Peach Brown Rot: Still in Search of an Ideal Management Option
Abstract
:1. Introduction
2. The Peach
2.1. Geography and Ecological Requirements of Peaches
2.2. Botany and Susceptibility of Peaches to Infection by Monilinia spp.
2.3. Economic Significance of Peaches in EU-28
3. Brown Rot
3.1. Monilinia spp.
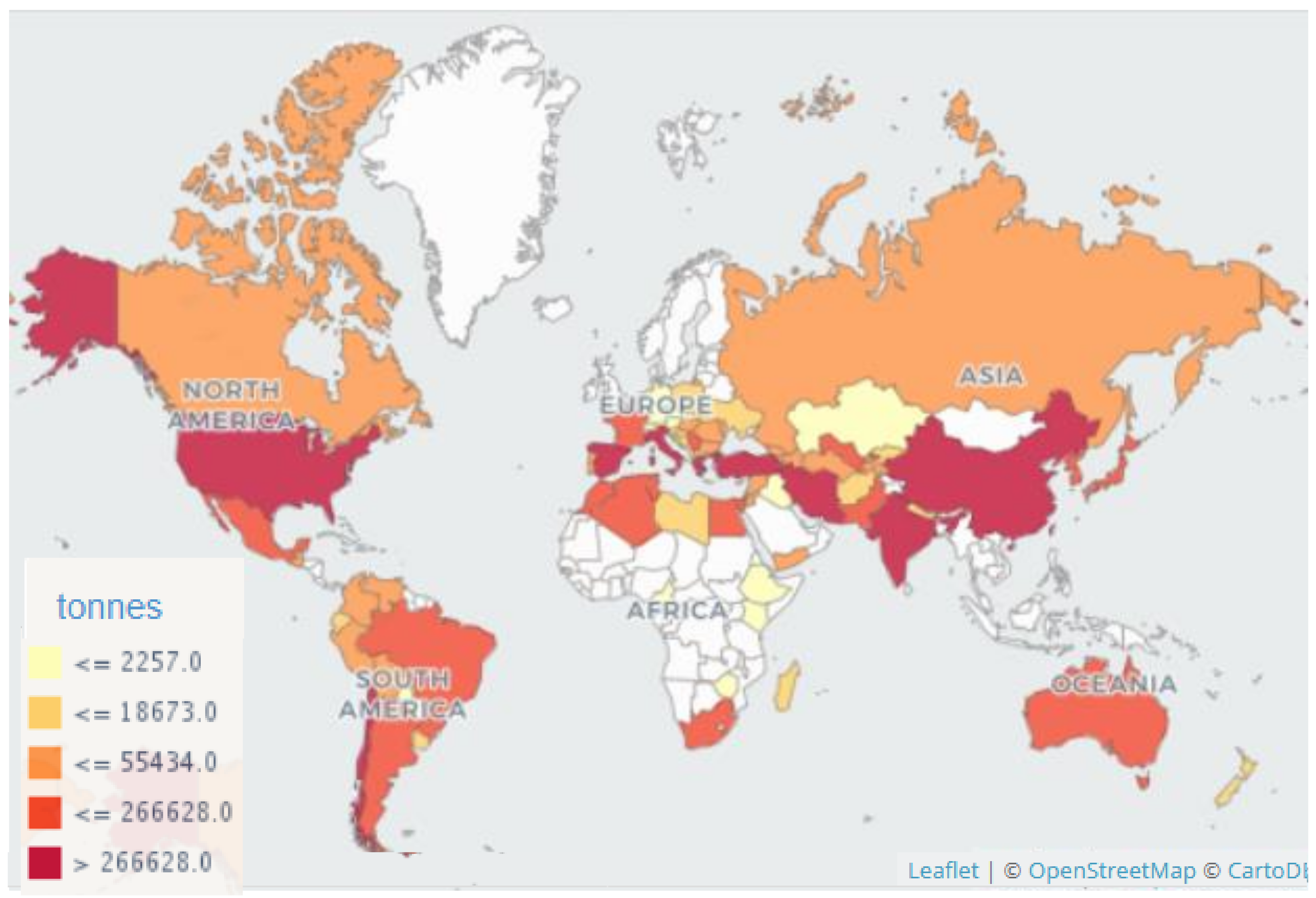
3.2. Geographical Distribution of the Species of Monilinia
3.3. Life Cycle of Species of Monilinia
3.4. Ecological Requirements of Monilinia Species
3.5. Characterization and Identification of Monilinia Species
3.5.1. Classical Methods
3.5.2. Molecular Methods
4. Host–Pathogen Interactions
5. Management Strategies to Control Brown Rot in Peaches
5.1. Agronomical Management
5.2. Biological Control Agents
5.3. Fungicide Treatments
5.4. Limitations in the Use of Conventional Fungicides
5.5. Botanical Fungicides
5.6. Physical Treatments
5.7. Host Resistance and Genetic Management
5.7.1. In Situ and Ex Situ Screening Methods to Evaluate Brown Rot Tolerance
5.7.2. Procedures for Spore Production and Inoculation in Lieu of Brown Rot Susceptibility Screening
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hummer, K.E.; Janick, J. Rosaceae: Taxonomy, Economic Importance, Genomics. In Genetics and Genomics of Rosaceae; Folta, K.M., Gardiner, S.E., Eds.; Springer: New York, NY, USA, 2009; pp. 1–17. [Google Scholar]
- Eurostat Statistics Explained. Agricultural Production—Crops. Available online: http://ec.europa.eu/eurostat/statisticsexplained/index.php/Agricultural_production-crops#Fruit (accessed on 7 August 2018).
- Usall, J.; Casals, C.; Sisquella, M.; Palou, L.; de Cal, A. Alternative technologies to control postharvest diseases of stone fruits. Stewart Postharvest Rev. 2015, 11, 1–6. [Google Scholar] [CrossRef]
- Rungjindamai, N.; Jeffries, P.; Xu, X.M. Epidemiology and management of brown rot on stone fruit caused by Monilinia laxa. Eur. J. Plant Pathol. 2014, 140, 1–17. [Google Scholar] [CrossRef]
- Villarino, M.; Egüen, B.; Melgarejo, P.; Lamarea, N.; Segarra, J.; Usall, J.; Melgarejo, P.; de Cal, A. Occurrence of the Monilinia laxa and M. fructigena after introduction of M. fructicola in peach orchards in Spain. Eur. J. Plant Pathol. 2013, 137, 835–845. [Google Scholar] [CrossRef]
- Mari, M.; Casalini, L.; Baraldi, E.; Bertolini, P.; Pretella, G.C. Susceptibility of apricot and peach fruit to Monilinia laxa during phenological stages. Postharvest Biol. Technol. 2003, 30, 105–109. [Google Scholar] [CrossRef]
- Guidarelli, M.; Zubini, P.; Nanni, V.; Bonghi, C.; Rasori, A.; Bertolini, P.; Baraldi, E. Gene expression analysis of peach fruit at different growth stages and with different susceptibility to Monilinia laxa. Eur. J. Plant Pathol. 2014, 140, 503–513. [Google Scholar] [CrossRef]
- Obi, V.I.; Barriuso, J.J.; Gogorcena, Y. Effects of pH and titratable acidity on the growth and development of Monilinia laxa (Aderh. & Ruhl.) in vitro and in vivo. Eur. J. Plant Pathol. 2018, 151, 781–790. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 8 August 2018).
- Seem, R.C. Disease incidence and severity relationships. Ann. Rev. Phytopathol. 1984, 22, 133–150. [Google Scholar] [CrossRef]
- Casals, C.; Segarra, J.; de Cal, A.; Lamarca, N.; Usall, J. Overwintering of Monilinia spp. on mummified stone fruit. J. Phytopathol. 2015, 163, 160–167. [Google Scholar] [CrossRef]
- Hrustić, J.; Mihajlović, M.; Grahovac, M.; Delibašić, G.; Bulajić, A.; Krstić, B.; Tanović, B. Genus Monilinia on pome and stone fruit species. Pestic. Phytomed. 2013, 27, 283–297. [Google Scholar] [CrossRef]
- Ritchie, D.F. Brown rot of stone fruits. Plant Health Instr. 2000. [Google Scholar] [CrossRef]
- Melgarejo, P.; Carillo, R.; Sagasta, E.M. Potential for biocontrol of Monilinia laxa in peach twigs. Crop Prot. 1986, 5, 422–426. [Google Scholar] [CrossRef]
- Kreidl, S.; Edward, J.; Villalta, O.N. Assessment of pathogenicity and infection requirements of Monilinia species causing brown rot of stone fruit in Australian orchards. Australas. Plant Pathol. 2015, 44, 419–430. [Google Scholar] [CrossRef]
- Gell, I.; de Cal, A.; Torres, R.; Usall, J.; Melgarejo, P. Conidial density of Monilinia spp. on peach fruit surfaces in relation to the incidences of latent infections and brown rot. Eur. J. Plant Pathol. 2009, 123, 415–424. [Google Scholar] [CrossRef]
- Martini, C.; Mari, M. Monilinia fructicola, Monilinia laxa (Monilinia rot, brown rot). In Postharvest Decay: Control Strategies; Academic Press Elsevier: London, UK, 2014; pp. 233–265. [Google Scholar]
- Egüen, B.; Melgarejo, P.; de Cal, A. Sensitivity of Monilinia fructicola from Spanish peach orchards to thiophanate-methyl, iprodione, and cyproconazole: Fitness analysis and competitiveness. Eur. J. Plant Pathol. 2015, 141, 789–801. [Google Scholar] [CrossRef]
- Teixidó, N. Brown Rot. 2018. Available online: http://www.biocomes.eu/ (accessed on 19 January 2018).
- Byrne, D.H.; Raseira, M.B.; Bassi, D.; Piagnani, M.C.; Gasic, K.; Reighard, G.L.; Moreno, M.A.; Pérez, S. Peach. In Fruit Breeding, Handbook of Plant Breeding; Badenes, M.L., Byrne, D.H., Eds.; Springer: Boston, MA, USA, 2012; pp. 505–570. [Google Scholar]
- Desmond, R.L.; Bassi, D. The Peach: Botany, Production and Uses; CABI: Wallingford, UK, 2008; 615p., ISBN 978-1-84593-386-9. [Google Scholar]
- Li, X.; Meng, X.; Jia, H.; Yu, M.; Ma, R.; Wang, L.; Aranzana, M.J. Peach genetic resources: Diversity, population structure and linkage disequilibrium. BMC Genet. 2013, 14, 84. [Google Scholar] [CrossRef] [PubMed]
- Charrier, G.; Ngao, J.; Saudreau, M.; Améglio, T. Effects of environmental factors and management practices on microclimate, winter physiology, and frost resistance in trees. Front. Plant Sci. 2015, 6, 259. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Tiedemann, A.V.; Teng, P.S. Climate change: Potential impact on plant diseases. Environ. Pollut. 2000, 108, 317–326. [Google Scholar] [CrossRef]
- Egilla, J.N.; Byrne, D.H. The search for peach root-stocks tolerant to alkalinity. Fruit Var. J. 1989, 43, 7–11. [Google Scholar]
- Bernhard, R.; Grasselly, C. Les pêchers × amandiers. L’Arboric. Fruit. 1981, 328, 37–42. [Google Scholar]
- Jiménez, S.; Pinochet, J.; Abadía, A.; Moreno, M.A.; Gogorcena, Y. Tolerance response to iron chlorosis of Prunus selections as rootstocks. HortScience 2008, 43, 304–309. [Google Scholar]
- Pinochet, J.; Calvet, C.; Hernández-Dorrego, A.; Bonet, A.; Felipe, A.; Moreno, M.A. Resistance of peach and plum rootstocks from Spain, France, and Italy to root-knot nematode Meloidogyne javanica. HortScience 1999, 34, 1259–1262. [Google Scholar]
- Gil-Albert, F. Tratado de Arboricultura Frutal, Vol IV: Técnicas de Mantenimiento del Suelo en Plantaciones Frutales; Mundi-Prensa Libros: Madrid, Spain, 1991; 109p., ISBN 978-84-8476-662-9. [Google Scholar]
- Cummings, G.A. Effect of soil pH and calcium amendments on peach yield, tree growth and longevity. Acta Hortic. 1989, 254, 179–184. [Google Scholar] [CrossRef]
- Font i Forcada, C.; Oraguzie, N.; Igartua, E.; Moreno, M.A.; Gogorcena, Y. Population structure and marker-trait association for pomological traits in peach and nectarine cultivars. Tree Genet. Genomes 2013, 9, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Font i Forcada, C.; Gradziel, T.M.; Gogorcena, Y.; Moreno, M.A. Phenotypic diversity among local Spanish and foreign peach and nectarine (Prunus persica (L.) Batsch) accessions. Euphytica 2014, 197, 261–277. [Google Scholar] [CrossRef]
- Bassi, D.; Moneté, R. Botany and Taxonomy. In The Peach: Botany, Production and Uses; Layne, D.R., Bassi, D., Eds.; CABI: Wallingford, UK, 2008; pp. 1–36. ISBN 978-1-84593-386-9. [Google Scholar]
- Lee, M.H.; Bostock, R.M. Induction, regulation and role in pathogenesis of appressoria in Monilinia fructicola. Phytopathology 2006, 96, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Gogorcena, Y.; Cantín, C.M.; Jiménez, S. Fruit tree crops. In Agricultural Sciences: Topics in Modern Agriculture Breeding; Studium Press LLC: Houston, TX, USA, 2010. [Google Scholar]
- Lombardo, V.A.; Osorio, S.; Borsani, J.; Lauxmann, M.A.; Bustamante, C.A.; Budde, C.O.; Andreo, C.S.; Lara, M.V.; Fernie, A.R.; Drincovich, M.F. Metabolic profiling during peach fruit development and ripening reveals the metabolic networks that underpin each developmental stage. Plant Physiol. 2011, 157, 1696–1710. [Google Scholar] [CrossRef] [PubMed]
- Thomidis, T.; Tsipouridis, C.; Darara, V. Seasonal variation of nutrient elements in peach fruits (cv. May Crest) and its correlation with development of brown rot (Monilinia laxa). Sci. Hortic. 2007, 111, 300–301. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). EU-28, Stone Fruit Annual. Available online: http://Stone Fruit Annual_Madrid_EU-28_8-22-2017.pdf (accessed on 19 January 2018).
- Ministerio de Agricultura, Pesca, Alimentación y Medio Ambiente (MAPAMA). Available online: http://www.mapama.gob.es/es/agricultura/temas/sanidad-vegetal/productos-fitosanitarios/registro/menu.asp (accessed on 6 February 2018).
- Oliveira-Lino, L.; Pacheco, I.; Mercier, V.; Faoro, F.; Bassi, D.; Bornard, I.; Quilot-Turion, B. Brown rot strikes Prunus fruit: An ancient fight almost always lost. J. Agric. Food Chem. 2016, 64, 4029–4047. [Google Scholar] [CrossRef] [PubMed]
- Dugan, M.F. The Identification of Fungi. An Illustrated Introduction with Keys, Glossary, and Guide to Literature; APS PRESS: Saint Paul, MN, USA, 2006; 176p., ISBN 2006 0-89054-336-4. [Google Scholar]
- Kew Royal Botany Gardens. Kew Mycology Collection. Available online: http://apps.kew.org/herbtrack/search? (accessed on 7 August 2018).
- Hrustić, J.; Delibasić, G.; Stanković, I.; Grahovać, M.; Krstić, B.; Bulajić, A.; Tanović, B. Monilinia spp. causing brown rot of stone fruit in Serbia. Plant Dis. 2015, 99, 709–717. [Google Scholar] [CrossRef]
- Žežlina, I.; Rot, M.; Kač, M.; Trdan, S. Causal agents of stone fruit diseases in Slovenia and the potential for diminishing their economic impact: A review. Plant Prot. Sci. 2016, 52, 149–157. [Google Scholar] [CrossRef]
- Pascal, T.; Levigneron, A.; Kervella, J.; Ngyen-The, C. Evaluation of two screening methods for resistance of apricot, plum and peach to Monilinia laxa. Prog. Temp. Fruit Breed. 1994, 77, 19–23. [Google Scholar] [CrossRef]
- Walter, M.; McLaren, G.F.; Fraser, J.A.; Frampton, C.M.; Boyd-Wilson, K.S.H.; Perry, J.H. Methods of screening apricot fruit for resistance to brown rot caused by Monilinia spp. Australas. Plant Pathol. 2004, 33, 541–547. [Google Scholar] [CrossRef]
- Villarino, M.; Melgarejo, P.; de Cal, A. Growth and aggresiveness factors affecting Monilinia spp. survival in peaches. Int. J. Food Microbiol. 2016, 227, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Obi, V.I.; Barriuso, J.J.; Moreno, M.A.; Giménez, R.; Gogorcena, Y. Optimizing protocols to evaluate brown rot (Monilinia laxa) susceptibility in peach and nectarine fruits. Australas. Plant Pathol. 2017, 46, 183–189. [Google Scholar] [CrossRef]
- Holb, I.J.; Szöke, S.; Abonyi, F. Temporal development and relationship amongst brown rot blossom blight, fruit blight and fruit rot in integrated and organic sour cherry orchards. Plant Pathol. 2013, 62, 799–808. [Google Scholar] [CrossRef] [Green Version]
- Cimen, I.; Ertugrul, B.B. Determination of mycoflora in almond plantations under drought conditions in South-eastern Anatolia project region, Turkey. Plant Pathol. J. 2007, 6, 82–86. [Google Scholar]
- Holb, I.J. Brown rot blight of pome and stone fruits: Symptom, disease cycle, host resistance, and biological control. Int. J. Hortic. Sci. 2008, 14, 15–21. [Google Scholar]
- Poniatowska, A.; Michalecka, M.; Bieleni, A. Characteristic of Monilinia spp. fungi causing brown rot of pome and stone fruits in Poland. Eur. J. Plant Pathol. 2013, 135, 855–865. [Google Scholar] [CrossRef]
- Holb, I.J.; Scherm, H. Temporal dynamics of brown rot in different apple management systems and importance of dropped fruit for disease development. Phytopathology 2007, 97, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Hrustić, J.; Grahovac, M.; Mihajlović, M.; Delibašić, G.; Ivanović, M.; Nikolić, M.; Tanović, B. Molecular detection of Monilinia fructigena as causal agent of brown rot on quince. Pestic. Phytomed. 2012, 27, 15–24. [Google Scholar] [CrossRef]
- Van Leeuwen, G.C.M.; Baayen, R.P.; Holb, I.J.; Jeger, M.J. Distinction of the Asiatic brown rot fungus Monilia polystroma sp. nov. from M. fructigena. Mycol. Res. 2002, 106, 444–451. [Google Scholar] [CrossRef]
- De Cal, A.; Egüen, B.; Melgarejo, P. Vegetative compatibility groups and sexual reproduction among Spanish Monilinia fructicola isolates obtained from peach and nectarine orchards, but not Monilinia laxa. Fungal Biol. 2014, 118, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Bernat, M.; Segarra, J.; Xu, X.M.; Casals, C.; Usall, J. Influence of temperature on decay, mycelium development and sporodochia production caused by Monilinia fructicola and M. laxa on stone fruits. Food Microbiol. 2017, 64, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Holtz, B.A.; Michailides, T.J.; Hong, C. Development of apothecia from stone fruit infected and stromatized by Monilinia fructicola in California. Plant Dis. 1998, 82, 1375–1380. [Google Scholar] [CrossRef]
- Zhu, X.-Q.; Niu, C.-W.; Chen, X.-Y.; Guo, L.-Y. Monilinia species associated with brown rot of cultivated apple and pear fruit in China. Plant Dis. 2016, 100, 2240–2250. [Google Scholar] [CrossRef]
- Vasić, M.; Vico, I. Distribution and characterization of Monilinia spp. causing apple fruit decay in Serbia. Plant Dis. 2018, 102, 359–369. [Google Scholar] [CrossRef]
- Petróczy, M.; Palkovics, L. First report of Monilia polystroma on apple in Hungary. Eur. J. Plant Pathol. 2009, 125, 343–347. [Google Scholar] [CrossRef]
- Hu, M.; Cox, K.D.; Schnabel, G.; Luo, C.X. Monilinia species causing brown rot of peach in China. PLoS ONE 2011, 6, e24990. [Google Scholar] [CrossRef] [PubMed]
- CABI. Distribution Maps of Plant Diseases. Available online: https://www.cabi.org/isc/ (accessed on 15 June 2018).
- Adaskaveg, J.E.; Schnabel, G.; Förster, H. Diseases of peach caused by fungi and fungal-like organisms: Biology, epidemiology and management. In The Peach: Botany, Production and Uses; Bassi, D., Layne, D., Eds.; CABI: Wallingford, UK, 2008; pp. 352–406. ISBN 9781845933869. [Google Scholar]
- Janisiewicz, W.J.; JurickIi, W.M.; Vico, I.; Peter, K.A.; Buyer, J.S. Culturable bacteria from plum fruit surfaces and their potential for controlling brown rot after harvest. Postharvest Biol. Technol. 2013, 76, 145–151. [Google Scholar] [CrossRef]
- Villarino, M.; Melgarejo, P.; Usall, J.; Segarra, J.; de Cal, A. Primary inoculum sources of Monilinia spp. in Spanish peach orchards and their relative importance in brown rot. Plant Dis. 2010, 94, 1048–1054. [Google Scholar] [CrossRef]
- Xu, X.M.; Bertone, C.; Berrie, A. Effects of wounding, fruit age and wetness duration on the development of cherry brown rot in the UK. Plant Pathol. 2007, 56, 114–119. [Google Scholar] [CrossRef]
- Nagarajan, S.; Singh, D.V. Long-distance dispersion of rust pathogens. Ann. Rev. Phytopathol. 1990, 28, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Bosshard, E.; Hilber-Bodmer, M.; Scharer, H.-J.; Bunter, M.; Duffy, B. First report of the quarantine brown rot pathogen Monilinia fructicola on imported stone fruits in Switzerland. Plant Dis. 2006, 90, 1554. [Google Scholar] [CrossRef]
- Ellis, M.A. Brown Rot of Stone Fruits. Available online: https://ohioline.osu.edu/factsheet/plpath-fru-29 (accessed on 29 January 2018).
- Hong, C.; Holtz, B.A.; Morgan, D.P.; Michailides, T.J. Significance of thinned fruit as a source of the secondary inoculum of Monilinia fructicola in California nectarine orchards. Plant Dis. 1997, 81, 519–524. [Google Scholar] [CrossRef]
- Kotleba, J. European and Mediterranean Plant Protection Organization (EPPO). Bull. OEPP/EPPO Bull. 2010, 40, 5–22. [Google Scholar] [CrossRef]
- Thomidis, T. Influence of relative virulence and latent infections on the development of Monilinia to Greek peach orchards. Crop Prot. 2017, 94, 159–165. [Google Scholar] [CrossRef]
- Fazekas, M.; Madar, A.; Sipiczki, M.; Miklós, I.; Holb, I.J. Genetic diversity in Monilinia laxa populations in stone fruit species in Hungary. World J. Microbiol. Biotechnol. 2014, 30, 1879–1892. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.P.; Bertolini, P. Effect of temperature during conidial formation of Monilinia laxa on conidial size, germination and infection of stored nectarines. J. Phytopathol. 1999, 147, 635–641. [Google Scholar] [CrossRef]
- Xu, X.M.; Robinson, J.D. Epidemiology of brown rot (Monilinia fructigena) on apple: Infection of fruits by conidia. Plant Pathol. 2000, 49, 201–206. [Google Scholar] [CrossRef]
- Gell, I.; de Cal, A.; Torres, R.; Usall, J.; Melgarejo, P. Relationship between the incidences of latent infections caused by Monilinia spp. and the incidence of brown rot of peach fruit: Factors affecting latent infection. Eur. J. Plant Pathol. 2008, 121, 487–498. [Google Scholar] [CrossRef]
- Casals, C.; Viñas, I.; Torres, R.; Griera, C.; Usall, J. Effect of temperature and water activity on in vitro germination of Monilinia spp. J. Appl. Microbiol. 2010, 108, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Angeli, S.S.; May de Mio, L.L.; Amorim, L. Comparative analysis of M. fructicola and M. laxa isolates from Brazil: Monocyclic components of peach brown rot. Cienc. Rural 2017, 47, 1–7. [Google Scholar] [CrossRef]
- European and Mediterranean plant protection organization (EPPO). Diagnostics on Monilinia fructicola, specific approval and amendment. EPPO Bull. 2009, 39, 337–343. [Google Scholar]
- De Cal, A.; Melgarejo, P. Effects of long-wave UV light on Monilinia growth and identification of species. Plant Dis. 1999, 83, 62–65. [Google Scholar] [CrossRef]
- Van Leeuwen, G.C.M.; Kesteren, H.A. Delineation of the three brown rot fungi of fruit crops (Monilinia spp.) on the basis of quantitative characteristics. Can. J. Bot. 1998, 76, 2042–2050. [Google Scholar] [CrossRef]
- European and Mediterranean plant protection organization (EPPO). Diagnostic protocols for regulated pests, Monilinia fructicola. EPPO Bull. 2003, 33, 281–288. [Google Scholar]
- Petróczy, M.; Szigethy, A.; Palkkovics, L. Monilinia species in Hungary: Morphology, culture characteristics, and molecular analysis. Trees 2012, 26, 153–164. [Google Scholar] [CrossRef]
- Côté, M.-J.; Tardif, M.-C.; Meldrum, A.J. Identification of Monilinia fructigena, M. fructicola, M. laxa, and Monilia polystroma on inoculated and naturally infected fruit using multiplex PCR. Plant Dis. 2004, 88, 1219–1225. [Google Scholar] [CrossRef]
- American Phytopathological Society. Brown Rot of Stone Fruits. Available online: https://www.apsnet.org/edcenter/intropp/lessons/fungi/ascomycetes/Pages/BrownRotStoneFruits.aspx (accessed on 7 August 2018).
- Gell, I.; Cubero, J.; Melgarejo, P. Two different PCR approaches for universal diagnosis of brown rot and identification of Monilinia spp. in stone fruit trees. J. Appl. Microbiol. 2007, 103, 2629–2637. [Google Scholar] [CrossRef] [PubMed]
- Van Brouwershaven, I.R.; Bruil, M.L.; van Leeuwen, G.C.M.; Kox, L.F.F. A real-time (TaqMan) PCR assay to differentiate Monilinia fructicola from other brown rot fungi of fruit crops. Plant Pathol. 2010, 59, 548–555. [Google Scholar] [CrossRef]
- Balodi, R.; Bisht, S.; Ghataka, A.; Rao, K.H. Plant disease diagnosis: Technological advancements and challenges. Indian Phytopath. 2017, 70, 275–281. [Google Scholar] [CrossRef]
- Forster, H.; Adaskaveg, J.E. Early brown rot infections in sweet cherry fruit are detected by Monilinia-specific DNA primers. Phytopathology 2000, 90, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Ioos, R.; Frey, P. Genomic variation within Monilinia laxa, M. fructigena, and M. fructicola, and application to species identified by PCR. Eur. J. Plant Pathol. 2000, 106, 337–373. [Google Scholar] [CrossRef]
- Boehm, E.W.A.; Ma, Z.; Michilides, T.J. Species-specific detention of Monilinia fructicola from California stone and flowers. Phytopathology 2001, 91, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Guinet, C.; Fourrier-Jeandel, C.; Cerf-Wendling, I.; Ioos, R. One-step detection of Monilinia fructicola, M. fructigena, and M. laxa on Prunus and Malus by a Multiplex Real-Time PCR assay. Plant Dis. 2016, 100, 2465–2474. [Google Scholar] [CrossRef]
- Papavasileiou, A.; Madesis, P.B.; Karaoglanidis, G.S. Identification and differentiation of Monilinia species causing brown rot of pome and stone fruit using high-resolution melting (HRM) analysis. Phytopathology 2016, 106, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Benitez, C.; Melgarejo, P.; de Cal, A. Detection of latent Monilinia infections in nectarine flowers and fruit by qPCR. Plant Dis. 2017, 101, 1002–1008. [Google Scholar] [CrossRef]
- Garcia-Benitez, C.; Melgarejo, P.; Beniusis, A.; Guinet, C.; Özben, S.; Degirnen, K.I.; Valente, M.T.; Riccioni, L.; de Cal, A. Proficiency of real-time PCR detection of latent Monilinia spp. infection in nectarine flowers and fruit. Phytopathol. Mediterr. 2017, 56, 242–250. [Google Scholar] [CrossRef]
- Ma, Z.; Luo, Y.; Michailides, T.J. Nested PCR assays for detection of Monilinia fructicola in stone fruit orchards and Botryosphaeria dothidea from pistachios in California. J. Phytopathol. 2003, 151, 312–322. [Google Scholar] [CrossRef]
- Miessner, S.; Stammler, G. Monilinia laxa, M. fructigena and M. fructicola: Risk estimation of resistance to QoI fungicides and identification of species with cytochrome b gene sequences. J. Plant Dis. Prot. 2010, 117, 162–167. [Google Scholar] [CrossRef]
- Hily, J.-M.; Singer, S.D.; Villani, S.M.; Cox, K.D. Characterization of the cytochrome b (cyt b) gene from Monilinia species causing brown rot of stone and pome fruit and its significance in the development of QoI resistance. Pest Manag. Sci. 2010, 67, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.H.; Yoshimura, M.A.; Holtz, B.A.; Michailides, T.J. Characterization and PCR-based detection of benzimidazole-resistant isolates of Monilinia laxa in California. Pest Manag. Sci. 2005, 61, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Lowe, C.-W.; Satterfield, B.A.; Nelson, D.B.; Thiriot, J.D.; Heder, M.J.; March, J.K.; Drake, D.S.; Lew, C.S.; Bunnell, A.J.; Moore, E.S.; et al. A quadruplex real-time PCR assay for the rapid detection and differentiation of the most relevant members of the B. pseudomallei Complex: B. mallei, B. pseudomallei, and B. thailandensis. PLoS ONE 2016, 11, e0164006. [Google Scholar] [CrossRef] [PubMed]
- Ivic, D.; Fazini, T.; Cole, J.; Novak, A. Monilinia species identified on peach and nectarine in Croatia, with the first record of Monilinia fructicola. Bull. OEPP/EPPO Bull. 2014, 44, 70–72. [Google Scholar] [CrossRef]
- Riccioni, L.; Valente, M.T. Comparison of different PCR tests to identify Monilinia fructicola. Bull. OEPP/EPPO Bull. 2015, 45, 33–40. [Google Scholar] [CrossRef]
- Raja, H.A.; Miller, A.N.; Pearce, C.J.; Oberlies, N.H. Fungal identification using molecular tools: A primer for the natural products research community. J. Nat. Prod. 2017, 80, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Benitez, C.; Melgarejo, P.; de Cal, A.; Fontaniella, B. Microscopic analyses of latent and visible Monilinia fructicola infections in nectarines. PLoS ONE 2016, 11, e0160675. [Google Scholar] [CrossRef] [PubMed]
- Papavasileiou, A.; Testempasis, S.; Michailides, T.J.; Karaoglanidis, G.S. Frequency of brown rot fungi on blossoms and fruit in stone fruit orchards in Greece. Plant Pathol. 2015, 64, 416–424. [Google Scholar] [CrossRef]
- Thomidis, T.; Exadaktylou, E. Effect of boron on the development of brown rot (Monilinia laxa) on peaches. Crop Prot. 2010, 29, 572–576. [Google Scholar] [CrossRef]
- Sisquella, M.; Casals, C.; Picouet, P.; Viñas, I.; Torres, R.; Usall, J. Immersion of fruit in water to improve radio frequency treatment to control brown rot in stone fruit. Postharvest Biol. Technol. 2013, 80, 31–36. [Google Scholar] [CrossRef]
- Pascual, S.; Rico, J.R.; de Cal, A.; Melgarejo, P. Eco-physiological factors affecting growth, sporulation and survival of the biocontrol agent Penicillium oxalicum. Mycopathologia 1997, 139, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Maharshi, A.R.; Thaker, V.S. Growth and development of plant pathogenic fungi in define media. Eur. J. Exp. Biol. 2012, 2, 44–54. [Google Scholar]
- Holb, I.J. Effect of acidity on growth rate and stroma formation of Monilinia fructigena and M. polystroma isolates. Int. J. Hortic. Sci. Hung. 2004, 10, 63–67. [Google Scholar]
- Romero-Arenas, O.; Damián-Huato, M.A.; Treviño, I.H.; Parraguire-Lezama, J.F.C.; Aragón-García, A.; Arellano, A.D. Effect of pH on growth of the mycelium of Trichoderma viride and Pleurotus ostreatus in solid cultivation mediums. Afr. J. Agric. Res. 2012, 7, 4724–4730. [Google Scholar] [CrossRef]
- Dirlewanger, E.; Moing, A.; Rothan, C.; Svanella, L.; Pronier, V.; Guye, A.; Plomion, C.; Monet, R. Mapping QTLs controlling fruit quality in peach (Prunus persica (L.) Batsch. Theor. App. Genet. 1999, 98, 18–31. [Google Scholar] [CrossRef]
- Tyl, C.; Sadler, G.D. pH and Titratable Acidity. In Food Analysis; Nielsen, S., Ed.; Springer: Cham, Switzerland, 2017; pp. 389–406. [Google Scholar]
- Lobit, P.; Soing, P.; Genard, M.; Habib, R. Theoretical analysis of relationships between composition, pH and titratable acidity of peach fruit. J. Plant Nutr. 2002, 25, 2775–2792. [Google Scholar] [CrossRef]
- Yamanaka, T. The effect of pH on the growth of saprotrophic and ectomycorrhizal ammonia fungi in vitro. Mycologia 2003, 95, 584–589. [Google Scholar] [CrossRef] [PubMed]
- De Cal, A.; España, P.S.; Martinez, F.; Egüen, B.; Chien-Ming, C.; Lee, M.H.; Melgarejo, P.; Prusky, D. Role of gluconic acid and pH modulation in virulence of Monilinia fructicola on peach fruit. Postharvest Biol. Technol. 2013, 86, 418–423. [Google Scholar] [CrossRef]
- Fazinic, T.; Lovrek, I.Z.; Ivic, D. Potential impact and management of Monilinia fructicola in an integrated peach orchard. Agric. Conspec. Sci. 2017, 82, 27–31. [Google Scholar]
- Minas, I.S.; Tanou, G.; Molassiotis, A. Environmental and orchard bases of peach fruit quality. Sci. Hortic. 2018, 235, 307–322. [Google Scholar] [CrossRef]
- Bevacqua, D.; Quilot-Turion, B.; Bolzoni, L. A model for temporal dynamics of brown rot spreading in fruit orchards. Phytopathology 2018, 108, 595–601. [Google Scholar] [CrossRef] [PubMed]
- De Cal, A.; Sagasta, E.M.; Melgarejo, P. Biological control of peach twig blight (Monilinia laxa) with Penicillium frequentans. Plant Pathol. 1990, 39, 612–618. [Google Scholar] [CrossRef]
- Larena, I.; Melgarejo, P. Biological Control of Monilinia laxa and Fusarium oxysporum f. sp. lycopersici by a lytic enzyme-producing Penicillium purpurogenum. Biol. Control 1996, 6, 361–367. [Google Scholar] [CrossRef]
- Dicklow, M.B. Biofungicides: Commercial Horticulture. Available online: https://ag.umass.edu/greenhouse-floriculture/fact-sheets/biofungicides (accessed on 17 February 2018).
- Bonaterra, A.; Mari, M.; Casalini, L.; Montesinos, E. Biological control of Monilinia laxa and Rhizopus stolonifer in postharvest of stone fruit by Pantoea agglomerans EPS125 and putative mechanisms of antagonism. Int. J. Food Microbiol. 2003, 84, 93–104. [Google Scholar] [CrossRef]
- Larena, I.; Torres, R.; de Cal, A.; Liñán, M.; Melgarejo, P.; Domenichini, P.; Bellini, A.; Mandein, J.F.; Lichou, J.; Ochoa de Eribe, X.; et al. Biological control of postharvest brown rot (Monilinia spp.) of peaches by field application of Epicoccum nigrum. Biol. Control. 2005, 32, 305–310. [Google Scholar] [CrossRef]
- Yánez-Mendizábal, V.; Zeriouh, H.; Viña, I.; Torres, R.; Usall, J.; de Vicente, A.; Pérez-García, A.; Teixidó, N. Biological control of peach brown rot (Monilinia spp.) by Bacillus subtilis CPA-8 is based on production of fengycin-like lipopeptides. Eur. J. Plant Pathol. 2012, 132, 609–619. [Google Scholar] [CrossRef]
- Guijarro, B.; Melgarejo, P.; Torres, R.; Lamarca, N.; Usall, J.; de Cal, A. Penicillium frequentans population dynamics on peach fruits after its applications against brown rot in orchards. Appl. Microbiol. Biotechnol. 2008, 104, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Gotor-Vila, A.; Teixidó, N.; Casals, C.; Torres, R.; de Cal, A.; Guijarro, B.; Usall, J. Biological control of brown rot in stone fruit using Bacillus amyloliquefaciens CPA-8 under field conditions. Crop Prot. 2017, 102, 72–80. [Google Scholar] [CrossRef] [Green Version]
- Giobbe, S.; Marceddu, S.; Scherm, B.; Zara, G.; Mazzarello, V.L.; Budroni, M.; Migheli, Q. The strange case of a biofilm-forming strain of Picia fermentans, which controls Monilinia brown rot on apple but is pathogenic on peach fruit. FEMS Yeast Res. 2007, 7, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Adaskaveg, J.E.; Gubler, D.; Michailides, T. Fungicides, Bactericides, and Biological Fro Deciduous Tree Fruit, Nut, Strawberry, and Vine Crops. Available online: http://ipm.ucanr.edu/PDF/PMG/fungicideefficacytiming.pdf. (accessed on 30 July 2018).
- De Cal, A.; Sagasta, E.M.; Melgarejo, P. Antifungal substances produced by Penicillium frequentans and their relationship to the biocontrol of Monilinia laxa. Phytopathology 1988, 78, 888–893. [Google Scholar] [CrossRef]
- Norton, G.W.; Rajotte, E.G.; Gapud, V. Participatory research in integrated pest management: Lessons from the IPM CRSP. Agric. Hum. Values 1999, 16. [Google Scholar] [CrossRef]
- Egüen, B.; Melgarejo, P.; de Cal, A. The effect of fungicide resistance on the structure of Monilinia laxa populations in Spanish peach and nectarine orchards. Eur. J. Plant Pathol. 2016, 145, 815–827. [Google Scholar] [CrossRef]
- Katan, T.; Shabi, E. Resistance to dicarboximide fungicides in laboratory isolates of Monilinia laxa. Neth. J. Plant Pathol. 1981, 87, 242. [Google Scholar]
- Cañez, U.M., Jr.; Ogawa, J.M. Parasitic fitness of benomyl-resistant and benomyl-sensitive Monilinia laxa. Phytopathology 1985, 20, 167–192. [Google Scholar]
- Pimentel, D. Environmental and economic costs of the application of pesticides primarily in the United States? Environ. Dev. Sustain. 2005, 7, 229–252. [Google Scholar] [CrossRef]
- Zehr, E.I. Control of brown rot in peach orchards. Plant Dis. 1982, 66, 1101–1105. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Wisniewski, M.; Droby, S.; Tian, S.; Norelli, H.V. Effect of heat treatment on inhibition of Monilinia fructicola and induction of disease resistance in peach fruit. Postharvest Biol. Technol. 2012, 65, 61–68. [Google Scholar] [CrossRef]
- Elmer, P.A.G.; Gaunt, R.E. The biological characteristics of dicarboximides resistant isolates of Monilinia fructicola from New Zeland stone orchards. Plant Pathol. 1994, 43, 130–137. [Google Scholar] [CrossRef]
- Chen, F.; Liu, X.; Schnabel, G. Field strains of Monilinia fructicola resistant to both MBC and DMI fungicides isolated from stone fruit orchards in the eastern United States. Plant Dis. 2013, 97, 1063–1068. [Google Scholar] [CrossRef]
- European Commission. Integrated Pest Management. Available online: https://ec.europa.eu/food/plant/pesticides/sustainable_use_pesticides/ipm_en (accessed on 28 July 2018).[Green Version]
- Solomon, R.D.J.; Kallidass, S.; Vimalan, J. Isolation, identification and study of antimicrobial property of a bioactive compound in an Indian medicinal plant Acalypha indica (Indian-nettle). World J. Microbiol. Biotechnol. 2005, 21, 1231–1236. [Google Scholar] [CrossRef]
- Suleiman, M.N.; Emua, S.A. Efficacy of four plant extracts in the control of root rot disease of cowpea (Vigna unguiculata [L.] Walp). Afr. J. Biotechnol. 2009, 8, 3806–3808. [Google Scholar]
- Khaled-Khodja, N.; Boulekbache-Makhlouf, L.; Madani, K. Phytochemical screening of antoxidant and antibacterial activities of methanolic extracts of some Lamiaceae. Ind. Crops Prod. 2014, 61, 41–48. [Google Scholar] [CrossRef]
- Yoon, M.-Y.; Cha, B.; Kim, J.-C. Recent trends in studies on botanical fungicides in Agriculture. Plant Pathol. J. 2013, 29, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Wani, A.H.; Bhat, M.Y.; Koka, J.A. Biological control of postharvest fungal rots of rosaceous fruits using microbial antagonists and plant extracts: A review. Czech Mycol. 2016, 68, 41–66. [Google Scholar]
- Wilson, C.L.; Solar, J.M.; El-Ghaouth, A.; Wisniewski, M.E. Rapid evaluation of plant extracts and essential oils for antifungal activity against Botrytis cinerea. Plant Dis. 1997, 81, 204–210. [Google Scholar] [CrossRef]
- Ziedan, E.H.E.; Farrag, E.S.H. Fumigation of peach fruits with essential oils to control postharvest decay. Res. J. Agric. Biol. Sci. 2008, 4, 512–519. [Google Scholar]
- Kouass, K.H.S.; Baiji, M.; Zhiri, A.; Lepoivre, P.; Jijaku, M.H. Evaluation of three essential oils as potential sources of botanical fungicide. Commun. Agric. Appl. Biol. Sci. 2010, 75, 525–529. [Google Scholar]
- Hassani, A.; Fathi, Z.; Ghosta, Y.; Abdollahi, A.; Meshkatalsadat, M.H.; Marandi, R.J. Evaluation of plant essential oils for control of postharvest brown and gray mold rots on apricot. J. Food Saf. 2012, 32, 94–101. [Google Scholar] [CrossRef]
- Goncalves, F.P.; Martins, M.C.; Junior, G.J.S.; Lourenco, S.A.; Amorim, L. Postharvest control of brown rot and Rhizopus rot in plums and nectarines using carnauba wax. Postharvest Biol. Technol. 2010, 58, 211–217. [Google Scholar] [CrossRef]
- Ganchev, D.A. Preliminarily study of antifungal activity of some active substances from plant origin according to Monilia fructigena. Bulg. J. Agric. Sci. 2007, 13, 679–682. [Google Scholar]
- Carović-Stanko, K.; Fruk, G.; Satovic, Z.; Ivić, D.; Politeo, O.; Sever, Z.; Grdiša, M.; Strikić, F.; Jemrić, T. Effects of Ocimum spp. essential oil on Monilinia laxa in-vitro. J. Essent. Oil Res. 2013, 25, 143–148. [Google Scholar] [CrossRef]
- Lopez-Reyes, J.G.; Spadaro, D.; Prelle, A.; Garbaldi, A.; Gullino, M.L. Efficacy of plant essential oils on postharvest control of rots caused by fungi on different stone fruits in vivo. J. Food Prot. 2013, 76, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Cindi, M.D.; Soundy, P.; Romanazzi, G.; Sivakumar, D. Different defence responses and brown rot control in two Prunus persica cultivars to essential oil vapours after storage. Postharvest Biol. Technol. 2016, 119, 9–17. [Google Scholar] [CrossRef]
- Jemric, T.; Ivic, D.; Fruk, G.; Matijas, H.S.; Cvjetkovic, B.; Bupic, M.; Pavkovic, B. Reduction of postharvest decay of peach and nectarine caused by Monilinia laxa using hot water dipping. Food Bioprocess Technol. 2011, 4, 149–154. [Google Scholar] [CrossRef]
- Casals, C.; Elmer, P.A.G.; Viñas, I.; Teixidó, N.; Sisquella, M.; Usall, J. The combination of curing with either chitosan or Bacillus subtilis CPA-8 to control brown rot infections caused by Monilinia fructicola. Postharvest Biol. Technol. 2012, 64, 126–132. [Google Scholar] [CrossRef]
- Bernat, M.; Segarra, J.; Casals, C.; Teixidó, N.; Torres, R.; Usall, J. Relevance of the main postharvest handling operations on the development of brown rot disease on stone fruits. J. Sci. Food Agric. 2017, 97, 5319–5326. [Google Scholar] [CrossRef] [PubMed]
- Spadoni, A.; Guidarelli, M.; Sanzani, S.M.; Ippolito, A.; Mari, M. Influence of hot water treatment on brown rot of peach and rapid fruit response to heat stress. Postharvest Biol. Technol. 2014, 94, 66–73. [Google Scholar] [CrossRef]
- Sisquella, M.; Viñas, I.; Picouet, P.; Torres, R.; Usall, J. Effect of host and Monilinia spp. variables on the efficacy of radio frequency treatment on peaches. Postharvest Biol. Technol. 2014, 87, 6–12. [Google Scholar] [CrossRef]
- Feliciano, A.; Feliciano, A.J.; Ogawa, J.M. Monilinia fructicola resistance in the peach cv. Bolinha. Phytopathology 1987, 77, 776–780. [Google Scholar] [CrossRef]
- Fresnedo-Ramírez, J.; Famula, T.R.; Gradziel, T.M. Application of a Bayesian ordinal animal model for the estimation of breeding values for the resistance to Monilinia fructicola (G. Winter) Honey in progenies of peach (Prunus persica (L.) Batsch). Breed. Sci. 2017, 67, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Gradziel, T.M. Changes in susceptibility to brown rot with ripening in three clingstone peach genotypes. J. Am. Soc. Hortic. Sci. 1994, 119, 101–103. [Google Scholar]
- Byrne, D.H. Trends in stone fruit cultivars development. Horticulture 2005, 15, 494–500. [Google Scholar]
- Vallad, G.E.; Goodman, R.M. Systemic acquired resistance and induced systemic resistance in conventional agriculture. Crop Sci. 2004, 44, 1920–1934. [Google Scholar] [CrossRef]
- Norton, G.A. Analysis of decision making in crop protection. Agro-Ecosystems 1976, 3, 27–44. [Google Scholar] [CrossRef]
- Dodds, K.; Coleman, A.; Browne, B. Orchard Plant Protection Guide for Deciduous Fruits in NSW, 2017–2018. Available online: http://www.dpi.nsw.gov.au/data/assets/pdf_file/0006/249729/orchard-plant-protection-guide-2017-18.pdf (accessed on 22 January 2018).
- Tarbath, M.P.; Measham, P.F.; Glen, M.; Barry, K.M. Host factors related to fruit rot of sweet cherry (Prunus avium L.) caused by Botrytis cinerea. Australas. Plant Pathol. 2014, 43, 513–522. [Google Scholar] [CrossRef]
- Compean, K.L.; Ynalvez, R.A. Antimicrobial activity of plant secondary metabolites. J. Med. Plant. Res. 2014, 8, 204–213. [Google Scholar]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Chérif, M.; Arfaoui, A.; Rhaiem, A. Phenolic compounds and their role in bio-control and resistance of chickpea to fungal pathogenic attacks. Tunis. J. Plant Prot. 2007, 2, 7–21. [Google Scholar]
- Prasad, D.; Singh, A.; Singh, K.P.; Bist, S.; Tewari, A.; Singh, U.P. The role of phenolic compounds in disease resistance in geranium. Arch. Phytopathol. Plant Prot. 2010, 43, 615–623. [Google Scholar] [CrossRef]
- Sanzani, S.M.; Schena, L.; Ippolito, A. Effectiveness of phenolic compounds against citrus green mould. Molecules 2014, 19, 12500–12508. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; Gil, M.I.; Cremin, P.; Waterhouse, A.L.; Hess-Pierce, B.; Kader, A.A. HPLC-DAD-ESIMS Analysis of phenolic compounds in nectarines, peaches, and plums. J. Agric. Food Chem. 2001, 4, 4748–4760. [Google Scholar] [CrossRef]
- Villarino, M.; Sandín-España, P.; Melgarejo, P.; de Cal, A. High chlorogenic and neochlorogenic acid levels in immature peaches reduce Monilinia laxa infection by interfering with fungal melanin biosynthesis. J. Agric. Food Chem. 2011, 59, 3205–3213. [Google Scholar] [CrossRef] [PubMed]
- Usall, J.; Ippolito, A.; Sisquella, M.; Neri, F. Physical treatment to control posthavest disease of fresh fruits and vegetables. Postharvest Biol. Technol. 2016, 122, 30–40. [Google Scholar] [CrossRef]
- Spiers, T.M.; Elmer, P.A.G.; Wood, P.N.; Regglinski, T.; Tate, K.G. Multiple strategies for effective pathogen control. N. Z. Plant 2005, 58, 62–67. [Google Scholar]
- Rubos, A.; Thomidis, T.; Tsipouridis, C.; Navrozidis, E.; Michailidou, O. Susceptibility of peach-nectarine cultivars on brown rot infections. Ann. Univ. Oradea Fascicle Environ. Prot. 2008, 13, 214–217. [Google Scholar]
- Martínez-Gómez, P.; Sánchez, P.R.; Rubio, M.; Dicenta, F.; Gradziel, T.M.; Sozzi, G.O. Application of recent biotechnologies to Prunus tree crop genetic improvement. Ciencia e Investigación Agraria 2005, 32, 73–96. [Google Scholar] [CrossRef]
- Martínez-García, P.J.; Parfitt, D.E.; Bostock, R.M.; Fresnedo-Ramírez, J.; Vazquez-Lobo, A.; Ogundiwin, E.A.; Crisosto, C.H. Application of genomic and quantitative genetic tools to identify candidate resistance genes for brown rot resistance in peach. PLoS ONE 2013, 8, e78634. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, I.; Bassi, D.; Eduardo, I.; Ciacciulli, A.; Pirona, R.; Rossini, L. QTL mapping for brown rot (Monilinia fructigena) resistance in an intraspecific peach (Prunus persica L. Batsch) F1 progeny. Tree Genet. Genomes 2014, 10, 1223–1242. [Google Scholar] [CrossRef]
- Mari, M.; Spadoni, A.; Ceredi, G. Alternative technologies to control postharvest diseases of kiwifruit. Stewart Postharvest Rev. 2015, 4, 1–5. [Google Scholar]
- Teksur, P.K. Alternative technologies to control postharvest diseases of pomegranate. Stewart Postharvest Rev. 2015, 4, 1–7. [Google Scholar] [CrossRef]
- Verde, I.; Jenkins, J.; Dondini, L.; Micali, S.; Pagliarani, G.; Vendramin, E.; Schmutz, J. The Peach v2.0 release: High-resolution linkage mapping and deep resequencing improve chromosome-scale assembly and contiguity. BMC Genom. 2017, 18, 225. [Google Scholar] [CrossRef] [PubMed]
- Gradziel, T.M.; Wang, D. Evaluation of brown rot resistance and its relation to enzymatic browning in clingstone peach germplasm. J. Am. Soc. Hortic. Sci. 1993, 118, 675–679. [Google Scholar]
- Christen, D.; Motry, L.; Devènes, G. Comparison of three different evaluation methods of Monilinia laxa impact on apricot flowers. Acta Hortic. 2012, 966, 143–147. [Google Scholar] [CrossRef]
- Obi, V.I.; Barriuso, J.J.; Saidani, F.; Aubert, C.; Gogorcena, Y. The tolerance of commercial peach cultivars to brown rot by Monilinia laxa is modulated by its antioxidant content? Sci. Hortic. submitted.
- Paunovic, S.A.; Paunovic, A.S. Investigation of peach germplasm (Prunus persica spp. 372 vulgaris = Vineyard peach) in-situ in Yugoslavia. Acta Hortic. 1996, 374, 201–207. [Google Scholar] [CrossRef]
- Biggs, A.R.; Miller, S.S. Comparative relative susceptibility of NE-183 apple cultivars to fruit rot pathogens in West Virginia. J. Am. Pomol. Soc. 2005, 59, 72–77. [Google Scholar]
- Biggs, A.R.; Northover, J. Early and late season susceptibility of peach fruits to Monilinia fructicola. Plant Dis. 1988, 72, 1070–1074. [Google Scholar] [CrossRef]
- Northover, J.; Biggs, A.R. Susceptibility of immature and mature sweet and sour cherries to Monilinia fructicola. Plant Dis. 1990, 74, 280–284. [Google Scholar] [CrossRef]
- Bassi, D.; Rizzo, M.; Cantoni, L. Assaying brown rot [(Monilinia laxa Aderh. & Ruhl. (Honey)] susceptibility in peach cultivars and progeny. Acta Hortic. 1998, 465, 715–721. [Google Scholar] [CrossRef]
- Jansch, M.; Frey, J.E.; Hilber-Bodmer, M.; Broggini, G.A.L.; Weger, J.; Schnabel, G.; Patocchi, A. SSR marker analysis of Monilinia fructicola from Swiss apricots suggests introduction of the pathogen from neighbouring countries and the United States. Plant Pathol. 2012, 61, 247–254. [Google Scholar] [CrossRef]
- Keske, C.; May-De Mio, L.L.; Amorim, L. Spatial pattern of brown rot within peach trees related to inoculum of Monilinia fructicola in organic orchard. J. Plant Pathol. 2013, 95, 67–73. [Google Scholar]
- Keske, C.; Amorim, L.; May-de Mio, L.L. Peach brown rot incidence related to pathogen infection at different stages of fruit development in an organic peach production system. Crop Prot. 2011, 30, 802–806. [Google Scholar] [CrossRef]






| Country | 2015–2016 | 2016–2017 | 2017–2018 |
|---|---|---|---|
| Spain | 1,581,510 | 1,475,849 | 1,487,444 |
| Italy | 1,408,504 | 1,262,127 | 1,362,749 |
| Greece | 777,160 | 788,120 | 910,000 |
| France | 217,146 | 207,004 | 214,800 |
| Characteristics/Pathogen | M. laxa | M. fructicola | M. fructigena | M. polystroma | Source |
|---|---|---|---|---|---|
| Conidia dimension | 11–13 µm × 8–9.5 µm | 12.5–14.5 µm × 8–10 µm | 17.5–20.5 µm × 10.5–12.5 µm | 13–17 µm × 9–10.5 µm | EPPO Bull [80]; van Leeuwen et al. [55] |
| Number of germ tube | 1/conidia | 1/conidia | 2/conidia | 2/conidia | EPPO Bull [80]; van Leeuwen et al. [55] |
| Form of germ tube | Short and twisted | Long and straight | Long and straight | Long and straight | EPPO Bull [80]; van Leeuwen et al. [55] |
| Size description | Smaller | Larger | Similar to M. laxa | Similar to M. fructigena | EPPO Bull [80]; van Leeuwen et al. [55] |
| Length of germ tube (>18 h at 22 °C) | 150–350 µm | 750–900 µm | 600–900 µm | 700–1000 µm | EPPO Bull [80]; van Leeuwen et al. [55] |
| Sporulation | Delayed and sparse | Quick, intense and abundant | Sparse | Sparse | EPPO Bull [80]; van Leeuwen et al. [55] |
| Sporulation range * | 0–3.7 | 2.8–5.3 | - | na | Hu et al. [62] |
| Mean sporulation * | 1.8 | 3.9 | na | na | Hu et al. [62] |
| Colony color | Hazel/Isabelline (greyish-brown) | Hazel/ Isabelline (greenish-brown) | Pale luteous (yellowish/creamy) | Pale luteous (yellowish/creamy) | EPPO Bull. [83]; Petróczy et al. [61]; Petróczy et al. [84] |
| Mycelium in distinct layers/colony rosetted | Resetting (mycelium in distinct layers on top of each) | No/rare | On distinct tufts; rings of aerial mycelium | Intense formation of black, stromatal plates initiated after 10–12 days incubation | van Leeuwen et al. [55]; EPPO Bull. [80]; Petróczy et al. [84] |
| Colony rosette with black arcs | Yes | No | No | No | EPPO Bull [80]; van Leeuwen et al. [55] |
| Concentric ring of spores | No | Yes | Sometimes | Sometimes | van Leeuwen et al. [55] |
| Colony margins | Serrulated/lobed | Not lobed but entire | Not lobed but entire | Not lobed but entire | van Leeuwen et al. [55]; Petróczy et al. [84] |
| Range of colony growth rate (mm/24 h) | 2–11 | 9–20 | 0–12 | nd | de Cal et al. [81]; van Leeuwen et al. [55] |
| Mean colony growth rate (mm/24 h) (in continuous darkness) | 6 | 13 | 3.7 | 7 | EPPO Bull [83]; Hu et al. [62]; Petróczy et al. [84] |
| Growth rating scale | Low | High | Low-moderate | Moderate | van Leeuwen et al. [55] |
| Biological Agents | Formulation | Target Pathogen | Disease | Application Phase | Effectiveness | Reference |
|---|---|---|---|---|---|---|
| Penicillium frequentans | P. frequentans | Monilinia laxa (Aderh. & Ruhl.) Honey | Twig blight | Preharvest | Effective and practical | de Cal et al. [121] |
| Penicillium purpurogenum | strain 828 | Monilinia laxa (Aderh. & Ruhl.) Honey and Fusarium oxysporum f. sp.lycopersici (Snyder & Hansen) | Shoot canker | Preharvest | Enhanced mycoparasitism | Larena and Melgarejo [122] |
| Pantoea agglomerans | EPS125 | Monilinia laxa (Aderh. & Ruhl.) Honey and Rhizopus stolonifer (Ehrenb., Fr.) | Brown rot | Postharvest | Potentially effective | Bonaterra et al. [124] |
| Epicoccum nigrum | Fresh conidia | Monilinia spp. | Brown rot | Preharvest (bloom and pre-harvest) | Reduced brown rot at post-harvest | Larena et al. [125] |
| Bacillus subtilis | CPA-8 | Monilinia laxa (Aderh. & Ruhl.) Honey and Monilinia fructicola (Wint.) Honey | Brown rot | Postharvest | Effective growth inhibition achieved | Yánez-Mendizábal et al. [126] |
| Penicillium frequentans | FOR1, FOR2 and 16 others | Monilinia spp. | Brown rot | Preharvest (blossom to harvest) | Good potential for development | Guijarro et al. [127] |
| Bacillus amyloliquefaciens | CPA-8 | Monilinia laxa (Aderh. & Ruhl.) Honey and Monilinia fructicola (Wint.) Honey | Brown rot | Preharvest | Potential alternative against Monilinia spp | Gotor-Vila et al. [128] |
| Formulation | Number of Comercial Products | Doses/Application | SP (Days)/MRL | Permissible Limit |
|---|---|---|---|---|
| Sulphur 80% + Cyproconazole 0.8% (WG) W/W | 1 | 0.1–0.2%/pulverization | 14/0.1 | 21/11/2018 |
| Cyproconazole 10% (WG) W/W | 1 | 0.01–0.02% | 14/0.1 | 12/09/2018 |
| Cyprodinil 37.5% + fludioxonil 25% (ESP) (WG) W/W | 1 | 0.8 kg/ha; 1 application | 7/2 | 30/04/2019 |
| Copper (II) hydroxide 35% (WG) W/W | 23 | 0.2–0.25% | NA/5 | 31/01/2020 |
| Iprodione 75% (WG) W/W | 1 | 0.1% at a maximum of 2 applications/season and less than 1 kg/ha | 14/10 | 31/10/2018 |
| Mancozeb 20% + Dicopper chloride trihydroxide 30% (WP) W/W | 20 | 2.5–3 kg/ha | 14/2 | 31/01/2020 |
| Mancozeb 75% (WG) W/W & 80% | 35 | 0.2% at a maximum of 4 applications/season and less than 2 kg/ha | 30/2 | 30/01/2020 |
| Thiophanate–methyl 50% (SC) W/V; 70% (WG) W/W | 4 | 0.09% at 1 application per season | 14/2 | 31/10/2019 |
| Mancozeb 8% + Cuprocalcium sulphate 20% (WP) W/W | 4 | 4–5 kg/ha | NA/2 | 31/01/2020 |
| Myclobutanil 4.5% (EW) W/V | 1 | 0.66–1.1% | 7/0.5 | 31/05/2021 |
| Dicopper chloride trihydroxide 11% + Cuprocalcium sulphate 10% (WP) W/W | 1 | 0.35–0.55%; 1.75–5.5 kg/ha to a maximum of 7.1 kg/ha per year | NA/5 | 31/01/2020 |
| Copper (I) oxide 40% (01) W/W | 12 | 0.65% at a maximum of 3.75 kg/ha per year | NA/5 | 31/01/2020 |
| Copper (I) oxide 50% (WP) W/W & 52% | 47 | 0.3%; 2.5 kg/ha | NA/5 | 31/01/2020 |
| Copper (I) oxide 70% (WG) W/W | 16 | 0.15%; 1.35 kg/ha | NA/5 | 31/01/2020 |
| Tribasic copper sulphate 40% (WG) W/W | 14 | 0.2–0.3%; 1–3.75 kg/ha per year | NA/5 | 31/01/2020 |
| Tebuconazole 25% (WG) W/W | 11 | 0.05–0.075%; 0.75 kg/ha | 7/0.6 | 31/08/2020 |
| Fenbuconazol 2.5% (EW) W/V | 2 | 0.2–0.6%; 3 L/ha | 3/0.5 | 30/04/2022 |
| Bacillus subtilis (Strain QST 713) 15.67% (5.13 × 1010 CFU/g (WP) W/W | 1 | 2.5–4 kg/ha | NA | 30/04/2019 |
| Total active ingredients applicable in preharvest peach and nectarine bio-fungicidal control (18) | ||||
| Fludioxonil 23% and 60% (SC) W/V | 2 | 0.3–0.4% | NA/10 | 12/31/2019 |
| Pyrimethanil 30% (GE) W/W | 1 | 6 g/tm | NA/5 | 30/04/2019 |
| Total active ingredients applicable in postharvest peach and nectarine bio-fungicidal control (2) | ||||
| Total number of commercial bio-fungicidal products allowed in Spanish peach market (197) | ||||
| Species | Common Name | Treatment Form | Disease | Target Pathogen | Fruit Type | Effectiveness | Reference |
|---|---|---|---|---|---|---|---|
| Mentha balsamea Wild and Ocimum basilicum L. | Peppermint and sweet basil | Essential oils | Yellowish, curl, brown blotch, white and soft rots syndromes | Rhizopus stolonifer (Ehrenb., Fr.); Monilinia fructicola (Wint.) Honey; Aspergillus niger Vantighm | Peach | High antifungal activity | Ziedan and Farrag [148] |
| Thymus vulgaris L., Eugenia caryophyllata L., and 2 others | Thyme and clavero | Essential oils | Brown and gray mold rots | Monilinia fructicola (Wint.) Honey and Botrytis cinerea (Pers. Fr.) | Apricot | Good antifungal activity | Hassani et al. [150] |
| Copernicia cerifera (Mill.) wax | Carnauba palm | Wax | Brown rot and Rhizopus rot | Monilinia fructicola (G. Winter) Honey and Rhizopus stolonifer (Ehrenb.) Vuill. | Plums and nectarines | Presents great potential | Goncalves et al. [151] |
| Apiaceae and Asteraceae families | NA | Active substances | Brown rot | Monilinia fructigena (Aderhold & Ruhl.) Honey | Stone fruits | Potentially effective | Ganchev [152] |
| Ocimum basilicum L., Ocimum tenuiflorum L. and 2 others | Basil and holy basil | Essential oils | Brown rot | Monilia laxa (Aderh. & Ruhl.) Honey | Peach and nectarine fruits | Potential antifungal properties | Carović-Stanko et al. [153] |
| Ocimumbasilicum L., Foeniculum officinale var. sativum (Bertol.) Arcangel plus 8 others | Basil and fennel | Essential oils | Brown rot and grey mold rot | Monilinia laxa (Aderh. & Ruhl.) Honey and Botrytis cinerea (Pers. Fr.) | Stone fruits | Variability in effectiveness | Lopez-Reyes et al. [154] |
| Thymus vulgaris L. and Laurus cinnamomum L. | Thyme and cinnamon | Essential oil vapours | Brown rot | Monilinia laxa (Aderh. & Ruhl.) Honey | Peach | Effective in preventive and curative treatments | Cindi et al. [155] |
| Treatments | Temperature | Period of Exposure | Effects | Reference |
|---|---|---|---|---|
| Hot water dipping (HWD) | 48 °C | 6/12 min | Reduced brown rot (BR) incidence and no significant loss of fruit quality | Jemric et al. [156] |
| Heat treatment (HT) | 40 °C | 5/10 min | Significant reduction in peach BR | Liu et al. [138] |
| Heat treatment (HT) 95% RH | 50 °C | 2 h | Proposed as potential strategy to control brown rot on peaches and nectarines | Casals et al. [157] |
| Radio frequency (RF) of dipping in hot water (HT) | 60 °C | 20 s | A 100% BRI reduction at 6 to 12 h after inoculation and 85.7%. BRI reduction at 0 to 48 h after inoculation as compared to untreated fruit | Spadoni et al. [159] |
| Radio frequency (RF) at 27.12 MHz of water immersion | 20 °C | 9 min | Controlled brown rot without adverse external and internal damage in both peaches and nectarines | Sisquella et al. [108] |
| Radio frequency (RF) at 27.12 MHz of exposition in air | 20 °C | 18 min | Brown rot incidence significantly reduced in both peaches and nectarines of different fruit size | Sisquella et al. [108] |
| Radio frequency (RF) at 27.12 MHz of water immersion | 40 °C | 4.5 min | Reduced BRI in stone fruits inoculated (0–48 h) before treatment and at all maturity levels evaluated in both peaches and nectarines without impaired fruit quality | Sisquella et al. [160] |
| Hydro cooling (HC) and water dump (WD) | 4 °C | 30 s/10 min | Reduced brown rot incidence by 50–77% when treated at 2/24 h of fruit harvest | Bernat et al. [158] |
| Authors | Fruit Type | Number of Fruit | Method of Inoculation | Fruit cheek | Source of Inoculum | Inoculum Density (cfu) | Inoculum Load | Incubation Period | Temperature/RH of Incubation | Susceptibility Variables |
|---|---|---|---|---|---|---|---|---|---|---|
| Biggs and Northover [190] | Peach | NA | UFIT | Randomly | PDA culture | 106–103 mL−1 | 30 µL (30,000 to 30 spores) | 144 h | 20 °C/60–95% | Disease severity score |
| Northover and Biggs [191] | Cherry | 10 | UFIT | Suture | PDA culture | 106–103 mL−1 | 30 µL (30,000 to 30 spores) | 144 h | 20 °C/60–95% | % BRI, lesion diameter |
| Gradziel and Wang [185] | Peach | 16 | UFIT/AIFIT | Most matured | PDA culture | 2 × 104 mL−1 | 10 µL (200 spores) | 72 h | 22 °C–25 °C/95% | Lesion diameter |
| Pascal et al. [45] | Peach, Plum Apricot | 10 | UFIT/AIFIT | Randomly | Natural fruit | 106 mL−1 | 20 µL (20,000 spores) | 240 h/120 h | 23 °C | % BRI, lesion diameter |
| Bassi et al. [192] | Peach | 15 | UFIT | Randomly | NA | 105 mL−1 | NA | 168 h | 25 ± 2 °C/95–100% | % BRI, lesion diameter |
| Walter et al. [46] | Apricot | 8 | UFIT/AIFIT | Randomly | Natural fruit | 1.5 × 104 mL−1 | 30 µL (450 spores) | 48 h/120 h | Ambient temperature/lightly misted with dH2O | Lesion area, spore counts, storage rot and cuticle thickness |
| Pacheco et al. [181] | Peach | 10 | UFIT/AIFIT | Sun-exposed fruit cheek | Peach fruit | 5 × 106 mL−1 | 10 μL (50,000 spores). | 120 h | 25 °C/high RH | % BRI, average rot diameter by scores |
| Obi et al. [48] | Peach | 20 | UFIT | Randomly | Peach fruit | 25 × 103 mL−1 | 25 µL (625 spores) | 120 h | 23 °C/50–60% | Lesion diameter, colonization diameter, % BRI, disease severity |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obi, V.I.; Barriuso, J.J.; Gogorcena, Y. Peach Brown Rot: Still in Search of an Ideal Management Option. Agriculture 2018, 8, 125. https://doi.org/10.3390/agriculture8080125
Obi VI, Barriuso JJ, Gogorcena Y. Peach Brown Rot: Still in Search of an Ideal Management Option. Agriculture. 2018; 8(8):125. https://doi.org/10.3390/agriculture8080125
Chicago/Turabian StyleObi, Vitus Ikechukwu, Juan José Barriuso, and Yolanda Gogorcena. 2018. "Peach Brown Rot: Still in Search of an Ideal Management Option" Agriculture 8, no. 8: 125. https://doi.org/10.3390/agriculture8080125
APA StyleObi, V. I., Barriuso, J. J., & Gogorcena, Y. (2018). Peach Brown Rot: Still in Search of an Ideal Management Option. Agriculture, 8(8), 125. https://doi.org/10.3390/agriculture8080125






