, 1020–1022. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Xiao, Y.; Jiang, C.; Cheng, L.; Guo, S.; Luo, C.; Wang, Y.; Jia, H. Proteomics analysis of a tobacco variety resistant to brown spot disease and functional characterization of NbMLP423 in Nicotiana benthamiana. Mol. Biol. Rep. 2023, 50, 4395–4409. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Guerra, I.R.; Beltrán Pineda, M.E.; Benavides Rozo, M.E. Characterization of Alternaria alternata and Alternaria scrophulariae Brown Spot in Colombian quinoa (Chenopodium quinoa). J. Fungi 2023, 9, 947. [Google Scholar] [CrossRef]
- Dube, J.; Truter, M.; Van Der Waals, J. First report of resistance to QoI fungicides in Alternaria alternata isolates from potato in South Africa. Plant Dis. 2014, 98, 1431. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.; Jo, M.; An, S.; Kim, Y.; Yoon, J.; Jeong, M.; Kim, E.; Choi, J.; Kim, Y. Isolation and identification of Alternaria alternata from potato plants affected by leaf spot disease in Korea: Selection of effective fungicides. J. Fungi 2024, 10, 53. [Google Scholar] [CrossRef]
- He, M.; Wang, Y.; Wu, E.J.; Shen, L.; Yang, L.; Wang, T.; Shang, L.; Zhu, W.; Zhan, J. Constraining evolution of Alternaria alternata resistance to a demethylation inhibitor (DMI) fungicide difenoconazole. Front. Microbiol. 2019, 10, 121–127. [Google Scholar] [CrossRef]
- Sun, C.; Li, F.; Wei, M.; Xiang, Z.; Chen, C.; Xu, D. Detection and biological characteristics of Alternaria alternata resistant to difenoconazole from Paris polyphylla var. chinensis, an indigenous medicinal herb. Plant Dis. 2021, 105, 1546–1554. [Google Scholar] [CrossRef]
- Avenot, H.F.; Solorio, C.; Morgan, D.P.; Michailides, T.J. Sensitivity and cross-resistance patterns to demethylation-inhibiting fungicides in California populations of Alternaria alternata pathogenic on pistachio. Crop Prot. 2016, 88, 72–78. [Google Scholar] [CrossRef]
- Feng, J.; Cai, L.; Li, T.; Wang, H.; Zhang, C. G462S substitution of AaCYP51 confers moderate resistance to tebuconazole in Alternaria alternata. Pest. Manag. Sci. 2025, 82, 8654. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, M.; Gai, Y.; Fu, H.; Zhang, B.; Ruan, R.; Chung, K.; Li, H. Thioredoxin and glutaredoxin systems required for oxidative stress resistance, fungicide sensitivity, and virulence of Alternaria alternata. Appl. Environ. 2018, 84, e00086-18. [Google Scholar] [CrossRef] [PubMed]
- Malandrakis, A.; Apostolidou, Z.; Markoglou, A.; Flouri, F. Fitness and cross-resistance of Alternaria alternata field isolates with specific or multiple resistance to single site inhibitors and mancozeb. Eur. J. Plant Pathol. 2015, 142, 489–499. [Google Scholar] [CrossRef]
- Malandrakis, A.; Apostolidou, Z.; Louka, D.; Markoglou, A.; Flouri, F. Biological and molecular characterization of field isolates of Alternaria alternata with single or double resistance to respiratory complex II and III inhibitors. Eur. J. Plant Pathol. 2018, 152, 199–211. [Google Scholar] [CrossRef]
- Pasche, J.S.; Piche, L.M.; Gudmestad, N.C. Effect of the F129L mutation in Alternaria solani on fungicides affecting mitochondrial respiration. Plant Dis. 2005, 89, 269–278. [Google Scholar] [CrossRef]
- Avenot, H.; Morgan, D.P.; Michailides, T.J. Resistance to pyraclostrobin, boscalid and multiple resistance to Pristine® (pyraclostrobin + boscalid) fungicide in Alternaria alternata causing alternaria late blight of pistachios in California. Plant Pathol. 2007, 88, 72–78. [Google Scholar] [CrossRef]
- Camiletti, B.X.; Lichtemberg, P.S.F.; Paredes, J.A.; Carraro, T.A.; Velascos, J.; Michailides, T.J. Characterization, pathogenicity, and fungicide sensitivity of Alternaria isolates associated with preharvest fruit drop in California citrus. Fungal Biol. 2022, 126, 277–289. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C. Multi-resistance to thiophanate-methyl, diethofencarb, and procymidone among Alternaria alternata populations from tobacco plants, and the management of tobacco brown spot with azoxystrobin. Phytoparasitica 2018, 46, 677–687. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Zhang, X.; Zhang, L.; Guo, M.; Xiang, L.; Cai, L.; Wang, F. Inhibitory activity of difenoconazole to Alternaria alternata from tobacco and the changes of phyllosphere microbes after its application. J. Henan Agric. Sci. 2023, 52, 94–103. [Google Scholar] [CrossRef]
- Kalia, A.; Gosal, S. Effect of pesticide application on soil microorganisms. Arch. Agron. Soil. Sci. 2011, 57, 569–596. [Google Scholar] [CrossRef]
- Zhu, C.; Xie, D.; Sui, Y.; Ni, C. Selection of a high-yield strain of agricultural antibiotic 120 by protoplast-fusion techniques. Chin. J. Biol. Control. 1990, 6, 18–22. [Google Scholar] [CrossRef]
- McErlean, M.; Liu, X.; Cui, Z.; Gust, B.; Van Lanen, S. Identification and characterization of enzymes involved in the biosynthesis of pyrimidine nucleoside antibiotics. Nat. Prod. Rep. 2021, 38, 1362–1407. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Cheng, Q.; Yao, F.; Wang, X.; Kong, L.; Cao, B.; Xu, M.; Lin, S.; Deng, Z.; Chooi, Y.-H. Biosynthesis of the pyrrolidine protein synthesis inhibitor anisomycin involves novel gene ensemble and cryptic biosynthetic steps. Proc. Natl. Acad. Sci. USA 2017, 114, 4135–4140. [Google Scholar] [CrossRef]
- Cao, B.; Yao, F.; Zheng, X.; Cui, D.; Shao, Y.; Zhu, C.; Deng, Z.; You, D. Genome mining of the biosynthetic gene cluster of the polyene macrolide antibiotic tetramycin and characterization of a P450 monooxygenase involved in the hydroxylation of the tetramycin B polyol segment. Chem. Bio Chem. 2012, 13, 2234–2242. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Liu, X.; Li, B.; Ma, Z. Characterization of sterol demethylation inhibitor-resistant isolates of Fusarium asiaticum and F. graminearum collected from wheat in China. Phytopathology 2009, 99, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Vega, B.; Dewdney, M.M. Distribution of QoI resistance in populations of tangerine-infecting Alternaria alternata in Florida. Plant Dis. 2014, 98, 67–76. [Google Scholar] [CrossRef]
- Cubeta, M.; Vilgalys, R. Population biology of the Rhizoctonia solani complex. Phytopathology 1997, 87, 480–484. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, L.; Ma, D.; Gao, Y.; Mu, W.; Liu, F. A bioactivity and biochemical analysis of iminoctadine tris (albesilate) as a fungicide against Corynespora cassiicola. Pest. Biochem. Physiol. 2019, 158, 121–127. [Google Scholar] [CrossRef]
- Tian, J.; Huang, B.; Luo, X.; Zeng, H.; Ban, X.; He, J.; Wang, Y. The control of Aspergillus flavus with Cinnamomum jensenianum Hand.-Mazz essential oil and its potential use as a food preservative. Food Chem. 2012, 130, 520–527. [Google Scholar] [CrossRef]
- Yang, Y.; Zuzak, K.; Feng, J. An improved simple method for DNA extraction from fungal mycelia. Can. J. Plant Pathol. 2016, 38, 476–482. [Google Scholar] [CrossRef]
- Pasquali, M.; Giraud, F.; Lasserre, J.; Planchon, S.; Hoffmann, L.; Bohn, T.; Renaut, J. Toxin induction and protein extraction from Fusarium spp. cultures for proteomic studies. Jove-J. Vis. Exp. 2010, 16, e1690. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, J.; Li, Y.; Li, R.; Hou, W.; Qu, Z.; Li, Y.; Zheng, P. Isolation, Identification and control effect of biocontrol bacteria against ginseng black spot disease. Chin. J. Biol. Control 2022, 38, 1308–1315. [Google Scholar] [CrossRef]
- Yang, X.; Gao, J.; Ma, G.; Zhang, J. Improvement on the calculation formula of plant disease control effect. J. Jilin Agric. Univ. 1999, 21, 46–48. [Google Scholar] [CrossRef]
- Peng, Q.; Li, X.; Li, G.; Hao, X.; Liu, X. Resistance risk assessment of mefentrifluconazole in Corynespora cassiicola and the control of cucumber target spot by a two-way mixture of mefentrifluconazole and prochloraz. Pest. Biochem. Physiol. 2024, 198, 105719. [Google Scholar] [CrossRef] [PubMed]
- Woudenberg, J.; Seidl, M.; Groenewald, J.; De Vries, M.; Stielow, J.; Thomma, B.; Crous, P. Alternaria section Alternaria: Species, formae speciales or pathotypes? Stud. Mycol. 2015, 82, 1–21. [Google Scholar] [CrossRef]
- Li, G.; Li, X.; Zeng, Y.; Liao, S.; Chen, Y.; Miao, J.; Peng, Q.; Liu, X. Three point mutations in AaCYP51 combined with induced overexpression of AaCYP51 conferred low-level resistance to mefentrifluconazole in Alternaria alternata. Pest. Biochem. Physiol. 2023, 197, 105677. [Google Scholar] [CrossRef]
- Duanis-Assaf, D.; Galsurker, O.; Davydov, O.; Maurer, D.; Feygenberg, O.; Sagi, M.; Poverenov, E.; Fluhr, R.; Alkan, N. Double-stranded RNA targeting fungal ergosterol biosynthesis pathway controls Botrytis cinerea and postharvest grey mould. Plant Biotechnol. J. 2022, 20, 226–237. [Google Scholar] [CrossRef]
- Feng, M.; Jin, Y.; Yang, S.; Joachim, A.M.; Ning, Y.; Mori-Quiroz, L.M.; Fromm, J.; Perera, C.; Zhang, K.; Werbovetz, K.A. Sterol profiling of Leishmania parasites using a new HPLC-tandem mass spectrometry-based method and antifungal azoles as chemical probes reveals a key intermediate sterol that supports a branched ergosterol biosynthetic pathway. Int. J. Parasitol.-Drug 2022, 20, 27–42. [Google Scholar] [CrossRef]
- Ratti, A.; Fassi, E.M.; Forlani, F.; Mori, M.; Villa, F.; Cappitelli, F.; Sgrignani, J.; Roda, G.; Cavalli, A.; Villa, S. Mechanistic insights into the antibiofilm mode of action of ellagic acid. Pharmaceutics 2023, 15, 1757. [Google Scholar] [CrossRef]
- Fan, K.; Wang, J.; Fu, L.; Li, X.; Zhang, Y.; Zhang, X.; Zhai, H.; Qu, J. Sensitivity of Botryosphaeria dothidea from apple to tebuconazole in China. Crop Prot. 2016, 87, 1–5. [Google Scholar] [CrossRef]
- Helps, J.; Lopez-Ruiz, F.; Zerihun, A.; Bosch, F. Do growers using solo fungicides affect the durability of disease control of growers using mixtures and alternations? The case of spot-form net blotch in western Australia. Phytopathology 2024, 114, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H. New chemical fungicides in relation to risk for resistance development. Trop. Plant Pathol. 2024, 49, 18–35. [Google Scholar] [CrossRef]
- Bi, Q.; Ma, Z. Sensitivity, resistance stability, and cross-resistance of Plasmopara viticola to four different fungicides. Crop Prot. 2016, 89, 265–272. [Google Scholar] [CrossRef]
- Li, T.; Li, H.; Liu, T.; Zhu, J.; Zhang, L.; Mu, W.; Liu, F. Evaluation of the antifungal and biochemical activities of mefentrifluconazole against Botrytis cinerea. Pest. Biochem. Physiol. 2021, 173, 104784. [Google Scholar] [CrossRef]
- Olaya, G.; Köller, W. Baseline sensitivities of Venturia inaequalis populations to the strobilurin fungicide kresoxim-methyl. Plant Dis. 1999, 83, 274–278. [Google Scholar] [CrossRef]
- McGrath, M.T. Efficacy of organic fungicides for downy mildew in field-grown sweet basil. Plant Dis. 2023, 107, 2467–2473. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cai, C.; Zhang, X.; Li, W.; Ma, Z.; Feng, J.; Liu, X.; Lei, P. Discovery of novel cinnamic acid derivatives as fungicide candidates. J. Agric. Food Chem. 2024, 72, 2492–2500. [Google Scholar] [CrossRef]
- Ghimire, B.; Aktaruzzaman, M.; Chowdhury, S.; Spratling, W.; Vermeer, C.; Buck, J.; Martinez-Espinoza, A.; Bahri, B. Sensitivity of Clarireedia spp. to benzimidazoles and dimethyl inhibitors fungicides and efficacy of biofungicides on dollar spot of warm season turfgrass. Front. Plant Sci. 2023, 14, 1155670. [Google Scholar] [CrossRef]
- Stridh, L.; Mostafanezhad, H.; Andersen, C.; Odilbekov, F.; Grenville-Briggs, L.; Lankinen, Å.; Liljeroth, E. Reduced efficacy of biocontrol agents and plant resistance inducers against potato early blight from greenhouse to field. J. Plant Dis. Prot. 2022, 129, 923–938. [Google Scholar] [CrossRef]
- Atri, A.; Banyal, D.; Bhardwaj, N.; Roy, A. Exploring the integrated use of fungicides, bio-control agent and biopesticide for management of foliar diseases (anthracnose, grey leaf spot and zonate leaf spot) of sorghum. Int. J. Pest. Manag. 2022, 70, 789–800. [Google Scholar] [CrossRef]
- Bessaire, T.; Savoy, M.; Ernest, M.; Christinat, N.; Badoud, F.; Desmarchelier, A.; Carrères, B.; Chan, W.; Wang, X.; Delatour, T. Enhanced surveillance of >1100 pesticides and natural toxins in food: Harnessing the capabilities of LC-HRMS for reliable identification and quantification. Foods 2024, 13, 3040. [Google Scholar] [CrossRef] [PubMed]
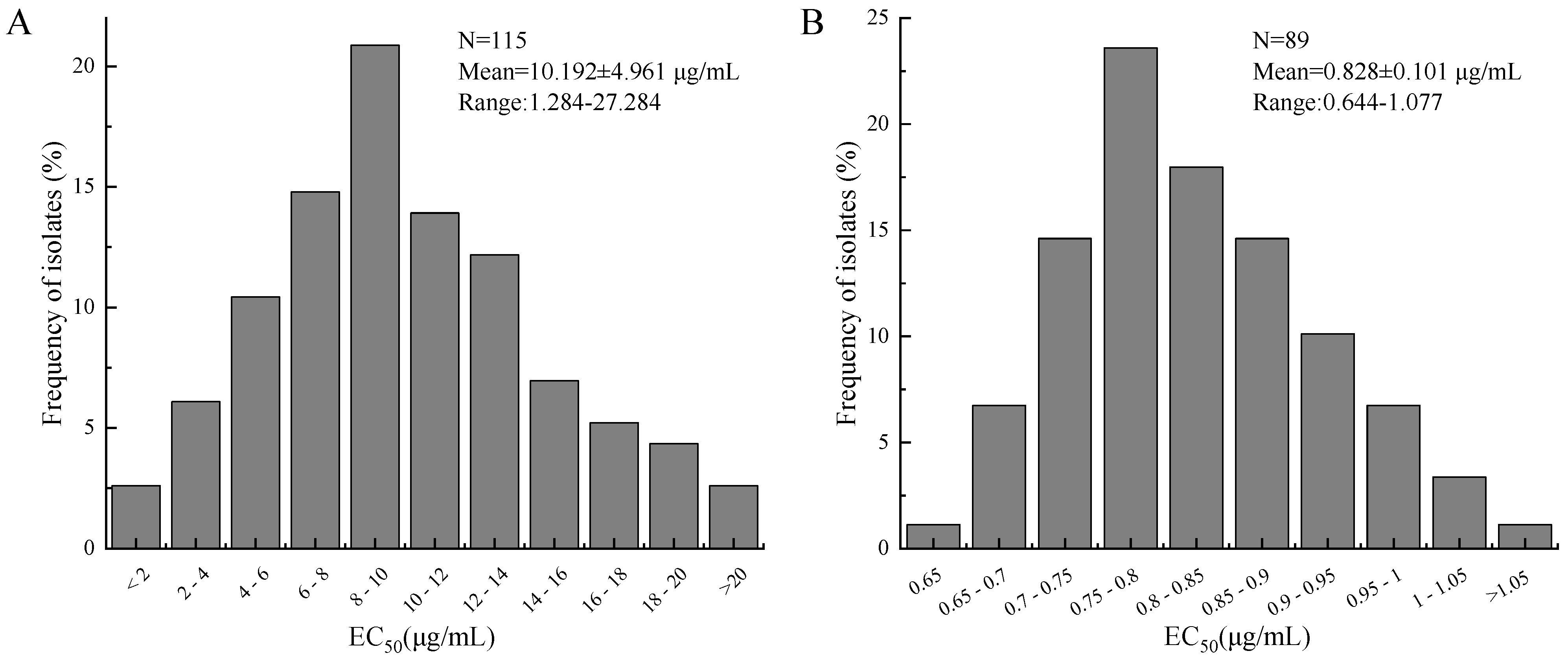
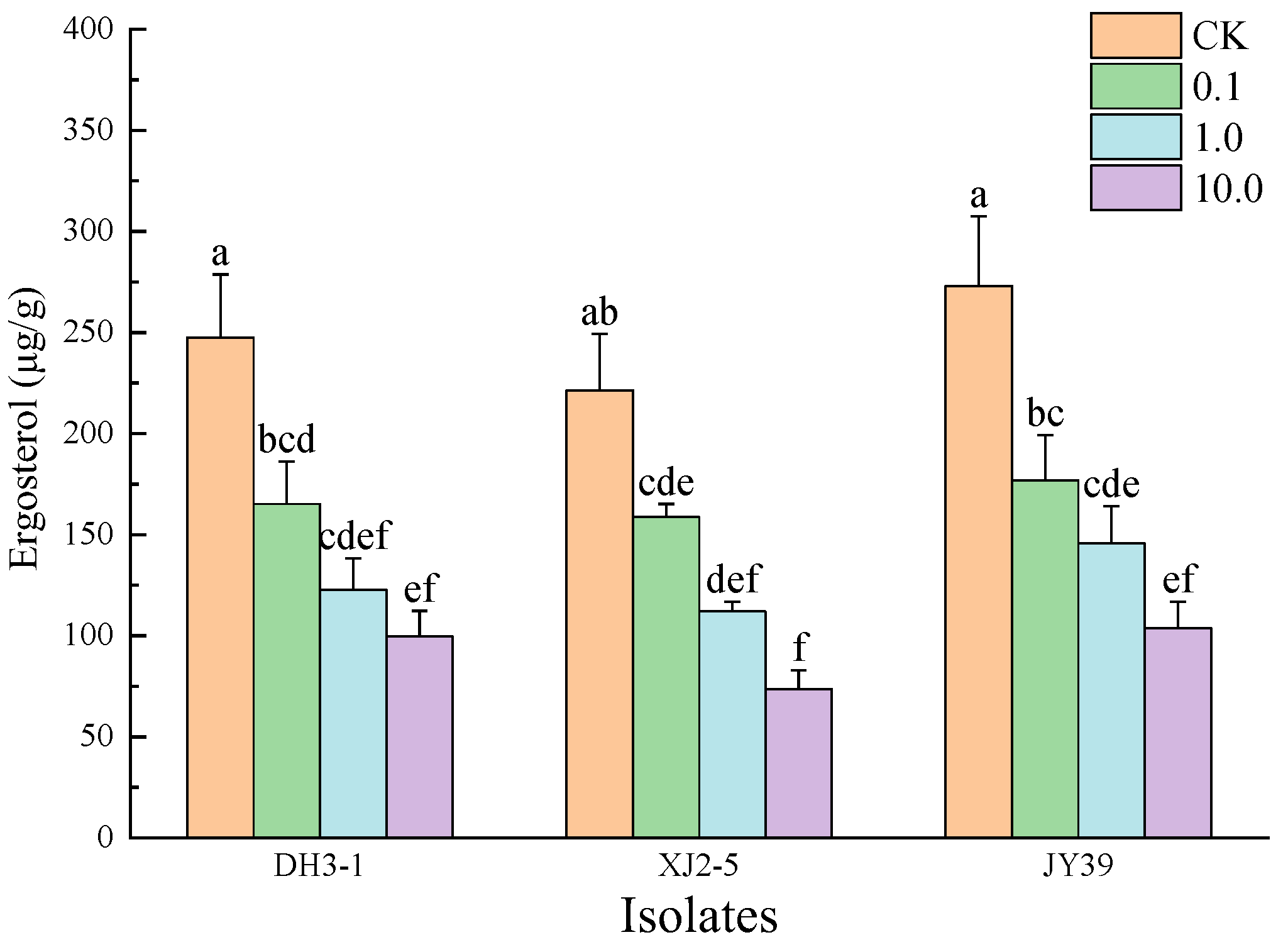
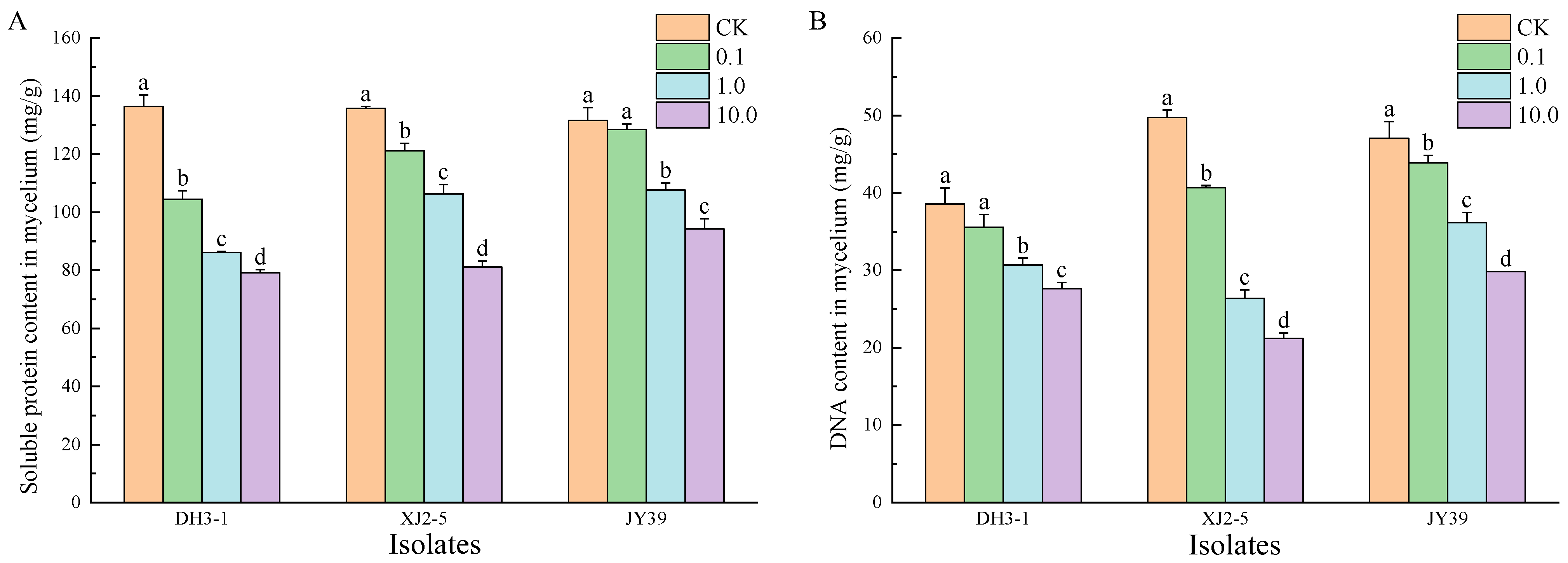
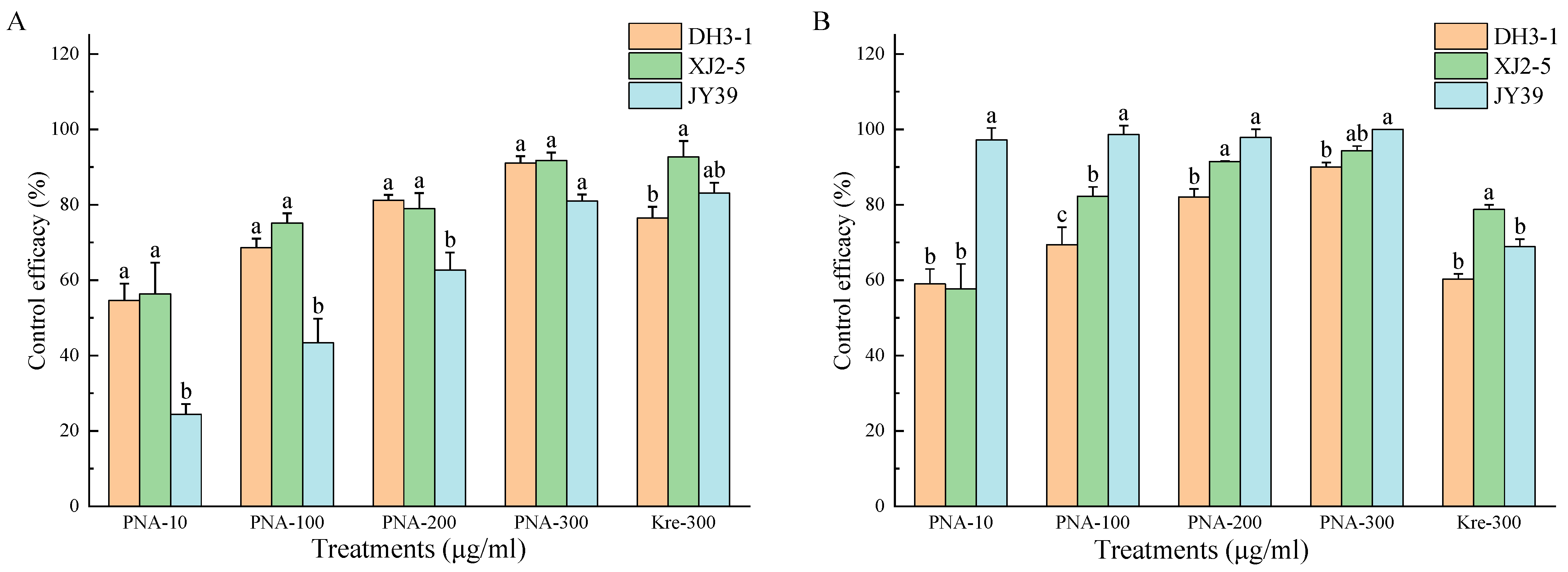
| Species | Test Methods | Isolates Number | EC50 (μg/mL) z | |
|---|---|---|---|---|
| Range | Mean ± SD y | |||
| A. alternata | Mycelial growth rate | 115 | 1.284–27.284 | 10.192 ± 4.961 b |
| A. tenuissima | 29 | 6.355–20.227 | 11.494 ± 3.379 b | |
| A. panax | 26 | 11.398–57.573 | 31.448 ± 12.846 a | |
| A. alternata | Conidia germination | 89 | 0.644–1.077 | 0.828 ± 0.101 c |
| A. tenuissima | 29 | 0.419–1.251 | 0.666 ± 0.199 c | |
| Fungicides | Treatment (μg/mL) | Protective Efficacy (%) | Curative Efficacy (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DH3-1 | XJ2-5 | JY39 | Average Efficacy | DH3-1 | XJ2-5 | JY39 | Average Efficacy | ||
| Pyrimidine nucleoside antibiotics | 100 | 68.63 ± 2.43 c | 75.13 ± 2.61 b | 43.45 ± 6.31 c | 62.40 ± 16.73 b | 69.38 ± 4.65 b | 82.26 ± 2.50 ab | 98.61 ± 2.41 a | 83.42 ± 14.65 ab |
| 200 | 81.18 ± 1.46 ab | 78.99 ± 4.11 b | 62.67 ± 4.69 b | 74.28 ± 10.11 ab | 82.07 ± 2.11 a | 91.49 ± 0.18 a | 97.86 ± 2.17 a | 90.47 ± 7.94 a | |
| 300 | 91.06 ± 1.85 a | 91.73 ± 2.09 a | 80.99 ± 1.72 a | 87.93 ± 6.02 a | 90.04 ± 1.17 a | 94.32 ± 1.24 a | 100.00 ± 0.00 a | 94.79 ± 5.00 a | |
| Kresoxim-methyl | 300 | 76.44 ± 2.98 bc | 92.66 ± 4.28 a | 83.05 ± 2.80 a | 84.05 ± 8.16 ab | 60.30 ± 1.37 c | 78.74 ± 1.25 b | 68.88 ± 2.01 b | 69.31 ± 9.23 b |
| Fungicide | Dosage (g a.i./hm2) | Control Efficacy (%) 7 Days Post Last Spray | Control Efficacy (%) 15 Days Post Last Spray | Control Efficacy (%) 30 Days Post Last Spray | |||
|---|---|---|---|---|---|---|---|
| Range z | Mean ± SD y | Range z | Mean ± SD y | Range z | Mean ± SD y | ||
| Pyrimidine nucleoside antibiotics 4% AS | 108 | 79.01–90.36 | 83.77 ± 6.08 a | 74.51–79.25 | 77.72 ± 2.78 ab | 51.92–79.01 | 64.20 ± 12.93 ab |
| 144 | 83.77–86.61 | 84.78 ± 1.59 a | 78.82–83.01 | 81.15 ± 2.13 ab | 56.73–68.51 | 64.26 ± 6.54 ab | |
| 180 | 81.68–89.29 | 85.62 ± 4.13 a | 83.43–85.11 | 84.07 ± 0.91 a | 67.15–76.10 | 72.55 ± 4.75 a | |
| Pyraclostrobin 25% SC | 112 | 74.55–81.15 | 78.65 ± 3.58 ab | 62.79–83.79 | 72.17 ± 10.68 bc | 52.48–52.81 | 52.67 ± 0.17 c |
| Azoxystrobin 25% SC | 94 | 64.51–77.78 | 70.81 ± 6.66 b | 56.62–73.20 | 64.58 ± 8.50 c | 45.61–62.00 | 53.18 ± 8.27 c |
| Kresoxim-methyl 30% WP | 220 | 69.63–82.72 | 74.74 ± 7.00 ab | 66.79–83.66 | 73.82 ± 8.78 abc | 47.12–60.06 | 53.56 ± 6.47 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, S.; Hu, M.; Chen, X.; Jiang, M.; Chen, C.; Lu, B.; Gao, J. Evaluation of the Potential of Pyrimidine Nucleoside Antibiotics Against Alternaria spp. Resistant to QoIs Fungicides: Insights for the Management of Ginseng Alternaria Leaf and Stem Blight Disease. Agriculture 2025, 15, 875. https://doi.org/10.3390/agriculture15080875
Shao S, Hu M, Chen X, Jiang M, Chen C, Lu B, Gao J. Evaluation of the Potential of Pyrimidine Nucleoside Antibiotics Against Alternaria spp. Resistant to QoIs Fungicides: Insights for the Management of Ginseng Alternaria Leaf and Stem Blight Disease. Agriculture. 2025; 15(8):875. https://doi.org/10.3390/agriculture15080875
Chicago/Turabian StyleShao, Shuai, Mingyuan Hu, Xiaolin Chen, Ming’en Jiang, Changqing Chen, Baohui Lu, and Jie Gao. 2025. "Evaluation of the Potential of Pyrimidine Nucleoside Antibiotics Against Alternaria spp. Resistant to QoIs Fungicides: Insights for the Management of Ginseng Alternaria Leaf and Stem Blight Disease" Agriculture 15, no. 8: 875. https://doi.org/10.3390/agriculture15080875
APA StyleShao, S., Hu, M., Chen, X., Jiang, M., Chen, C., Lu, B., & Gao, J. (2025). Evaluation of the Potential of Pyrimidine Nucleoside Antibiotics Against Alternaria spp. Resistant to QoIs Fungicides: Insights for the Management of Ginseng Alternaria Leaf and Stem Blight Disease. Agriculture, 15(8), 875. https://doi.org/10.3390/agriculture15080875








