Response of the Durum Wheat Cultivar Um Qais (Triticum turgidum subsp. durum) to Salinity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experiment
2.2. Greenhouse Experiment
2.3. Experimental Measurements
2.4. Statistical Analysis
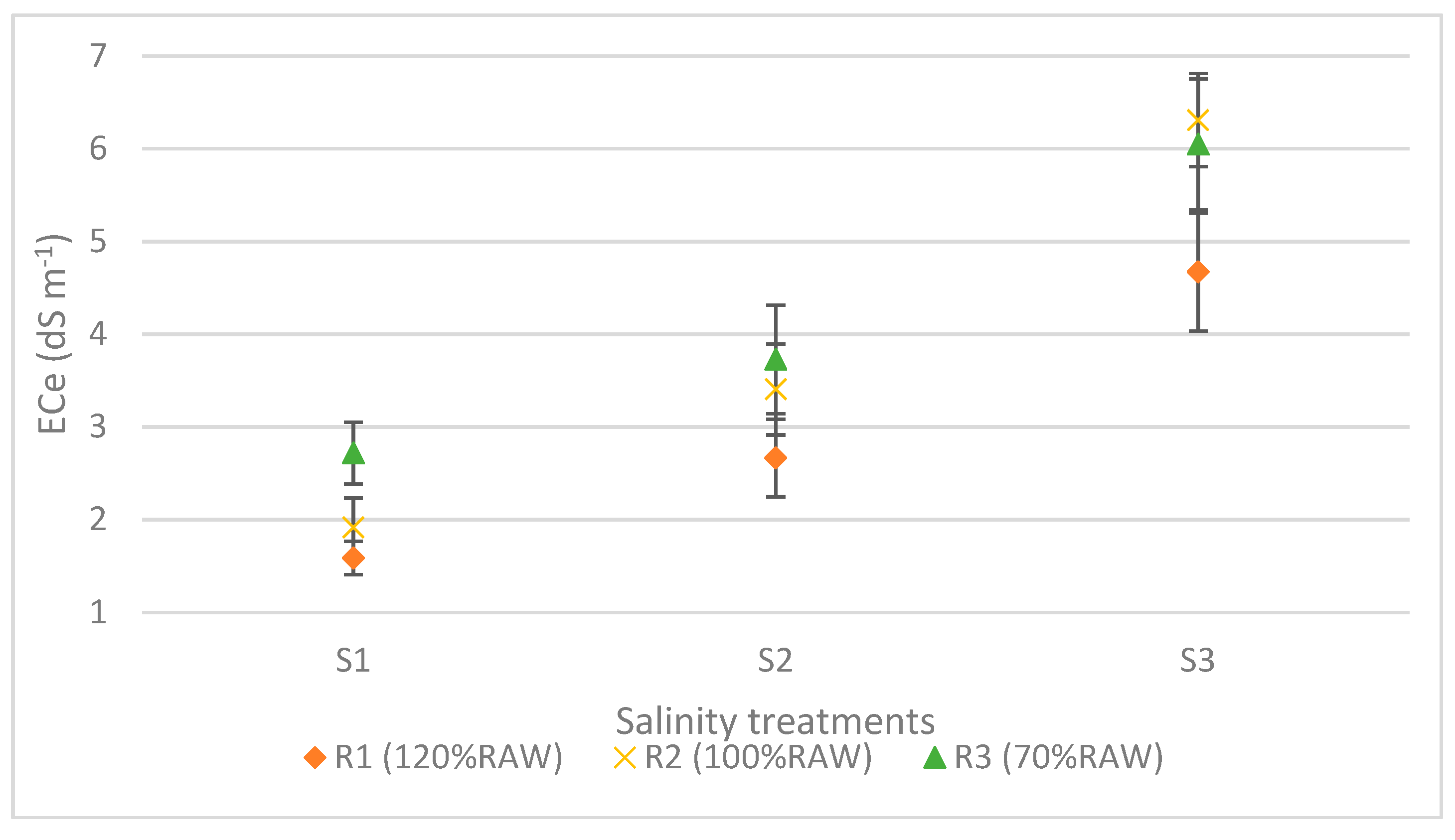
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Flowers, T. Plants and Salinity. J. Exp. Bot. Pref. 2006, 57, IV. [Google Scholar] [CrossRef]
- Läuchli, A.; Grattan, S. Plant Growth and Development Under Salinity Stress. In Advances in Molecular Breeding Toward Drought and Salt Tolerant Crops; Jenks, M.A., Hasegawa, P.M., Jain, S.M., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 1–32. [Google Scholar] [CrossRef]
- Hajiboland, R. Role of Arbuscular Mycorrhiza in Amelioration of Salinity. In Salt Stress in Plants; Ahmad, P., Azooz, M.M., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2013; pp. 301–354. [Google Scholar] [CrossRef]
- Pimentel, D.; Berger, B.; Filiberto, D.; Newton, M.; Wolfe, B.; Karabinakis, E.; Clark, S.; Poon, E.; Abbett, E.; Nandaopal, S. Water Resources, Agriculture and the Environment. BioScience 2004, 54, 909–918. [Google Scholar] [CrossRef]
- Machado, R.; Serralheiro, R. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Jamil, A.; Riaz, S.; Ashraf, M.; Foolad, R. Gene expression profiling of plants under salt stress. Crit. Rev. Plant Sci. 2011, 30, 435–458. [Google Scholar] [CrossRef]
- AbuAisha, E. Permanent Use of Water in Irrigation in Jordan’s Valley. In The Second International Conference of Water; The Jordanian Engineers Syndicate and The Ministry of Water and Irrigating: Amman, Jordan, 2001. [Google Scholar]
- Miyamoto, S.; Chacon, A.; Hossain, M.; Martinez, I. Soil salinity of urban turf areas irrigated with saline water. Landsc. Urban Plan 2005, 71, 233–241. [Google Scholar] [CrossRef]
- Ammari, T.G.; Tahhan, R.; Abubaker, S.; Al-Zu’Bi, Y.; Tahboub, A.; Ta’Any, R.; Abu-Romman, S.; Al-Manaseer, N.; Stietiya, M.H. Soil Salinity Changes in the Jordan Valley Potentially Threaten Sustainable Irrigated Agriculture. Pedosphere 2013, 23, 376–384. [Google Scholar] [CrossRef]
- Ekmekci, B.; Elkoca, O.; Tekkaya, E.; Erden, A. Residual stress state and hardness depth in electric discharge machining: De-ionized water as dielectric liquid. Mach. Sci. Technol. 2005, 9, 39–61. [Google Scholar] [CrossRef]
- Webber, H.A.; Madramootoo, C.A.; Bourgault, M.; Horst, M.G.; Stulina, G.; Smith, D.L. Adapting the CROPGRO Model for Saline Soils: The Case for a Common Bean Crop. Irrig. Sci. 2010, 28, 317–329. [Google Scholar] [CrossRef]
- Al-Zu’bi, Y.; Al-Kharabsheh, A. Multicriteria analysis for water productivity in the Jordan Valley. Water Int. 2003, 28, 501–511. [Google Scholar] [CrossRef]
- Munns, R. Comparative Physiology of Salt and Water Stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Greenway, H.; Munns, R. Mechanisms of Salt Tolerance in Nonhalophytes. Annu. Rev. Plant Physiol. 1980, 31, 149–190. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Rengasamy, P.; Mcdonald, G.K. The response of barely to salinity stress differs between hydroponics and soil systems. Funct. Plant Biol. 2010, 37, 621–633. [Google Scholar] [CrossRef]
- Borrelli, M.G.; Fragasso, M.; Nigro, F.; Platani, C.; Papa, R.; Beleggia, R.; Trono, D. Analysis of metabolic and mineral changes in response to salt stress in durum wheat (Triticum turgidum ssp. durum) genotypes, which differ in salinity tolerance. Plant Physiol. Biochem. 2018, 133, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Porceddu, E.; Damania, B.; Qualset, O. Genetics and breeding of durum wheat. In Proceedings of the International Symposium on Genetics and Breeding of Durum Wheat, Rome, Italy, 27–30 May 2013. [Google Scholar]
- Elings, A.; Nachit, M. Durum wheat landraces from Syria. I. Agro-ecological and morphological characterization. Euphytica 1991, 53, 211–224. [Google Scholar] [CrossRef]
- Jaradat, A. Phenotypic divergence for morphological and yield-related traits among landrace genotypes of durum wheat from Jordan. Euphytica 1991, 52, 155–164. [Google Scholar] [CrossRef]
- Munns, R.; Passioura, J.B.; Guo, J.; Chazen, O.; Cramer, G.R. water relations and leaf expansoin: Importance of time scale. J. Exp. Bot. 2000, 51, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Kabbaj, H.; Sall, A.; Al-Abdallat, A.; Geleta, M.; Amri, A.; Matlouf, A.; Belkadi, B.; Ortiz, R.; Bassi, F. Genetic Diversity within a Global Panel of Durum Wheat (Triticum durum) Landraces and Modern Germplasm Reveals the History of Alleles Exchange. Plant Sci. 2017, 8, 1495–1504. [Google Scholar] [CrossRef]
- Al-Rjoub, F.A.; Al-Samarrai, M.A. Growth and Yield Responses of Three Durum Wheat Cultivars Subjected to Four Levels of Available Soil Moisture. Agric. Sci. 2006, 33, 153–164. [Google Scholar]
- Thalji, T.; Shalaldeh, G. Screening Wheat and Barely Genotypes for Salinity Resistance. J. Agron. 2007, 6, 647–650. [Google Scholar]
- Katerji, N.; Mastrorilli, M.; van Hoon, J.W.; Lahmer, F.Z.; Hamdy, A.; Oweis, T. Durum wheat and barley productivity in saline-drought environments. Eur. J. Agron. 2009, 31, 1–9. [Google Scholar] [CrossRef]
- Dura, S.; Duwayri, M.; Nachit, M. Detection of molecular markers associated with yield and yield components in durum wheat (Triticum turgidum L. var. durum Desf.). Afr. J. Agric. Res. 2013, 8, 2118–2128. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). The State of Food and Agriculture; FAO: Rome, Italy, 1993. [Google Scholar]
- Maas, E.V.; Hoffman, G.J. Crop Salt Tolerance-Current Assessment. J. Irrig. Drain. Div. 1977, 103, 115–134. [Google Scholar]
- El-Hendawy, S.E.; Hu, Y.; Yakout, G.M.; Awad, A.M.; Hafiz, S.E.; Schmidhalter, U. Evaluating Salt Tolerance of Wheat Genotypes Using Multiple Parameters. Eur. J. Agron. 2005, 22, 243–253. [Google Scholar] [CrossRef]
- Munns, R.; Hare, R.A.; James, R.A.; Rebetzke, G.J. Genitic variation for improving the salt tolerance of durum wheat. Aust. J. Res. 2000, 51, 69–74. [Google Scholar] [CrossRef]
- Prathapar, S.A.; Qureshi, A.S. Modelling the Effects of Deficit Irrigation on Soil Salinity, Depth to Water Table and Transpiration in Semi-arid Zones with Monsoonal Rains. Int. J. Water Resour. Dev. 1999, 15, 141–159. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration, Guidelines for Computing Crop Water Requirements; FAO Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998. [Google Scholar]
- Day, P.R. Particle Fractionation and Particle-Size Analysis. In Methods of Soil Analysis. Part 1. Physical and Mineralogical Properties, Including Statistics of Measurement and Sampling; Black, C.A., Ed.; Agron. Monogr. 9.1; ASA, SSSA: Madison, WI, USA, 1965; pp. 545–567. [Google Scholar] [CrossRef]
- Gardner, W.H. Water Content. In Methods of Soil Analysis. Part 1. Physical and Mineralogical Properties, Including Statistics of Measurement and Sampling; Black, C.A., Ed.; Agron. Monogr 1965. 9.1; ASA, SSSA: Madison, WI, USA, 1965; pp. 82–127. [Google Scholar] [CrossRef]
- Blake, G.R. Bulk Density1. In Methods of Soil Analysis. Part 1. Physical and Mineralogical Properties, Including Statistics of Measurement and Sampling; Black, C.A., Ed.; Agron. Monogr 1965. 9.1; ASA, SSSA: Madison, WI, USA, 1965; pp. 374–390. [Google Scholar] [CrossRef]
- Rhoades, J.D.; Chanduvi, F.; Lesch, S. Soil Salinity Assessment: Methods of Interpretation of Electrical Conductivity Measurements; FAO: Rome, Italy, 1999. [Google Scholar]
- Chapman, H.D. Cation-Exchange Capacity 1. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, 2nd ed.; Norman, A.G., Ed.; Agron. Monogr 1965. 9.2; ASA, SSSA: Madison, WI, USA, 1965; pp. 891–901. [Google Scholar] [CrossRef]
- Bower, C.A.; Wilcox, L.V. Soluble Salts 1. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Norman, A.G., Ed.; Agron. Monogr.1965. 9.2; ASA, SSSA: Madison, WI, USA, 1965; pp. 933–951. [Google Scholar] [CrossRef]
- Olsen, S.R.; Dean, L.A. Phosphorus 1. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, 2nd ed.; Norman, A.G., Ed.; Agron. Monogr 1965. 9.2; ASA, SSSA: Madison, WI, USA, 1965; pp. 1035–1049. [Google Scholar] [CrossRef]
- Bremner, J.M. Total Nitrogen 1. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, 2nd ed.; Norman, A.G., Ed.; Agron. Monogr 1965. 9.2; ASA, SSSA: Madison, WI, USA, 1965; pp. 1149–1178. [Google Scholar] [CrossRef]
- Rani, B.; Sharma, V.K. Standarisation of methodology for obtaining the desired salt stress environment for salinity effect observation in rice seedlings. Int. J. Environ. Sci. 2015, 6, 232. [Google Scholar] [CrossRef]
- Ben Ahmed, C.; Magdich, S.; Ben Rouina, B.; Boukhris, M.; Ben Abdullah, F. Saline Water Irrigation Effects on Soil Salinity Distribution and Some Physiological Responses of Field Grown Chemlali Olive. J. Environ. Manag. 2012, 113, 538–544. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R.; Maas, E.V. Plant salt tolerance. Agric. Salin. Assess. Manag. 2012, 2, 405–459. [Google Scholar]
- Barlow, K.M.; Christy, B.P.; O’Leary, G.J.; Riffkin, P.A.; Nuttall, J.G. Simulating the impact of extreme heat and frost events on wheat crop production: A review. Field Crops Res. 2015, 171, 109–119. [Google Scholar] [CrossRef]
- Singh, K.N.; Chatrath, R. Salinity tolerance. In Application of Physiology in Wheat Breeding; Reynolds, M.P., Monasterio, J.I.O., McNab, A., Eds.; CIMMYT: Mexico City, Mexico, 2001; pp. 101–110. [Google Scholar]
- Steppuhn, K.; Volkmar, K.M.; Miller, P.R. Comparing canola foeld pea, dry bean, and durum crops grown in saline media. Crop Sci. 2001, 41, 1827–1833. [Google Scholar] [CrossRef]
- Saqib, M.; Akhtar, J.; Qureshi, R.H. Pot study on wheat growth in saline and waterlogged compacted soil I. Grain yield and yield components. Soil Till. Res. 2004, 77, 169–177. [Google Scholar] [CrossRef]
- Dikgwatlhe, S.B.; Ceronio, G.M.; van Rensburg, L.D. Wheat (Triticum aestivum, L.) Growth and Yield Response to Saline Irrigation Water under Controlled Conditions. S. Afr. J. Plant Soil 2008, 25, 172–177. [Google Scholar] [CrossRef]
- Colmer, T.D.; Flowers, T.J.; Munns, R. Use of wild relatives to improve salt tolerance in wheat. J. Exp. Bot. 2006, 57, 1059–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| a. Soil Chemical Properties | |
|---|---|
| Cl (ppm) | 0.33 ± 0.05 |
| Na (ppm) | 41.98 ± 6.52 |
| K (ppm) | 54.55 ± 0.04 |
| Ca (ppm) | 120 ± 0.65 |
| P (ppm) | 42 ± 4.10 |
| Mg (ppm) | 105.60 ± 1.24 |
| HCO3 (ppm) | 231.86 ± 10.01 |
| Organic C (%) | 0.23 ± 0.01 |
| N (%) | 0.50 ± 0.05 |
| CEC (cmolkg−1) | 18.60 ± 0.48 |
| SAR | 0.83 ± 0.02 |
| ECe (dS m−1) | 4.00 ± 0.23 |
| pH | 8.00 ± 0.09 |
| b. Soil Physical Properties | |
| Clay (%) | 16 ± 0.48 |
| Sand (%) | 73 ± 0.65 |
| Upper limit (cm3 cm−3) | 0.19 ± 0.01 |
| Lower limit (cm3 cm−3) | 0.09 ± 0.01 |
| Saturated water content (cm3 cm−3) | 0.38 ± 0.02 |
| Bulk density (g cm−3) | 1.58 ± 0.11 |
| Ca (ppm) | Mg (ppm) | Cl (ppm) | Na (ppm) | SAR | pH | ECiw (dS m−1) | |
|---|---|---|---|---|---|---|---|
| Field exp. | 92 ± 2.10 | 40.8 ± 0.47 | 0.35 ± 0.01 | 240 ± 0.91 | 5.22 ± 0.05 | 7.8 ± 0.04 | 2 ± 0.31 |
| Greenhouse exp. | 28 ± 0.46 | 31.2 ± 2.08 | 0.054 ± 0.01 | 170 ± 0.57 | 5.230 ± 0.05 | 7.53 ± 0.14 | 0.68 ± 0.05 |
| Tillering | Flag Leaf | Maturity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DAP | LAI | P.HEIGHT | DAP | LAI | P. HEIGHT | DAP | LAI | P. HEIGHT | |
| a. Field Experiment | |||||||||
| S1R1 control | 19 | 0.40 ± 0.11 | 31 ± 0.75 | 53 | 5.03 ± 0.41 | 84 ± 0.75 | 94 | 2.89 ± 0.58 | 103 ± 2.53 |
| S1R2 | 19 | 0.32 ± 0.07 | 31 ± 0.48 | 53 | 5.18 ± 0.34 | 86 ± 0.29 | 94 | 2.53 ± 0.25 | 104 ± 1.32 |
| S1R3 | 19 | 0.38 ± 0.09 | 31 ± 0.63 | 53 | 4.50 ± 0.19 | 85 ± 0.29 | 94 | 2.41 ± 0.46 | 106± 1.81 |
| S2R1 | 19 | 0.47 ± 0.08 | 32 ± 0.48 | 53 | 5.06 ± 0.18 | 84 ± 0.29 | 94 | 2.85 ± 0.18 | 104 ± 1.81 |
| S2R2 | 19 | 0.44 ± 0.03 | 32 ± 0.63 | 53 | 4.36 ± 0.09 | 85 ± 1.04 | 94 | 2.25 ± 0.36 | 103 ± 2.63 |
| S2R3 | 19 | 0.20 ± 0.01 | 32 ± 0.48 | 53 | 4.09 ± 0.10 * | 85 ± 0.63 | 94 | 2.11 ± 0.04 | 103 ± 0.45 |
| S3R1 | 19 | 0.27 ± 0.02 | 30 ± 0.25 | 53 | 3.96 ± 0.16 * | 83 ± 0.29 | 94 | 2.41 ± 0.38 | 103 ± 1.43 |
| S3R2 | 19 | 0.31 ± 0.09 | 30 ± 0.29 | 53 | 3.81 ± 0.20 * | 81 ± 0.41 | 94 | 2.23 ± 0.03 | 101 ± 1.19 |
| S3R3 | 19 | 0.21 ± 0.03 | 31 ± 0.48 | 53 | 3.73 ± 0.18 * | 81 ± 0.41 | 94 | 1.84 ± 0.26 | 098 ± 1.09 |
| b. Greenhouse | |||||||||
| S1 control | 26 | NA | 41 ± 0.48 | 70 | NA | 97 ± 0.35 | 98 | NA | 106 ± 2.39 |
| S2 | 26 | NA | 39 ± 0.48 * | 70 | NA | 84 ± 0.28 * | 98 | NA | 98 ± 1.94 * |
| S3 | 26 | NA | 35 ± 0.25 * | 70 | NA | 79 ± 0.42 * | 98 | NA | 90 ± 2.46 * |
| S4 | 26 | NA | 35 ± 0.25 * | 70 | NA | 73 ± 0.50 * | 96 | NA | 85 ± 0.75 * |
| Treatments | Grains Number m−2 | Grain Yield kg ha−1 | Above-Ground Biomass kg ha−1 |
|---|---|---|---|
| a. Field Experiment | |||
| S1R1 | 6131 ± 150 | 3311 ± 81 | 9353 ± 241 |
| S1R2 | 6124 ± 248 | 3307 ± 134 | 9436 ± 471 |
| S1R3 | 6048 ± 247 | 3265 ± 133 | 9391 ± 407 |
| S2R1 | 6130 ± 181 | 3310 ± 97 | 9930 ± 293 |
| S2R2 | 5773 ± 210 | 3117 ± 11 | 9665 ± 353 |
| S2R3 | 5390 ± 136 | 2911 ± 73 * | 9024 ± 227 |
| S3R1 | 5825 ± 92 | 3145 ± 50 | 9752 ± 155 |
| S3R2 | 5508 ± 105 | 2754 ± 52 * | 8813 ± 169 |
| S3R3 | 4963 ± 80 | 2430 ± 11 * | 8021 ± 38 * |
| b. Greenhouse Experiment | |||
| S1 | 2742 ± 228 | 1448 ± 103 | 4576 ± 355 |
| S2 | 2288 ± 192 | 1148 ± 94 * | 4110 ± 352 |
| S3 | 2568 ± 141 | 1100 ± 52 * | 4129 ± 207 |
| S4 | 2242 ± 73 * | 878 ± 67 * | 3365 ± 109 * |
| Treatments | DF | Mean | CV (%) | SE | F | p-Value | F crit |
|---|---|---|---|---|---|---|---|
| a. Field Experiment | |||||||
| Irrigation water salinity (S) | 2 | NA | NA | NA | 19.82709 * | 9.8 × 10−8 * | 3.109311 |
| Irrigation water amount (R) | 2 | NA | NA | NA | 2.288454 | 0.107945 | 3.109311 |
| Interaction | 4 | NA | NA | NA | 0.127075 | 0.972231 | 2.484441 |
| Number of grains (m−2) | 35 | 5766 | 9 | 82.3 | 5.581253 * | 0.000322 * | 2.305313 |
| Grain yield (kg ha−1) | 35 | 3061 | 11 | 55.8 | 11.13967 * | 8.34 × 10−7 * | 2.305313 |
| Aboveground biomass (kg ha−1) | 35 | 9265 | 8 | 125.9 | 3.981641 * | 0.003164 * | 2.305313 |
| Plant height (cm) | 35 | 102.7 | 4 | 0.60 | 1.552936 | 0.185889 | 2.305313 |
| LAI | 35 | 4.40 | 15 | 0.10 | 6.164087 * | 0.000151 * | 2.305313 |
| b. Greenhouse Experiment | |||||||
| Number of grains (m−2) | 15 | 2460 | 0.00 | 2.3 | 1.958419 | 0.174143 | 3.490295 |
| Grain yield (kg ha−1) | 15 | 1143 | 22 | 64.1 | 8.175895 * | 0.003123 * | 3.490295 |
| Aboveground biomass kg ha−1 | 15 | 4045 | 17 | 167.1 | 3.287114 | 0.058261 | 3.490295 |
| Plant height (cm) | 15 | 94.3 | 10 | 2.3 | 16.5935 * | 0.000853 * | 4.066181 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamdi, L.; Suleiman, A.; Hoogenboom, G.; Shelia, V. Response of the Durum Wheat Cultivar Um Qais (Triticum turgidum subsp. durum) to Salinity. Agriculture 2019, 9, 135. https://doi.org/10.3390/agriculture9070135
Hamdi L, Suleiman A, Hoogenboom G, Shelia V. Response of the Durum Wheat Cultivar Um Qais (Triticum turgidum subsp. durum) to Salinity. Agriculture. 2019; 9(7):135. https://doi.org/10.3390/agriculture9070135
Chicago/Turabian StyleHamdi, Luma, Ayman Suleiman, Gerrit Hoogenboom, and Vakhtang Shelia. 2019. "Response of the Durum Wheat Cultivar Um Qais (Triticum turgidum subsp. durum) to Salinity" Agriculture 9, no. 7: 135. https://doi.org/10.3390/agriculture9070135
APA StyleHamdi, L., Suleiman, A., Hoogenboom, G., & Shelia, V. (2019). Response of the Durum Wheat Cultivar Um Qais (Triticum turgidum subsp. durum) to Salinity. Agriculture, 9(7), 135. https://doi.org/10.3390/agriculture9070135





