Tetramine in the Salivary Glands of Marine Carnivorous Snails: Analysis, Distribution, and Toxicological Aspects
Abstract
:1. Introduction
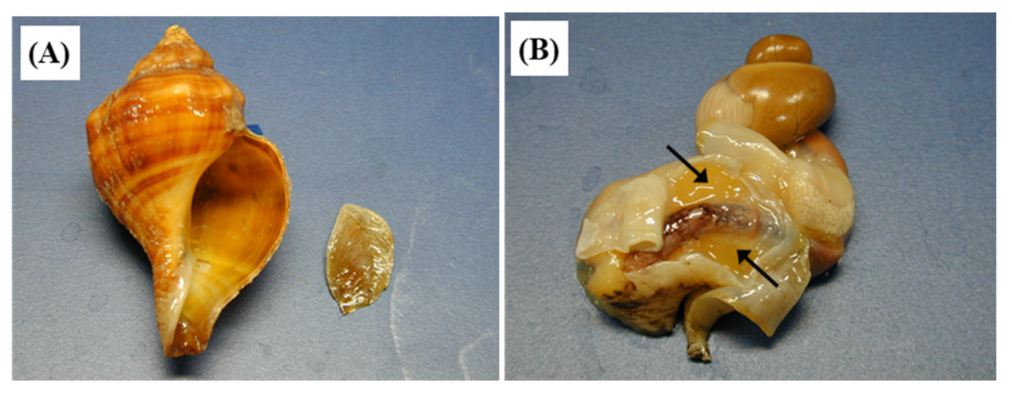
2. Anatomical Descriptions of Salivary Glands of Neptunea Species
3. Analytical Methods
4. Distribution in Marine Snails
4.1. Marine Snails Containing High Amounts of Tetramine
4.2. Seasonal Variation of Tetramine Concentration
| Order | Family | Species | Tetramine Content in Salivary Gland (mg/g) | Reference |
|---|---|---|---|---|
| Caenogastropoda | Batillariidae | Batillaria multiformis | <0.01 | [55] |
| Littorinimorpha | Naticidae | Neverita didyma | <0.01 | [55] |
| Charoniidae | Charonia lampas | 0.003–0.031 | [19] | |
| Ranellidae | Monoplex parthenopeum | <0.01 | [55] | |
| Fusitriton oregonensis | 0.064–4.0 | [35,38,56] | ||
| Fusitriton galea | 0.01 | [55] | ||
| Neogastropoda | Austrosiphonidae | Kelletia lischkei | 0.01 | [55] |
| Buccinidae | Buccinum aniwanum | 0.0007 | [56] | |
| Buccinum bayani | <0.01 | [34] | ||
| Buccinum inclytum | 0.00294–0.00340 | [56] | ||
| Buccinum leucostoma | <0.01 | [34] | ||
| Buccinum middendorffi | 0.0012–0.45 | [46,55,56] | ||
| Buccinum mirandum | 0.04 | [55] | ||
| Buccinum opisoplectum | 0.1 | [55] | ||
| Buccinum striatissimum | 0.03–0.05 | [55] | ||
| Buccinum tenuissimum | 0.0299–0.186 | [56] | ||
| Buccinum tsubai | <0.01 | [34] | ||
| Buccinum verkruzeni | <0.01 | [34] | ||
| Neptunea amianta | 11.81 | [55] | ||
| Neptunea antiqua | 0.75–4.476 | [32,33] | ||
| Neptunea arthritica | 0.85–12 | [34,35,38,40,41,46,55] | ||
| Neptunea cumingii | 6.3–15 | [57] | ||
| Neptunea decemcostata | 1.28 | [46] | ||
| Neptunea frater | 0.91–0.94 | [56] | ||
| Neptunea heros | 1.95–3.73 | [56] | ||
| Neptunea intersculpta * | 0.17–9.75 | [34,38,40,41,43,47] | ||
| Neptunea kuroshio | 2.67–3.58 | [40] | ||
| Neptunea lamellosa | 0.27–9.41 | [34,53,56] | ||
| Neptunea lyrata | 0.64–14.8 | [19,58] | ||
| Neptunea polycostata | 0.16–4.9 | [34,35,46,56] | ||
| Neptunea purpurea | 1.72–7.4 | [56] | ||
| Neptunea vinosa | 0.373–6.96 | [55,56] | ||
| Japeuthria ferrea | 0.05 | [56] | ||
| Siphonalia cassidariaeformis | 0.117–0.135 | [56] | ||
| Siphonalia fusoides | 0.204 | [56] | ||
| Fasciolariidae | Leucozonia smaragdula | 0.08 | [56] | |
| Fusinus forceps salisburyi | 0.0675 | [56] | ||
| Melongenidae | Hemifusus tuba | 4.5–8.8 | [55] | |
| Muricidae | Drupa rubisidaeus | 0.19 | [55] | |
| Mancinella siro | 0.42 | [55] | ||
| Rapana venosa | 0.0057–0.04 | [19,55,56] | ||
| Reishia bronni | 0.09 | [55] | ||
| Babyloniidae | Babylonia japonica | 0.08–0.13 | [55] | |
| Babylonia zeylanica | 0.25 | [55] | ||
| Turbinellidae | Vasum ceramicum | <0.01 | [55] |
4.3. Tetramine in Fusitriton oregonensis and Hemifusus tuba
5. Pharmacological Properties
5.1. Absorption, Distribution, and Excretion
5.2. Toxicity
6. Food Poisoning
6.1. Occurrence Situation
6.2. General Symptoms
6.3. Serious Symptoms in Patients with Kidney Dysfunction
6.4. Prevention of Poisoning
7. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turner, A.H.; Craik, D.J.; Kaas, Q.; Schroeder, C.I. Bioactive compounds isolated from neglected predatory marine gastropods. Mar. Drugs 2018, 16, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noguchi, T.; Arakawa, O. Tetrodotoxin-distribution and accumulation in aquatic organisms, and cases of human intoxication. Mar. Drugs 2008, 6, 220–242. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, T.; Onuki, K.; Arakawa, O. Tetrodotoxin poisoning due to pufferfish and gastropods, and their intoxication mechanism. ISRN Toxicol. 2011, 2011, 276939. Available online: https://downloads.hindawi.com/archive/2011/276939.pdf (accessed on 17 December 2021). [CrossRef] [PubMed] [Green Version]
- Bane, V.; Lehane, M.; Dikshit, M.; O’Riordan, A.; Furey, A. Tetrodotoxin: Chemistry, toxicity, source, distribution and detection. Toxins 2014, 6, 693–755. [Google Scholar] [CrossRef] [Green Version]
- Kosuge, T.; Tsuji, K.; Hirai, K.; Fukuyama, T.; Nukaya, H.; Ishida, H. Isolation and structure determination of a new marine toxin, neosurugatoxin, from the Japanese ivory shell, Babylonia japonica. Tetrahedron Lett. 1981, 22, 3417–3420. [Google Scholar] [CrossRef]
- Kosuge, T.; Tsuji, K.; Hirai, K.; Yamaguchi, K.; Okamoto, T.; Iitaka, Y. Isolation of a new toxin, prosurugatoxin, from the toxic Japanese ivory shell, Babylonia japonica. Chem. Pharm. Bull. 1985, 33, 2890–2895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosuge, T.; Tsuji, K.; Hirai, K.; Fukuyama, T. First evidence of toxin production by bacteria in a marine organism. Chem. Pharm. Bull. 1985, 33, 3059–3061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akondi, K.B.; Muttenthaler, M.; Dutertre, S.; Kaas, Q.; Craik, D.J.; Lewis, R.J.; Alewood, P.F. Discovery, synthesis, and structure-activity relationships of conotoxins. Chem. Rev. 2014, 114, 5815–5847. [Google Scholar] [CrossRef]
- Gao, B.; Peng, C.; Yang, J.; Yi, Y.; Zhang, J.; Shi, O. Cone snails: A big store of conotoxins for novel drug discovery. Toxins 2017, 9, 397. [Google Scholar] [CrossRef] [Green Version]
- Himaya, S.W.A.; Lewis, R.J. Venomics-accelerated cone snail venom peptide discovery. Int. J. Mol. Sci. 2018, 19, 788. [Google Scholar] [CrossRef] [Green Version]
- Duque, H.M.; Dias, S.C.; Franco, O.L. Structural and functional analyses of cone snail toxins. Mar. Drugs 2019, 17, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miljanich, G.P. Ziconotide: Neuronal calcium channel blocker for treating severe chronic pain. Curr. Med. Chem. 2004, 11, 3029–3040. [Google Scholar] [CrossRef]
- Rigo, F.K.; Dalmolin, G.D.; Trevisan, G.; Tonello, R.; Silva, M.A.; Rossato, M.F.; Klafke, J.Z.; Cordeiro, M.N.; Castro, C.J., Jr.; Montijo, D.; et al. Effect of ω-conotoxin MVIIA and Phαlβ on paclitaxel-induced acute and chronic pain. Pharmacol. Biochem. Behav. 2013, 114, 16–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisapoor, S.S.; Jamili, S.; Shahbazzadeh, D.; Mostafavi, P.G.; Bagheri, K.P. A new, high yield, rapid, and cost-effective protocol to deprotection of cysteine-rich conopeptide, omega-conotoxin MVIIA. Chem. Biol. Drug Des. 2016, 87, 687–693. [Google Scholar] [CrossRef]
- Erspamer, V.; Benati, O. Identification of murexine as β-[imidazolyl-(4)]-acryl-choline. Science 1953, 117, 161–162. [Google Scholar] [CrossRef]
- Roseghini, M.; Severini, C.; Erspamer, G.F.; Erspamer, V. Choline esters and biogenic amines in the hypobranchial gland of 55 molluscan species of the neogastropod Muricoidea superfamily. Toxicon 1996, 34, 33–55. [Google Scholar] [CrossRef]
- Whittaker, V.P. βl, β2-Dimethylacrylylcholine, a new naturally occurring pharmacologically active ester of choline. Biochem. J. 1957, 66, 35P. [Google Scholar]
- Kelley, W.P.; Wolters, A.W.; Sack, J.T.; Jockusch, R.A.; Jurchen, J.C.; Williams, E.R.; Sweedler, J.V.; Gilly, W.F. Characterization of a novel gastropod toxin (6-bromo-2-mercaptotryptamine) that inhibits shaker K channel activity J. Biol. Chem. 2003, 278, 34934–34942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiomi, K.; Mizukami, M.; Shimakura, K.; Nagashima, Y. Toxins in the salivary gland of some marine carnivorous gastropods. Comp. Biochem. Physiol. 1994, 107, 427–432. [Google Scholar] [CrossRef]
- Shiomi, K.; Kawashima, Y.; Mizukami, M.; Nagashima, Y. Properties of proteinaceous toxins in the salivary gland of the marine gastropod (Monoplex echo). Toxicon 2002, 40, 563–571. [Google Scholar] [CrossRef]
- Kawashima, Y.; Nagai, H.; Ishida, M.; Nagashima, Y.; Shiomi, K. Primary structure of echotoxin 2, an actinoporin-like hemolytic toxin from the salivary gland of the marine gastropod Monoplex Echo. Toxicon 2003, 42, 491–497. [Google Scholar] [CrossRef]
- Gunji, K.; Ishizaki, S.; Shiomi, K. Cloning of complementary and genomic DNAs encoding echotoxins, proteinaceous toxins from the salivary gland of marine gastropod Monoplex Echo. Protein J. 2010, 29, 487–492. [Google Scholar] [CrossRef]
- Anthoni, U.; Bohlin, L.; Larsen, C.; Nielsen, P.; Nielsen, N.H.; Christophersen, C. Tetramine: Occurrence in marine organisms and pharmacology. Toxicon 1989, 27, 707–716. [Google Scholar] [CrossRef]
- West, D.J.; Andrews, E.B.; Bowman, D.; McVean, A.R.; Thorndyke, M.C. Toxins from some poisonous and venomous marine snails. Comp. Biochem. Physiol. 1996, 113, 1–10. [Google Scholar] [CrossRef]
- Whittle, K.; Gallacher, S. Marine toxins. Br. Med. Bull. 2000, 56, 236–253. [Google Scholar] [CrossRef]
- Dolan, L.C.; Matulka, R.A.; Burdock, G.A. Naturally occurring food toxins. Toxins 2010, 2, 2289–2332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modica, M.V.; Holford., M. The Neogastropoda: Evolutionary innovations of predatory marine snails with remarkable pharmacological potential. In Evolutionary Biology-Concepts, Molecular and Morphological Evolution; Pontarotti, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 249–270. [Google Scholar]
- Takasaki, S.; Konta, T.; Shiomi, K.; Kubota, I. Quiz page October 2009. Tetramine poisoning. Neurologic symptoms in a dialysis patient after ingesting seafood. Am. J. Kidney Dis. 2009, 54, A37–A39. [Google Scholar]
- Yeo, I.H.; Lim, J.H. Critical tetramine poisoning after sea snail ingestion in a patient on peritoneal dialysis: A case report. Medicina 2021, 57, 564. [Google Scholar] [CrossRef] [PubMed]
- Ponte, G.; Modica, M.V. Salivary glands in predatory mollusks: Evolutionary considerations. Front. Physiol. 2017, 8, 580. [Google Scholar] [CrossRef] [Green Version]
- Andrews, E.B. The fine structure and function of the salivary glands of Nucella lapillus (gastropoda: Muricidae). J. Moll. Stud. 1991, 57, 111–126. [Google Scholar] [CrossRef]
- Power, A.J.; Keegan, B.F.; Nolan, K. The seasonality and role of the neurotoxin tetramine in the salivary glands of the red whelk Neptunea antiqua (L.). Toxicon 2002, 40, 419–425. [Google Scholar] [CrossRef]
- Fänge, R. The salivary gland of Neptune antiqua. Ann. N. Y. Acad. Sci. 1960, 90, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Shindo, T.; Ushiyama, H.; Kan, K.; Saito, H.; Kuwahara, Y.; Uehara, S.; Yasuda, K. Study on contents of tetramine in salivary gland, meat and internal organs of buccinid gastropods (Mollusca). J. Food Hyg. Soc. Jpn. 2000, 41, 17–22. [Google Scholar] [CrossRef]
- Tazawa, T.; Ishige, M.; Ueno, K.; Kuwahara, Y.; Ouchi, S. Study on tetramine content in salivary gland of gastropods—Comparison between mouse bioassay and ion chromatography methods. Rep. Hokkaido Inst. Pub. Health 2001, 51, 83–86. [Google Scholar]
- Emmelin, N.; Fänge, R. Comparison between biological effects of neurine and a salivary gland extract of Neptunea antiqua. Acta Zool. 1958, 39, 47–52. [Google Scholar] [CrossRef]
- Asano, M.; Ito, M. Occurrence of tetramine and choline compounds in the salivary gland of a marine gastropod Neptunea arthritica, Bernardi. Tohuku J. Agric. Res. 1959, 10, 209–227. [Google Scholar]
- Asano, M.; Itoh, M. Salivary poison of a marine gastropod Neptunea arthritica Bernardi and the seasonal variation of its toxicity. Ann. N. Y. Acad. Sci. 1960, 90, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Kungswan, A.; Noguchi, T.; Kanoh, S.; Hashimoto, K. Assay method for tetramine in carnivorous gastropods. Nippon Suisan Gakkaishi 1986, 52, 881–884. [Google Scholar] [CrossRef]
- Fujii, R.; Moriwaki, M.; Tanaka, K.; Ogawa, T.; Mori, E.; Saito, M. Spectrophotometric determination of tetramine in carnivorous gastropods with tetrabromophenolphthalein ethyl ester. J. Food Hyg. Soc. Jpn. 1992, 33, 237–240. [Google Scholar] [CrossRef] [Green Version]
- Saitoh, H.; Oikawa, K.; Takano, T.; Kamimura, K. Determination of tetramethylammonium ion in shellfish by ion chromatography. J. Chromatogr. 1983, 281, 397–402. [Google Scholar] [CrossRef]
- Shindo, T.; Ushiyama, H.; Kan, K.; Saito, H.; Kuwahara, Y.; Uehara, S.; Yasuda, K. Determination of tetramine in gastropods (Mollusca) by ion chromatography and effect of cooking. J. Food Hyg. Soc. Jpn. 2000, 41, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Hashizume, K.; Toda, C.; Yasui, T.; Nagano, H. Determination of tetramine in Neptunea intersculpta by high performance liquid chromatography. Eisei Kagaku 1987, 33, 179–184. [Google Scholar] [CrossRef]
- Anthoni, U.; Christophersen, C.; Nielsen, P.H. Simultaneous identification and determination of tetramine in marine snails by proton nuclear magnetic resonance spectroscopy. J. Agric. Food Chem. 1989, 37, 705–707. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Thibault, P.; Tazawa, T.; Quilliam, M.A. Analysis of tetramine in sea snails by capillary electrophoresis-tandem mass spectrometry. J. Chromatogr. A 1997, 781, 555–564. [Google Scholar] [CrossRef]
- Kawashima, Y.; Nagashima, Y.; Shiomi, K. Determination of tetramine in marine gastropods by liquid chromatography/electrospray ionization-mass spectrometry. Toxicon 2004, 44, 185–191. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, K.J.; Suzuki, T.; Kim, C.M.; Lee, J.Y.; Mok, J.S.; Lee, T.S. Identification of tetramine, a toxin in whelks, as the cause of a poisoning incident in Korea and the distribution of tetramine in fresh and boiled whelk (Neptunea intersculpta). J. Food Prot. 2009, 72, 1935–1940. [Google Scholar] [CrossRef]
- Ackermann, D.; Holtz, F.; Reinwein, H. Reindarstellung und Konstitutionsemitteelung des Tetramins, eines Giftes aus Aktina Equina. Z. Biol. 1923, 78, 113–120. [Google Scholar]
- Welsh, J.H.; Prock, P.B. Quaternary ammonium bases in the coelenterates. Biol. Bull. 1958, 115, 551–561. [Google Scholar] [CrossRef]
- Honma, T.; Shiomi, K. Peptide toxins in sea anemones: Structural and functional aspects. Mar. Biotechnol. 2006, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Anderluh, G.; Maček, P. Cytolytic peptide and protein toxins from sea anemones (Anthozoa: Actiniaria). Toxicon 2002, 40, 111–124. [Google Scholar] [CrossRef]
- Asano, M. Studies of the toxic substances contained in marine animals I. Locality of the poison of Neptunea (Barbitonia) arthritica Bernardi. Bull. Japan. Soc. Sci. Fish. 1952, 17, 73–77. [Google Scholar] [CrossRef] [Green Version]
- Anthoni, U.; Bohlin, L.; Larsen, C.; Nielsen, P.; Nielsen, N.H.; Christophersen, C. The toxin tetramine from the “edible” whelk Neptunea Antiqua. Toxicon 1989, 27, 717–723. [Google Scholar] [CrossRef]
- Power, A.J.; Keegan, B.F. Seasonal patterns in the reproductive activity of the red whelk, Neptunea antiqua (Mollusca: Prosobranchia) in the Irish Sea. J. Mar. Biol. Ass. U.K. 2001, 81, 243–250. [Google Scholar] [CrossRef]
- Kawashima, Y.; Nagashima, Y.; Shiomi, K. Toxicity and tetramine contents of salivary glands from carnivorous gastropods. J. Food Hyg. Soc. Jpn. 2002, 43, 385–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshinaga-Kiriake, A.; Ishizaki, S.; Nagashima, Y. Tetramine contents in the salivary glands from 16 species of marine carnivorous gastropods collected along Japanese coasts. Food Hyg. Saf. Soc. 2021, 62, 203–208. [Google Scholar]
- Eto, S.; Isshiki, K.; Momozono, Y.; Yano, T.; Sakuma, T.; Miyazaki, A. Measurement of tetramine contents in shellfish, Neptunea Cumingii. Eisei Kagaku 1989, 35, 476–478. [Google Scholar] [CrossRef]
- Tazawa, T.; Ishige, M.; Ueno, K.; Kuwahara, Y.; Ouchi, S. Study on tetramine content in salivary gland of sea snails (Part II). Rep. Hokkaido Inst. Public Health 2004, 54, 63–64. [Google Scholar]
- Fujinaga, K.; Oyama, Y. Reproductive ecology of the neptune whelk Neptunea polycostata with special reference to maturity size, reproductive cycle, and sex ratio. Nippon Suisan Gakkaishi 2007, 73, 256–262. [Google Scholar] [CrossRef]
- Tsubaki, H.; Komai, T. Intestinal absorption of tetramethylammonium and its derivatives in rats. J. Pharm.-Dyn. 1986, 9, 747–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsubaki, H.; Nakajima, E.; Shigehara, E.; Komai, T.; Shindo, H. The relation between structure and distribution of quaternary ammonium ions in mice and rats. simple tetraalkylammonium and a series of m-substituted trimethylphenylammonium ions. J. Pharm.-Dyn. 1986, 9, 737–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neef, C.; Oosting, R.; Meijer, D.K. Structure-pharmacokinetics relationship of quaternary ammonium compounds. Elimination and distribution characteristics. Naunyn-schmiedeberg’s Arch. Pharmacol. 1984, 328, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Gebber, G.L.; Volle, R.L. Mechanisms involved in ganglionic blockade induced by tetramethylammonium. J. Pharmacol. Exp. Ther. 1966, 152, 18–28. [Google Scholar]
- Henry, A.J. The toxic principle of Courbonia virgata: Its isolation and identification as a tetramethylammonium salt. Brit. J. Pharmacol. 1948, 3, 187–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiomi, K.; Horiguchi, Y.; Kaise, T. Acute toxicity and rapid excretion in urine of tetramethylarsonium salts found in some marine animals. Appl. Organomet. Chem. 1988, 2, 385–389. [Google Scholar] [CrossRef]
- Fleming, C. Case of poisoning from red whelk. Br. Med. J. 1971, 3, 250–251. [Google Scholar] [CrossRef] [Green Version]
- Reid, T.M.; Gould, I.M.; Mackie, I.M.; Ritchie, A.H.; Hobbs, G. Food poisoning due to the consumption of red whelks (Neptunea antiqua). Epidemiol. Infect. 1988, 101, 419–423. [Google Scholar] [CrossRef] [Green Version]
- Watson-Wright, W.M.; Sims, G.G.; Smyth, C.; Gillis, M.; Maher, M.; Trottier, T.; Van Sinclair, D.E.; Gilgan, M. Identification of tetramine as toxin causing food poisoning in Atlantic Canada following consumption of whelks Neptunea decemcostata. In Recent Advances in Toxinology Research; Gopalakrishnakone, P., Tan, C.K., Eds.; University of Singapore: Singapore, 1992; Volume 2, pp. 551–561. [Google Scholar]




| LC | Column | Nucleosil 100-10SA (0.46 × 25 cm, Macherey-Nagel) |
| Injection volume | 10 µL | |
| Eluent | 0.03 M pyridine-formic acid buffer (pH 3.1) containing 20% methanol | |
| Flow rate | 1 mL/min | |
| MS | Ionization | Electrospray ionization |
| Polarity | Positive | |
| Monitor ion | m/z 74 (molecular ion) | |
| Cone voltage | 30 V |
| Experimental Animal | Route | Lethal Dose or LD50 (mg/kg) | Reference |
|---|---|---|---|
| Rat | Oral | 45–50 *1 | [53] |
| Intraperitoneal | 15 *1 | [53] | |
| Mouse | Oral | 16 *2 | [65] |
| Intraperitoneal | 11 *2 | [65] | |
| Subcutaneous | 7.4–14.7 *3 | [64] |
| Causative Gastropod | No. of Incident | No. of Patient |
|---|---|---|
| Neptunea intersculpta * | 23 | 54 |
| Neptunea arthritica | 13 | 20 |
| Neptunea arthritica and Neptunea bulbacea | 1 | 2 |
| Neptunea intersculpta or Neptunea amianta | 1 | 1 |
| Neptunea polycostata | 3 | 10 |
| Neptunea lamellose | 3 | 8 |
| Fusitriton oregonensis | 2 | 17 |
| Neptunea bulbacea | 1 | 2 |
| Unidentified (possibly Neptunea species) | 25 | 40 |
| Total | 72 | 154 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiomi, K. Tetramine in the Salivary Glands of Marine Carnivorous Snails: Analysis, Distribution, and Toxicological Aspects. J. Mar. Sci. Eng. 2022, 10, 6. https://doi.org/10.3390/jmse10010006
Shiomi K. Tetramine in the Salivary Glands of Marine Carnivorous Snails: Analysis, Distribution, and Toxicological Aspects. Journal of Marine Science and Engineering. 2022; 10(1):6. https://doi.org/10.3390/jmse10010006
Chicago/Turabian StyleShiomi, Kazuo. 2022. "Tetramine in the Salivary Glands of Marine Carnivorous Snails: Analysis, Distribution, and Toxicological Aspects" Journal of Marine Science and Engineering 10, no. 1: 6. https://doi.org/10.3390/jmse10010006
APA StyleShiomi, K. (2022). Tetramine in the Salivary Glands of Marine Carnivorous Snails: Analysis, Distribution, and Toxicological Aspects. Journal of Marine Science and Engineering, 10(1), 6. https://doi.org/10.3390/jmse10010006





