A Contemporary Introduction to Essential Oils: Chemistry, Bioactivity and Prospects for Australian Agriculture
Abstract
:1. Introduction
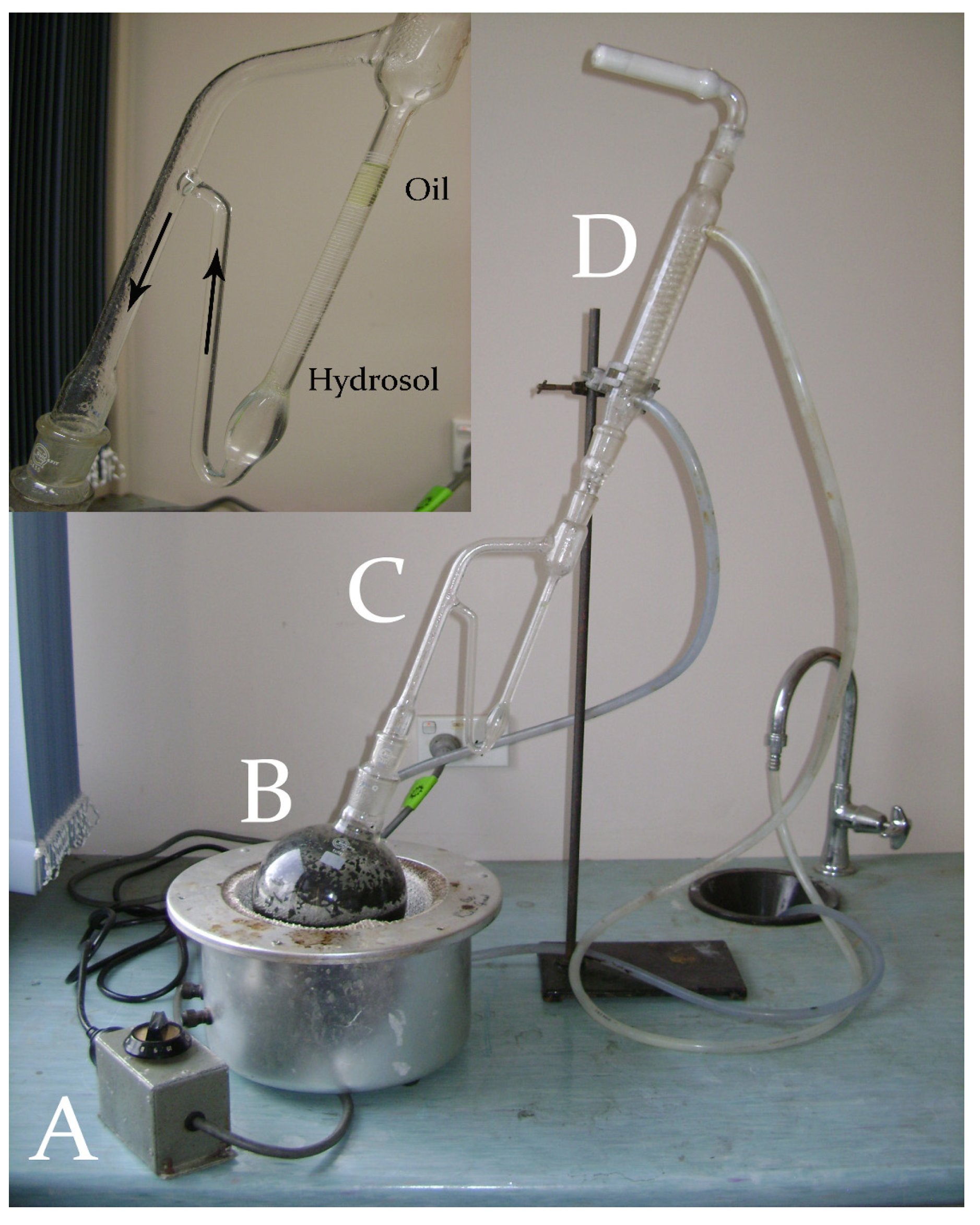
1.1. Terminology of Essential Oils and Methodologies of Production
“product obtained from natural raw material, either by distillation with water and steam, or from the epicarp of citrus fruits by mechanical processing, or by dry distillation”.
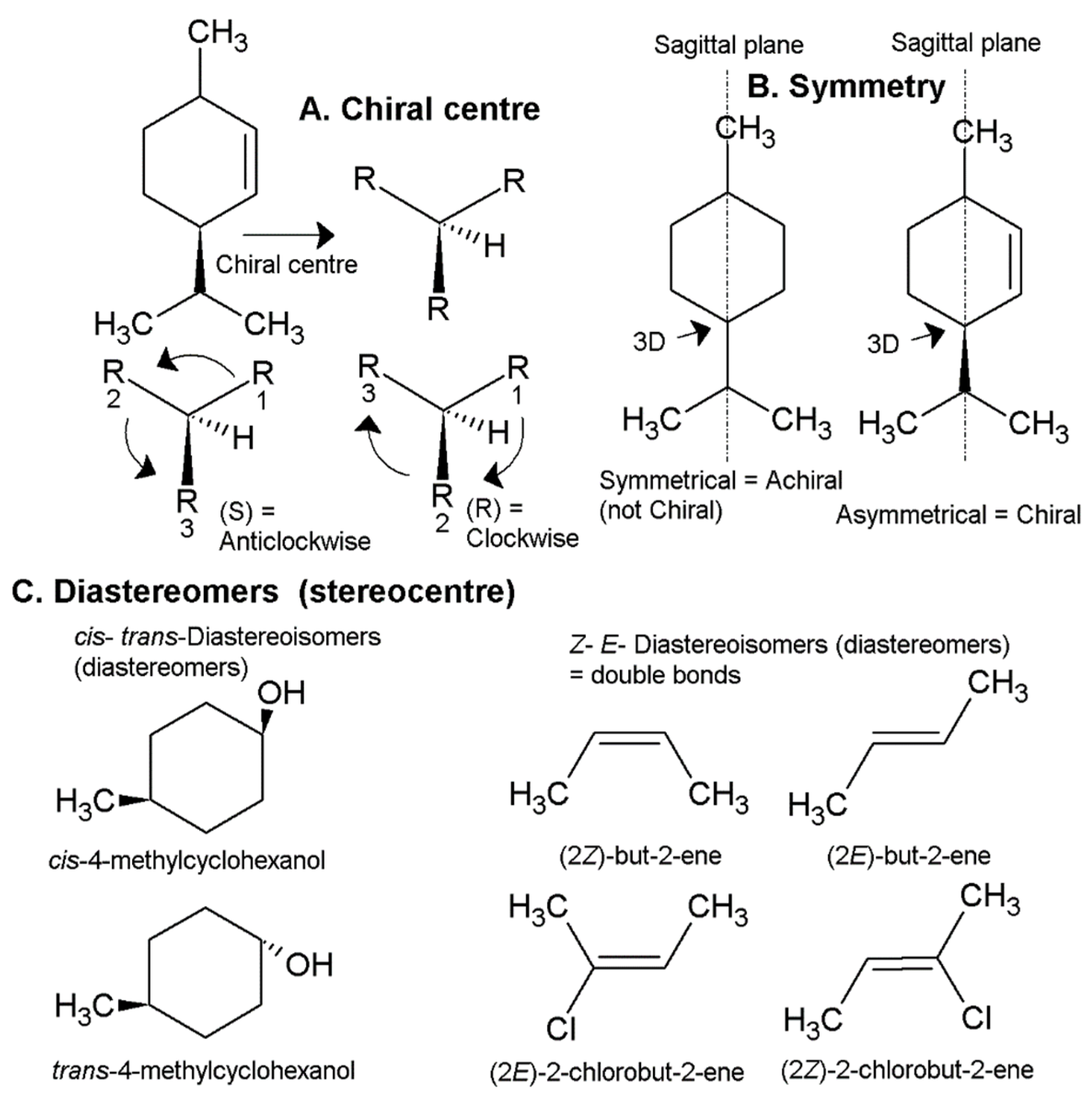
1.2. Chemistry, Chirality and Stereochemistry of Essential Oils

1.3. Chemical Analysis and Standardisation/Legislation of Essential Oils


“Essential oil obtained by steam distillation of the foliage and terminal branchlets of Melaleuca alternifolia (Maiden et Betche) Cheel, Melaleuca linariifolia Smith, and Melaleuca dissitiflora F. Mueller, as well as other species of Melaleuca provided that the oil obtained conforms to the requirements given in this International Standard”.
| Reference to International Standard (ISO) | Australian Standard (AS) | ||
|---|---|---|---|
| 212 | Essential oils—Sampling | 4550 | Essential oils—Sampling |
| 11024 | Essential oils—General guidance on chromatographic profiles | 5025 | Essential oils—General guidance on chromatographic profiles |
| 11024-1 | Part 1: Preparation of chromatographic profiles for presentation in standards | 5025.1 | Part 1: Preparation of chromatographic profiles for presentation in standards |
| 11024-2 | Part 2: Utilization of chromatographic profiles of samples of essential oils | 5025.2 | Part 2: Utilization of chromatographic profiles of samples of essential oils |
1.4. Biosynthesis and Subjective Classification of Essential Oils
1.5. Essential Oils in Agriculture
“Academic researchers tend to be more concerned about maintaining the rigour of science, judged by their peers in journals and conference proceedings, rather than research that contributes directly to the exploitation of essential oils and development of the industry”.[50]
2. Pharmacology of Well-Known Essential Oil Components of World-Wide Origin
2.1. Bioactivity Testing

2.2. Pharmacological Character of Internationally Recognized Essential Oils
| Essential Oil Types Described by Franchomme and Penoel | ||
|---|---|---|
| Alcohols and Phenols (hydroxyl group) | Coumarins | Ether-Oxides |
| Methoxycoumarins | Acetophenones | Hydroquinones |
| Non-Terpenoid Hydrocarbons | Acids | Oxides |
| Terpenoid and Non-Terpenoid esters | Ketones; | Lactones |
| Phenol and Methyl-Ether | Phthalides | Aldehydes |
| Bi- or Multifunctional Compositions | Acids and Esters | Terpenes (hydrocarbons) |
| Nitrogen Compositions | Sulfur Compounds | - |

3. More on Essential Oils in the Australian Context
3.1. Historical Uses of the Australian Essential Oils
“there is nothing more delightful in the approach, on a winter evening, to a township where Cypress pine is used as a fuel. Its delicious perfume is borne on the air for miles, and is often the first intimation that the weary traveller experiences that he is approaching a human habitation, and that his long journey is drawing to a close”.[103]
3.2. Today’s Essential Oil Industry
3.3. Recent Innovation in Australian Essential Oils
3.4. Ethnopharmacology of Aromatic Medicinal Plants Used Traditionally by Aboriginal Australians
3.5. Phytochemical and Chemotaxonomic Investigations
“This is a quite oddity! This specimen does not match any known Zieria taxon. It appears to be allied to 3 closely related species; Z. furfuracea, Z. granulata and Z. smithii”.
| Species | Chemotype | Use |
|---|---|---|
| Geijera parviflora | geijerene (46)/pregeijerene (45) (and germacrene D) | Commercial plantation: Insect repellent, topical analgaesia (linalool content). “Australian Green Lavender”. |
| Geijera parviflora | osthole (35), isopsoralen (33), xanthyletine (34) | Commercial plantations: therapeutic effects |
| Zieria floydii | car-3-en-2-one (48) | Commercial plantations: Chemical scaffold for further drug development and antimicrobial activities |
| Prostanthera prunelloides | maaliol (55) | Commercial plantations: Medicinal applications consistent with the Indian Valeriana willichii |
| Prostanthera rotundifolia, P. centralis | prostantherol (54) | Commercial plantations: Antimicrobial activities |
| Eremophila dalyana | NA | Essential oil requires characterisation—useful in topical applications to treat fungal or bacterial infections. Also an effective decongestant in coughs and colds. |
| Eremophila deserti | ngaione | Commercial plantation: antifungal treatment |
| Eremophila deserti | methoxymyodesert-3-ene | Commercial plantation: chemical scaffold |
| Eremophila longifolia | isomenthone (41)/menthone (39) | Commercial plantation: topical, gastrointestinal for antimicrobial activities, topical for muscle aches and pains, active in applications for treatment of thrush (Candida) |
| Eremophila longifolia | fenchyl- (22)/bornyl acetate (20) | Commerical plantations: possible activity in gastrointestinal disease, possible activity in aromatherapy for headache sufferers |
| Eremophila longifolia | Limonene (3)/sabinene/α-terpinolene, (−)-genifuranal (43) | Commercial plantations: derive (−)-genifuranal for therapeutic effects (i.e., treatment of MRSA) |
| Callitris glaucophylla | NA | (1) Bioactive γ-lactones; ferruginol, pisiferal, pisiferol. (2) Occurrence of slightly hydrophilic antibiotic highly active against S. aureus (MRSA) and B. subtilis—requires purification and structure elucidation. Medicinal applications consistent with the Japanese species Chamaecyparis pisifera |
4. Conclusions: Suggested Areas for Further Research
Acknowledgments
Author Contributions
Appendix


Introduction to Line Structures and Chiral Concepts Used in Organic Chemistry

Conflicts of Interest
References
- Asakawa, Y.; Ludwiczuk, A.; Nagashima, F. Chemical Constituents of Bryophytes: Bio- and Chemical Diversity, Biological Activity, and Chemosystematics: 95 (Progress in the Chemistry of Organic Natural Products); Springer: New York, NY, USA, 2012. [Google Scholar]
- Schnaubelt, K. Medical Aromatherapy: Healing with Essential Oils, 1st ed.; Frog Books: Berkeley, Canada, 1999. [Google Scholar]
- ISO. International Standards Organisation—Home Page. Available online: http://www.iso.org/iso/home.htm (accessed on 12 December 2014).
- Fadel, O.; Ghazi, Z.; Mouni, L.; Benchat, N.; Ramdani, M.; Amhamdi, H.; Wathelet, J.P.; Asehraou, A.; Charof, R. Comparison of microwave-assisted hydrodistillation and traditional hydrodistillation methods for the Rosmarinus eriocalyx essential oils from eastern Morocco. J. Mater. Environ. Sci. 2011, 2, 112–117. [Google Scholar]
- Asghari, J.; Touli, K.C.; Mazaheritehrani, M. Microwave-assisted hydrodistillation of essential oils from Echinophora platyloba Dc. J. Med. Plants Res. 2012, 6, 4475–4480. [Google Scholar]
- Mohamadi, M.; Shamspur, T.; Mostafavi, A. Comparison of microwave-assisted distillation and conventional hydrodistillation in the essential oil extraction of flowers of Rosa damascena Mill. J. Essent. Oil Res. 2013, 25, 55–61. [Google Scholar] [CrossRef]
- Stewart, D. Chemistry of Essential Oils Made Simple: Godʼs Love Manifest in Molecules; NAPSAC Reproductions: Marble Hill, MO, USA, 2005. [Google Scholar]
- Kostadinovic, S.; Jovanov, D.; Mirhosseini, H. Comparative investigation of cold pressed essential oils from peel of different mandarin varieties. IIOAB J. 2011, 3, 7–14. [Google Scholar]
- Markley, K.S.; Nelson, E.K.; Sherman, S.M. Some wax-like constituents from expressed oil from the peel of florida grapefruit, Citrus grandis. Food Research Division and Fertlizer Investigations, Bureau of Chemistry and Soils, United States Department of Agriculture, Washington. J. Biol. Chem. 1937, 118, 433–441. [Google Scholar]
- Sadgrove, N.; Jones, G.L. A possible role of partially pyrolysed essential oils in Australian Aboriginal traditional ceremonial and medicinal smoking applications of Eremophila longifolia (R. Br.) F. Muell (Scrophulariaceae). J. Ethnopharmacol. 2013, 147, 638–644. [Google Scholar] [CrossRef]
- Braithwaite, M.; Vuuren, V.S.F.; Viljoen, A.M. Validation of smoke inhalation therapy to treat microbial infections. J. Ethnopharmacol. 2008, 119, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Sadgrove, N.; Jones, G.L.; Greatrex, B.W. Isolation and characterisation of (−)-genifuranal: The principal antimicrobial component in traditional smoking applications of Eremophila longifolia (Scrophulariaceae) by Australian Aboriginal peoples. J. Ethnopharmacol. 2014, 154, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Guenther, E. The Essential Oils—vol 1: History—Origin in Plants—Production—Analysis; Van Nostrand: New York, NY, USA, 1948. [Google Scholar]
- Stewart, D. Healing Oils of the Bible; Care Publications: Marble Hill, MO, USA, 2003. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.; Garbe, D. Common Fragrance and Flavor Materials. Preparation, Properties and Uses; VCH Verlagsgesellschaft: Weinheim, UK, 1985. [Google Scholar]
- Sell, C. Chemistry of essential oils. In Handbook of Essential Oils: Science, Technology, and Applications; Başer, K.H.C., Buchbauer, G., Eds.; CRC Press, Taylor and Francis Group: London, UK, 2010. [Google Scholar]
- Moussaieff, A.; Rimmerman, N.; Bregman, T.; Straiker, A.; Felder, C.C.; Shoham, S.; Kashman, Y.; Huang, S.M.; Lee, H.; Shohami, E.; et al. Incensole acetate, an incense component, elicits psychoactivity by activating TRPV3 channels in the brain. FASEB J. 2008, 22, 3024–3034. [Google Scholar]
- Moussaieff, A.; Shein, N.A.A.; Tsenter, J.; Grigoriadis, S.; Simeonidou, C.; Alexandrovich, A.G.; Trembovler, V.; Ben-Neriah, Y.; Schmitz, M.L.; Fiebich, B.L.; et al. Incensole acetate: A novel neuroprotective agent isolated from Boswellia carterii. J. Cereb. Blood Flow Metab. 2008, 28, 1341–1352. [Google Scholar] [CrossRef]
- Moussaieff, A.; Shoham, E.; Kashman, Y.; Fride, E.; Schmitz, M.L.; Renner, F.; Fiebich, B.L.; Munoz, E.; Ben-Neriah, Y.; Mechoulam, R. Incensole acetate, a novel anti-inflammatory compound isolated from Boswellia resin, inhibits nuclear factor-κB activation. Mol. Pharm. 2007, 72, 1657–1664. [Google Scholar] [CrossRef]
- Başer, K.H.C.; Demirci, F. Chemistry of essential oils. In Fragrance and Flavours: Chemistr, Bioprocessing and Sustainability, 1st ed.; Berger, R.G., Ed.; Springer: Leipzig, Germany, 2007. [Google Scholar]
- Della, E.W.; Jefferies, P.R. The chemistry of Eremophila Species. 111. The essential oil of Eremophila longifolia F. Muell. Aust. J. Chem. 1961, 14, 663–664. [Google Scholar]
- Sainsbury, M. Aromatic Chemistry; Oxford University Press: New York, NY, USA, 1992. [Google Scholar]
- Clayden, J.; Greeves, N.; Warren, S. Organic Chemistry, 2nd ed.; Oxford University Press Inc.: New York, NY, USA, 2012. [Google Scholar]
- Kelvin, W.T. The Molecular Tactics of a Crystal; Clarendon Press: London, UK, 1894. [Google Scholar]
- König, W.A.; Hochmuth, D.H. Enantioselectie gas chromatography in flavor and fragrant analysis: Strategies for the identification of know and unknown plant volatiles. J. Chromatogr. Sci. 2004, 42, 423–439. [Google Scholar] [CrossRef] [PubMed]
- Sadgrove, N.; Telford, I.R.H.; Greatrex, B.W.; Dowell, A.; Jones, G.L. Dihydrotagetone, an unusual fruity ketone, is found in enantiopure and enantioenriched forms in additional australian native taxa of Phebalium (Rutaceae: Boronieae). Nat. Prod. Commun. 2013, 8, 737–740. [Google Scholar]
- Leitereg, T.J.; Guadagni, D.G.; Harris, J.; Mon, T.R.; Teranishi, R. Chemical and sensory data supporting the difference between the odors of the enantiomeric carvones. J. Agric. Food Chem. 1971, 19, 785–787. [Google Scholar] [CrossRef]
- Zellner, B.D.A.; Dugo, P.; Dugo, G.; Mondello, L. Analysis of Essential Oils. In Handbook of Essential Oils: Science, Technology and Applications; Başer, K.H.C., Buchbauer, G., Eds.; CRC Press, Taylor and Francis Group: London, UK, 2010. [Google Scholar]
- Reineccius, G.A. Flavour-Isolation Techniques. In Fragrance and Flavours: Chemistr, Bioprocessing and Sustainability, 1st ed.; Berger, R.G., Ed.; Springer: Leipzig, Germany, 2007. [Google Scholar]
- Mosandl, M. Enantioselective and Isotope Analysis—Key Steps to Flavour Authentication. In Fragrance and Flavours: Chemistr, Bioprocessing and Sustainability, 1st ed.; Berger, R.G., Ed.; Springer: Leipzig, Germany, 2007. [Google Scholar]
- Australian Standards—Home Page. Available online: http://www.standards.org.au/Pages/default.aspx (accessed on 12 December 2014).
- Association Française de Normalisation—Home Page. Available online: http://www.afnor.org/en (accessed on 12 December 2014).
- British Pharmacopoeia—Home Page. Available online: https://www.pharmacopoeia.gov.uk/reference-standards.php (accessed on 12 December 2014).
- Adams, T.B.; Taylor, S.V. Safety evaluation of essential oils: A constituent-based approach. In Handbook of Essential Oils: Science, Technology and Applications; Başer, K.H.C., Buchbauer, G., Eds.; CRC Press, Taylor and Francis Group: London, UK, 2010. [Google Scholar]
- Tisserand, R.; Balacs, T. Essential Oil Safety: A Guide for Health Care Professionals; Churchill livingstone: New York, NY, USA, 1995. [Google Scholar]
- Schnaubelt, K. Advanced Aromatherapy: The Science of Essential Oil Therapy; Healing Art Press: Rochester, VT, USA, 1995. [Google Scholar]
- Sadgrove, N.; Gonçalves-Martins, M.; Jones, G.L. Chemogeography and antimicrobial activity of essential oils from Geijera parviflora and Geijera salicifolia (Rutaceae): Two traditional australian medicinal plants. Phytochemistry 2014, 104, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Therapeutic Goods Administration—Home Page. Available online: https://www.tga.gov.au/ (accessed on 12 December 2014).
- Bowles, J.E. The Chemistry of Aromatherapeutic Oils; Allen and Unwin: Crows Nest, NSW Australia, 2003. [Google Scholar]
- Poucher, W.A. Poucherʼs Perfumes, Cosmetics and Soaps, 9th ed.; Chapman & Hall: London, UK, 1993. [Google Scholar]
- Kusari, S.; Spiteller, M. Metabolomics of Endophytic Fungi Producing Associated Plant Secondary Metabolites: Progress, Challenges and Opportunities. In Metabolomics; Roessner, U., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Emiliani, G.; Mengoni, A.; Maida, I.; Perrin, E.; Chiellini, C.; Fondi, M.; Gallo, E.; Gori, L.; Maggini, V.; Vannacci, A.; et al. Linking bacterial endophytic communities to essential oils: Clues from Lavandula angustifolia Mill. Evid. Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef]
- Mucciarelli, M.; Camusso, W.; Maffei, M.; Panicco, P.; Bichi, C. Volatile terpenoids of endophyte-free and infected peppermint (Mentha piperita L.): Chemical partitioning of a symbiosis. Microb. Ecol. 2007, 54, 685–696. [Google Scholar]
- Gersbach, P.V. The essential oil secretory structures of Prostanthera ovalifolia (Lamiaceae). Ann. Bot. 2002, 89, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Schrader, J. Microbial flavour production. In Fragrance and Flavours: Chemistr, Bioprocessing and Sustainability, 1st ed.; Berger, R.G., Ed.; Springer: Leipzig, Germany, 2007. [Google Scholar]
- Noma, Y.; Asakawa, Y. Biotransformation of monoterpenoids by microorganisms, insects, and mammals. In Handbook of Essential Oils: Science, Technology and Applications; Başer, K.H.C., Buchbauer, G., Eds.; CRC Press, Taylor and Francis Group: London, UK, 2010. [Google Scholar]
- Asakawa, Y.; Noma, Y. Biotransformation of sesquiterpenoids, ionones, damascones, adamantanes, and aromatic compounds by green algae, fungi, and mammals. In Handbook of Essential Oils: Science, Technology and Applications; Başer, K.H.C., Buchbauer, G., Eds.; CRC Press, Taylor and Francis Group: London, UK, 2010. [Google Scholar]
- Scragg, A.H. The production of flavours by plant cell cultures. In Fragrance and Flavours: Chemistr, Bioprocessing and Sustainability, 1st ed.; Berger, R.G., Ed.; Springer: Leipzig, Germany, 2007. [Google Scholar]
- Hunter, M. Essential Oils: Art, Agriculture, Science, Industry and Entrepreneurship (a Focus on the Asia-Pacific Region); Nova Science Publishers, Inc.: New York, NY, USA, 2009. [Google Scholar]
- Blazquez, M.A. Role of natural essential oils in sustainable agriculture and food preservation. J. Sci. Res. Rep. 2014, 3, 1843–1860. [Google Scholar]
- Başer, K.H.C.; Franz, C. Essential oils used in veterinary medicine. In Handbook of Essential Oils: Science, Technology and Applications; Başer, K.H.C., Buchbauer, G., Eds.; CRC Press, Taylor and Francis Group: London, UK, 2010. [Google Scholar]
- Kloucek, P.; Frankova, A.; Smid, J. Effect of Warm Air Flow and Reduced Pressure on Antibacterial Activity of Essential Oil Vapors. In Proceedings of the 42th ed International Symposium on Essential Oils, Antalya, Turkey, 11–14 September 2011.
- Sangwan, N.S.; Farooqi, A.H.A.; Shabih, F.; Sangwan, R.W. Regulation of essential oil production in plants. Plant Growth Regul. 2001, 34, 3–21. [Google Scholar] [CrossRef]
- Schwab, W. Genetic engineering of plants and microbial cells for flavour production. In Fragrance and Flavours: Chemistr, Bioprocessing and Sustainability, 1st ed.; Berger, R.G., Ed.; Springer: Leipzig, Germany, 2007. [Google Scholar]
- Sadgrove, N.J.; Jones, G.L.; University of New England, Armidale, NSW, Australia. Unpublished work. 2008.
- Buchbauer, G. Chapter 9. Biological activities of essential oils. In Handbook of Essential Oils: Science, Technology and Applications; Başer, K.H.C., Buchbauer, G., Eds.; CRC Press, Taylor and Francis Group: London, UK, 2010. [Google Scholar]
- Koroch, A.R.; Juliani, H.R.; Zygadlo, J.A. Bioactivity of essential oils and their components. In Fragrance and Flavours: Chemistr, Bioprocessing and Sustainability, 1st ed.; Berger, R.G., Ed.; Springer: Leipzig, Germany, 2007. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard—Tenth Edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009; Volume 29, pp. 1–54. [Google Scholar]
- Mann, C.M.; Markham, J.L. A new method for determining the minimum inhibitory concentration of essential oils. J. Appl. Microbiol. 1998, 84, 538–544. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Testing for Bacteria that Grow Aerobically Approved Standard—Eight Edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009; Volume 29, pp. 1–66. [Google Scholar]
- Sadgrove, N.; Mijajlovic, S.; Tucker, D.J.; Watson, K.; Jones, G.L. Characterization and bioactivity of essential oils from novel chemotypes of Eremophila longifolia (F. Muell) (Myoporaceae): A highly valued traditional Australian medicine. Flavour Fragr. J. 2011, 26, 341–350. [Google Scholar]
- Van Vuuren, S.F.; Viljoen, A.M. Plant-Based antimicrobial studies—Methods and approaches to study the interaction between natural products. Plant. Med. 2011, 77, 1168–1182. [Google Scholar] [CrossRef]
- Yi, A.-K.; Yoon, J.-G.; Hong, S.-C.; Redford, T.W.; Krieg, A.M. Lipopolysaccharide and CPG DNA synergise to tumor necrosis factor-alpha production through activation of NF-κB. Int. Immunol. 2001, 13, 1391–1404. [Google Scholar] [CrossRef] [PubMed]
- Ashley, J.W.; McCoy, E.M.; Clements, D.A.; Shi, Z.; Chen, T. Development of cell-based high-throughput assays for the identification of inhibitors of receptor activator of nuclear factor-kappa B signalling. Assay Drug Dev. Technol. 2011, 9, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Semple, S.J.; Reynolds, G.D.; OʼLeary, M.C.; Flower, R.L. Screening of Australian medicinal plants for antiviral activity. J. Ethnopharmacol. 1998, 60, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Schill, H.; Grange, R.L.; Porzelle, A.; Johns, J.P.; Parsons, P.G.; Gordon, V.A.; Reddell, P.W.; Williams, C.M. Anticancer agents from the Australain tropical rainforest: Spiroacetals EBC-23, 24, 25, 72, 73, 75 and 76. Chem. A Eur. J. 2009, 15, 11307–11318. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The frap assay. Anal. Biochem. 1996, 239, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.L.; Fong, W.F.; Redburn, J.; Griffiths, L.R. Fluorescence detection of plant extracts that affect neuronal voltage-gated Ca2+ channels. Eur. J. Pharm. Sci. 2002, 15, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, H.-J.; Germann, T.; Gillen, C.; Hatt, H.; Jostock, R. Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using fluorometric imaging plate reader (FLIPR) assay. Br. J. Pharmacol. 2004, 141, 173–745. [Google Scholar] [CrossRef]
- Van Vuuren, S.F.; du Toit, L.C.; Parry, A.; Pillay, V.; Choonara, Y.E. Encapsulation of essential oils within a polymeric liposomal formulation for enhancement of antimicrobial efficacy. Nat. Prod. Commun. 2010, 5, 1401–1408. [Google Scholar] [PubMed]
- Karlsen, J. Encapsulation and other programmed release techniques for essential oils and volatile terpenes. In Handbook of Essential Oils: Science, Technology and Applications; Başer, K.H.C., Buchbauer, G., Eds.; CRC Press, Taylor and Francis Group: London, UK, 2011. [Google Scholar]
- Sadgrove, N.; Jones, G.L. Medicinal compounds, chemically and biologically characterised from extracts of Australian Callitris endlicheri and C. glaucophylla (Cupressaceae): Used traditionally in Aboriginal and colonial pharmacopoeia. J. Ethnopharmacol. 2014, 153, 872–883. [Google Scholar]
- Elisabetsky, E.; Coelho de Souza, G.P.; Santos, M.A.C.; Siqueira, I.R.; Amador, T.A. Sedative properties of linalool. Fitoterapia 1995, 66, 407–414. [Google Scholar]
- Horak, S.; Koschak, A.; Stuppner, H.; Striessnig, J. Use-dependent block of voltage-gated Cav2.1 Ca2+ channels by petasins and eudesmol isomers. J. Pharmacol. Exp. Ther. 2009, 330, 220–226. [Google Scholar]
- Asakura, K.; Kanemasa, T.; Minagawa, K.; Kagawa, K.; Ninomiya, M. The nonpeptide alpha-eudesmol from Juniperus virginiana Linn. (Cupressaceae) inhibits omega-agatoxin IVA-sensitive calcium currents and synaptosomal 45Ca2+ uptake. Brain Res. 1999, 823, 169–176. [Google Scholar]
- Hongratanaworakit, T.; Heuberger, E.; Buchbauer, G. Evaluation of the effects of east indian sandalwood oil on alpha-santalol on humans after transdermal absorption. Plant. Med. 2004, 70, 3–7. [Google Scholar] [CrossRef]
- Sadgrove, N.; Jones, G.L. Antimicrobial activity of essential oils and solvent extracts from Zieria species (Rutaceae). Nat. Prod. Commun. 2013, 8, 741–745. [Google Scholar]
- Maia, M.F.; Moore, S.J. Plant-based insect repellents: A review of their efficacy, development and testing. Malar. J. 2011, 10 (Suppl. 1), S11. [Google Scholar] [CrossRef] [Green Version]
- Sadgrove, N.; Hitchock, M.; Watson, K.; Jones, G.L. Chemical and biological characterization of novel essential oils from Eremophila bignoniiflora (F. Muell) (Myoporaceae): A traditional Aboriginal Australian bush medicine. Phytother. Res. 2013, 27, 1508–1516. [Google Scholar]
- Wang, J.; Cai, Y.; Wu, Y. Antiinflammatory and analgesic activity of topical administration of Siegesbeckia pubescens. Pak. J. Pharm. Sci. 2008, 21, 89–91. [Google Scholar] [PubMed]
- Semnani-Morteza, K.; Saeedi, M.; Hamidian, M. Anti-inflammatory and analgesic activity of the topical preparation of Glaucium grandiflorum. Fitoterapia 2004, 75, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Kalbhen, D. Abbo Nutmeg as a narcotic. A contribution to the chemistry of pharmacology of nutmeg (Myristica fragrans). Angew. Chem. Int. 1971, 10, 370–374. [Google Scholar]
- Beyer, J.; Ehlers, D.; Maurer, H.H. Abuse of nutmeg (Myristica fragrans Houtt.): Studies on the metabolism and the toxicologic detection of its ingredients elemicin, myristicin, and safrole in rat and human urine using gas chromagrography/mass spectrometery. Ther. Drug Monit. 2006, 28, 568–575. [Google Scholar]
- Baker, R.T.; Smith, H.G. On a new species of Prostanthera and its essential oil. J. Proc. R. Soc. NSW 1912, 46, 103–110. [Google Scholar]
- Southwell, I.A.; Tucker, D.J. cis-Dihydroagarofuran from Prostanthera sp. aff. ovalifolia. Phytochemistry 1993, 22, 857–862. [Google Scholar] [CrossRef]
- Dellar, J.E.; Cole, M.D.; Gray, A.I.; Gibbons, S.; Waterman, P.G. Antimicrobial sesquiterpenes from Postanthera aff. melissifolia and P. rotundifolia. Phytochemistry 1994, 36, 957–960. [Google Scholar] [CrossRef]
- Pala-Paul, J.; Copeland, L.M.; Brophy, J.J.; Goldsack, R.J. Essential oil composition of two variants of Prostanthera lasianthos Labill. from Australia. Biochem. Syst. Ecol. 2006, 34, 48–55. [Google Scholar] [CrossRef]
- Lassak, E.V.; McCarthy, T. Australian Medicinal Plants; Methuen Australia Pty Ltd.: North Rhyde, Australia, 2011. [Google Scholar]
- Lassak, E.V.; Pinhey, J.T. The constituents of Eriostemon Trachyphyllus. The structure of trachyphyllin, a new coumarin. Aust. J. Chem. 1969, 22, 2175–2185. [Google Scholar]
- Lahey, F.N.; MacLeod, J.K. The coumarins of Geijera parviflora Lindl. Aust. J. Chem. 1967, 20, 1943–1955. [Google Scholar] [CrossRef]
- Carotti, A.; Carrieri, A.; Chimichi, S.; Boccalini, M.; Cosimelli, B.; Gnerre, C.; Carotti, A.; Carrupt, P.-A.; Testa, B. Natural and synthetic geiparvarins are strong and selective MOA-B inhibitors. Synthesis and sar studies. Bioorg. Med. Chem. Lett. 2002, 12, 3551–3555. [Google Scholar] [CrossRef]
- Tanaka, K.; Pescitelli, G.; Di Bari, L.; Xiao, T.L.; Nakanishi, K.; Armstrong, D.W.; Berova, N. Absolute stereochemistry of dihydrofuroangelicins bearing C-8 substituted double bonds: A combined chemical/exciton chirality protocol. Organic Biomol. Chem. 2004, 2, 48–58. [Google Scholar] [CrossRef]
- Hutt, A.J. Chirality and pharmacokinetics: An area of neglected dimensionality? Durg Metab. Drug Interact. 2007, 22, 79–112. [Google Scholar]
- Maiden, J.H. The Useful Native Plants of Australia; Alexander Bros Vic: Sydney, Australia, 1889. [Google Scholar]
- Cribb, A.B.; Cribb, J.W. Wild Medicine in Australia; William Collins, Pty, Ltd.: Sydney, Austrilia, 1981. [Google Scholar]
- Cribb, A.B.; Cribb, J.W. Useful Wild Plants in Australia; William Collins Pty Ltd.: Sydney, Australia, 1981. [Google Scholar]
- Behnam, S.; Farzaneh, M.; Ahmadzadeh, M.; Tehrani, A.S. Composition and antifungal activity of essential oils of Mentha piperita and Lavandula angustifolia on post-harvest phytopathogens. Commun. Agric. Appl. Biol. Sci. 2006, 71, 1321–1326. [Google Scholar] [PubMed]
- Barr, A. Traditional Bush Medicines: An Aboriginal Pharmacopoeia; Greenhouse publications Pty Ltd.: Richmond Vic, Australia, 1988. [Google Scholar]
- Jirovetz, L.; Buchbauer, G.; Denkova, Z.; Stoyanova, A.; Murgov, I.; Gearon, V.; Birkbeck, S.; Schmidt, E.; Geissler, M. Comparative study on the antimicrobial activities of different sandalwood essential oils of various origin. Flavour Fragr. J. 2006, 21, 465–468. [Google Scholar] [CrossRef]
- Thompson, J.; Johnson, L.A.S. Callitris glaucophylla, Australiaʼs white cypress pine—A new name for an old species. Telopea 1986, 2, 731. [Google Scholar] [CrossRef]
- Low, T. Bush Medicine: A Pharmacopoeia of Natural Remedies; Greenhouse publications Pty Ltd.: Richmond Vic, Australia, 1990. [Google Scholar]
- Oprava, A.; Leach, D.N.; Beattie, K.; Connellan, P.; Forster, P.I.; Leach, G.; Buchbauer, G.; Shepherd, K.; Deseo, M. Chemical composition and biological activity of the essential oils from native Australian Callitris species. Plant. Med. 2010, 76. [Google Scholar] [CrossRef]
- McKern, H.H.G. Arthur de Ramon Penfold. J. Proc. R. Soc. N.S.W. 1980, 113, 100. [Google Scholar]
- McKern, H.H.G. Arthur de Ramon Penfold, 1890–1980. Chem. Aust. 1981, 48, 327. [Google Scholar]
- Guenther, E. The Essential Oils—Vol 1–6; Van Nostrand Company, Inc.: New York, NY, USA, 1948; Volume 2. [Google Scholar]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (tea tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Plummer, J.A.; Wann, J.M.; Spadek, Z.E. Intraspecific variation in oil components of Boronia megastigma Nees. (Rutaceae) flowers. Ann. Bot. 1999, 83, 253–262. [Google Scholar] [CrossRef]
- Beattie, K.; Waterman, P.G.; Forster, P.I.; Thompson, D.R.; Leach, D.N. Chemical composition and cytotoxicity of oils and eremophilanes derived from various parts of Eremophila mitchellii Benth. (Myoporaceae). Phytochemistry 2011, 71, 400–408. [Google Scholar] [CrossRef]
- Thomas, J.; Narkowicz, C.K.; Jacobson, G.A.; Davies, N.W. An examination of the essential oils of tasmanian Kunzea ambigua, other Kunzea spp. and commercial Kunzea oil. J. Essent. Oil Res. 2010, 22, 381–385. [Google Scholar] [CrossRef]
- Trevena, G. Essentially Australia. Available online: https://essentiallyaustralia.com.au/about-us/ (accessed on 12 December 2014).
- Amri, I.; Mancini, E.; de Martino, L.; Marandino, A.; Lamia, H.; Mohsen, H.; Bassem, J.; Scognamiglio, M.; Reverchon, E.; de Feo, V. Chemical composition and biological activities of the essential oils from three Melaleuca species grown in Tunisia. Int. J. Mol. Sci. 2012, 13, 16580–16591. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.E.; Tucker, D.J.; Alter, D.; Watson, K.; Jones, G.L. Intraspecific variation in essential oil composition of Eremophila longifolia F. Muell (Myoporaceae): Evidence for three chemotypes. Phytochemistry 2010, 71, 1521–1527. [Google Scholar]
- Sadgrove, N.; Jones, G.L. Cytogeography of essential oil chemotypes of Eremophila longifolia F. Muell (Schrophulariaceae). Phytochemistry 2014, 105, 43–51. [Google Scholar] [CrossRef]
- Murray, D.; Kamilaroi Tribe, Collarenebri, NSW, Australia. Personal communication, 2014.
- Sadgrove, N.; Jones, G.L. Chemical and biological characterisation of solvent extracts and essential oils from leaves and fruit of two Australian species of Pittosporum (Pittosporaceae) used in Aboriginal medicinal practice. J. Ethnopharmacol. 2013, 145, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Bäcker, C.; Jenett-Siems, K.; Bodtke, A.; Lindequist, U. Polyphenolic compounds from the leaves of Pittosporum angustifolium. Biochem. Syst. Ecol. 2014, 55, 101–103. [Google Scholar] [CrossRef]
- Bäcker, C.; Jenett-Siems, K.; Siems, K.; Wurster, M.; Bodtke, A.; Chamseddin, C.; Crüsemann, M.; Lindequist, U. Triterpene glycosides from the leaves of Pittosporum angustifolium. Plant. Med. 2013, 79, 1461–1469. [Google Scholar] [CrossRef]
- Bäcker, C.; Jenett-Siems, K.; Siems, K.; Wurster, M.; Bodtke, A.; Lindequist, U. Cytotoxic saponins from the seeds of Pittosporum angustifolium. Zeitzchrift Naturforschung. C J. Biosci. 2014, 69, 191–198. [Google Scholar]
- Vesoul, J.; Cock, I.E. An examination of the medicinal potential of Pittosporum phylliraeoides: Toxicity, antibacterial and antifungal activities. Pharmacogn. Commun. 2011, 1, 8–17. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Telford, I.R.H.; Greatrex, B.W.; Jones, G.L. Composition and antimicrobial activity of essential oils from the Phebalium squamulosum species complex (Rutaceae) in New South Wales, Australia. Phytochemistry 2014, 97, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.L.; Jones, G.L.; Sadgrove, N. Volatiles from the rare australian desert plant Prostanthera centralis B.J.Conn (Lamiaceae): Chemical composition and antimicrobial activity. Agriculture 2014, 4, 308–316. [Google Scholar]
- Sah, S.P.; Mathela, C.S.; Chopra, K. Valeriana wallichii DC (maaliol chemotype): Antinociceptive studies on experimental animal models and possible mechanism of action. Pharmacologia 2012, 3, 432–437. [Google Scholar] [CrossRef]
- Lassak, E.V. New essential oils from the Australian flora (October 1980)—Perfumes and flavours symphony of nature. In Proceedings of the 8th International Congress of Essential Oils—Paper No. 120, Fedarom Grasse, France; 1980; pp. 409–415. [Google Scholar]
- Hellyer, R.O. Occurence of maaliol, elemol, and globulol in some australian essential oils. Aust. J. Chem. 1962, 15, 157–157. [Google Scholar] [CrossRef]
- Lassak, E.V.; Southwell, I.A. Essential oils isolates from the australian flora. Int. Flavours Food Addit. 1977, 8, 126–132. [Google Scholar]
- Buchi, G.; Wittenau, S.V.; White, D.M.; Terpenes, X. The constitution of maaliol. J. Am. Chem. Soc. 1959, 81, 1968–1980. [Google Scholar] [CrossRef]
- Santos, F.A.; Rao, V.S.N. Antiinflammatory and antinociceptive effects of 1,8-cineole a terpenoid oxide present in many plant essential oils. Phytother. Res. 2000, 14, 240–244. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadgrove, N.; Jones, G. A Contemporary Introduction to Essential Oils: Chemistry, Bioactivity and Prospects for Australian Agriculture. Agriculture 2015, 5, 48-102. https://doi.org/10.3390/agriculture5010048
Sadgrove N, Jones G. A Contemporary Introduction to Essential Oils: Chemistry, Bioactivity and Prospects for Australian Agriculture. Agriculture. 2015; 5(1):48-102. https://doi.org/10.3390/agriculture5010048
Chicago/Turabian StyleSadgrove, Nicholas, and Graham Jones. 2015. "A Contemporary Introduction to Essential Oils: Chemistry, Bioactivity and Prospects for Australian Agriculture" Agriculture 5, no. 1: 48-102. https://doi.org/10.3390/agriculture5010048
APA StyleSadgrove, N., & Jones, G. (2015). A Contemporary Introduction to Essential Oils: Chemistry, Bioactivity and Prospects for Australian Agriculture. Agriculture, 5(1), 48-102. https://doi.org/10.3390/agriculture5010048





