The Simulation of Stomatal Aperture Size on the Upper and Lower Epidermis of Gynura formosana Kitam Leaves Based on Cellular Automata
Abstract
:1. Introduction
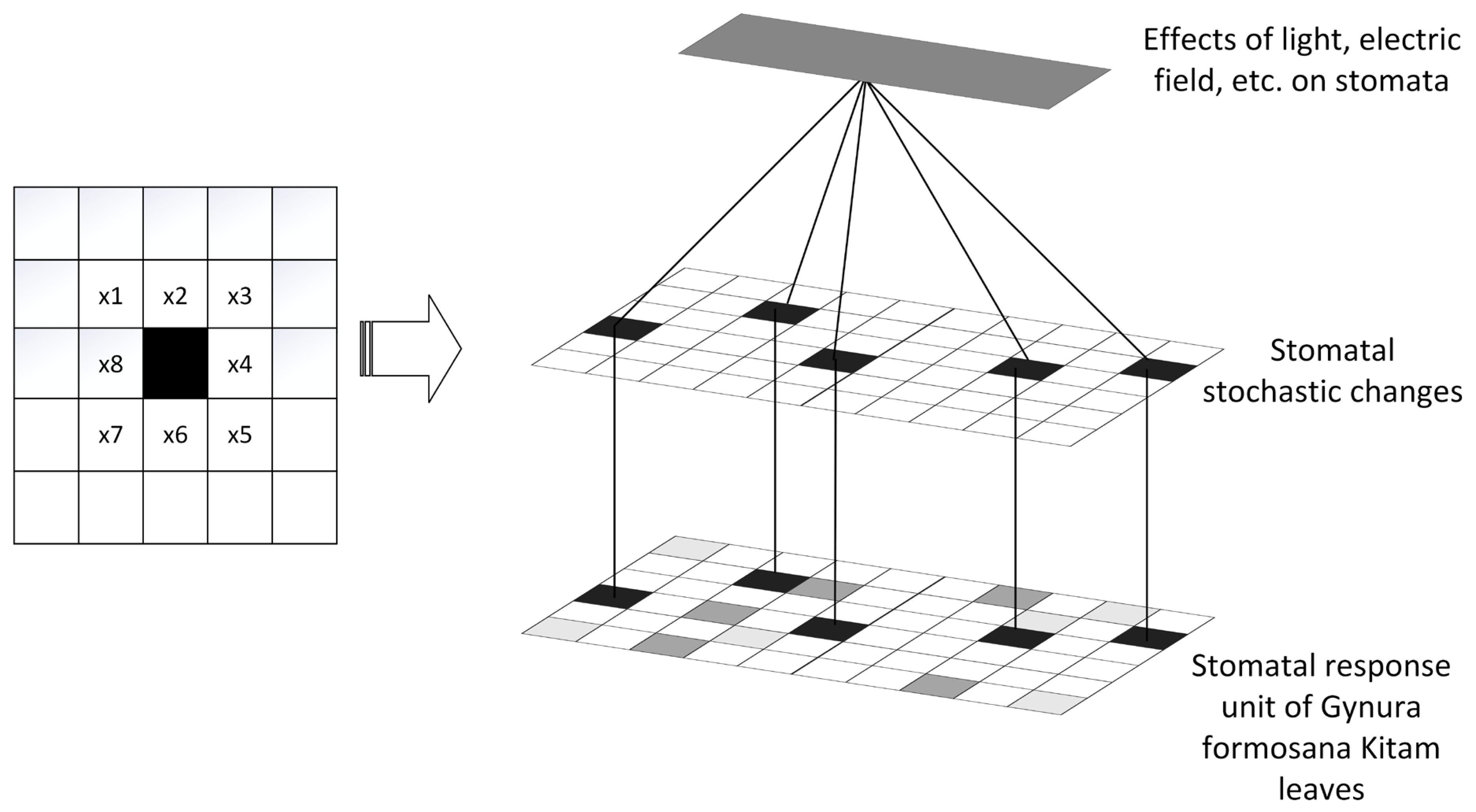
2. A Framework for Evaluating the Stomatal Opening Size of the Upper and Lower Epidermis of Gynura formosana Kitam Leaves Based on Cellular Automata
2.1. Cellular Automaton Model
2.2. Basic Composition of Stomatal Opening Size on the Upper and Lower Epidermis of Gynura formosana Kitam Leaves Based on Cellular Automata
2.2.1. The Expression Form of Stomatal Opening and Closing in Gynura formosana Kitam Leaves
2.2.2. The Size of Stomatal Openings on the Upper and Lower Epidermis of the Leaves of the Gynura formosana Kitam, and the Cellular Automata and Their Cellular Space
3. Materials and Methods
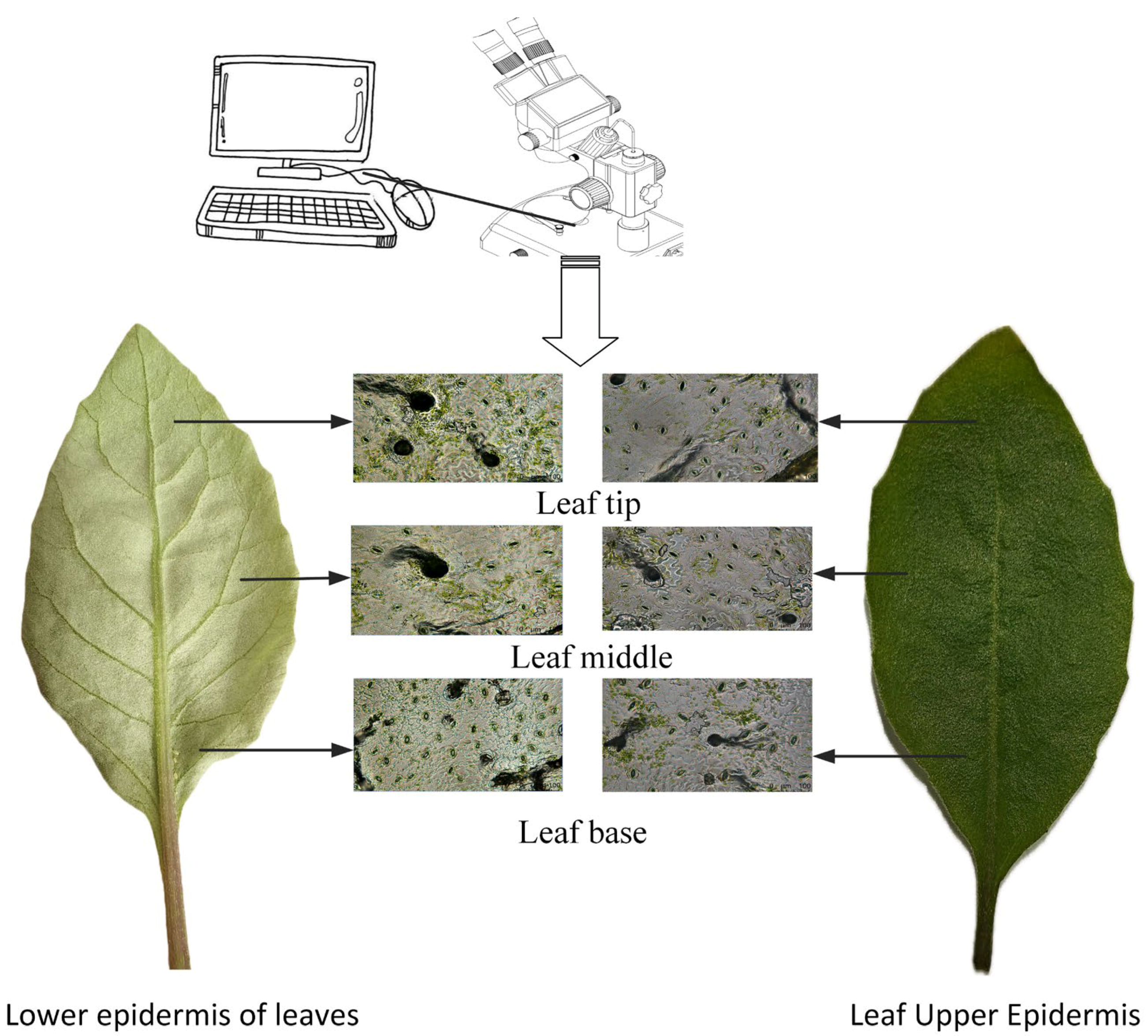
3.1. Materials
Data Source
3.2. Methods
3.2.1. Intelligent Adaptive Environmental Model Construction of Stomatal Opening Size on the Upper and Lower Epidermis of Gynura formosana Kitam Leaves
3.2.2. Changes in Domain Rules
3.2.3. Model Pre-Configuration
3.2.4. Formulation of Rule Changes
4. Case Simulation and Comparative Analysis
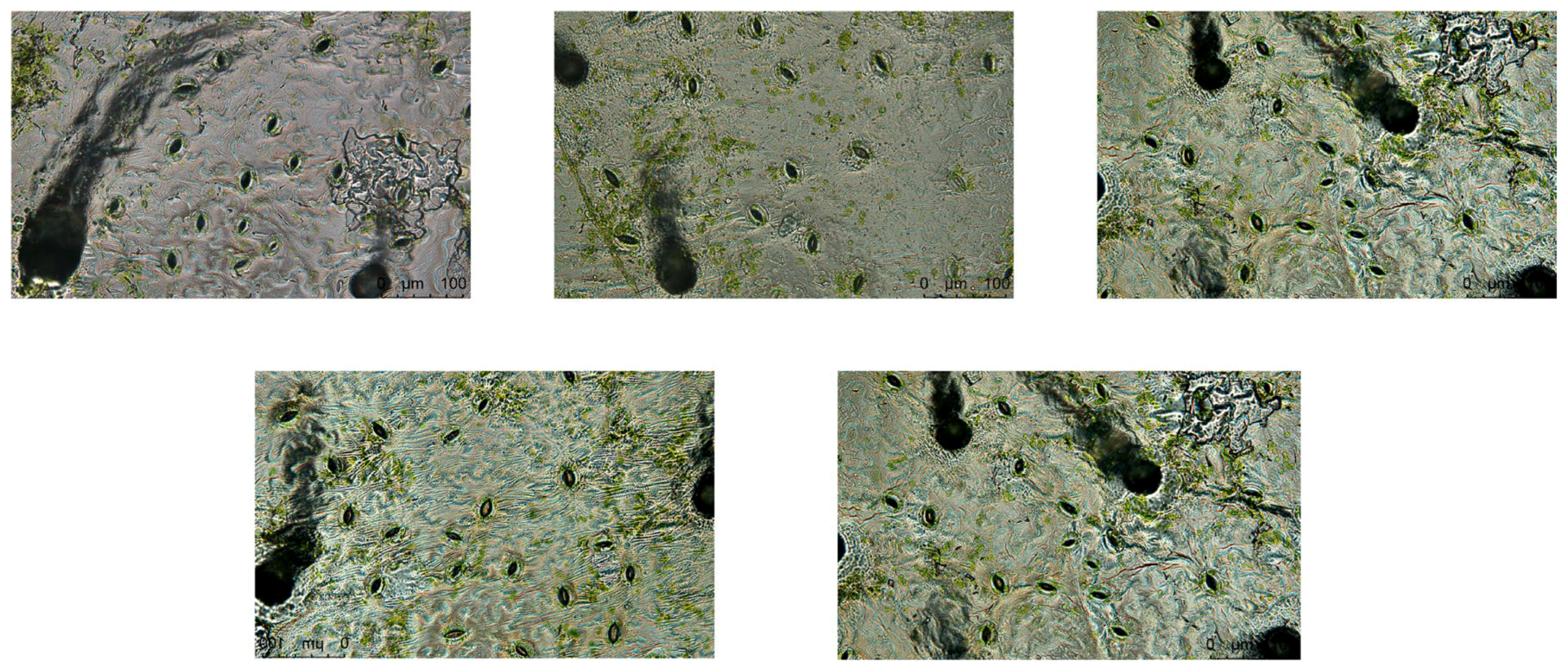
4.1. Selection of Stomatal Regions on the Upper and Lower Epidermis of Gynura formosana Kitam Leaves
4.2. Comparative Analysis of Stomatal Aperture Changes on the Upper and Lower Epidermis of Gynura formosana Kitam Leaves
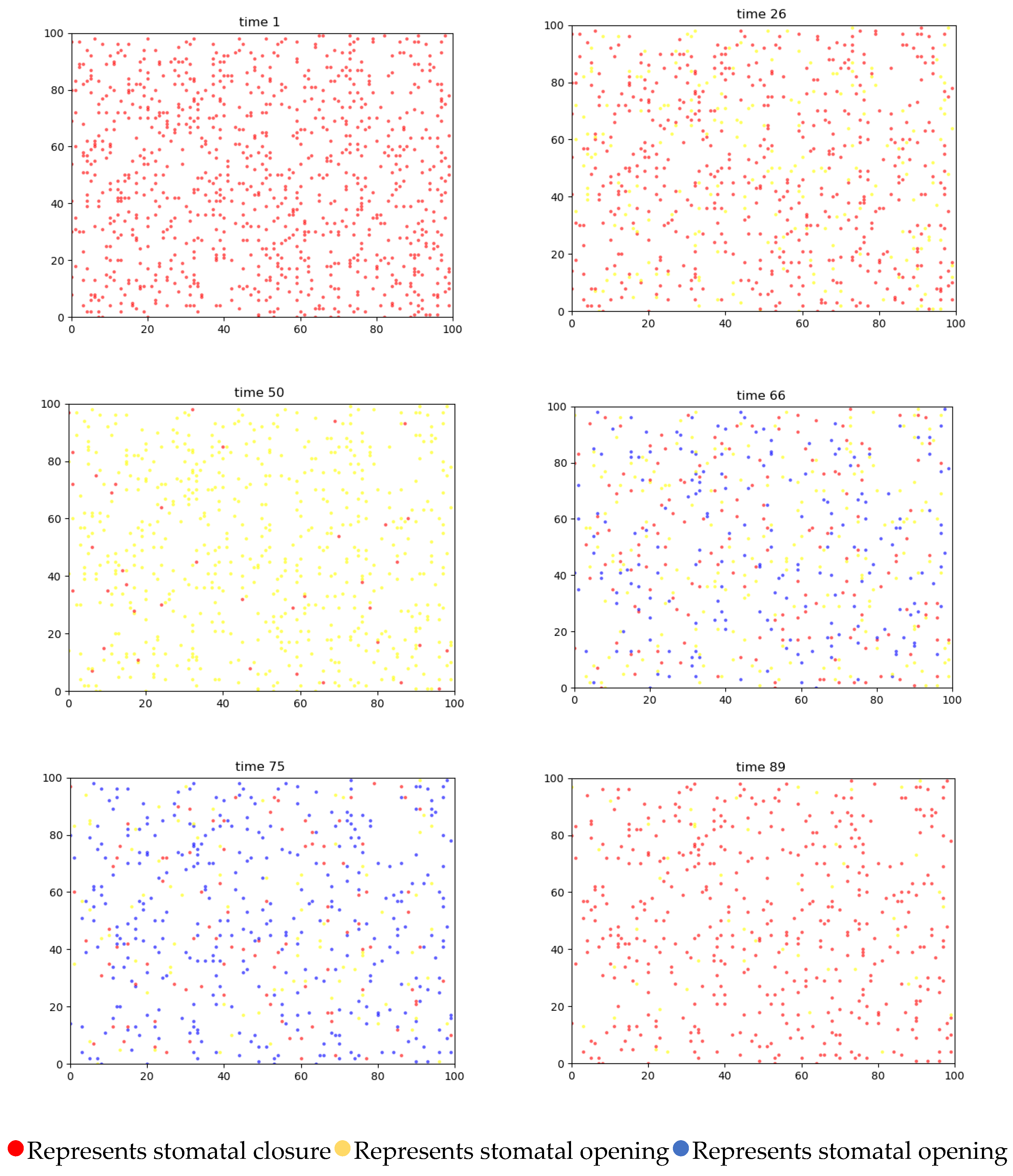
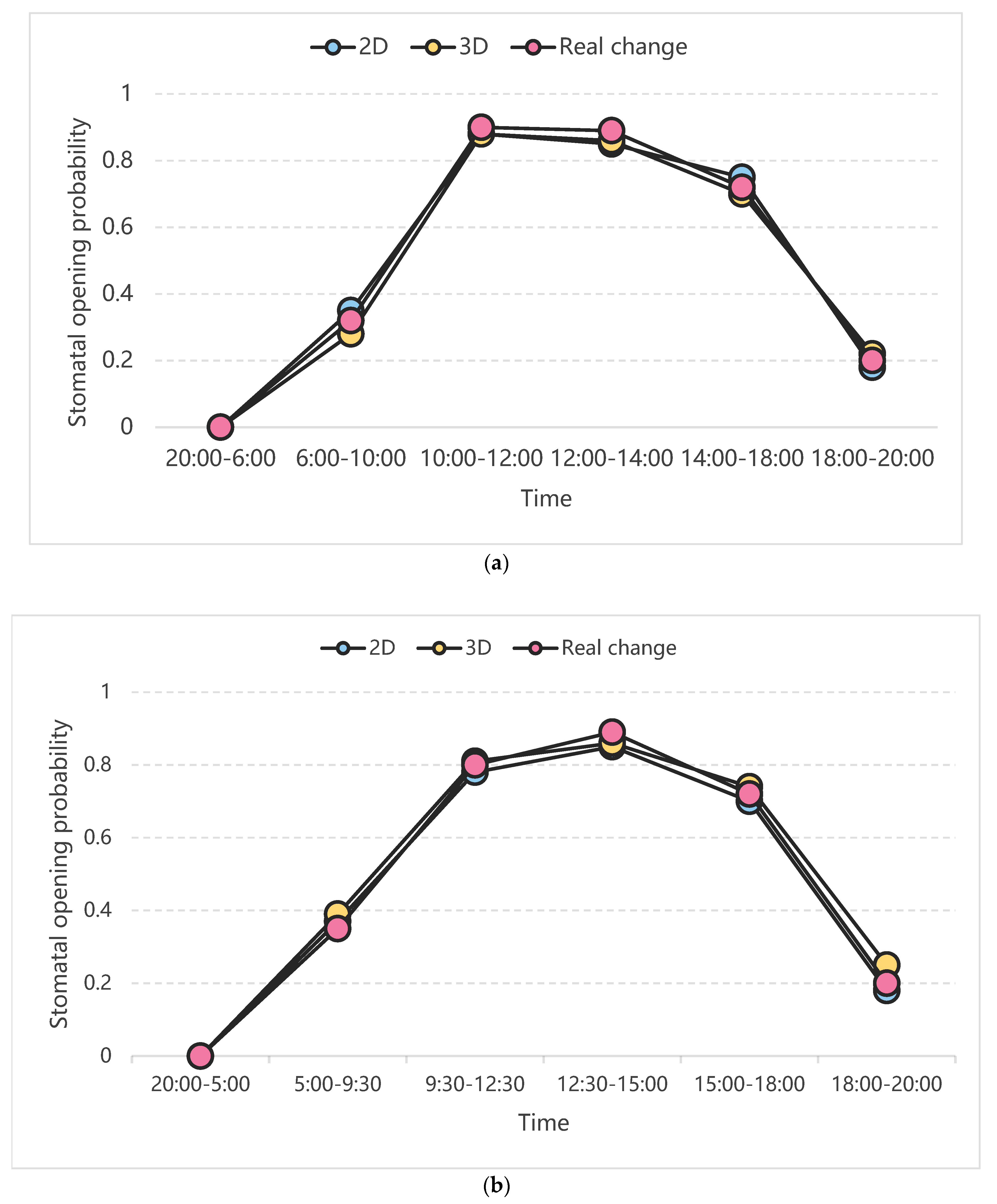
4.3. Simulation and Analysis
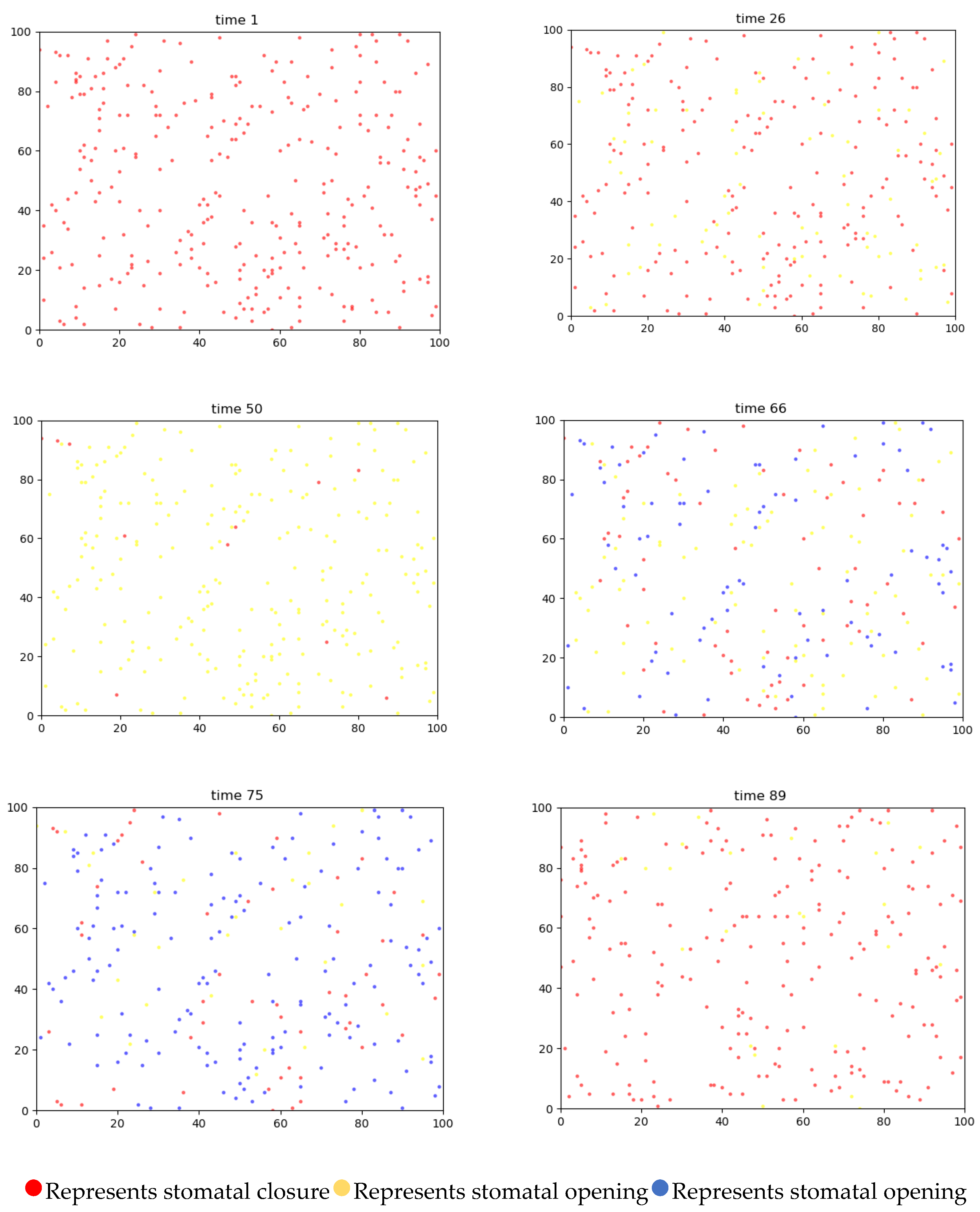
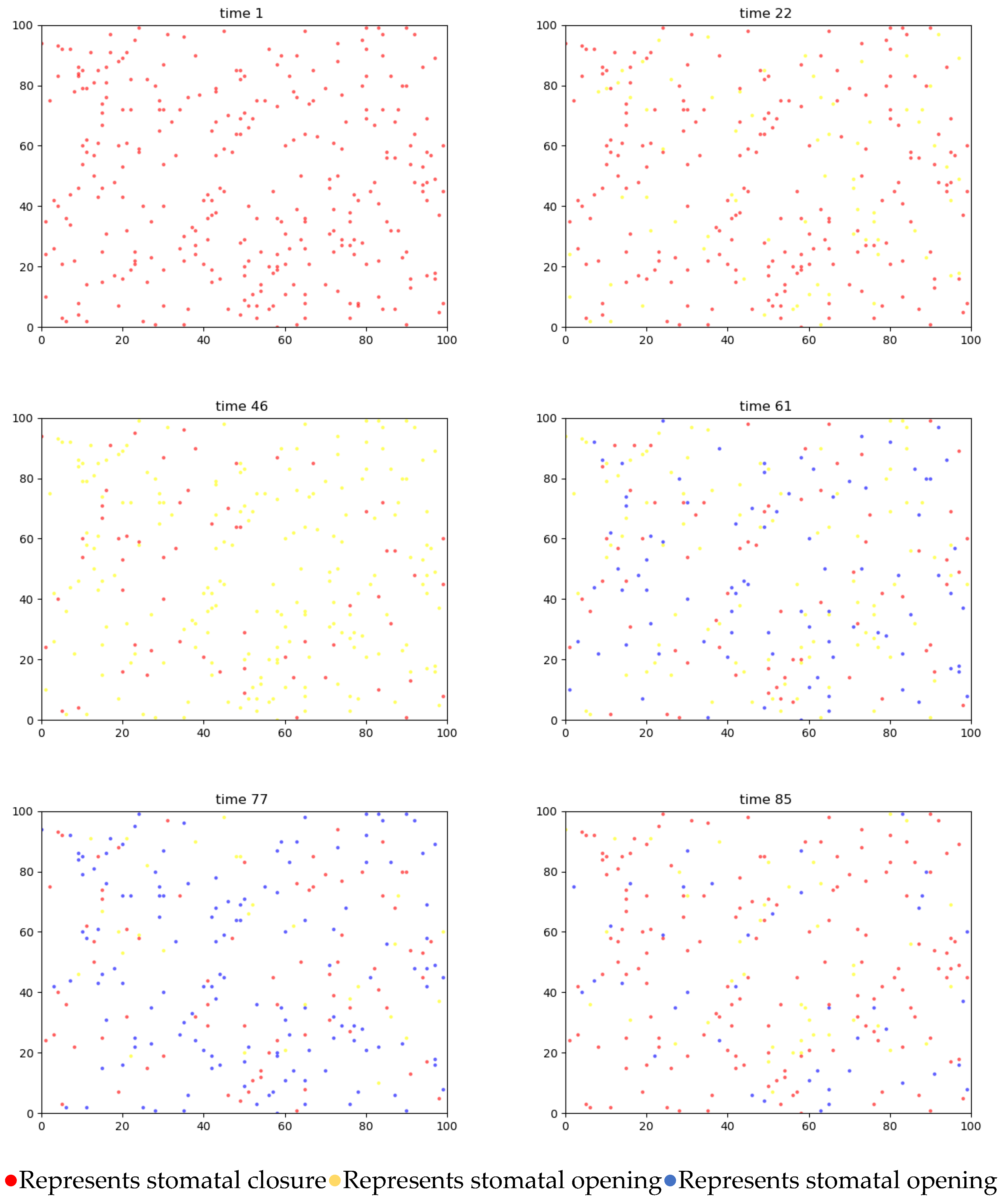
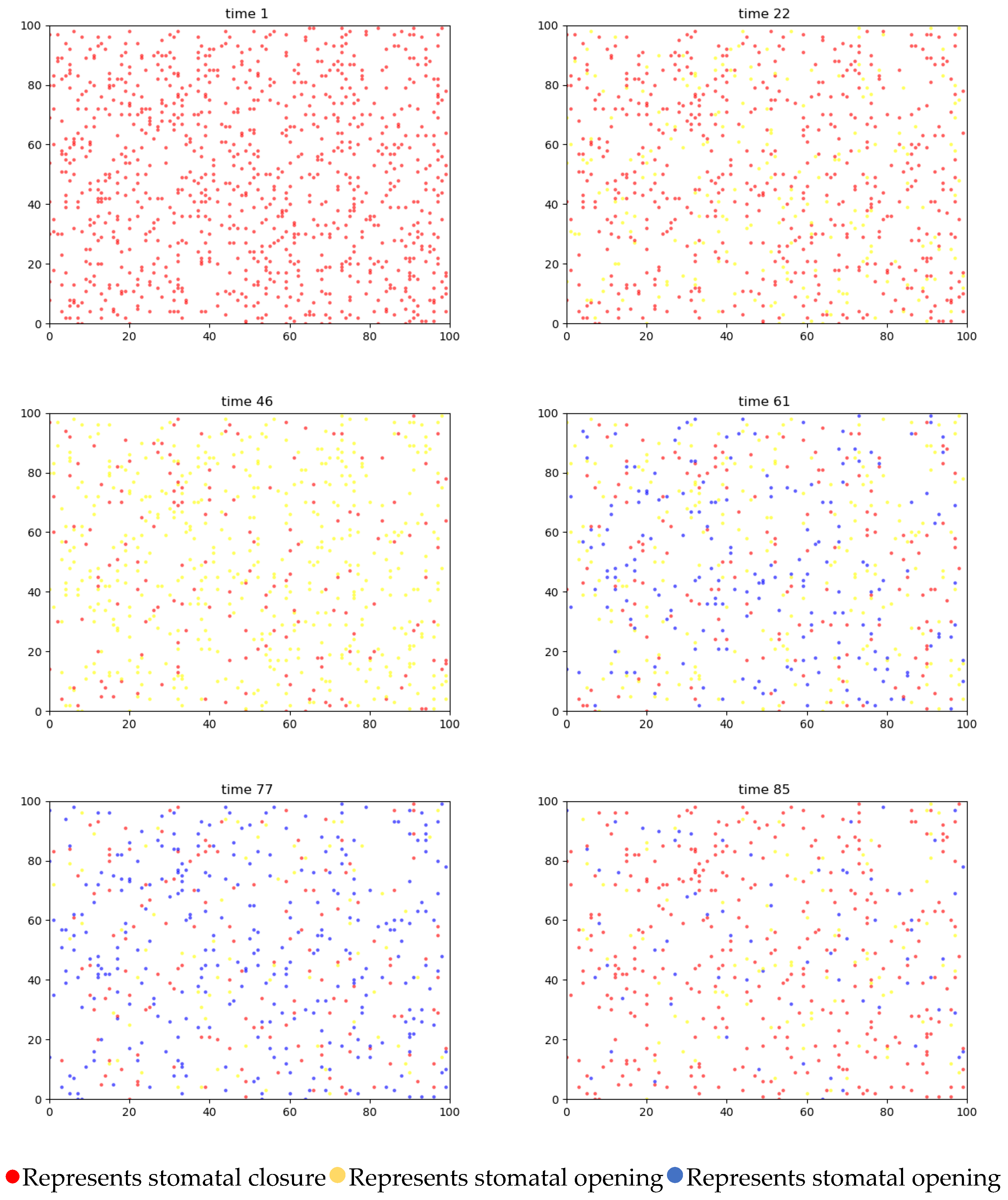
4.3.1. Two-Dimensional Simulation of Stomatal Opening Changes in the Upper and Lower Epidermis of Gynura formosana Kitam Leaves
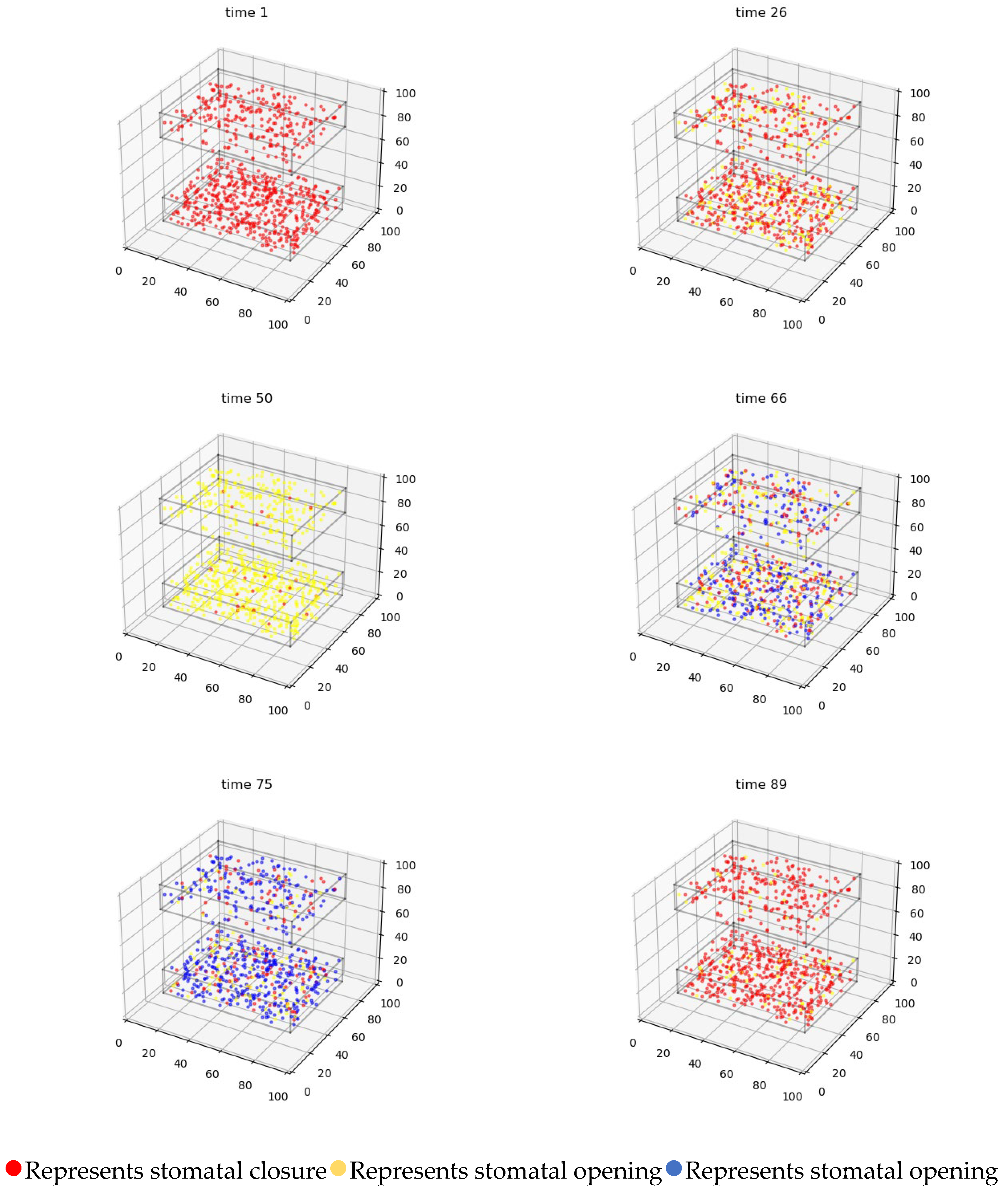
4.3.2. Three-Dimensional Simulation of Changes in Stomatal Opening on the Upper and Lower Epidermis of Gynura formosana Kitam Leaves
4.4. Simulation Analysis of Changes in Stomatal Opening on the Upper and Lower Epidermis of Gynura formosana Kitam Leaves
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, Y.; Xu, X.; Huang, Z.; Lin, Z.; Luo, X.; Shi, Y. Effects of total flavonoids from C. alba on the growth, proliferation and apoptosis of hepatocellular carcinoma Hep G2 cells. J. Xiamen Univ. (Nat. Sci. Ed.) 2019, 58, 48–55. [Google Scholar]
- Lin, Y.; Huang, T.; Lin, Y.; Shen, Y. First record of Phytophthora drechsleri on Gynura formosana. Australas. Plant Dis. Notes 2019, 14, 16. [Google Scholar] [CrossRef]
- Ma, J.; Liu, X.; Chen, J.; Lu, Y.; Shi, Y. Study on the anti-inflammatory activity and mechanism of different extracted parts of Gynura formosana Kitam. J. Anhui Agric. Univ. 2018, 45, 309–315. [Google Scholar]
- Ma, J.; Guo, C.; Pan, Y.; Lin, D.; Qiu, L.; Wen, L. Antioxidant and anti-inflammatory activities of ethyl acetate extract of Gynura formosana (Kitam) leaves. Exp. Ther. Med. 2017, 14, 2303–2309. [Google Scholar] [CrossRef]
- Fetter, K.C.; Eberhardt, S.; Barclay, R.S.; Wing, S.; Keller, S.R. Stomata Counter: A neural network for automatic stomata identification and counting. New Phytol. 2019, 223, 1671–1681. [Google Scholar] [CrossRef]
- Laga, H.; Shahinnia, F.; Fleury, D. Image-based Plant Stomata Phenotyping. In Proceedings of the 3th International Conference on Controle, Automation, Robotics and Vision (ICARCV 2014), Singapore, 10–12 December 2014. [Google Scholar]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef]
- Fanourakis, D.; Giday, H.; Hyldgaard, B.; Bouranis, D.; Körner, O.; Ottosen, C.-O. Low air humidity during cultivation promotes stomatal closure ability in rose. Eur. J. Hortic. Sci. 2019, 84, 245–252. [Google Scholar] [CrossRef]
- Fanourakis, D.; Bouranis, D.; Tsaniklidis, G.; Nejad, A.R.; Ottosen, C.-O.; Woltering, E.J. Genotypic and phenotypic differences in fresh weight partitioning of cut rose stems: Implications for water loss. Acta Physiol. Plant. 2020, 42, 48. [Google Scholar] [CrossRef]
- Fanourakis, D.; Giday, H.; Li, T.; Kambourakis, E.; Ligoxigakis, E.K.; Papadimitriou, M.; Strataridaki, A.; Bouranis, D.; Fiorani, F.; Heuvelink, E.; et al. Antitranspirant compounds alleviate the mild-desiccation-induced reduction of vase life in cut roses. Postharvest Biol. Technol. 2016, 117, 110–117. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, L.; Zhao, H.; Jiang, Z.; Cai, J. Differences in Near Isohydric and Anisohydric Behavior of Contrasting Poplar Hybrids (I-101 (Populus alba L.) × 84K(Populus alba L. × Populus glandulosa Uyeki)) under Drought-Rehydration Treatments. Forests 2020, 11, 402. [Google Scholar] [CrossRef]
- Jarvis, P.G. The interpretation of the variations in leaf water potential and stomatal conductance found in canopies in the field. Philos. Trans. R. Soc. 1976, 273, 593–610. [Google Scholar]
- Ball, T.; Woodrow, I.E.; Berry, J.A. Model predicting stomatal conductance and its contribution to the control of photosynthesis under different environmental conditions. In Progress in Photosynthesis; Biggens, J., Ed.; Springer: Dordrecht, The Netherlands, 1987; pp. 221–224. [Google Scholar]
- Wang, Y.; Zhou, L.; Lv, Y. Vehicle lane-changing rules based on metric automata traffic flow model. China J. Highw. 2008, 89–93. [Google Scholar]
- Zhao, Y.; Wang, X.; Huan, J.; Wang, Z.; Zhang, F.; Wang, X. Visual simulation of evacuation under earthquake damage scenarios. Sci. Technol. Eng. 2022, 22, 1549–1557. [Google Scholar]
- Yan, J.; Wang, L.; Cheng, L. Dynamic safety evaluation method of evacuated individuals in building fire scene. Sci. Technol. Eng. 2021, 21, 6526–6532. [Google Scholar]
- Wu, F.; Li, C.; Lin, L.; Zhu, J. A study on a simulation algorithm for crowd evacuation based on metacellular automata. J. Yanbian Univ. (Nat. Sci. Ed.) 2019, 45, 329–334. [Google Scholar]
- Cheng, M. Analysis of near-natural urban forest landscape creation in Beijing’s far suburban plains. For. Surv. Plan. 2018, 43, 175–178. [Google Scholar]
- Xu, S.; Gao, Y. Practice of wetland plant landscape creation based on multiple habitats—An example of aquatic plant area in Hangzhou Botanical Garden. Chin. Gard. 2022, 38 (Suppl. S1), 50–53. [Google Scholar]
- Zhu, X. Research on key technologies for the construction of country parks in Shanghai. China Gard. 2019, 35 (Suppl. S2), 103–106. [Google Scholar]
- Takahashi, S.; Monda, K.; Negi, J.; Konishi, F.; Ishikawa, S.; Hashimoto-Sugimoto, M.; Goto, N.; Iba, K. Natural variation in stomatal responses to environmental changes among Arabidopsis thaliana ecotypes. PLoS ONE 2015, 10, e0117449. [Google Scholar] [CrossRef]
- Peel, J.R.; Mandujano Sánchez, M.C.; López Portillo, J.; Golubov, J. Stomatal density, leaf area and plant size variation of Rhizophora mangle (Malpighiales: Rhizophoraceae) along a salinity gradient in the Mexican Caribbean. Rev. Biol. Trop. 2017, 65, 701–712. [Google Scholar] [CrossRef]
- Sumathi, M.; Bachpai, V.K.W.; Deeparaj, B.; Mayavel, A.; Dasgupta, M.G.; Nagarajan, B.; Rajasugunasekar, D.; Sivakumar, V.; Yasodha, R. Quantitative trait loci mapping for stomatal traits in interspecific hybrids of Eucalyptus. J. Genet. 2018, 97, 323–329. [Google Scholar] [CrossRef]
- Haus, M.J.; Kelsch, R.D.; Jacobs, T.W. Application of Optical Topometry to Analysis of the Plant Epidermis. Plant Physiol. 2015, 169, 946–959. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Fernandes, S.B.; Mayfield-Jones, D.; Erice, G.; Choi, M.; E Lipka, A.; Leakey, A.D.B. Optical topometry and machine learning to rapidly phenotype stomatal patterning traits for QTL mapping in maize. bioRxiv 2020. [Google Scholar] [CrossRef]
- Shi, X.; Song, Y.; Shi, X.; Lu, W.; Zhao, Y.; Zhou, Z.; Chai, J.; Liu, Z. Deep Learning for Stomatal Opening Recognition in Gynura formosana Kitam Leaves. Agronomy 2024, 14, 2622. [Google Scholar] [CrossRef]
- Chang, C.L.; Zhang, Y.J.; Gdong, Y.Y. Cellular automata for edge detection of images. In Proceedings of the 2004 International Conference on Machine Learning and Cybernetics (IEEE Cat. No. 04EX826), Shanghai, China, 26–29 August 2004; IEEE: New York, NY, USA, 2004; Volume 6, pp. 3830–3834. [Google Scholar]
- Yeh, A.G.O.; Li, X. A constrained CA model for the simulation and planning of sustainable urban forms by using GIS. Environ. Plan. B Plan. Des. 2001, 28, 733–753. [Google Scholar] [CrossRef]
- Wang, A.-L.; Wang, T.-H.; Liu, C.-G.; Hu, Z.-W. Study on CA-based structural topology design—Simulation of topology creation for planar thin plate reinforcement. Mech. Strength 2001, 181–186. [Google Scholar]
- Wu, D.; Xu, F.; Guo, W.; Wang, R.; Zhang, Z. Comparison of factors and models affecting summer stomatal conductance of common green plants in northern Chinese cities. J. Ecol. 2007, 10, 4141–4148. [Google Scholar]
- Yep, P.; Yu, Q. Mechanistic modeling of stomatal conductance in plants. J. Plant Ecol. 2009, 33, 772–782. [Google Scholar]



| Intelligent Agent Integration | Stomatal Sum |
|---|---|
| Total number of stomata | sum |
| stomatal closure | sum1 |
| Stomatal opening (small) | Sum2 |
| Stomatal opening (middle) | Sum3 |
| Stomatal opening (large) | Sum4 |
| Software | Hardware |
| Python 3.11.4 | CPU: I7-14650HX |
| Matplotlib 3.7.1 | GPU: RTX4060 |
| Numpy 1.24.3 | Operating system: Windows 11 |
| Simulate | Time Period | Number of Iterations (Every 5 min) | Simulated Stomatal Opening Rate (%) | True Stomatal Opening Rate (%) | Error Rate (%) | Number of Training Sessions | Simulated Stomatal Opening Rate (%) | True Stomatal Opening Rate (%) | Error Rate (%) |
|---|---|---|---|---|---|---|---|---|---|
| 2D | 20:00–6:00 | 120 | 0.00 | 0.00 | 0.00 | 100 | 0.05 | 0.00 | 0.05 |
| 6:00–10:00 | 168 | 0.35 | 0.32 | 0.03 | 0.28 | 0.32 | 0.04 | ||
| 10:00–12:00 | 192 | 0.88 | 0.90 | 0.02 | 0.87 | 0.90 | 0.03 | ||
| 12:00–14:00 | 216 | 0.85 | 0.89 | 0.04 | 0.88 | 0.89 | 0.01 | ||
| 14:00–18:00 | 264 | 0.75 | 0.72 | 0.03 | 0.76 | 0.72 | 0.04 | ||
| 18:00–20:00 | 288 | 0.18 | 0.20 | 0.02 | 0.16 | 0.20 | 0.04 | ||
| 3D | 20:00–6:00 | 120 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.02 | |
| 6:00–10:00 | 168 | 0.28 | 0.32 | 0.04 | 0.29 | 0.32 | 0.03 | ||
| 10:00–12:00 | 192 | 0.88 | 0.90 | 0.02 | 0.85 | 0.90 | 0.05 | ||
| 12:00–14:00 | 216 | 0.86 | 0.89 | 0.03 | 0.86 | 0.89 | 0.03 | ||
| 14:00–18:00 | 264 | 0.70 | 0.72 | 0.02 | 0.68 | 0.72 | 0.04 | ||
| 18:00–20:00 | 288 | 0.22 | 0.20 | 0.02 | 0.15 | 0.20 | 0.05 |
| Simulate | Time Period | Number of Iterations (Every 5 min) | Simulated Stomatal Opening Rate (%) | True Stomatal Opening Rate (%) | Error Rate (%) | Number of Training Sessions | Simulated Stomatal Opening Rate (%) | True Stomatal Opening Rate (%) | Error Rate (%) |
|---|---|---|---|---|---|---|---|---|---|
| 2D | 20:00–5:00 | 108 | 0.00 | 0.00 | 0.00 | 100 | 0.02 | 0.00 | 0.02 |
| 5:00–9:30 | 162 | 0.37 | 0.35 | 0.02 | 0.30 | 0.35 | 0.05 | ||
| 9:30–12:30 | 198 | 0.78 | 0.80 | 0.02 | 0.81 | 0.80 | 0.01 | ||
| 12:30–15:00 | 228 | 0.85 | 0.89 | 0.04 | 0.84 | 0.89 | 0.05 | ||
| 15:00–18:00 | 264 | 0.70 | 0.72 | 0.02 | 0.75 | 0.72 | 0.03 | ||
| 18:00–20:00 | 288 | 0.18 | 0.20 | 0.02 | 0.22 | 0.20 | 0.02 | ||
| 3D | 20:00–5:00 | 108 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.02 | |
| 5:00–9:30 | 162 | 0.39 | 0.35 | 0.03 | 0.37 | 0.35 | 0.02 | ||
| 9:30–12:30 | 198 | 0.81 | 0.80 | 0.01 | 0.84 | 0.80 | 0.04 | ||
| 12:30–15:00 | 228 | 0.86 | 0.89 | 0.03 | 0.84 | 0.89 | 0.05 | ||
| 15:00–18:00 | 264 | 0.74 | 0.72 | 0.02 | 0.69 | 0.72 | 0.03 | ||
| 18:00–20:00 | 288 | 0.25 | 0.20 | 0.05 | 0.22 | 0.20 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Song, Y.; Shi, X.; Li, P.; Wang, Y.; Jia, L.; Liu, Z. The Simulation of Stomatal Aperture Size on the Upper and Lower Epidermis of Gynura formosana Kitam Leaves Based on Cellular Automata. Agriculture 2025, 15, 878. https://doi.org/10.3390/agriculture15080878
Shi X, Song Y, Shi X, Li P, Wang Y, Jia L, Liu Z. The Simulation of Stomatal Aperture Size on the Upper and Lower Epidermis of Gynura formosana Kitam Leaves Based on Cellular Automata. Agriculture. 2025; 15(8):878. https://doi.org/10.3390/agriculture15080878
Chicago/Turabian StyleShi, Xinlong, Yanbo Song, Xiaojing Shi, Penghui Li, Yun Wang, Liyan Jia, and Zhenyu Liu. 2025. "The Simulation of Stomatal Aperture Size on the Upper and Lower Epidermis of Gynura formosana Kitam Leaves Based on Cellular Automata" Agriculture 15, no. 8: 878. https://doi.org/10.3390/agriculture15080878
APA StyleShi, X., Song, Y., Shi, X., Li, P., Wang, Y., Jia, L., & Liu, Z. (2025). The Simulation of Stomatal Aperture Size on the Upper and Lower Epidermis of Gynura formosana Kitam Leaves Based on Cellular Automata. Agriculture, 15(8), 878. https://doi.org/10.3390/agriculture15080878




