Daidzein Promotes the Proliferation of Porcine Mammary Epithelial Cells Through the mTOR Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Chemicals
2.2. Cell Culture and Treatment
2.3. Cell Viability Assay Using CCK-8
2.4. Cell Viability Assay Using EdU
2.5. Western Blot Analysis
2.6. Statistical Analysis
3. Results
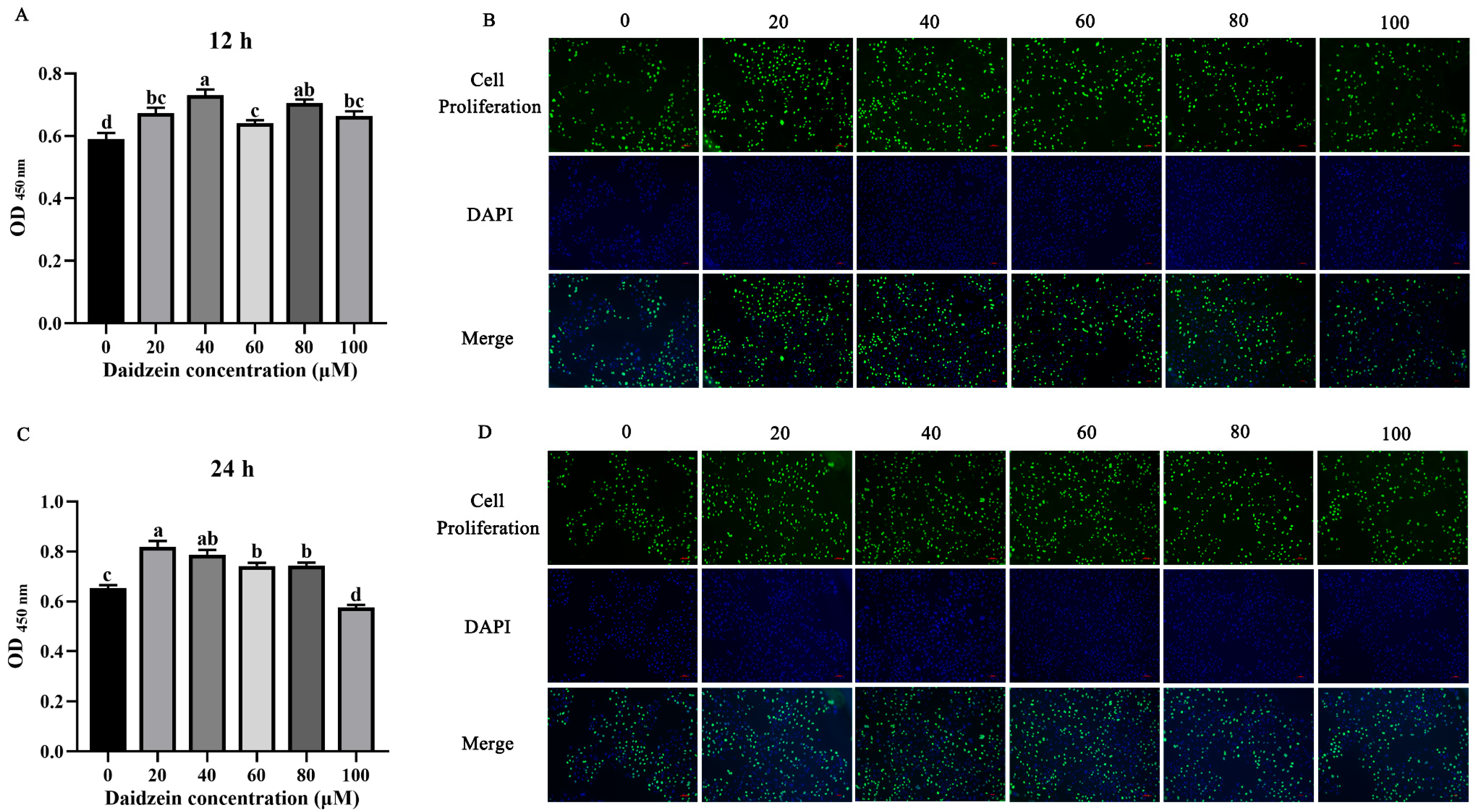
3.1. Effect of Daidzein Supplementation on PMEC Proliferation
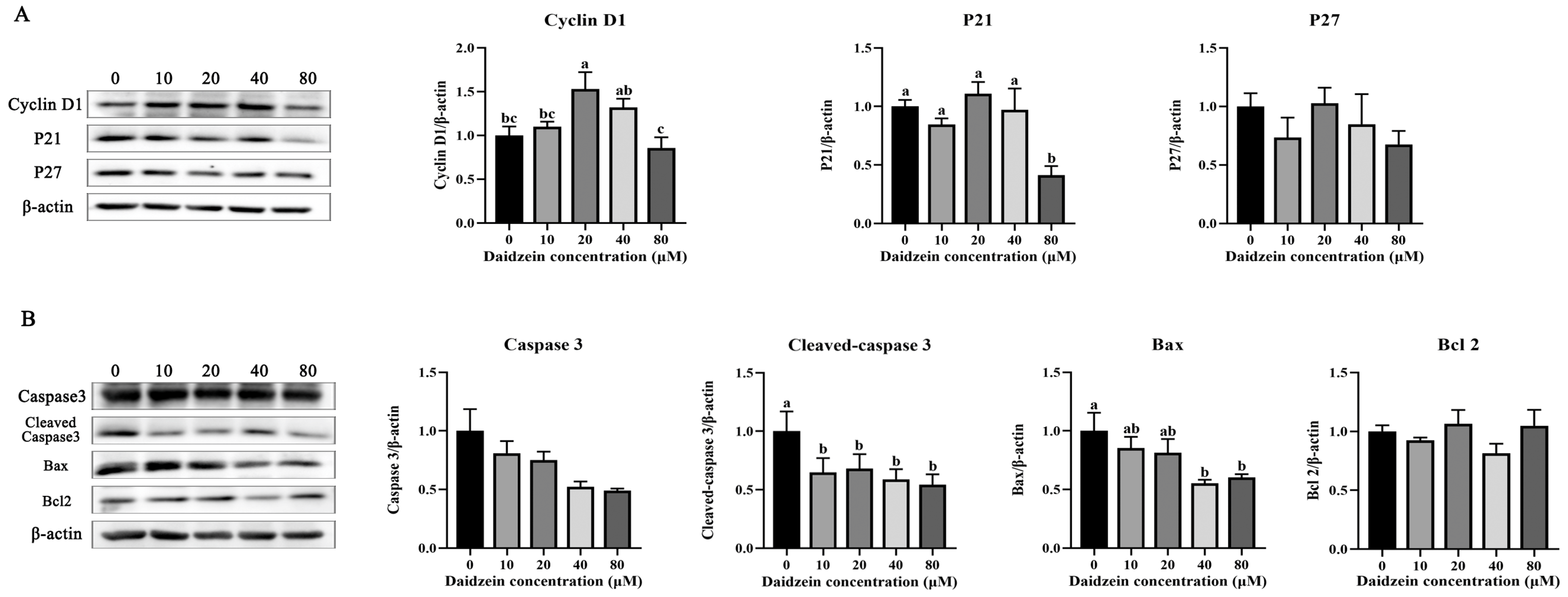
3.2. Effects of Daidzein Supplementation on the PMEC Cycle and Apoptosis
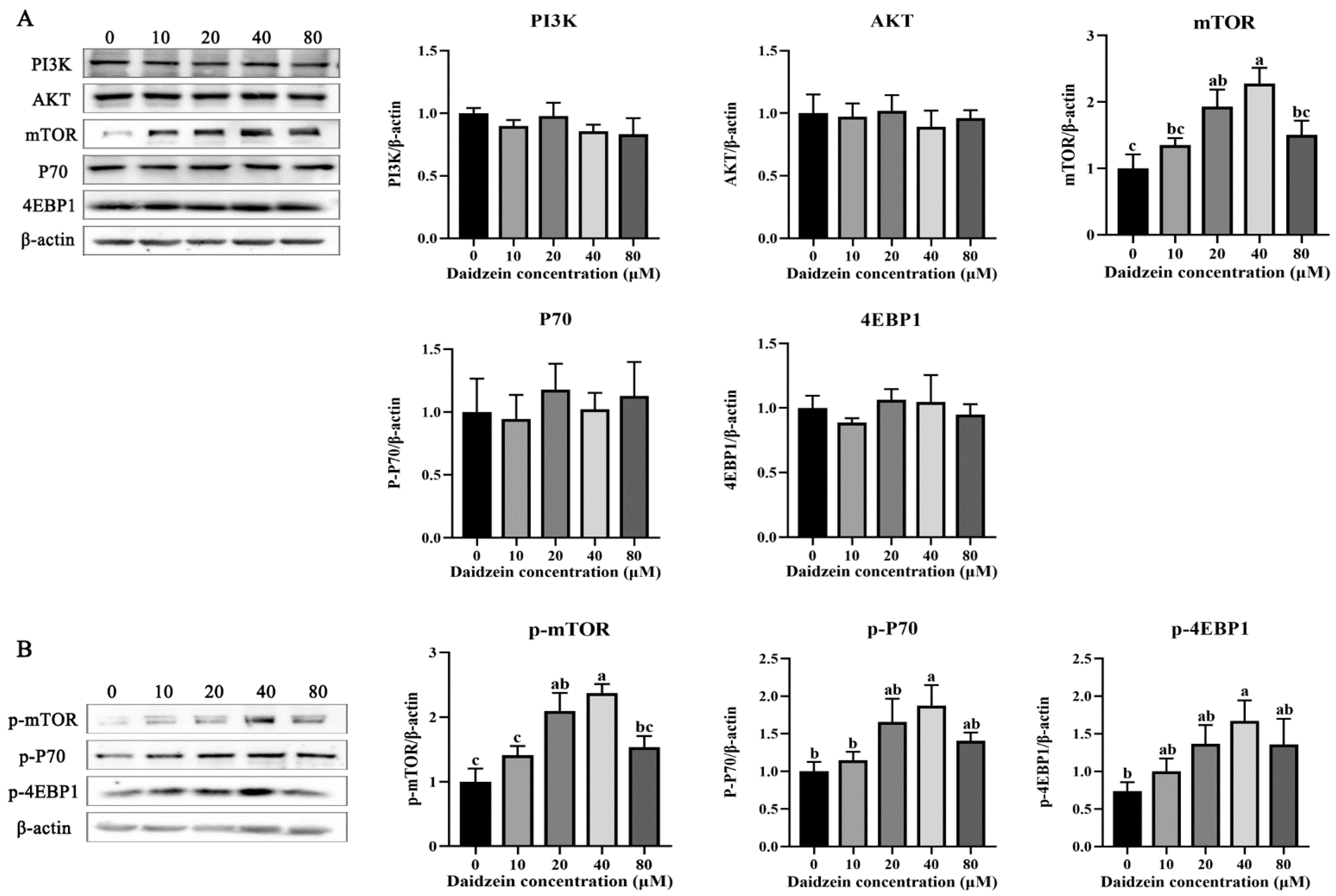
3.3. Effects of Daidzein Supplementation on PI3K/AKT/mTOR Signaling
3.4. Effects of Rapamycin Supplementation on Cell Viability
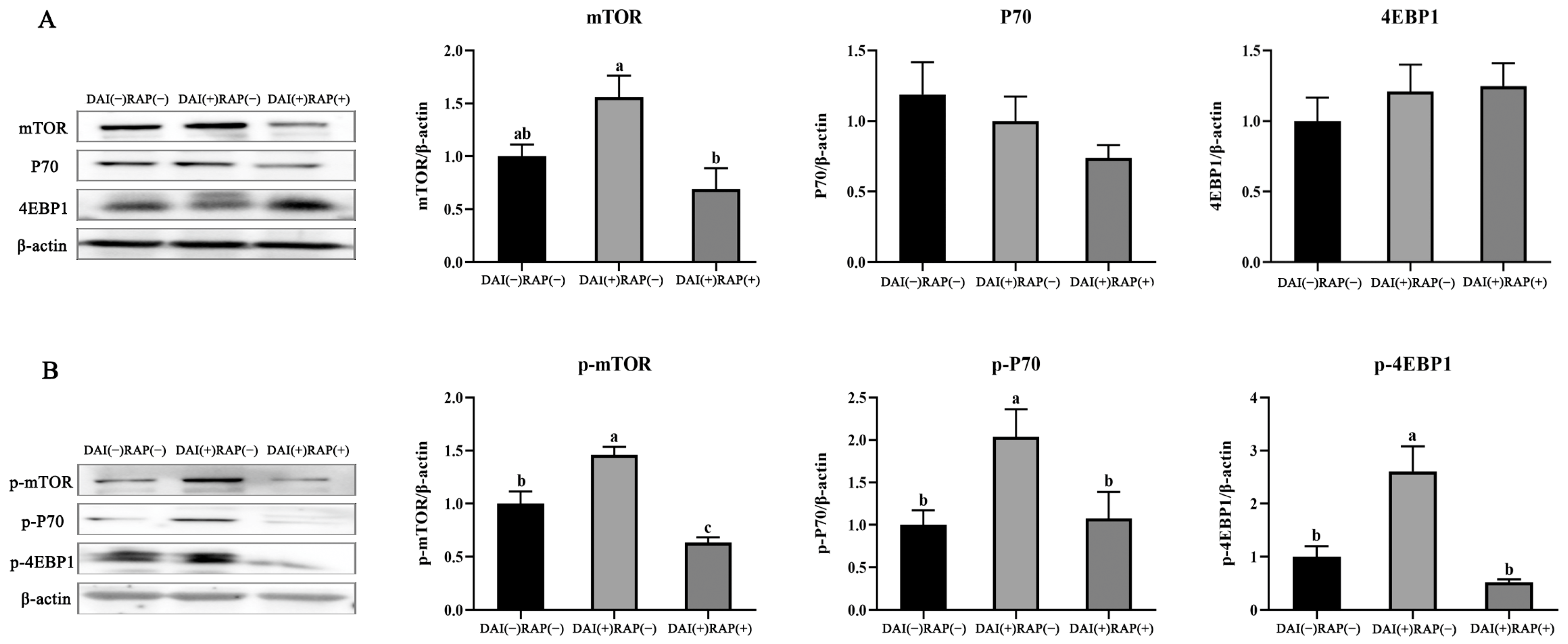
3.5. Effects of Daidzein and Rapamycin on mTOR Signaling and Downstream Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tokach, M.D.; Menegat, M.B.; Gourley, K.M.; Goodband, R.D. Review: Nutrient Requirements of the Modern High-Producing Lactating Sow, with an Emphasis on Amino Acid Requirements. Animal 2019, 13, 2967–2977. [Google Scholar] [CrossRef]
- Gormley, A.; Jang, K.B.; Garavito-Duarte, Y.; Deng, Z.; Kim, S.W. Impacts of Maternal Nutrition on Sow Performance and Potential Positive Effects on Piglet Performance. Animals 2024, 14, 1858. [Google Scholar] [CrossRef]
- Thomas, L.L.; Goodband, R.D.; Tokach, M.D.; Woodworth, J.C.; DeRouchey, J.M.; Dritz, S.S. Effect of Parity and Stage of Gestation on Growth and Feed Efficiency of Gestating Sows. J. Anim. Sci. 2018, 96, 4327–4338. [Google Scholar] [CrossRef]
- Thiengpimol, P.; Koonawootrittriron, S.; Suwanasopee, T. Assessing Reproductive Performance and Predictive Models for Litter Size in Landrace Sows under Tropical Conditions. Anim. Biosci. 2024, 37, 1333–1344. [Google Scholar] [CrossRef]
- Sampath, V.; Han, K.; Kim, I.H. Impact of Artificial Colostrum Supplement on the Growth Performance and Blood Profile in Piglets. Anim. Nutr. Feed Technol. 2022, 22, 665–672. [Google Scholar] [CrossRef]
- Farmer, C. The Gestating and Lactating Sow; Wageningen Academic Publishers: Wageningen, The Netherlands, 2015. [Google Scholar]
- Zhang, S.; Chen, F.; Zhang, Y.; Lv, Y.; Heng, J.; Min, T.; Li, L.; Guan, W. Recent Progress of Porcine Milk Components and Mammary Gland Function. J. Anim. Sci. Biotechnol. 2018, 9, 77. [Google Scholar] [CrossRef]
- Slepicka, P.F.; Somasundara, A.V.; Santos, C.D. The Molecular Basis of Mammary Gland Development and Epithelial Differentiation. Semin. Cell Dev. Biol. 2021, 114, 93–112. [Google Scholar] [CrossRef]
- Che, L.; Xu, M.; Gao, K.; Wang, L.; Yang, X.; Wen, X.; Xiao, H.; Jiang, Z. Effects of Dietary Valine Supplementation during Late Gestation on the Reproductive Performance and Mammary Gland Development of Gilts. J. Anim. Sci. Biotechnol. 2020, 11, 15. [Google Scholar] [CrossRef]
- Wang, B.; Shi, L.; Men, J.; Li, Q.; Hou, X.; Wang, C.; Zhao, F. Controlled Synchronization of Prolactin/STAT5 and AKT1/mTOR in Bovine Mammary Epithelial Cells. Vitr. Cell. Dev. Biol.-Anim. 2020, 56, 243–252. [Google Scholar] [CrossRef]
- Liu, G.Y.; Sabatini, D.M. mTOR at the Nexus of Nutrition, Growth, Ageing and Disease. Nat. Rev. Mol. Cell. Biol. 2020, 21, 246. [Google Scholar] [CrossRef]
- Feyera, T.; Theil, P.K. Energy and Lysine Requirements and Balances of Sows during Transition and Lactation: A Factorial Approach. Livest. Sci. 2017, 201, 50–57. [Google Scholar] [CrossRef]
- Moreira, L.P.; Menegat, M.B.; Barros, G.P.; Bernardi, M.L.; Wentz, I.; Bortolozzo, F.P. Effects of Colostrum, and Protein and Energy Supplementation on Survival and Performance of Low-birth-weight Piglets. Livest. Sci. 2017, 202, 188–193. [Google Scholar] [CrossRef]
- Ferrari, C.V.; Sbardella, P.E.; Bernardi, M.L.; Coutinho, M.L.; Vaz, I.S., Jr.; Wentz, I.; Bortolozzo, F.P. Effect of Birth Weight and Colostrum Intake on Mortality and Performance of Piglets after Cross-fostering in Sows of Different Parities. Prev. Vet. Med. 2014, 114, 259–266. [Google Scholar] [CrossRef]
- Wang, Q.; Ge, X.; Tian, X.; Zhang, Y.; Zhang, J.; Zhang, P. Soy Isoflavone: The Multipurpose Phytochemical (Review). Biomed. Rep. 2013, 1, 697–701. [Google Scholar] [CrossRef]
- Cools, S.; Broeck, W.V.; Vanhaecke, L.; Heyerick, A.; Bossaert, P.; Hostens, M.; Opsomer, G. Feeding Soybean Meal Increases the Blood Level of Isoflavones and Reduces the Steroidogenic Capacity in Bovine Corpora Lutea, without Affecting Peripheral Progesterone Concentrations. Anim. Reprod. Sci. 2014, 144, 79–89. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, L.; Deng, S.; Liang, M. Daidzein Ameliorates LPS-induced Hepatocyte Injury by Inhibiting Inflammation and Oxidative Stress. Eur. J. Pharmacol. 2020, 885, 173399. [Google Scholar] [CrossRef]
- Kao, T.; Chen, B.H. Functional Components in Soybean Cake and Their Effects on Antioxidant Activity. J. Agric. Food. Chem. 2006, 54, 7544–7555. [Google Scholar] [CrossRef]
- Yu, F.; Liu, Z.; Tong, Z.; Zhao, Z.; Liang, H. Soybean Isoflavone Treatment Induces Osteoblast Differentiation and Proliferation by Regulating Analysis of Wnt/β-catenin Pathway. Gene 2015, 573, 273–277. [Google Scholar] [CrossRef]
- Hüser, S.; Guth, S.; Joost, H.G.; Soukup, S.T.; Köhrle, J.; Kreienbrock, L.; Diel, P.; Lachenmeier, D.W.; Eisenbrand, G.; Vollmer, G.; et al. Effects of Isoflavones on Breast Tissue and the Thyroid Hormone System in Humans: A Comprehensive Safety Evaluation. Arch. Toxicol. 2018, 92, 2703–2748. [Google Scholar] [CrossRef]
- Mortensen, A.; Kulling, S.E.; Schwartz, H.; Rowland, I.; Ruefer, C.E.; Rimbach, G.; Cassidy, A.; Magee, P.; Millar, J.; Hall, W.L.; et al. Analytical and Compositional Aspects of Isoflavones in Food and Their Biological Effects. Mol. Nutr. Food. Res. 2009, 2, S266–S309. [Google Scholar] [CrossRef]
- Lamartiniere, C.; Wang, J. Prepubertal Genistein Exposure Suppresses Mammary Cancer Development and Alters Proteomic Signature in Adult Rats. Toxicol. Lett. 2010, 196, S173–S174. [Google Scholar] [CrossRef]
- Yu, M.; Qi, H.; Gao, X. Daidzein Promotes Milk Synthesis and Proliferation of Mammary Epithelial Cells via the Estrogen Receptor A-dependent NfκB1 Activation. Anim. Biotechnol. 2022, 33, 43–52. [Google Scholar] [CrossRef]
- Dahanayaka, S.; Rezaei, R.; Porter, W.W.; Johnson, G.A.; Burghardt, R.C.; Bazer, F.W.; Hou, Y.Q.; Wu, Z.L.; Wu, G. Technical Note: Isolation and Characterization of Porcine Mammary Epithelial Cells. J. Anim. Sci. 2015, 93, 5186–5193. [Google Scholar] [CrossRef]
- Che, L.; Liu, L.; Xu, M.; Fan, Z.; Niu, L.; Chen, Y.; Chang, X.; Zhou, P.; Li, M.; Deng, H.; et al. Valine Metabolite, 3-Hydroxyisobutyrate, Promotes Lipid Metabolism and Cell Proliferation in Porcine Mammary Gland Epithelial Cells. Front. Nutr. 2025, 11, 1524738. [Google Scholar] [CrossRef]
- Alshehri, M.M.; Sharifi-Rad, J.; Herrera-Bravo, J.; Jara, E.L.; Salazar, L.A.; Kregiel, D.; Uprety, Y.; Akram, M.; Iqbal, M.; Martorell, M.; et al. Therapeutic Potential of Isoflavones with an Emphasis on Daidzein. Oxid. Med. Cell. Longev. 2021, 9, 6331630. [Google Scholar] [CrossRef]
- Liu, J.; Fu, Y.; Zhou, S.; Zhao, P.; Zhao, J.; Yang, Q.; Wu, H.; Ding, M.; Li, Y. Comparison of the Effect of Quercetin and Daidzein on Production Performance, Anti-oxidation, Hormones, and Cecal Microflora in Laying Hens during the Late Laying Period. Poultry Sci. 2023, 102, 102674. [Google Scholar] [CrossRef]
- Obianwuna, U.E.; Chang, X.; Wu, R.; Zhang, H.; Qiu, K.; Wu, S. Effect of Dietary Daidzein Supplementation on Reproductive Performance, Egg Quality and Bone Mineralization in Laying Hens at First-cycle of Production via Reproductive-growth Axis. Ital. J. Anim. Sci. 2024, 23, 1169–1180. [Google Scholar] [CrossRef]
- Jin, X.; Sun, J.; Yu, B.; Wang, Y.; Sun, W.J.; Yang, J.; Huang, S.H.; Xie, W.L. Daidzein Stimulates Osteogenesis Facilitating Proliferation, Differentiation, and Antiapoptosis in Human Osteoblast-Like Mg-63 Cells via Estrogen Receptor-Dependent MEK/ERK and PI3K/Akt Activation. Nutr. Res. 2017, 42, 20–30. [Google Scholar] [CrossRef]
- Chen, W.F.; Wong, M.S. Genistein Enhances Insulin-like Growth Factor Signaling Pathway in Human Breast Cancer (MCF-7) cells. J. Clin. Endocrinol. Metab. 2004, 89, 2351–2359. [Google Scholar] [CrossRef]
- Ma, X.; Cui, Y.; Tian, Z.; Yu, M. Effect of Soybean Isoflavones on Proliferation and Related Gene Expression of Sow Mammary Gland Cells In Vitro. Animals 2022, 12, 3241. [Google Scholar] [CrossRef]
- Park, J.; Choi, H.; Shim, K. Inhibition of GSK3β Promotes Proliferation and Suppresses Apoptosis of Porcine Muscle Satellite Cells. Animals 2022, 12, 3328. [Google Scholar] [CrossRef]
- Dong, J.; Wang, Z.; Fei, F.; Jiang, Y.; Jiang, Y.; Guo, L.; Liu, K.; Cui, L.; Meng, X.; Li, J.; et al. Selenium Enhances the Growth of Bovine Endometrial Stromal Cells by PI3K/AKT/GSK-3β and Wnt/β-Catenin Pathways. Vet. Sci. 2024, 11, 674. [Google Scholar] [CrossRef]
- Qiao, J.; Tang, C.; Xie, M.; Gong, M.; Fu, C.; Cheng, Z.; Chen, Z.; Mei, A.; Bo, Y.; Zhao, M.; et al. Aberrant activation of the mTOR signaling pathway in Rasmussen encephalitis. Sci. Rep. 2025, 15, 6347. [Google Scholar] [CrossRef]
- Szwed, A.; Kim, E.; Jacinto, E. Regulation and Metabolic Functions of mTORC1 and mTORC2. Physiol. Rev. 2021, 101, 1371–1426. [Google Scholar] [CrossRef]
- Ragupathi, A.; Kim, C.; Jacinto, E. The mTORC2 Signaling Network: Targets and Cross-talks. Biochem. J. 2024, 481, 45–91. [Google Scholar] [CrossRef]
- Bar-Peled, L.; Sabatini, D.M. Regulation of mTORC1 by Amino Acids. Trends Cell Biol. 2014, 24, 400–406. [Google Scholar] [CrossRef]
- Che, L.; Xu, M.; Gao, K.; Wang, L.; Yang, X.; Wen, X.; Xiao, H.; Jiang, Z.; Wu, D. Valine Supplementation During Late Pregnancy in Gilts Increases Colostral Protein Synthesis Through Stimulating mTOR Signaling Pathway in Mammary Cells. Amino Acids 2019, 51, 1547–1559. [Google Scholar] [CrossRef]
- Cuyàs, E.; Corominas-Faja, B.; Joven, J.; Menendez, J.A. Cell Cycle Regulation by the Nutrient-sensing Mammalian Target of Rapamycin (mTOR) Pathway. Methods Mol. Biol. 2014, 1170, 113–144. [Google Scholar]
- Sencan, S.; Tanriover, M.; Ulasli, M.; Karakas, D.; Ozpolat, B. UV Radiation Resistance-Associated Gene (Uvrag) Promotes Cell Proliferation, Migration, Invasion by Regulating Cyclin-Dependent Kinases (Cdk) and Integrin-Β/Src Signaling in Breast Cancer Cells. Mol. Cell. Biochem. 2021, 476, 2075–2084. [Google Scholar] [CrossRef]
- Mori, S.; Nada, S.; Kimura, H.; Tajima, S.; Takahashi, Y.; Kitamura, A.; Oneyama, C.; Okada, M. The mTOR Pathway Controls Cell Proliferation by Regulating the FoxO3a Transcription Factor via SGK1 Kinase. PLoS ONE 2014, 9, e88891. [Google Scholar] [CrossRef]
- Gitego, N.; Agianian, B.; Mak, O.W.; Mv, V.K.; Cheng, E.H.; Gavathiotis, E. Chemical Modulation of Cytosolic BAX Homodimer Potentiates BAX Activation and Apoptosis. Nat. Commun. 2023, 14, 8381. [Google Scholar] [CrossRef] [PubMed]
- Czabotar, P.E.; Garcia-Saez, A.J. Mechanisms of BCL-2 Family Proteins in Mitochondrial Apoptosis. Nat. Rev. Mol. Cell Biol. 2023, 24, 732–748. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Jiang, X.; Liu, Y.; Jiang, X.; Che, L.; Lin, Y.; Zhuo, Y.; Feng, B.; Fang, Z.; Hua, L.; et al. Silibinin Alleviates Lipopolysaccharide Induced Inflammation in Porcine Mammary Epithelial Cells via mTOR/NF-κB Signaling Pathway. Mol. Nutr. Food. Res. 2023, 67, e2200715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Zhang, J.; Qi, X.; Cui, Y.; Yin, K.; Lin, H. Cadmium Induced Inflammation and Apoptosis of Porcine Epididymis via Activating RAF1/MEK/ERK and NF-κB Pathways. Toxicol. Appl. Pharm. 2021, 415, 115449. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Liu, L.; Duan, W.; Niu, L.; Cheng, H.; Du, C.; Li, M.; Huo, W.; Deng, H.; Zhou, P.; et al. Daidzein Promotes the Proliferation of Porcine Mammary Epithelial Cells Through the mTOR Signaling Pathway. Agriculture 2025, 15, 930. https://doi.org/10.3390/agriculture15090930
Xu M, Liu L, Duan W, Niu L, Cheng H, Du C, Li M, Huo W, Deng H, Zhou P, et al. Daidzein Promotes the Proliferation of Porcine Mammary Epithelial Cells Through the mTOR Signaling Pathway. Agriculture. 2025; 15(9):930. https://doi.org/10.3390/agriculture15090930
Chicago/Turabian StyleXu, Mengmeng, Le Liu, Wenjing Duan, Lizhu Niu, He Cheng, Chenyang Du, Mengyun Li, Wenying Huo, Hongyu Deng, Pan Zhou, and et al. 2025. "Daidzein Promotes the Proliferation of Porcine Mammary Epithelial Cells Through the mTOR Signaling Pathway" Agriculture 15, no. 9: 930. https://doi.org/10.3390/agriculture15090930
APA StyleXu, M., Liu, L., Duan, W., Niu, L., Cheng, H., Du, C., Li, M., Huo, W., Deng, H., Zhou, P., Chen, W., & Che, L. (2025). Daidzein Promotes the Proliferation of Porcine Mammary Epithelial Cells Through the mTOR Signaling Pathway. Agriculture, 15(9), 930. https://doi.org/10.3390/agriculture15090930




