Carbon and Nitrogen Contents Driven by Organic Matter Source within Pichavaram Wetland Sediments
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. Factors Driving Carbon and Nutrient Contents in Sediments
3.2. Sedimentary Organic Matter Sources in Sediments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kathiresan, K.; Alikunhi, N.M. Tropical coastal ecosystems: Rarely explored for their interaction. Ecologia 2011, 1, 1–22. [Google Scholar] [CrossRef]
- Lee, S.Y.; Primavera, J.H.; Dahdouh-Guebas, F.; McKee, K.; Bosire, J.O.; Cannicci, E.; Diele, K.; Fromard, F.; Koedam, N.; Marchand, C.; et al. Ecological role and services of tropical mangrove systems: An assessment. Global Ecol. Biogeogr. 2014, 23, 726–743. [Google Scholar] [CrossRef]
- Alongi, D.M. Mangrove forests: Resilience, protection from tsunamis, and response to global climate change. Est. Coast. Shelf Sci. 2008, 76, 339–349. [Google Scholar] [CrossRef]
- Marois, D.E.; Mitsch, W.J. Coastal protection from tsunamis and cyclones by mangrove wetlands: A review. Int. J. Biodivers. Sci. Ecosyst. Serv. Mgt. 2015, 11, 71–83. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon Cycling and Storage in Mangrove Forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef]
- Sanders, C.J.; Eyre, B.D.; Santos, I.R.; Machado, W.; Luiz-Silva, W.; Smoak, J.M.; Breithaupt, J.L.; Ketterer, M.E.; Sanders, L.; Marotta, H.; et al. Elevated rates of organic carbon, nitrogen, and phosphorus accumulation in a highly impacted mangrove wetland. Geophys. Res. Lett. 2014, 41, 2475–2480. [Google Scholar] [CrossRef]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Breithaupt, J.L.; Smoak, J.M.; Smith, T.J.; Sanders, C.J.; Hoare, A. Organic carbon burial rates in mangrove sediments: Strengthening the global budget. Global Biogeochem. Cycles 2012, 26, GB3011. [Google Scholar] [CrossRef]
- McLeod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef] [Green Version]
- Chmura, G.L.; Anisfeld, S.C.; Cahoon, D.R.; Lynch, J.C. Global carbon sequestration in tidal, saline wetland soils. Glob. Biogeochem. Cycles 2003, 17. [Google Scholar] [CrossRef]
- Chen, G.; Azkab, M.H.; Chmura, G.L.; Chen, S.; Sastrosuwondo, P.; Ma, Z.; Dharmawan, I.W.E.; Yin, X.; Chen, B. Mangroves as a major source of soil carbon storage in adjacent seagrass meadows. Sci. Rep. 2017, 7, srep42406. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.; Libardoni, B.G.; Sanders, C.J. Factors influencing organic carbon accumulation in mangrove ecosystems. Biol. Lett. 2018, 14, 20180237. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, E.; Bouillon, S.; Dittmar, T.; Marchand, C. Organic carbon dynamics in mangrove ecosystems: A review. Aquat. Bot. 2008, 89, 201–219. [Google Scholar] [CrossRef] [Green Version]
- Pérez, A.; Machado, W.; Gutierrez, D.; Smoak, J.M.; Breithaupt, J.L.; Saldarriaga, M.S.; Sanders, L.; Marotta, H.; Sanders, C.J. Carbon and nutrient accumulation in mangrove sediments affected by multiple environmental changes. J. Soils Sediments 2020, 20, 2504–2509. [Google Scholar] [CrossRef]
- SFR. Mangroves cover: India State of Forest Report; Forest Survey of India: Dehradun, India, 2015; pp. 63–67. [Google Scholar]
- Naidu, A.S. Some aspects of texture, mineralogy and geochemistry of modern deltaic sediments of the Godavari River, India. Ph.D. Thesis, Andhra University, Visakhapatnam, India, 1968; p. 150. [Google Scholar]
- Ranjan, R.K.; Ramanathan, A.L.; Singh, G.; Chidambaram, S. Heavy metal enrichments due to tsunami in Pichavaram mangrove sediments. Environ. Mont. Assess. 2008, 147, 389–411. [Google Scholar] [CrossRef] [PubMed]
- Jeyabal, G.; Ramasamy, S. Down core variation in sediment characteristics and trace element geochemistry of a core sample in Pichavaram mangrove area, Tamil Nadu southeast coast of India. EnviroGeoChimica. Acta 2014, 1, 206–214. [Google Scholar]
- Hemminga, M.A.; Slim, F.J.; Kazungu, J.; Ganssen, G.M.; Nieuwenhuize, J.; Kruyt, N.M. Carbon outwelling from a mangrove forest with adjacent seagrass beds and coral reefs (Gazi Bay, Kenya). Mar. Ecol. Prog. Ser. 1994, 106, 291–302. [Google Scholar] [CrossRef]
- Chmura, G.L. What do we need to assess the sustainability of the tidal salt marsh carbon sink? Ocean Coast. Manag. 2013, 83, 25–31. [Google Scholar] [CrossRef]
- Duarte, C.M. Reviews and syntheses: Hidden forests, the role of vegetated coastal habitats in the ocean carbon budget. Biogeosciences 2017, 14, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Kathiresan, K. A review of studies on Pichavaram mangrove, southeast India. Hydrobiology 2000, 430, 185–205. [Google Scholar] [CrossRef]
- Ranjan, R.K.; Routh, J.; Ramanathan, A.L. Bulk organic matter characteristics in the Pichavaram mangrove—Estuarine complex, south-eastern India. Appl. Geochem. 2010, 25, 1176–1186. [Google Scholar] [CrossRef]
- Naidu, A.S.; Mowatt, T.C.; Somayajulu, B.L.K.; Rao, K.S. Characteristics of clay minerals in the bed loads of major rivers of India. In SCOPE/UNEP Sonderband Heft 58; University of Hamburg: Hamburg, Germany, 1985; pp. 559–568. [Google Scholar]
- Pérez, A.; Gutiérrez, D.; Saldarriaga, M.; Sanders, C.J. Hydrological controls on the biogeochemical dynamics of a Peruvian mangrove system. Hydrobiologia 2017, 803, 69–86. [Google Scholar] [CrossRef]
- Kennedy, P.; Kennedy, H.; Papadimitriou, S. The effect of acidification on the determination of organic carbon, total nitrogen and their stable isotopic composition in algae and marine sediment. Rapid Commun. Mass Spectrom. 2005, 19, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Schlacher, T.A.; Connolly, R.M.; Kurle, C. Effects of acid treatment on carbon and nitrogen stable isotope ratios in ecological samples: A review and synthesis. Methods Ecol. Evol. 2014, 5, 541–550. [Google Scholar] [CrossRef] [Green Version]
- Naidu, A.S.; Cooper, L.W.; Grebmeier, J.M.; Whitledge, T.E.; Hameedi, M.J. The continental margin of the North Bering-Chukchi Sea: Distribution, sources, fluxes, and burial rates of organic carbon. In The Arctic Ocean Carbon Cycle: Present and Past; Stein, R., Macdonald, R.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 193–203. [Google Scholar]
- Machado, W.; Sanders, C.J.; Santos, I.; Sanders, L.; Silva-Filho, E.; Luiz-Silva, W. Mercury dilution by autochthonous organic matter in a fertilized mangrove wetland. Environ. Pollut. 2016, 213, 30–35. [Google Scholar] [CrossRef]
- Pérez, A.; Machado, W.; Gutierrez, D.; Stokes, D.; Sanders, L.; Smoak, J.M.; Santos, I.; Sanders, C.J. Changes in organic carbon accumulation driven by mangrove expansion and deforestation in a New Zealand estuary. Estuar. Coast. Shelf Sci. 2017, 192, 108–116. [Google Scholar] [CrossRef]
- Mazda, Y.; Wolanski, E.; King, B.; Sase, A.; Ohtsuka, D.; Magi, M. Drag force due to vegetation in mangrove swamps and saltmarsh. Mangrove Salt Marsh. 1997, 1, 193–199. [Google Scholar] [CrossRef]
- Kathiresan, K.; Bingham, B.L. Biology of mangroves and mangrove ecosystems. Adv. Mar. Biol. 2001, 40, 84–254. [Google Scholar]
- Ramanathan, A.L. Sediment characteristics of the Pichavaram mangrove environment. Indian J. Mar. Sci. 1997, 26, 319–322. [Google Scholar]
- Ranjan, R.K.; Routh, J.; Ramanathan, A.L.; Klump, J.V. Elemental and stable isotope records of organic matter input and its fate in the Pichavaram mangrove-estuarine sediments (Tamil Nadu, India). Mar. Chem. 2011, 126, 163–172. [Google Scholar] [CrossRef]
- Bianchi, T.S. Biogeochemistry of Estuaries; OXFORD University Press: Oxford, UK, 2007; p. 706. [Google Scholar]
- Keil, G.R.; Mayer, L.M. Mineral matrices and organic matter. In Treatise on Geochemistry, 2nd ed.; Falkowski, P.G., Freeman, K.H., Eds.; Elsevier Ltd.: New York, NY, USA, 2014; Volume 12, pp. 337–359. [Google Scholar]
- IPCC. Climate Change 2020, The Physical Scientific Basis: Summary for Policymakers. Available online: http://www.ipcc.ch (accessed on 29 December 2021).
- Holland, D. On the rocks: The challenges of predicting sea level rise. EOS 2015, 96, 9–12. [Google Scholar] [CrossRef]
- Kirwan, M.L.; Temmerman, S.; Skeehan, E.E.; Guntenspergen, G.R.; Fagherazzi, S. Overestimation of marsh vulnerability to sea level rise Matthew. Nat. Clim. Chang. 2016, 6, 253–260. [Google Scholar] [CrossRef]
- Peck, E.K.; Wheatcroft, R.A.; Brophy, L.S. Controls on Sediment Accretion and Blue Carbon Burial in Tidal Saline Wetlands: Insights From the Oregon Coast, USA. J. Geophys. Res. Biogeosci. 2020, 125, e2019JG005464. [Google Scholar] [CrossRef]
- Jennerjahn, T.C.; Ittekot, V. Relevance of mangroves for the production and depositionof organic matter along tropical continental margins. Naturwissenschaften 2002, 89, 21–30. [Google Scholar] [CrossRef]
- Fry, B.; Sherr, E.B. δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems. Contrib. Mar. Sci. 1984, 27, 13–47. [Google Scholar]
- Boutton, T.W. Stable carbon isotope ratios of natural materials, II: Atmospheric, terrestrial, marine, and freshwater environments. In Carbon Isotope Techniques; Coleman, D.C., Fry, B., Eds.; Academic Press Inc.: San Diego, CA, USA, 1991; pp. 177–185. [Google Scholar]
- Middelburg, J.J. Stable isotopes dissect aquatic food webs from the top to the bottom. Biogeosciences 2014, 11, 2357–2371. [Google Scholar] [CrossRef] [Green Version]
- Rullkötter, J. Organic Matter: The Driving Force for Early Diagenesis. In Marine Geochemistry; Shulz, H.D., Zabel, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 125–168. [Google Scholar]
- Schultz, D.; Calder, J.A. Organic carbon 13C/12C variations in estuarine sediments. Geochim. Cosmochim. Acta 1976, 40, 381–385. [Google Scholar] [CrossRef]
- Jiménez-Arias, J.L.; Morris, E.; Rubio-de-Inglés, M.J.; Peralta, G.; García-Robledo, E.; Corzo, A.; Papaspyrou, S. Tidal elevation is the key factor modulating burial rates and composition of organic matter in a coastal wetland with multiple habitats. Sci. Total Environ. 2020, 724, 138205. [Google Scholar] [CrossRef]
- Maher, D.T.; Santos, I.R.; Golsby-Smith, L.; Gleeson, J.; Eyre, B.D. Ground water derived dissolved inorganic and organic carbon exports from a mangrove tidal creek: The missing mangrove carbon sink? Limnol. Oceanogr. 2013, 58, 475–488. [Google Scholar] [CrossRef]
- Rezende, E.C.; Lacerda, D.L.; Ovalle, A.R.C.; Silva, R.C.A.; Martinelli, A.L. Nature of POC transport in a mangrove ecosystem: A carbon isotopic study. Estuar. Coast. Shelf Sci. 1990, 30, 641–645. [Google Scholar] [CrossRef]
- Middelburg, J.J.; Nieuwenhuize, J.; Lubberts, R.K.; van de Plassche, O. Organic carbon isotope systematics of coastal marshes. Estuar. Coast. Shelf Sci. 1997, 45, 681–687. [Google Scholar] [CrossRef]
- Watanabe, K.; Kuwae, T. How organic carbon derived from multiple sources contributes to carbon sequestration processes in a shallow coastal system? Glob. Chang. Biol. 2015, 21, 2612–2623. [Google Scholar] [CrossRef]
- Santos, R.; Duque-Núñez, N.; de los Santos, C.B.; Martins, M.; Carrasco, A.R.; Veiga-Pires, C. Superficial sedimentary stocks and sources of carbon and nitrogen in coastal vegetated assemblages along a flow gradient. Sci. Rep. 2019, 9, 610. [Google Scholar] [CrossRef]
- Muzuka, A.N.N.; Shunula, J.P. Stable isotope composition of organic carbon and nitrogen of two mangrove stands along the Tanzanian coastal zone. Est. Coast. Shelf Sci. 2006, 66, 447–458. [Google Scholar] [CrossRef]
- McKee, K.; Feller, I.C.; Popp, M. Mangrove isotopic (δ15N and δ13C) fractionation across a nitrogen vs. phosphorus limitation gradient. Ecology 2002, 83, 1065–1075. [Google Scholar] [CrossRef]
- Müller, A.; Mathesius, U. The palaeoenvironments of coastal lagoons in the southern Baltic Sea, I. The application of sedimentary C org/N ratios as source indicators of organic matter. Palaeogeogr. Palaeoclimatol. Palaeoecol 1999, 145, 17–32. [Google Scholar] [CrossRef]
- Andrews, J.E.; Greenaway, A.M.; Dennis, P.F. Combined carbon isotope and C/N ratios as indicators of source and fate of organic matter in a poorly flushed, tropical estuary: Hunts Bay, Kingston Harbour, Jamaica. Estuar Coast Shelf Sci. 1998, 46, 743–756. [Google Scholar] [CrossRef]
- Kendall, C.; Elliott, E.M.; Wankel, S.D. Tracing anthropogenic inputs of nitrogen to ecosystems. In Stable Isotopes in Ecology and Environmental Science; Michener, R.H., Lajtha, K., Eds.; Blackwell Publishing: Hoboken, NJ, USA, 2007; pp. 375–449. [Google Scholar]
- Bouillon, S.; Frankignoulle, M.; Velimirov, B.; Eiler, A.; Abril, G.; Etcheber, H.; Borges, A. Inorganic and organic biogeochemistry in the Gautami Godavari estuary (Andhra Pradesh, India) during pre-monsoon: The local impact of extensive mangrove forests. Glob. Biogeochem. Cycles 2003, 17, 1114–1127. [Google Scholar] [CrossRef] [Green Version]
- Ricart, A.M.; Pérez, M.; Romero, J. Landscape configuration modulates carbon storage in seagrass sediments. Estuar. Coast. Shelf Sci. 2017, 185, 69–76. [Google Scholar] [CrossRef]
- Ricart, A.M.; York, P.H.; Bryant, C.V.; Rasheed, M.A.; Ierodiaconou, D.; Macreadie, P.I. High variability of Blue Carbon storage in seagrass meadows at the estuary scale. Sci. Rep. 2020, 10, 5865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
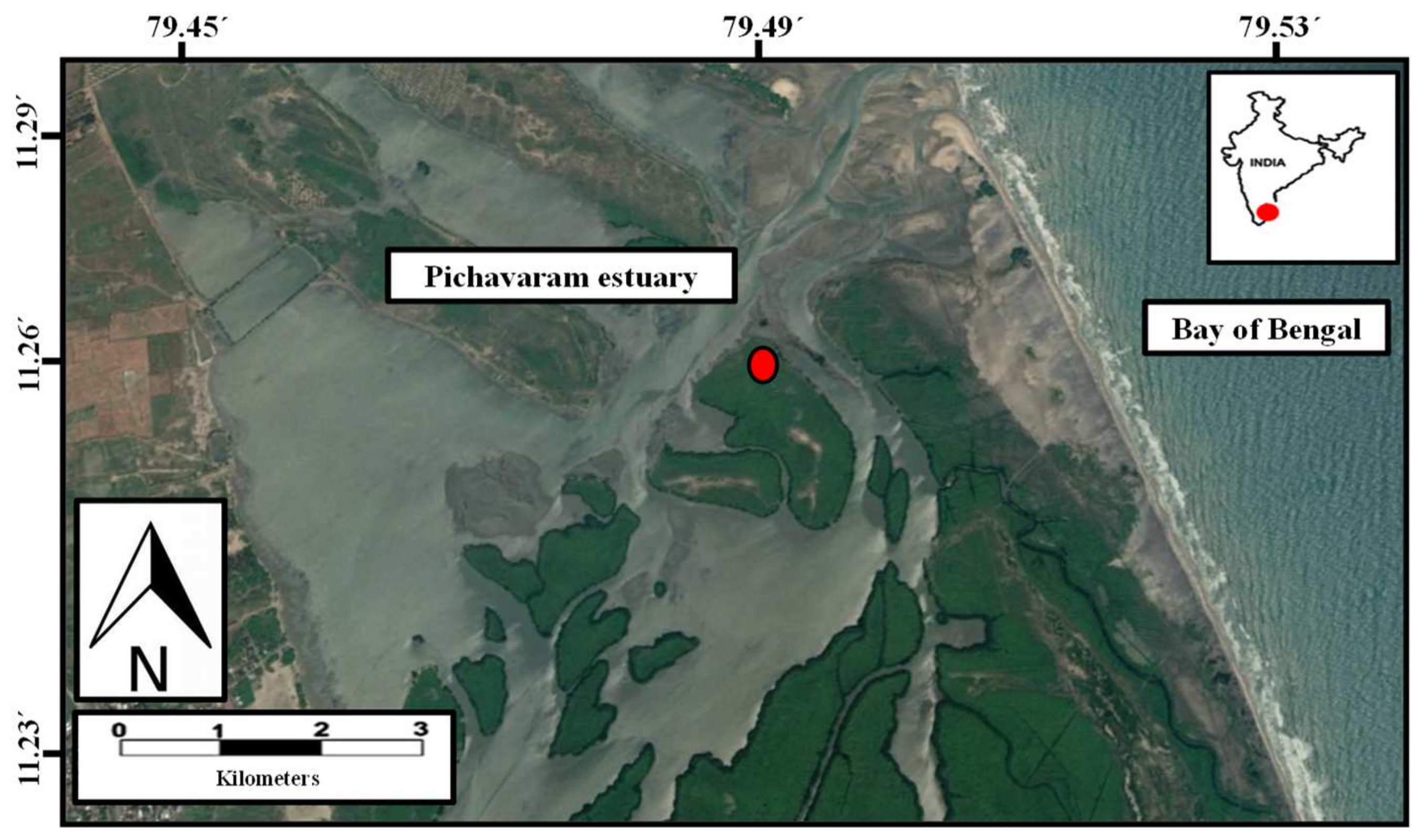
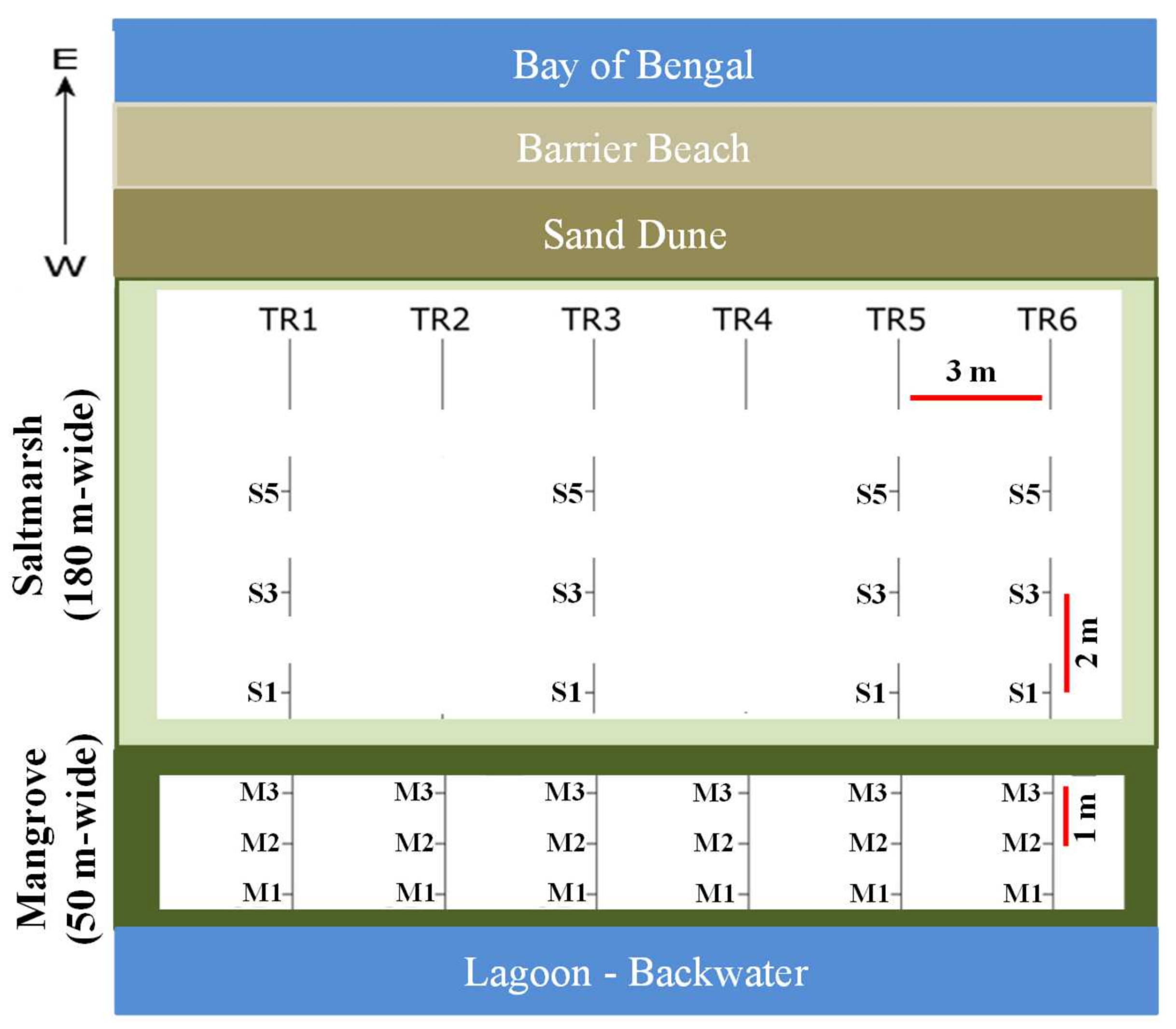

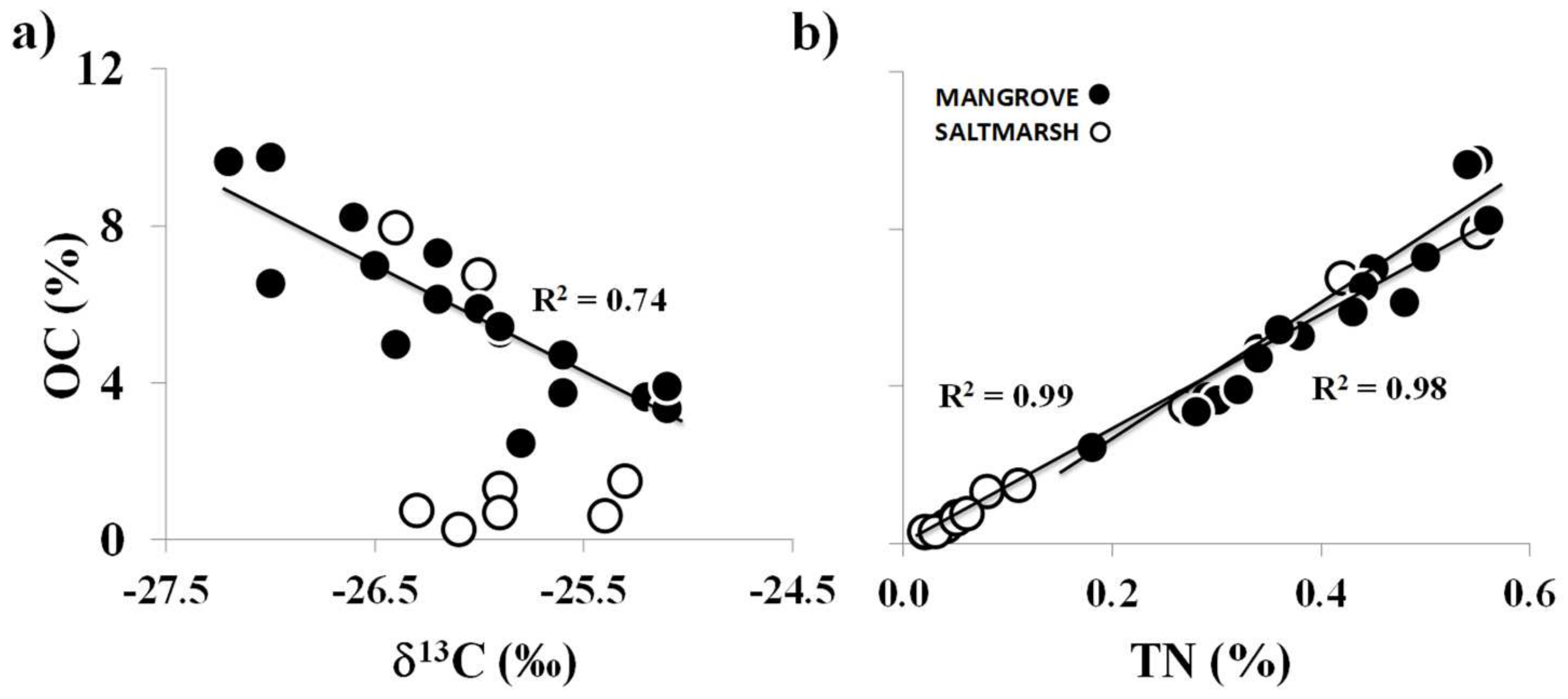
| Stations | OC (%) | TN (%) | OC/TN | δ13C (‰) | δ15N (‰) | Silt and Clay (%) | |
|---|---|---|---|---|---|---|---|
| Mangrove zone | TR1-M1 | 6.1 | 0.5 | 11.0 | −26.2 | +3.6 | 75.0 |
| TR1-M2 | 7.0 | 0.5 | 13.0 | −26.5 | +2.9 | 81.8 | |
| TR1-M3 | 3.7 | 0.3 | 11.0 | −25.6 | +2.6 | 61.9 | |
| TR2-M1 | 7.3 | 0.5 | 13.0 | −26.2 | +3.5 | 63.6 | |
| TR2-M2 | 9.7 | 0.6 | 15.0 | −27.0 | +3.6 | 63.6 | |
| TR2-M3 | 9.6 | 0.5 | 15.0 | −27.2 | +3.4 | 72.2 | |
| TR3-M1 | 8.2 | 0.6 | 13.0 | −26.6 | +2.9 | 84.2 | |
| TR3-M2 | 5.0 | 0.3 | 13.0 | −26.4 | +3.5 | 57.7 | |
| TR3-M3 | 4.7 | 0.3 | 12.0 | −25.6 | +3.5 | 72.9 | |
| TR4-M1 | 6.5 | 0.4 | 13.0 | −27.0 | +3.7 | 72.7 | |
| TR4-M2 | 5.3 | 0.4 | 12.0 | −25.9 | +3.5 | 70.0 | |
| TR4-M3 | 3.6 | 0.3 | 11.0 | −25.2 | +3.7 | 77.1 | |
| TR5-M1 | 2.5 | 0.2 | 12.0 | −25.8 | +3.9 | 57.1 | |
| TR5-M2 | 3.3 | 0.3 | 10.0 | −25.1 | +4.4 | 70.5 | |
| TR5-M3 | 3.9 | 0.3 | 10.0 | −25.1 | +4.0 | 36.4 | |
| TR6-M1 | 5.9 | 0.4 | 12.0 | −26.0 | +3.2 | 95.5 | |
| TR6-M2 | 5.4 | 0.4 | 13.0 | −25.9 | +3.4 | 81.2 | |
| TR6-M3 | 3.9 | 0.3 | 10.0 | −25.1 | +4.0 | 7.4 | |
| Saltmarsh zone | TR1-S1 | 1.3 | 0.1 | 14.0 | −25.9 | +3.6 | 15.5 |
| TR1-S3 | 0.7 | 0.1 | 12.0 | −25.9 | +3.4 | 9.8 | |
| TR1-S5 | 3.5 | 0.3 | 11.0 | −23.2 | +3.7 | 26.9 | |
| TR3-S1 | 0.3 | 0.0 | 12.0 | −26.1 | +2.5 | 4.0 | |
| TR3-S3 | 0.3 | 0.0 | 12.0 | −23.9 | +3.6 | 3.7 | |
| TR3-S5 | 1.5 | 0.1 | 12.0 | −25.3 | +2.4 | 12.5 | |
| TR5-S1 | 0.5 | 0.0 | 10.0 | −23.0 | +3.1 | 8.1 | |
| TR5-S3 | 0.3 | 0.0 | 10.0 | −21.6 | +3.8 | 3.9 | |
| TR5-S5 | 7.9 | 0.6 | 12.0 | −26.4 | +3.1 | 45.0 | |
| TR6-S1 | 0.6 | 0.1 | 11.0 | −25.4 | +3.6 | 11.8 | |
| TR6-S3 | 0.8 | 0.1 | 11.0 | −26.3 | +3.5 | 10.4 | |
| TR6-S5 | 6.8 | 0.4 | 14.0 | −26.0 | +3.3 | 71.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naidu, S.A.; Kathiresan, K.; Simonson, J.H.; Blanchard, A.L.; Sanders, C.J.; Pérez, A.; Post, R.M.; Subramoniam, T.; Naidu, R.A.; Narender, R. Carbon and Nitrogen Contents Driven by Organic Matter Source within Pichavaram Wetland Sediments. J. Mar. Sci. Eng. 2022, 10, 53. https://doi.org/10.3390/jmse10010053
Naidu SA, Kathiresan K, Simonson JH, Blanchard AL, Sanders CJ, Pérez A, Post RM, Subramoniam T, Naidu RA, Narender R. Carbon and Nitrogen Contents Driven by Organic Matter Source within Pichavaram Wetland Sediments. Journal of Marine Science and Engineering. 2022; 10(1):53. https://doi.org/10.3390/jmse10010053
Chicago/Turabian StyleNaidu, Sathy A., Kandasamy Kathiresan, Jeffrey H. Simonson, Arny L. Blanchard, Christian J. Sanders, Alexander Pérez, Ruth M. Post, Thanumalaya Subramoniam, Raj A. Naidu, and Rajendran Narender. 2022. "Carbon and Nitrogen Contents Driven by Organic Matter Source within Pichavaram Wetland Sediments" Journal of Marine Science and Engineering 10, no. 1: 53. https://doi.org/10.3390/jmse10010053







