Short-Term Response of Soil Microbial Community to Field Conversion from Dryland to Paddy under the Land Consolidation Process in North China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Field and Design
2.2. Soil Sample Collection and Analysis
2.3. DNA Purification, PCR amplification, and High-Throughput Sequencing
2.4. Data Analysis and Processing
3. Results and Analysis
3.1. Impact of Dryland-to-Paddy Conversion on Soil Physicochemical Properties
3.2. Impact of Dryland-to-Paddy Conversion on Soil Microbial Community
3.2.1. Impact of Dryland-to-Paddy Conversion on Microbial Community Structure and Diversity
3.2.2. Impact of Dryland-to-Paddy Conversion on the Soil Microbial Community Composition
3.3. Impact of Soil Physicochemical Properties on the Microbial Community Structure of Consolidated Soil
4. Discussion
4.1. Impact of Conversion of Dryland to Paddy on Soil Eco-Environment
4.2. Changes in Microbial Community Structure and Function After the Dryland-to-Paddy Conversion
4.3. Uncertainty of Ecological iImpacts of Dryland-to-Paddy Conversion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Akala, V.; Lal, R. Potential of mine land reclamation for soil organic carbon sequestration in Ohio. Land Degrad.Dev. 2015, 11, 289–297. [Google Scholar] [CrossRef]
- Lyu, M.; Xie, J.; Ukonmaanaho, L.; Jiang, M.; Li, Y.; Chen, Y.; Yang, Z.; Zhou, Y.; Lin, W.; Yang, Y. Land use change exerts a strong impact on deep soil C stabilization in subtropical forests. J. Soils Sediments 2017, 17, 2305–2317. [Google Scholar] [CrossRef]
- Cox, P.M.; Betts, R.A.; Jones, C.D.; Spall, S.A.; Totterdell, I.J. Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 2000, 408, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Crowther, T.W.; Todd-Brown, K.E.; Rowe, C.W.; Wieder, W.R.; Carey, J.C.; Machmuller, M.B.; Snoek, B.; Fang, S.; Zhou, G.; Allison, S.D. Quantifying global soil carbon losses in response to warming. Nature 2016, 540, 104. [Google Scholar] [CrossRef] [PubMed]
- Arneth, A.; Sitch, S.; Pongratz, J.; Stocker, B.; Ciais, P.; Poulter, B.; Bayer, A.; Bondeau, A.; Calle, L.; Chini, L. Historical carbon dioxide emissions caused by land-use changes are possibly larger than assumed. Nat. Geosci. 2017, 10, 79. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, E.; Baldock, J.; Xing, H. Potential soil organic carbon stock and its uncertainty under various cropping systems in Australian cropland. Soil Res. 2014, 52, 463–475. [Google Scholar] [CrossRef]
- Wu, H.; Guo, Z.; Gao, Q.; Peng, C. Distribution of soil inorganic carbon storage and its changes due to agricultural land use activity in China. Agric. Ecosyst. Environ. 2009, 129, 413–421. [Google Scholar] [CrossRef]
- Jin, X.; Shao, Y.; Zhang, Z.; Resler, L.M.; Campbell, J.B.; Chen, G.; Zhou, Y. The evaluation of land consolidation policy in improving agricultural productivity in China. Sci. Rep. 2017, 7, 2792. [Google Scholar] [CrossRef]
- Jin, X.; Ding, N.; Zhang, Z.; Zhou, Y.; Yang, X. Inter-provincial allocation of land consolidation fund and effects of land consolidation in China. Trans. Chin. Soc. Agric. Eng. 2012, 28, 1–9. [Google Scholar]
- Sharifi, A.; Gorji, M.; Asadi, H.; Pourbabaee, A.A. Land leveling and changes in soil properties in paddy fields of Guilan province, Iran. Paddy Water Environ. 2014, 12, 139–145. [Google Scholar] [CrossRef]
- Brye, K.R.; Slaton, N.A.; Norman, R.J. Soil Physical and Biological Properties as Affected by Land Leveling in a Clayey Aquert. Soil Sci. Soc. Am. J. 2006, 70, 631–642. [Google Scholar] [CrossRef]
- Brye, K.R.; Slaton, N.A.; Mozaffari, M.; Savin, M.C.; Norman, R.J.; Miller, D.M. Short-Term Effects of Land Leveling on Soil Chemical Properties and Their Relationships with Microbial Biomass. Soil Sci. Soc. Am. J. 2004, 68, 924–934. [Google Scholar] [CrossRef]
- Crecente, R.; Alvarez, C.; Fra, U. Economic, social and environmental impact of land consolidation in Galicia. Land Use Policy 2002, 19, 135–147. [Google Scholar] [CrossRef]
- Legrand, F.; Picot, A.; Cobo-Díaz, J.F.; Carof, M.; Chen, W.; Le Floch, G. Effect of tillage and static abiotic soil properties on microbial diversity. Appl. Soil Ecol. 2018, 132, 135–145. [Google Scholar] [CrossRef]
- Ma, A.; Zhuang, X.; Wu, J.; Cui, M.; Lv, D.; Liu, C.; Zhuang, G. Ascomycota members dominate fungal communities during straw residue decomposition in arable soil. PLoS ONE 2013, 8, e66146. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Yu, Z.; Shi, Y.; Chu, H.; Jin, J.; Liu, X.; Wang, G. Soil carbon content drives the biogeographical distribution of fungal communities in the black soil zone of northeast China. Soil Biol. Biochem. 2015, 83, 29–39. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Freeman, C.; Ostle, N.J. Microbial contributions to climate change through carbon cycle feedbacks. ISME J. 2008, 2, 805–814. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, D.; Sparrow, A.; Gregorich, E.; Novis, P.; Elberling, B.; Greenfield, L. Redistributed lacustrine detritus as a spatial subsidy of biological resources for soils in an Antarctic dry valley. Geoderma 2008, 144, 86–92. [Google Scholar] [CrossRef]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Peñuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant–microbial–soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef]
- Kolis, K.; Hiironen, J.; Riekkinen, K.; Vitikainen, A. Forest land consolidation and its effect on climate. Land Use Policy 2017, 61, 536–542. [Google Scholar] [CrossRef]
- Dong, J.; Xiao, X.; Zhang, G.; Menarguez, M.; Choi, C.; Qin, Y.; Luo, P.; Zhang, Y.; Moore, B. Northward expansion of paddy rice in northeastern Asia during 2000–2014. Geophys. Res. Lett. 2016, 43, 3754–3761. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Xia, F.; Bao, H.X.H. Strategic planning framework for land consolidation in China: A top-level design based on SWOT analysis. Habitat Int. 2015, 48, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Suleiman, A.K.A.; Manoeli, L.; Boldo, J.T.; Pereira, M.G.; Roesch, L.F.W. Shifts in soil bacterial community after eight years of land-use change. Syst. Appl. Microbiol. 2013, 36, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zeng, Q.; Yu, G. The influence of land consolidation on biomass and ecological environment. Res. J. Appl. Sci. Eng. Technol. 2014, 7, 3656–3662. [Google Scholar] [CrossRef]
- He, X.Y.; Su, Y.R.; Liang, Y.M.; Chen, X.B.; Zhu, H.H.; Wang, K.L. Land reclamation and short-term cultivation change soil microbial communities and bacterial metabolic profiles. J. Sci. Food Agric. 2012, 92, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, J.; Wei, D.; Zhu, P.; Cui, X.A.; Zhou, B.; Chen, X.; Jin, J.; Liu, X.; Wang, G. Effects of over 30-year of different fertilization regimes on fungal community compositions in the black soils of northeast China. Agric. Ecosys. Environ. 2017, 248, 113–122. [Google Scholar] [CrossRef]
- Kang, S.; Eltahir, E.A. North China Plain threatened by deadly heatwaves due to climate change and irrigation. Nat. Commun. 2018, 9, 2894. [Google Scholar] [CrossRef]
- Yu, G.; Feng, J.; Che, Y.; Lin, X.; Hu, L.; Yang, S. The identification and assessment of ecological risks for land consolidation based on the anticipation of ecosystem stabilization: A case study in Hubei Province, China. Land Use Policy 2010, 27, 293–303. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Long, H. Analysis of arable land loss and its impact on rural sustainability in Southern Jiangsu Province of China. J. Environ. Manag. 2010, 91, 646–653. [Google Scholar] [CrossRef]
- Long, H. Land consolidation: An indispensable way of spatial restructuring in rural China. J. Geogr. Sci. 2014, 24, 211–225. [Google Scholar] [CrossRef]
- Chen, F.; Yu, M.; Zhu, F. Rethinking Rural Transformation Caused by Comprehensive Land Consolidation: Insight from Program of Whole Village Restructuring in Jiangsu Province, China. Sustainability 2018, 10, 2029. [Google Scholar] [CrossRef]
- Du, X.; Zhang, X.; Jin, X. Assessing the effectiveness of land consolidation for improving agricultural productivity in China. Land Use Policy 2018, 70, 360–367. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, W.; Ma, J.; Yang, Y.; Zhang, S.; Chen, R. Experimental study on the effects of underground CO2 leakage on soil microbial consortia. Int. J. Greenh. Gas. Control. 2017, 63, 241–248. [Google Scholar] [CrossRef]
- Mao, Y.; Sang, S.; Liu, S.; Jia, J. Spatial distribution of pH and organic matter in urban soils and its implications on site-specific land uses in Xuzhou, China. Comptes Rendus Biol. 2014, 337, 332–337. [Google Scholar] [CrossRef]
- Li, X.; Yu, M.; Ma, J.; Luo, Z.; Chen, F.; Yang, Y. Identifying the Relationship between Soil Properties and Rice Growth for Improving Consolidated Land in the Yangtze River Delta, China. Sustainability 2018, 10, 3072. [Google Scholar] [CrossRef]
- Schoeneberger, P.J. Field Book for Describing and Sampling Soils; USDA Natural Resources Conservation Service: Lincoln, NE, USA, 1998.
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef] [Green Version]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Drenovsky, R.; Vo, D.; Graham, K.; Scow, K. Soil water content and organic carbon availability are major determinants of soil microbial community composition. Microb. Ecol. 2004, 48, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, W.; Gu, X. Changes resulting from a land consolidation project (LCP) and its resource–environment effects: A case study in Tianmen City of Hubei Province, China. Land Use Policy 2014, 40, 74–82. [Google Scholar] [CrossRef]
- Jiang, G.; Wang, X.; Yun, W.; Zhang, R. A new system will lead to an optimal path of land consolidation spatial management in China. Land Use Policy 2015, 42, 27–37. [Google Scholar]
- Wang, J.; Yan, S.; Guo, Y.; Li, J.; Sun, G. The effects of land consolidation on the ecological connectivity based on ecosystem service value: A case study of Da’an land consolidation project in Jilin province. J. Geogr. Sci. 2015, 25, 603–616. [Google Scholar] [CrossRef]
- Parfitt, J.M.B.; Timm, L.C.; Reichardt, K.; Pinto, L.F.S.; Pauletto, E.A.; Castilhos, D.D. Chemical and biological attributes of a lowland soil affected by land leveling. Pesqui. Agropecu. Bras. 2013, 48, 1489–1497. [Google Scholar] [CrossRef] [Green Version]
- Oppermann, B.; Michaelis, W.; Blumenberg, M.; Frerichs, J.; Schulz, H.; Schippers, A.; Beaubien, S.; Krüger, M. Soil microbial community changes as a result of long-term exposure to a natural CO2 vent. Geochim. Cosmochim. Acta 2010, 74, 2697–2716. [Google Scholar] [CrossRef]
- Roth, P.J.; Lehndorff, E.; Cao, Z.H.; Zhuang, S.; Bannert, A.; Wissing, L.; Schloter, M.; Kögel-Knabner, I.; Amelung, W. Accumulation of nitrogen and microbial residues during 2000 years of rice paddy and non-paddy soil development in the Yangtze River Delta, China. Glob. Chang. Biol. 2011, 17, 3405–3417. [Google Scholar] [CrossRef]
- Yan, X.; Zhou, H.; Zhu, Q.; Wang, X.; Zhang, Y.; Yu, X.; Peng, X. Carbon sequestration efficiency in paddy soil and upland soil under long-term fertilization in southern China. Soil Tillage Res. 2013, 130, 42–51. [Google Scholar] [CrossRef]
- Mulqueen, J.; Rodgers, M.; Scally, P. Phosphorus transfer from soil to surface waters. Agric. Water Manag. 2004, 68, 91–105. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, S.; Li, R.; Zhang, J.; Liu, Y.; Lv, L.; Zhu, H.; Wu, W.; Li, W. Plant cultivars imprint the rhizosphere bacterial community composition and association networks. Soil Biol. Biochem. 2017, 109, 145–155. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Eldridge, D.J.; Hamonts, K.; Reich, P.B.; Singh, B.K. Experimentally testing the species-habitat size relationship on soil bacteria: A proof of concept. Soil Biol. Biochem. 2018, 123, 200–206. [Google Scholar] [CrossRef] [Green Version]
- Unger, I.M.; Goyne, K.W.; Kremer, R.J.; Kennedy, A.C. Microbial community diversity in agroforestry and grass vegetative filter strips. Agrofor. Syst. 2013, 87, 395–402. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, G.; Harhangi, H.R.; Zhu, B.; Jetten, M.S.; Yin, C.; Op den Camp, H.J. Co-occurrence and distribution of nitrite-dependent anaerobic ammonium and methane-oxidizing bacteria in a paddy soil. FEMS Microbiol. Let. 2012, 336, 79–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahrawat, K.L. Organic matter accumulation in submerged soils. Adv. Agron. 2004, 81, 170–203. [Google Scholar]
- Jiang, Y.; Liang, Y.; Li, C.; Wang, F.; Sui, Y.; Suvannang, N.; Zhou, J.; Sun, B. Crop rotations alter bacterial and fungal diversity in paddy soils across East Asia. Soil Biol. Biochem. 2016, 95, 250–261. [Google Scholar] [CrossRef] [Green Version]
- Welbaum, G.E.; Sturz, A.V.; Dong, Z.; Nowak, J. Managing soil microorganisms to improve productivity of agro-ecosystems. Crit. Rev. Plant. Sci. 2004, 23, 175–193. [Google Scholar] [CrossRef]
- Kragelund, C.; Caterina, L.; Borger, A.; Thelen, K.; Eikelboom, D.; Tandoi, V.; Kong, Y.; Van Der Waarde, J.; Krooneman, J.; Rossetti, S. Identity, abundance and ecophysiology of filamentous Chloroflexi species present in activated sludge treatment plants. FEMS Microbiol. Ecol. 2007, 59, 671–682. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, R.; Gaspar, H.; Gonzalez, J.M.; Clara, M.I.; Santana, M.M. Copper and temperature modify microbial communities, ammonium and sulfate release in soil. J. Plant. Nutr. Soil Sci. 2015, 178, 953–962. [Google Scholar] [CrossRef]
- He, F.; Yang, B.; Wang, H.; Yan, Q.; Cao, Y.; He, X. Changes in composition and diversity of fungal communities along Quercus mongolica forests developments in Northeast China. Appl. Soil Ecol. 2016, 100, 162–171. [Google Scholar] [CrossRef]
- Sterkenburg, E.; Bahr, A.; Durling, M.B.; Clemmensen, K.E.; Lindahl, B.D. Changes in fungal communities along a boreal forest soil fertility gradient. New Phytol. 2015, 207, 1145–1158. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Mo, X.; Lin, Z.; Xu, Y.; Ji, J.; Wen, G.; Richey, J. Crop yield responses to climate change in the Huang-Huai-Hai Plain of China. Agric. Water Manag. 2010, 97, 1195–1209. [Google Scholar] [CrossRef]
- Georgescu, M.; Lobell, D.; Field, C. Potential impact of US biofuels on regional climate. Geophys. Res. Lett. 2009, 36, L21806. [Google Scholar] [CrossRef]
- Kreye, C.; Dittert, K.; Zheng, X.; Zhang, X.; Lin, S.; Tao, H.; Sattelmacher, B. Fluxes of methane and nitrous oxide in water-saving rice production in north China. Nutr. Cycl. Agroecosyst. 2007, 77, 293–304. [Google Scholar] [CrossRef]
- Yang, R.; Ti, C.; Li, F.; Deng, M.; Yan, X. Assessment of N2O, NOx and NH3 emissions from a typical rural catchment in Eastern China. Soil Sci. Plant Nutr. 2010, 56, 86–94. [Google Scholar] [CrossRef]
- Qin, H.; Tang, Y.; Shen, J.; Wang, C.; Chen, C.; Yang, J.; Liu, Y.; Chen, X.; Li, Y.; Hou, H. Abundance of transcripts of functional gene reflects the inverse relationship between CH4 and N2O emissions during mid-season drainage in acidic paddy soil. Biol. Fertil. Soils 2018, 54, 885–895. [Google Scholar] [CrossRef]
- Galford, G.L.; Melillo, J.M.; Kicklighter, D.W.; Cronin, T.W.; Cerri, C.E.; Mustard, J.F.; Cerri, C.C. Greenhouse gas emissions from alternative futures of deforestation and agricultural management in the southern Amazon. Proc. Natl. Acad. Sci. USA 2010, 107, 19649–19654. [Google Scholar] [CrossRef] [Green Version]
- Zheng, C.; Liu, J.; Cao, G.; Kendy, E.; Wang, H.; Jia, Y. Can China cope with its water crisis?—Perspectives from the North China Plain. Groundwater 2010, 48, 350–354. [Google Scholar] [CrossRef]
- Hu, Y.; Moiwo, J.P.; Yang, Y.; Han, S.; Yang, Y. Agricultural water-saving and sustainable groundwater management in Shijiazhuang Irrigation District, North China Plain. J. Hydrol. 2010, 393, 219–232. [Google Scholar] [CrossRef]
- Piao, S.; Ciais, P.; Huang, Y.; Shen, Z.; Peng, S.; Li, J.; Zhou, L.; Liu, H.; Ma, Y.; Ding, Y. The impacts of climate change on water resources and agriculture in China. Nature 2010, 467, 43. [Google Scholar] [CrossRef]
- Ding, T.; Qian, W. Geographical patterns and temporal variations of regional dry and wet heatwave events in China during 1960–2008. Adv. Atmos. Sci. 2011, 28, 322–337. [Google Scholar] [CrossRef]
- Kueppers, L.M.; Snyder, M.A.; Sloan, L.C. Irrigation cooling effect: Regional climate forcing by land-use change. Geophys. Res. Lett. 2007, 34, L3703. [Google Scholar] [CrossRef]
- Qian, Y.; Huang, M.; Yang, B.; Berg, L.K. A modeling study of irrigation effects on surface fluxes and land–air–cloud interactions in the Southern Great Plains. J. Hydrometeorol. 2013, 14, 700–721. [Google Scholar] [CrossRef]
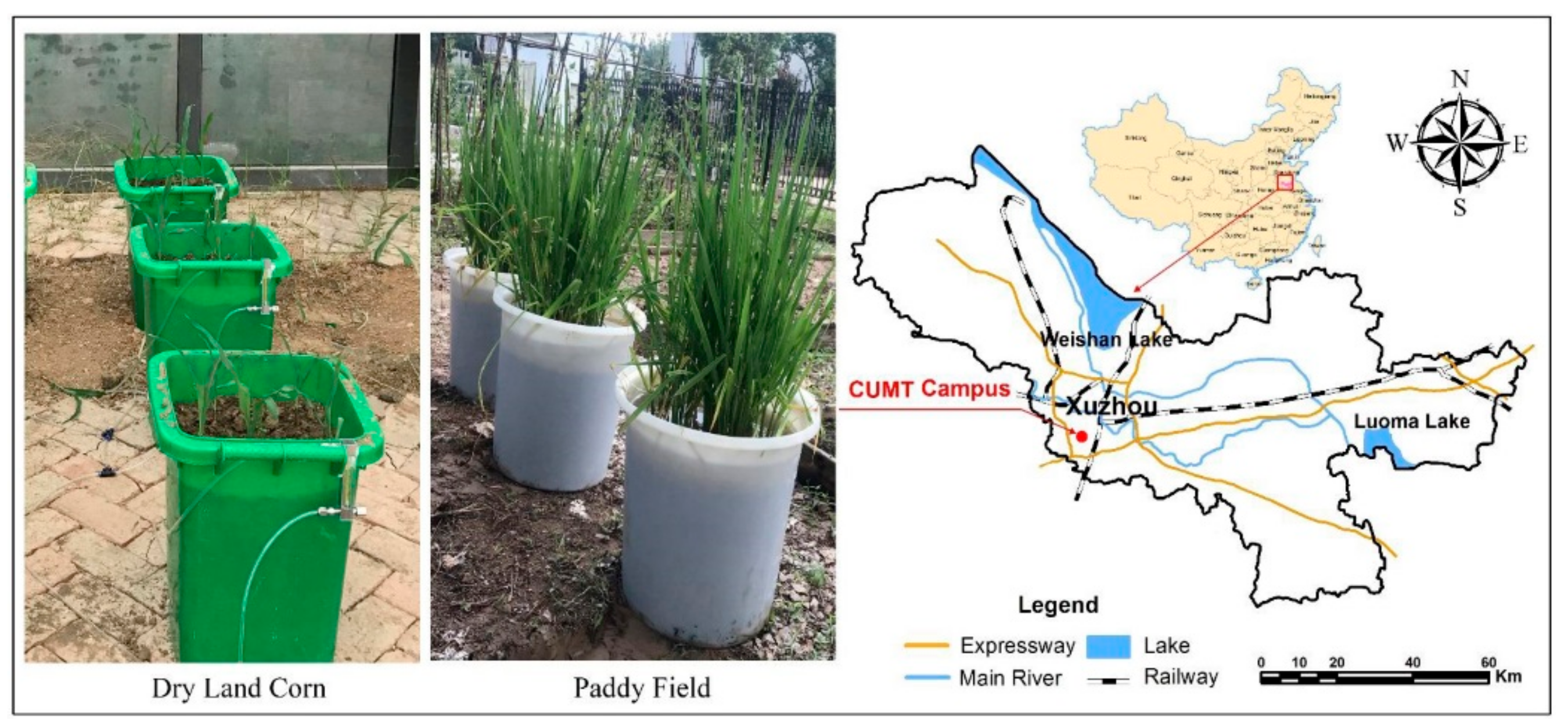



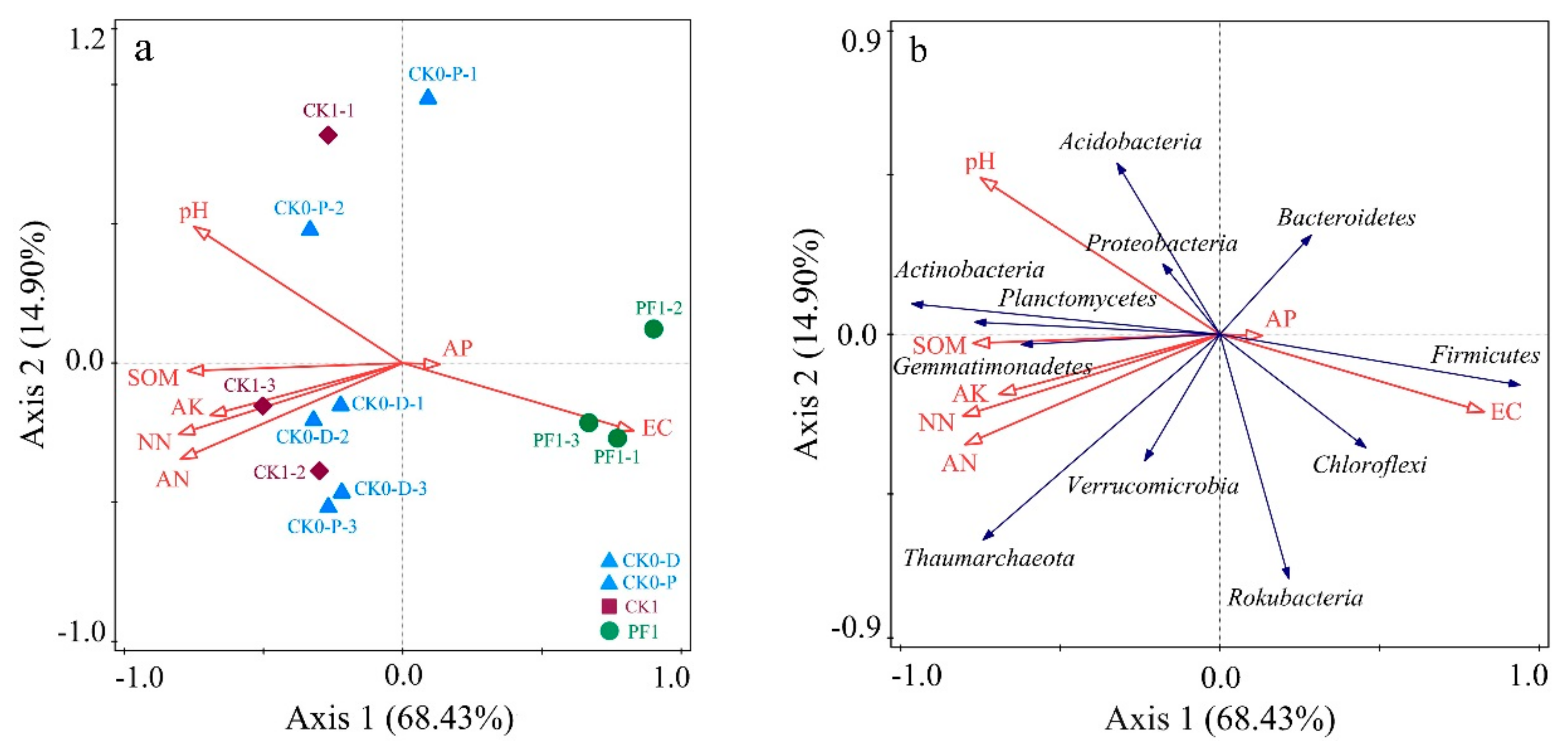
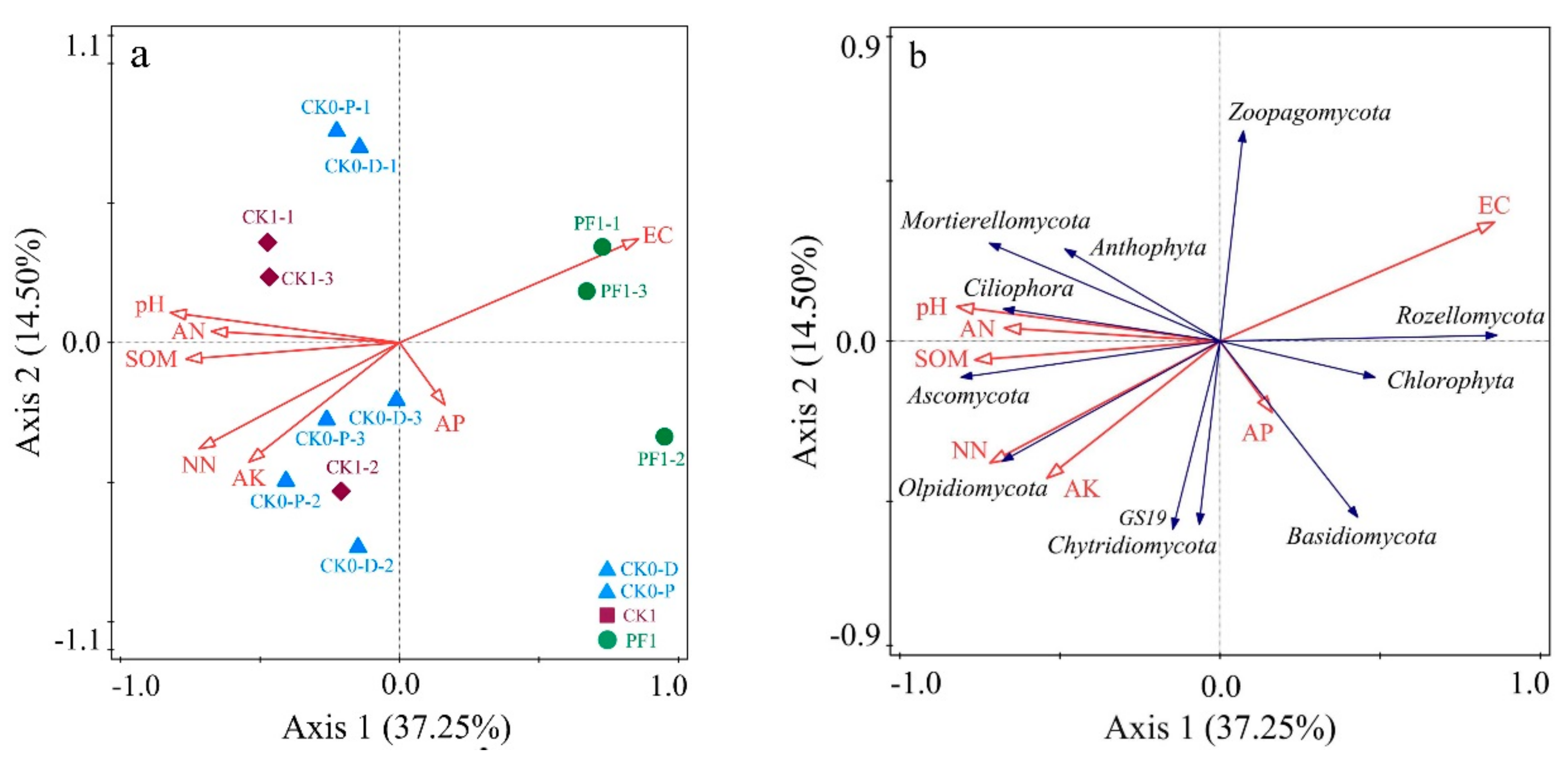
| Soil Properties | Dry Land Before Experiment | Dry Land Corn CK1 | Paddy Field PF1 | |
|---|---|---|---|---|
| CK0-D | CK0-P | |||
| pH | 8.62 ± 0.13b | 8.43 ± 0.23b | 8.65 ± 0.06b | 8.15 ± 0.01a |
| EC (mS·cm−3) | 17.25 ± 8.94a | 16.85 ± 4.96a | 15.13 ± 3.68a | 31.54 ± 2.72b |
| SOM (g·kg−1) | 23.00 ± 3.51b | 21.55 ± 7.12b | 21.10 ± 2.42b | 13.05 ± 1.19a |
| NN (mg·kg−1) | 4.59 ± 0.26b | 4.14 ± 0.43b | 4.31 ± 0.52b | 3.30 ± 0.27a |
| AN (mg·kg−1) | 1.33 ± 0.15b | 1.33 ± 0.17b | 1.36 ± 0.20b | 0.93 ± 0.07a |
| AK (mg·kg−1) | 30.88 ± 8.79a | 30.41 ± 8.67a | 29.51 ± 2.74a | 23.25 ± 1.58a |
| AP (mg·kg−1) | 77.89 ± 6.54a | 76.22 ± 4.79a | 68.82 ± 7.61a | 76.59 ± 2.62a |
| Sequencing Type | Diversity Index | Dry Land Before Experiment | Dry Land Corn CK1 | Paddy Field PF1 | |
|---|---|---|---|---|---|
| CK0-D | CK0-P | ||||
| Bacteria | Chao1 | 2396 ± 348a | 2528 ± 720a | 2771 ± 654a | 2297 ± 748a |
| ACE | 2472 ± 419a | 2578 ± 725a | 2818 ± 650a | 2338 ± 757a | |
| Shannon | 10.03 ± 0.20b | 9.99 ± 0.30b | 10.10 ± 0.24b | 9.22 ± 0.43a | |
| Simpson | 0.997 ± 0.00b | 0.997 ± 0.00b | 0.997 ± 0.00b | 0.989 ± 0.07a | |
| Fungus | Chao1 | 345.25 ± 116.2a | 364.09± 104.8a | 406.8 ± 83.0a | 243.9 ± 66.8a |
| ACE | 345.45 ± 115.3a | 364.05 ± 105.1a | 407.1 ± 91.0a | 244.5 ± 66.0a | |
| Shannon | 6.28 ± 0.45b | 6.12 ± 0.35b | 5.64 ± 0.54b | 4.56 ± 0.43a | |
| Simpson | 0.955 ± 0.03b | 0.957 ± 0.03b | 0.933 ± 0.04ab | 0.858 ± 0.68a | |
| Bacteria | Fungi | ||||
|---|---|---|---|---|---|
| Variable | r | p | Variable | r | p |
| pH | 0.538 | 0.004 ** | pH | 0.367 | 0.013 * |
| EC | 0.576 | 0.002 ** | EC | 0.484 | 0.004 ** |
| SOM | 0.438 | 0.006 ** | SOM | 0.345 | 0.014 * |
| NN | 0.359 | 0.021 * | NN | 0.408 | 0.004 ** |
| AN | 0.351 | 0.012 * | AN | 0.393 | 0.007 ** |
| AP | −0.282 | 0.967 | AP | −0.202 | 0.912 |
| AK | 0.253 | 0.039 * | AK | 0.167 | 0.126 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Ma, J.; Yang, Y.; Hou, H.; Liu, G.-J.; Chen, F. Short-Term Response of Soil Microbial Community to Field Conversion from Dryland to Paddy under the Land Consolidation Process in North China. Agriculture 2019, 9, 216. https://doi.org/10.3390/agriculture9100216
Li X, Ma J, Yang Y, Hou H, Liu G-J, Chen F. Short-Term Response of Soil Microbial Community to Field Conversion from Dryland to Paddy under the Land Consolidation Process in North China. Agriculture. 2019; 9(10):216. https://doi.org/10.3390/agriculture9100216
Chicago/Turabian StyleLi, Xiaoxiao, Jing Ma, Yongjun Yang, Huping Hou, Gang-Jun Liu, and Fu Chen. 2019. "Short-Term Response of Soil Microbial Community to Field Conversion from Dryland to Paddy under the Land Consolidation Process in North China" Agriculture 9, no. 10: 216. https://doi.org/10.3390/agriculture9100216
APA StyleLi, X., Ma, J., Yang, Y., Hou, H., Liu, G. -J., & Chen, F. (2019). Short-Term Response of Soil Microbial Community to Field Conversion from Dryland to Paddy under the Land Consolidation Process in North China. Agriculture, 9(10), 216. https://doi.org/10.3390/agriculture9100216






