Photosynthesis and Yellow Vine Syndrome of American Cranberry
Abstract
:1. Introduction

2. Photosynthesis
3. Yellow Vine Syndrome of Cranberry
3.1. Effect of Shade Treatment
| Unshaded yellow vine leaves | Shaded yellow vine leaves | Change (%) | |
|---|---|---|---|
| Chl a (mg/g) | 0.99 ± 0.05 | 1.10 ± 0.06 | +11.1 |
| Chl b (mg/g) | 0.72 ± 0.04 | 0.82 ± 0.04 | +13.9 |
| Chl a/ Chl b | 1.37 ± 0.06 | 1.34 ± 0.06 | −0.02 |

| Sample Conditions | Fv/Fm | “Area” (×105 unit) | PI |
|---|---|---|---|
| Unshaded yellow vine leaves | 0.72 ± 0.10 | 1.9 ± 0.9 | 0.32 ± 0.20 |
| Shaded yellow vine leaves | 0.85 ± 0.01 | 2.1 ± 0.4 | 0.35 ± 0.06 |
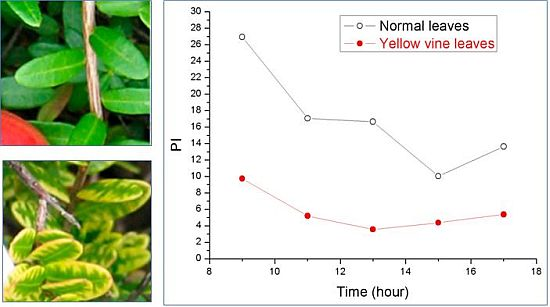
3.2. Photosynthetic Activity
| Normal leaves (mg/g fresh weight) | Yellow vine syndrome leaves (mg/g fresh weight) | Change (%) | |
|---|---|---|---|
| Chl a | 1.30 ± 0.09 | 0.99 ± 0.10 | 23.8 |
| Chl b | 0.93 ± 0.06 | 0.72 ± 0.09 | 22.6 |
| Chl a/Chl b ratio | 1.40 | 1.40 | 0 |
| FV/FM | Area | RC/ABS | PI | |
|---|---|---|---|---|
| Normal leave | 100 ± 5 | 100 ± 15 | 100 ± 8 | 100 ± 25 |
| Yellow vine | 72 ± 4 | 40 ± 6 | 43 ± 5 | 11 ± 3 |

3.3. Working Model and Hypothesis
4. Conclusions
Acknowledgments
References
- Cranberry production: A guide for Massachusetts, 2008. UMass Cranberry Station Web site. Available online: http://scholarworks.umass.edu/cgi/viewcontent.cgi?article=1000&context=cranberry_prod_guide (accessed on 18 January 2011).
- Kalgaonkar, S.; Gross, H.B.; Yokoyama, W.; Keen, C.L. Effects of a flavonol-rich diet on select cardiovascular parameters in a golden syrian hamster model. J. Med. Food 2010, 13, 108–115. [Google Scholar] [CrossRef]
- Neto, C.C.; Amoroso Jon, W.; Liberty Anne, M. Anticancer activities of cranberry phytochemicals: An update. Mol. Nutr. Food Res. 2008, 52, S18–S27. [Google Scholar]
- Neto, C.C. Cranberry and its phytochemicals: A review of in vitro anticancer studies. J. Nutr. 2007, 137, 186–193. [Google Scholar]
- Lipson, S.M.; Cohen, P.; Zhou, J.; Burdowski, A.; Stotzky, G. Cranberry cocktail juice, cranberry concentrates, and proanthocyanidins reduce reovirus infectivity titers in African green monkey kidney epithelial cell cultures. Mol. Nutr. Food Res. 2007, 51, 752–758. [Google Scholar] [CrossRef]
- Deyhim, F.; Patil, B.S.; Villarreal, A.; Lopez, E.; Garcia, K.; Rios, R.; Garcia, C.; Gonzales, C.; Mandadi, K. Cranberry juice increases antioxidant status without affecting cholesterol homeostasis in orchidectomized rats. J. Med. Food 2007, 10, 49–53. [Google Scholar] [CrossRef]
- Imsande, J. Iron, sulfur, and chlorophyll deficiencies: A need for an integrative approach in plant physiology. Physiol. Plant 1998, 103, 139–144. [Google Scholar]
- Abadia, J. Leaf responses to iron deficiency: A review. J. Plant Nutr. 1992, 15, 1699–1713. [Google Scholar] [CrossRef]
- Davies, J.P.; Grossman, A.R. Responses to deficiencies in macronutrients. In Molecular Biology of Chloroplasts and Mitochondria in Chlamydomonas; Rochaix, J.-D., Goldschmidt-Clermont, M., Merchant, S., Eds.; Springer: New York, NY, USA, 1998; pp. 613–635. [Google Scholar]
- Cornic, G.; Massacci, A. Leaf photosynthesis under drought stress. In Photosynthesis and the Environment; Baker, N.R., Ed.; Springer: New York, NY, USA, 1996; pp. 347–366. [Google Scholar]
- Pshybytko, N.L.; Kruk, J.; Kabashnikova, L.F.; Strzalka, K. Function of plastoquinone in heat stress reactions of plants. Biochim. Biophys. Acta 2008, 1777, 1393–1399. [Google Scholar] [CrossRef]
- Gombos, Z.; Murata, N. Genetic engineering of the unsaturation of membrane glycerolipid: effects on the ability of the photosynthetic machinery to tolerate temperature stress. In Lipids in Photosynthesis: Structure, Function and Genetics; Siegenthaler, P.-A., Murata, N., Eds.; Springer: New York, NY, USA, 1998; pp. 249–262. [Google Scholar]
- Kasahara, M.; Wada, M. Chloroplast avoidance movement. Ann. Rev. Plant Biol. 2005, 13, 267–282. [Google Scholar]
- Tevini, M. Plant responses to ultraviolet radiation stress. In Chlorophyll a Fluorescence; Papageorgiou, G.C., Govindjee, Eds.; Springer: New York, NY, USA, 2004; pp. 605–621. [Google Scholar]
- Demetriou, G.; Neonaki, C.; Navakoudis, E.; Kotzabasis, K. Salt stress impact on the molecular structure and function of the photosynthetic apparatus—The protective role of polyamines. Biochim. Biophys. Acta 1767, 272–280. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Ann. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Mommer, L.; Visser, E.J.W. Underwater photosynthesis in flooded terrestrial plants: A matter of leaf plasticity. Anna. Bot. 2005, 96, 581–589. [Google Scholar] [CrossRef]
- Sack, L.; Holbrook, N.M. Leaf hydraulics. Ann. Rev. Plant Biol. 2006, 57, 361–381. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Flooding stress: Acclimations and genetic diversity. Ann. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef]
- Nishida, I.; Murata, N. Chilling sensitivity in plants and cyanobacteria: The crucial contribution of membrane lipids. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 541–568. [Google Scholar] [CrossRef]
- DeMoranville, C.; Lampinen, B. Cranberry Station Newsletter; UMass Cranberry Station: East Wareham, MA, USA, 1999; Volume 8, pp. 2–3. [Google Scholar]
- Blankenship, R.E. Molecular Mechanisms of Photosynthesis; Blackwell Science: Bristol, UK, 2002. [Google Scholar]
- Brudvig, G.W. Water oxidation chemistry of photosystem II. Philos. Trans. R. Soc. B 2008, 363, 1211–1219. [Google Scholar] [CrossRef]
- Rutherford, A.W.; Boussac, A. Biochemistry: Water photolysis in biology. Science 2004, 303, 1782–1784. [Google Scholar] [CrossRef]
- Kramer, D.M. The photonic smart grid of the chloroplast in action. Proc. Natl. Acad. Sci. USA 2010, 107, 2729–2730. [Google Scholar] [CrossRef]
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef]
- Bailey, S.; Grossman, A. Photoprotection in cyanobacteria: regulation of light harvesting. Photochem. Photobiol. 2008, 84, 1410–1420. [Google Scholar] [CrossRef]
- Vener, A.V. Environmentally modulated phosphorylation and dynamics of proteins in photosynthetic membranes. Biochim. Biophys. Acta 2007, 1767, 449–457. [Google Scholar]
- Kumudini, S. Effect of radiation and temperature on cranberry photosynthesis and characterization of diurnal change in photosynthesis. J. Am. Soc. Hort. Sci. 2004, 129, 106–111. [Google Scholar]
- Vanden Heuvel, J.E.; Davenport, J.R. Effect of light, temperature, defoliation, and fruiting on carbon assimilation and partitioning in potted cranberry. HortScience 2005, 40, 1699–1704. [Google Scholar]
- Forney, C.F.; Kalt, W.; Abrams, S.R.; Owen, S.J. Effects of postharvest light and ABA treatments on the composition of late-harvested white cranberry fruit. Acta Hortic. 2009, 810, 799–806. [Google Scholar]
- Zhou, Y.; Singh, B.R. Effect of light on anthocyanin levels in submerged, harvested cranberry fruit. J. Biomed. Biotechnol. 2004, 5, 259–263. [Google Scholar] [CrossRef]
- Sicuranza, J.; Jeranyama, P.; Hou, H.J.M.; DeMoranville, C. Shade effects on chlorophyll content and nutrient content of cranberry vines exhibiting yellow vine symptoms. HortScience 2009, 44, 553. [Google Scholar]
- Wei, Z.; Jeranyama, P.; Zhang, F.; DeMoranville, C.; Hou, H.J.M. Probing the mechanisms of the yellow vine syndrome development in cranberry: Shade effect. HortScience 2010, 45, 1345–1348. [Google Scholar]
- Tanaka, R.; Tanaka, A. Tetrapyrrole biosynthesis in higher plants. Annu. Rev. Plant Biol. 2007, 58, 321–346. [Google Scholar] [CrossRef]
- Ito, H.; Yokono, M.; Tanaka, R.; Tanaka, A. Identification of a novel vinyl reductase gene essential for the biosynthesis of monovinyl chlorophyll in Synechocystis sp. PCC6803. J. Biol. Chem. 2008, 283, 9002–9011. [Google Scholar] [CrossRef]
- Masuda, T. Recent overview of the Mg branch of the tetrapyrrole biosynthesis leading to chlorophylls. Photosynth. Res. 2008, 96, 121–143. [Google Scholar] [CrossRef]
- Nedbal, L.; Samson, G.; Whitmarsh, J. Redox state of a one-electron component controls the rate of photoinhibition of photosystem II. Proc. Natl. Acad. Sci. USA 1992, 89, 7929–7933. [Google Scholar]
- Tracewell, C.A.; Cua, A.; Stewart, D.H.; Bocian, D.F.; Brudvig, G.W. Characterization of carotenoid and chlorophyll photooxidation in photosystem II. Biochemistry 2001, 40, 193–203. [Google Scholar]
- Telfer, A.; Barber, J. Evidence for the photoinduced oxidation of the primary electron donor P680 in the isolated photosystem II reaction center. FEBS Lett. 1989, 246, 223–228. [Google Scholar] [CrossRef]
- Hou, J.; Kuang, T.; Peng, D.; Tang, C.; Tang, P. The photodamage and protective role of pheophytin a in the photosystem II reaction center against light-induced damage. Prog. Nat. Sci. 1996, 6, 489–493. [Google Scholar]
- Melis, A. Photosystem-II damage and repair cycle in chloroplasts: What modulates the rate of photodamage in vivo? Trends Plant Sci. 1999, 4, 130–135. [Google Scholar] [CrossRef]
- Hou, J.-M.; Kuang, T.-Y.; Peng, D.-C.; Tang, C.-Q.; Tang, P.-S. Photoinduced damage of the photosystem II primary electron donor P680. In Photosynthesis: Mechanisms and Effects; Garab, G., Ed.; Kluwers Academic Publishers: Norwell, MA, USA, 1998; Volume 3, pp. 2119–2122. [Google Scholar]
- Telfer, A.; Bishop, S.M.; Phillips, D.; Barber, J. Isolated photosynthetic reaction center of photosystem II as a sensitizer for the formation of singlet oxygen. Detection and quantum yield determination using a chemical trapping technique. J. Biol. Chem. 1994, 269, 13244–13253. [Google Scholar]
- Krause, G.H.; Weis, E. Chlorophyll fluorescence and photosynthesis: The basics. Annu. Rev.Plant Physiol. Plant Mol. Biol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Bazzaz, F.A. From leaves to ecosystems: Using chlorophyll fluorescence to assess photosynthesis and plant function in ecological studies. In Chlorophyll a Fluorescence; Papageorgiou, G.C., Govindjee, Eds.; Springer: New York, NY, USA, 2004; pp. 737–755. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence; Papageorgiou, G.C., Govindjee, Eds.; Springer: New York, NY, USA, 2004; pp. 321–362. [Google Scholar]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Adams, W.W., III; Demmig-Adams, B. Chlorophyll fluorescence as a tool to monitor plant response to the environment. In Chlorophyll a Fluorescence; Papageorgiou, G.C., Govindjee, Eds.; Springer: New York, NY, USA, 2004; pp. 583–604. [Google Scholar]
- Srivastava, A.; Strasser, R.J.; Govindjee. Greening of peas: Parallel measurements of 77 K emission spectra, OJIP chlorophyll a fluorescence transient, period four oscillation of the initial fluorescence level, delayed light emission, and P700. Photosynthetica 1999, 37, 365–392. [Google Scholar] [CrossRef]
- Kruger, G.H.J.; Tsimilli-Michael, M.; Strasser, R.J. Light stress provokes plastic and elastic modifications in structure and function of photosystem II in camellia leaves. Physiol. Plant. 1997, 101, 265–277. [Google Scholar] [CrossRef]
- Strasser Reto, J. Energy pipeline model. Prog. Bot. Res. 1987, 2, 717–720. [Google Scholar]
- Toth, S.Z.; Schansker, G.; Strasser, R.J. A noninvasive assay of the plastoquinone pool redox state based on the OJIP transient. Photosynth. Res. 2007, 93, 193–203. [Google Scholar] [CrossRef]
- De las Rivas, J.; Abadia, A.; Abadia, J. A new reversed-phase HPLC method resolving all major higher plant photosynthetic pigments. Plant Physiol. 1989, 91, 190–192. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Borrmann, D.; Castelhano de Andrade, J.; Lanfer-Marquez, U.M. Chlorophyll degradation and formation of colorless chlorophyll derivatives during soybean (glycine max L. Merill) seed maturation. J. Agric. Food Chem. 2009, 57, 2030–2034. [Google Scholar]
- Shioi, Y.; Fukae, R.; Sasa, T. Chlorophyll analysis by high-performance liquid chromatography. Biochim. Biophys. Acta 1983, 722, 72–79. [Google Scholar] [CrossRef]
- DeMoranville, C. Cranbery best management practice adoption and conservation farm planning in Massachusetts. Hort. Tech. 2006, 16, 393–397. [Google Scholar]
- Zhang, F.; Wei, Z.; Jeranyama, P.; Demoranville, C.; Hou, H.J.M. A significant loss in photosynthetic activity associated with the yellow vine syndrome of cranberry. HortScience 2011, 49, 901–907. [Google Scholar]
- DeMoranville, C.; Howes, B.; Schlezinger, D.; White, D. Cranberry phosphorus management: How changes in practice can reduce output in drainage water. Acta Hortic. 2009, 810, 633–640. [Google Scholar]
- Oguchi, R.; Jia, H.; Barber, J.; Chow, W.S. Recovery of photoinactivated photosystem II in leaves: Retardation due to restricted mobility of photosystem II in the thylakoid membrane. Photosynth. Res. 2008, 98, 621–629. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hou, H.J.M. Photosynthesis and Yellow Vine Syndrome of American Cranberry. Agriculture 2012, 2, 125-138. https://doi.org/10.3390/agriculture2020125
Hou HJM. Photosynthesis and Yellow Vine Syndrome of American Cranberry. Agriculture. 2012; 2(2):125-138. https://doi.org/10.3390/agriculture2020125
Chicago/Turabian StyleHou, Harvey J. M. 2012. "Photosynthesis and Yellow Vine Syndrome of American Cranberry" Agriculture 2, no. 2: 125-138. https://doi.org/10.3390/agriculture2020125
APA StyleHou, H. J. M. (2012). Photosynthesis and Yellow Vine Syndrome of American Cranberry. Agriculture, 2(2), 125-138. https://doi.org/10.3390/agriculture2020125