Estimation of Phytoplankton Size Classes in the Littoral Sea of Korea Using a New Algorithm Based on Deep Learning
Abstract
:1. Introduction
2. Data and Methods
2.1. Field Observation Data
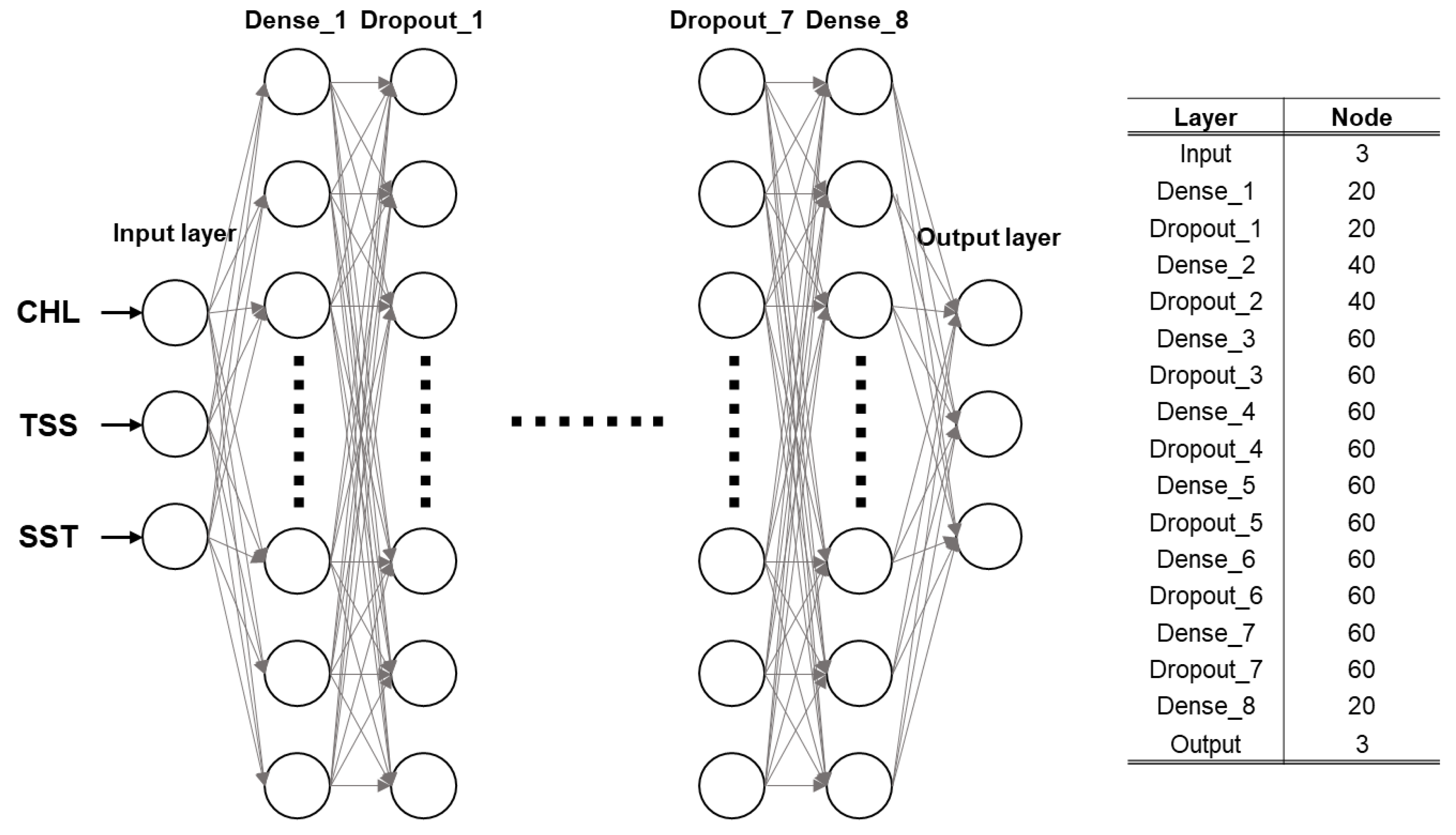
2.2. Training and Model Structure
2.3. Satellite Data
3. Results and Discussion
3.1. Results of DNN-Based Model for PSCs
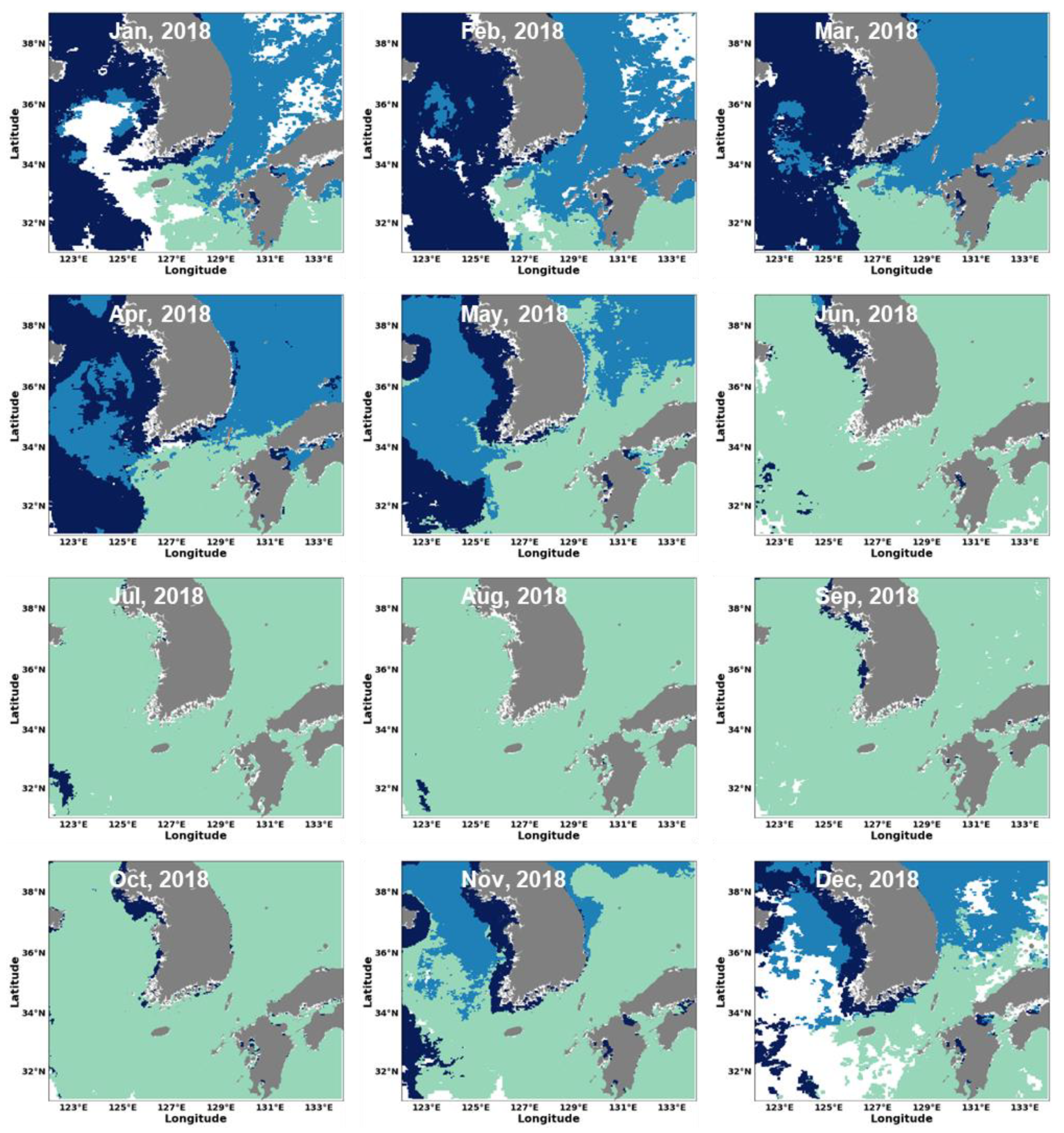
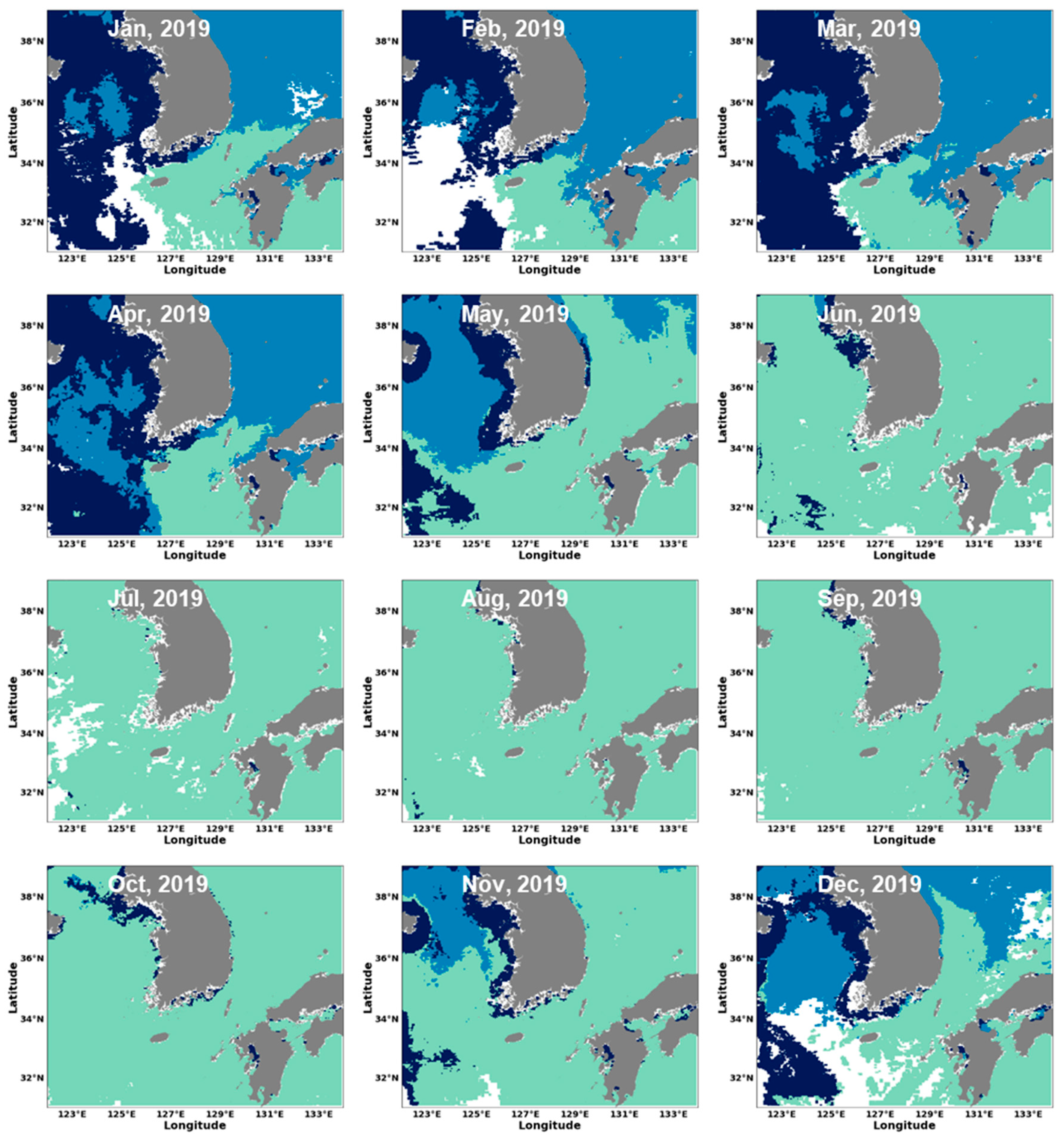
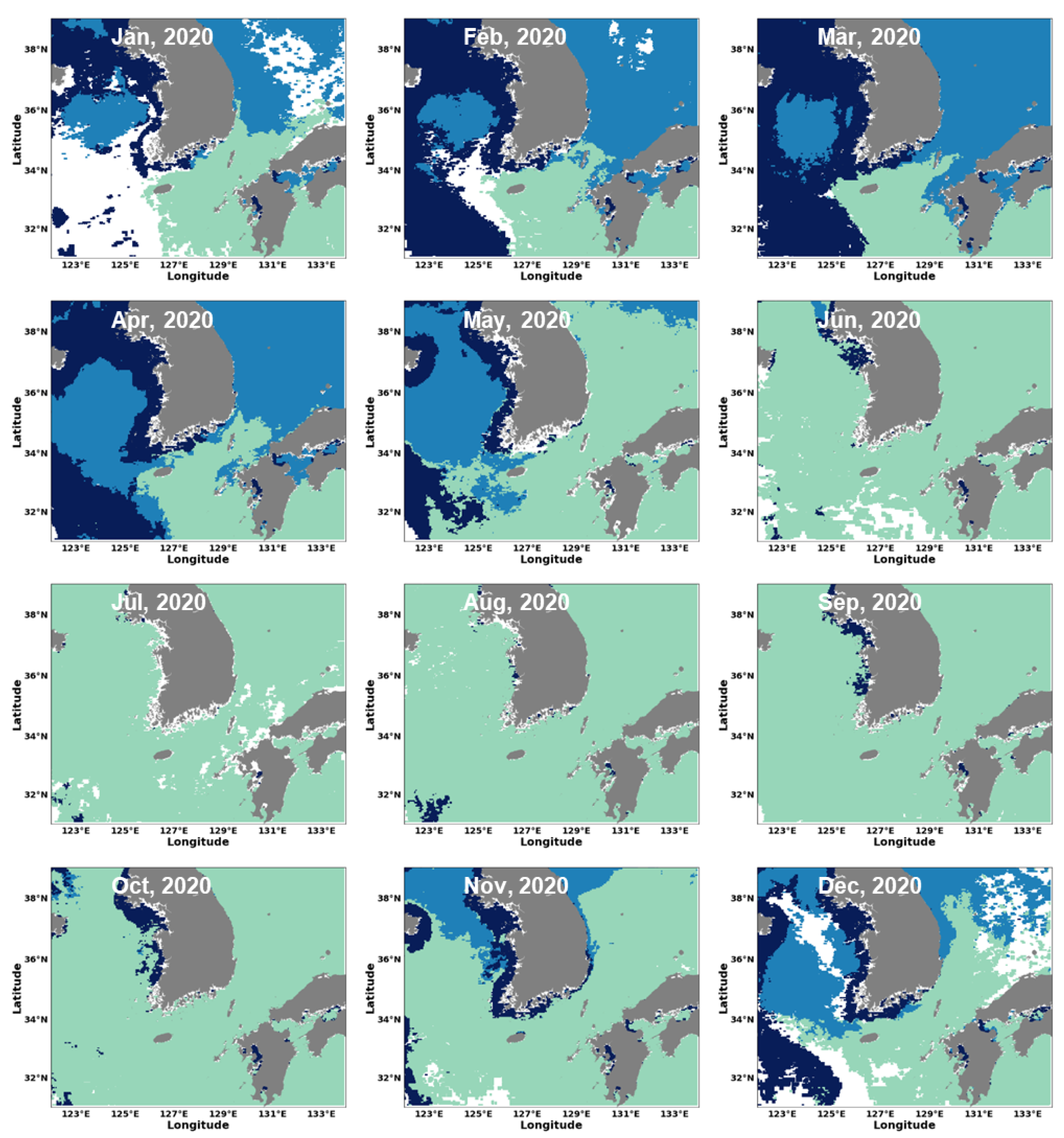
3.2. Estimation of Phytoplankton Size Classes in the Littoral Sea of South Korea Using Satellite
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Alelio, D.; Libralato, S.; d’Alcalà, M.R. Ecological-network models link diversity, structure and function in the plankton food-web. Sci. Rep. 2016, 6, 21806. [Google Scholar] [CrossRef] [Green Version]
- D’Alelio, D.; Rampone, S.; Cusano, L.M.; Morfino, V.; Russo, L.; Saneverino, N.; Cloern, J.E.; Lomas, M.W. Machine learning identifies a strong association between warming and reduced primary productivity in an oligotrophic ocean gyre. Sci. Rep. 2020, 10, 3287. [Google Scholar] [CrossRef] [Green Version]
- Harris, G. Phytoplankton Ecology: Structure, Function and Fluctuation; Chapman and Hall: London, UK, 1986. [Google Scholar]
- Belkin, I.M. Rapid warming of Large Marine Ecosystems. Prog. Oceanogr. 2009, 81, 207–213. [Google Scholar] [CrossRef]
- Kang, J.J.; Jang, H.K.; Lim, J.-H.; Lee, D.; Lee, J.-H.; Bae, H.; Lee, C.H.; Kang, C.-K.; Lee, S.H. Characteristics of different size phytoplankton for primary production and biochemical compositions in the western East/Japan Sea. Front. Microbiol. 2020, 11, 560102. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Batten, S.; Sasaoka, K.; Sasai, Y.; Sugisaki, H. Influence of the Pacific Decadal Oscillation on phytoplankton phenology and community structure in the western North Pacific. Geophys. Res. Lett. 2012, 39, 2–7. [Google Scholar] [CrossRef]
- Doney, S.C.; Ruckelshaus, M.; Duffy, J.E.; Barry, J.P.; Chan, F.; English, C.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N.; et al. Climate change impacts on marine ecosystems. Ann. Rev. Mar. Sci. 2012, 4, 11–37. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Joo, H.T.; Lee, J.H.; Lee, J.H.; Kang, J.J.; Lee, H.W.; Lee, D.; Kang, C.K. Seasonal carbon uptake rates of phytoplankton 494 in the northern East/Japan Sea. Deep. Res. Part II Top. Stud. Oceanogr. 2017, 143, 45–53. [Google Scholar] [CrossRef]
- Agawin, N.; Duarte, C.; Agustí, S. Nutrient and temperature control of the contribution of picoplankton to phytoplankton biomass and production. Limnol. Oceanogr. 2000, 45, 591–600. [Google Scholar] [CrossRef]
- Morán, X.A.G.; López-urrutia, Á.; Calvo-díaz, A.; Li, W.K. Increasing importance of small phytoplankton in a warmer ocean. Glob. Change Biol. 2010, 16, 1137–1144. [Google Scholar] [CrossRef]
- Hilligsøe, K.M.; Richardson, K.; Bendtsen, J.; Sørensen, L.L.; Nielsen, T.G.; Lyngsgaard, M.M. Linking phytoplankton community size composition with temperature, plankton food web structure and sea–air CO2 flux. Deep Sea Res. Part I Oceanogr. Res. Pap. 2011, 58, 826–838. [Google Scholar] [CrossRef]
- Mousing, E.A.; Ellegaard, M.; Richardson, K. Global patterns in phytoplankton community size structure—Evidence for a direct temperature effect. Mar. Ecol. Prog. Ser. 2014, 497, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Legendre, L.; Rassoulzadegan, F. Food-web mediated export of biogenic carbon in oceans: Hydrodynamic control. Mar. Ecol. Prog. Ser. 1996, 145, 179–193. [Google Scholar] [CrossRef] [Green Version]
- Falkowski, P.G.; Oliver, M.J. Mix and match: How climate selects phytoplankton. Nat. Rev. Microbiol. 2007, 5, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Finkel, Z.V.; Beardall, J.; Flynn, K.J.; Quigg, A.; Rees, T.A.V.; Raven, J.A. Phytoplankton in a changing world: Cell size and elemental stoichiometry. J. Plankton Res. 2010, 32, 119–137. [Google Scholar] [CrossRef] [Green Version]
- Marañón, E.; Cermeño, P.; Latasa, M.; Tadonléké, R.D. Temperature, resources, and phytoplankton community size structure in the ocean. Limnol. Oceanogr. 2012, 57, 1266–1278. [Google Scholar] [CrossRef]
- Liu, H.; Liu, X.; Xiao, W.; Laws, E.A.; Huang, B. Spatial and temporal variations of satellite-derived phytoplankton size classes using a three-component model bridged with temperature in marginal seas of the western pacific ocean. Prog. Oceanogr. 2021, 191, 102511. [Google Scholar] [CrossRef]
- Brewin, R.J.W.; Sathyendranath, S.; Jackson, T.; Barlow, R.; Brotas, V.; Airs, R.; Lamont, T. Influence of light in the mixed-layer on the parameters of a threecomponent model of phytoplankton size class. Remote Sens. Environ. 2015, 168, 437–450. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, W.C.; Kim, H.C.; Jo, N.; Kim, K.; Lee, D.; Kang, J.J.; Sim, B.-R.; Kwon, J.-I.; Lee, S.H. Temporal and Spatial Variations of the Biochemical Composition of Phytoplankton and Potential Food Material (FM) in Jaran Bay, South Korea. Water 2020, 12, 3093. [Google Scholar] [CrossRef]
- Alvain, S.; Moulin, C.; Dandonneau, Y.; Breon, F.M. Remote sensing of phytoplankton groups in case 1 waters from global SeaWiFS imagery. Deep-Sea Res. I 2005, 52, 1989–2004. [Google Scholar] [CrossRef] [Green Version]
- IOCCG. Phytoplankton Functional Types from Space; Sathyendranath, S., Ed.; Reports of the International Ocean-Colour Coordinating Group, No. 15; IOCCG: Dartmouth, Canada, 2014. [Google Scholar]
- Zhang, H.; Wang, S.; Qiu, Z.; Sun, D.; Ishizaka, J.; Sun, S.; He, Y. Phytoplankton size class in the East China Sea derived from MODIS satellite data. Biogeosciences 2018, 15, 4271–4289. [Google Scholar] [CrossRef]
- Mouw, C.; Hardman-Mountford, N.J.; Alvain, S.; Bracher, A.; Brewin, R.; Bricaud, A.; Ciotti, A.M.; Devred, E.; Fujiwara, A.; Hirata, T.; et al. A consumer’s guide to satellite remote sensing of multiple phytoplankton groups in the global ocean. Front. Mar. Sci. 2017, 4, 41. [Google Scholar] [CrossRef] [Green Version]
- Ciotti, A.M.; Lewis, M.R.; Cullen, J.J. Assessment of the relationships between dominant cell size in natural phytoplankton communities and the spectral shape of the absorption coefficient. Limnol. Oceanogr. 2002, 47, 404–417. [Google Scholar] [CrossRef] [Green Version]
- Hirata, T.; Aiken, J.; Hardman-Mountford, N.; Smyth, T.; Barlow, R. An absorption model to determine phytoplankton size classes from satellite ocean colour. Remote Sens. Environ. 2008, 112, 3153–3159. [Google Scholar] [CrossRef]
- Kostadinov, T.S.; Siegel, D.A.; Maritorena, S. Retrieval of the particle size distribution from satellite ocean color observations. J. Geophys. Res. Ocean. 2009, 114, C09015. [Google Scholar] [CrossRef]
- Roy, S.; Sathyendranath, S.; Bouman, H.; Platt, T. The global distribution of phytoplankton size spectrum and size classes from their light-absorption spectra derived from satellite data. Remote Sens. Environ. 2013, 139, 185–197. [Google Scholar] [CrossRef]
- Garver, S.A.; Siegel, D.A.; Greg, M.B. Variability in near-surface particulate absorption spectra: What can a satellite ocean color imager see? Limnol. Oceanogr. 1994, 39, 1349–1367. [Google Scholar] [CrossRef]
- Sun, D.; Huan, Y.; Wang, S.; Qiu, Z.; Ling, Z.; Mao, Z.; He, Y. Remote sensing of spatial and temporal patterns of phytoplankton assemblages in the Bohai Sea, Yellow Sea, and east China sea. Water Res. 2019, 157, 119–133. [Google Scholar] [CrossRef]
- Brewin, R.J.W.; Sathyendranath, S.; Hirata, T.; Lavender, S.J.; Barciela, R.M.; Hardman-Mountford, N.J. A three-component model of phytoplankton size class for the Atlantic Ocean. Ecol. Model 2010, 221, 1472–1483. [Google Scholar] [CrossRef]
- Hirata, T.; Hardman-Mountford, N.J.; Brewin, R.J.W.; Aiken, J.; Barlow, R.; Suzuki, K.; Isada, T.; Howell, E.; Hashioka, T.; Noguchi-Aita, M.; et al. Synoptic relationships between surface Chlorophyll-a and diagnostic pigments specific to phytoplankton functional types. Biogeosciences 2011, 8, 311–327. [Google Scholar] [CrossRef] [Green Version]
- Brewin, R.J.W.; Ciavatta, S.; Sathyendranath, S.; Jackson, T.; Tilstone, G.; Curran, K.; Airs, R.L.; Cummings, D.; Brotas, V.; Organelli, E.; et al. Uncertainty in ocean-color estimates of chlorophyll for phytoplankton groups. Front. Mar. Sci. 2017, 4, 104. [Google Scholar] [CrossRef]
- Ward, B.A. Temperature-correlated changes in phytoplankton community structure are restricted to polar waters. PLoS ONE 2015, 10, e0135581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.; Liu, H.; Zhao, W.; Shi, T.; Hu, Z.; Li, Q.; Wu, G. Comparison of machine learning techniques in inferring phytoplankton size classes. Remote Sens. 2018, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Biological and Chemical Methods for Seawater Analysis; Pergamon Press: Oxford, UK, 1984. [Google Scholar]
- Moon, J.-E.; Ahn, Y.-H.; Ryu, J.-H.; Palanisamy, S. Development of Ocean environmental algorithms for Geostationary ocean color imager. Korea J. Remote Sens. 2010, 26, 198–207. [Google Scholar]
- Jang, H.K.; Youn, S.H.; Joo, H.; Kim, Y.; Kang, J.J.; Lee, D.; Jo, N.; Kim, K.; Kim, M.-J.; Kim, S.; et al. First Concurrent Measurement of Primary Production in the Yellow Sea, the South Sea of Korea, and the East/Japan Sea, 2018. J. Mar. Sci. Eng. 2021, 9, 1237. [Google Scholar] [CrossRef]
- Yamada, K.; Ishizaka, J.; Yoo, S.; Kim, H.-C.; Chiba, S. Seasonal and interannual variability of sea surface chlorophyll a concentration in the Japan/East Sea (JES). Prog. Oceanogr. 2004, 61, 193–211. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lee, Y.-W.; Kim, G. Hydrographically mediated patterns of photosynthetic pigments in the East/Japan Sea: Low N:P ratios and cyanobacterial dominance. J. Mar. Syst. 2010, 82, 72–79. [Google Scholar] [CrossRef]
- Kwak, J.H.; Hwang, J.; Choy, E.J.; Park, H.J.; Kang, D.-J.; Lee, T.; Chang, K.-I.; Kim, K.-R.; Kang, C.-K. Monthly measured primary and new productivities in the Ulleung Basin as a biological “hot spot” in the East/Japan Sea. Biogeosciences 2013, 10, 4405–4417. [Google Scholar] [CrossRef] [Green Version]
- Kwak, J.H.; Lee, S.H.; Hwang, J.; Suh, Y.S.; Park, H.J.; Chang, K.I.; Kim, K.-R.; Kang, C.K. Summer primary productivity and phytoplankton community composition driven by different hydrographic structures in the East/Japan Sea and the Western Subarctic Pacific. J. Geophys. Res. Ocean. 2014, 119, 4505–4519. [Google Scholar] [CrossRef]
- Kang, J.J.; Joo, H.; Lee, J.H.; Lee, J.H.; Lee, H.W.; Lee, D.; Kang, C.K.; Yun, M.S.; Lee, S.H. Comparison of biochemical compositions of phytoplankton during spring and fall seasons in the northern East/Japan Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 143, 73–81. [Google Scholar] [CrossRef]
- Kwak, J.H.; Han, E.; Lee, S.H.; Park, H.J.; Kim, K.R.; Kang, C.K. A consistent structure of phytoplankton communities across the warm–cold regions of the water mass on a meridional transect in the East/Japan Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 143, 36–44. [Google Scholar] [CrossRef]
- Jo, N.; Kang, J.J.; Park, W.G.; Lee, B.R.; Yun, M.S.; Lee, J.H.; Kim, S.M.; Lee, D.; Joo, H.; Lee, J.H.; et al. Seasonal variation in the biochemical compositions of phytoplankton and zooplankton communities in the southwestern East/Japan Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 143, 82–90. [Google Scholar] [CrossRef]
- Lee, M.; Kim, Y.B.; Park, C.H.; Baek, S.H. Characterization of Seasonal Phytoplankton Pigments and Functional Types around Offshore Island in the East/Japan Sea, Based on HPLC Pigment Analysis. Sustainability 2022, 14, 5306. [Google Scholar] [CrossRef]
- Sun, X.; Shen, F.; Liu, D.; Bellerby, R.G.; Liu, Y.; Tang, R. In situ and satellite observations of phytoplankton size classes in the entire continental shelf sea, China. J. Geophys. Res. Ocean. 2018, 123, 3523–3544. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Youn, S.H.; Oh, H.J.; Kang, J.J.; Lee, J.H.; Lee, D.; Kim, K.; Jang, H.K.; Lee, J.; Lee, S.H. Spatiotemporal variation in phytoplankton community driven by environmental factors in the northern East China Sea. Water 2020, 12, 2695. [Google Scholar] [CrossRef]
- Fu, M.; Wang, Z.; Li, Y.; Li, R.; Sun, P.; Wei, X.; Lin, X.; Guo, J. Phytoplankton biomass size structure and its regulation in the Southern Yellow Sea (China): Seasonal variability. Cont. Shelf Res. 2009, 29, 2178–2194. [Google Scholar] [CrossRef]
- Kang, J.J.; Min, J.O.; Kim, Y.; Lee, C.H.; Yoo, H.; Jang, H.K.; Kim, M.-J.; Oh, H.-J. Lee, S.H. Vertical Distribution of Phytoplankton Community and Pigment Production in the Yellow Sea and the East China Sea during the Late Summer Season. Water 2021, 13, 3321. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.H.; Kang, J.J.; Lee, J.H.; Lee, H.W.; Kang, C.K.; Lee, S.H. River discharge effects on the contribution of small-sized phytoplankton to the total biochemical composition of POM in the Gwangyang Bay, Korea. Estuar. Coast. Shelf Sci. 2019, 226, 106293. [Google Scholar] [CrossRef]
- Shaha, D.C.; Cho, Y.-K. Comparison of empirical models with intensively observed data for prediction of salt intrusion in the Sumjin River estuary, Korea. Hydrol. Earth Syst. Sci. 2009, 13, 923–933. [Google Scholar] [CrossRef]
- Ye, H.; Tang, D. A three component model of phytoplankton size classes for the south china sea. Malays. J. Sc. 2013, 32, 325–332. [Google Scholar]
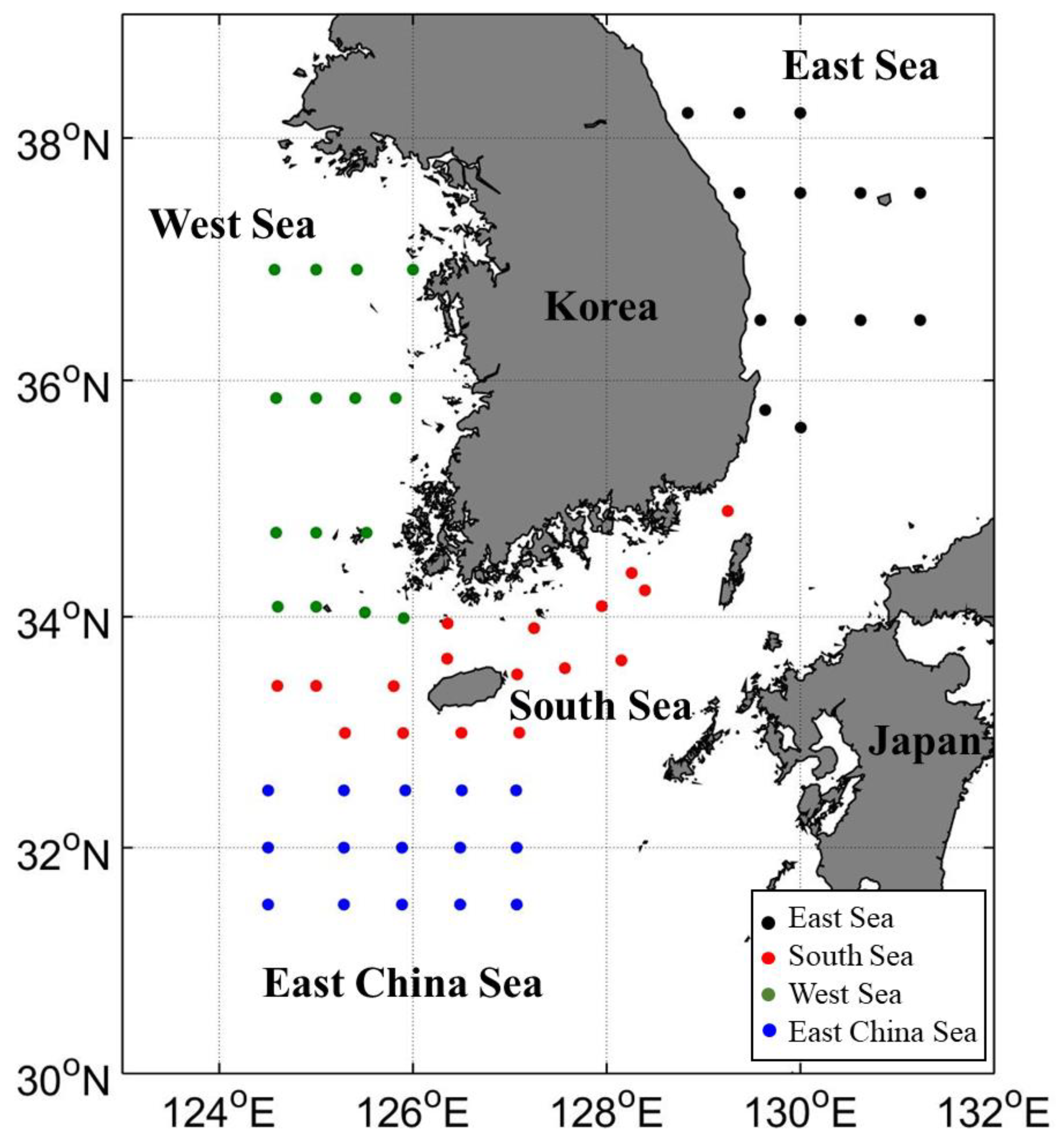

| East Sea | West Sea | South Sea | East China Sea | Sum | |
|---|---|---|---|---|---|
| Microsize phytoplankton (>20 µM) | 39 | 13 | 21 | 53 | 126 |
| Nanosize phytoplankton (2–20 µM) | 42 | 39 | 8 | 10 | 99 |
| Picosize phytoplankton (0.7–2 µM) | 54 | 115 | 86 | 51 | 306 |
| Total | 135 | 167 | 115 | 114 | 531 |
| In Situ Results | |||
|---|---|---|---|
| True | False | ||
| Model results | True | True Positive (TP) | False Positive (FP) |
| False | False Negative (FN) | True Negative (TN) | |
| Data | Precision (%) | Recall (%) | F1_Score | Accuracy (%) | |
|---|---|---|---|---|---|
| Training data (210) | Micro-size phytoplankton (70) | 37.1 | 65.0 | 47.3 | 55.7 |
| Nano-size phytoplankton (70) | 44.3 | 67.4 | 53.5 | ||
| Pico-size phytoplankton (70) | 85.7 | 48.4 | 61.9 | ||
| Validation data (30) | Micro-size phytoplankton (10) | 20.0 | 66.7 | 30.8 | 46.7 |
| Nano-size phytoplankton (10) | 30.0 | 60.0 | 40.0 | ||
| Pico-size phytoplankton (10) | 90.0 | 40.9 | 56.3 | ||
| Test data (291) | Micro-size phytoplankton (46) | 34.8 | 45.7 | 39.5 | 70.5 |
| Nano-size phytoplankton (19) | 42.1 | 17.8 | 25.0 | ||
| Pico-size phytoplankton (226) | 80.1 | 85.8 | 82.8 | ||
| Field Measurments | New AI Algorithm (This Study) | Aph Algorithm | Three-Component Model |
|---|---|---|---|
| Study area | 67.3% | 54.3% | 13.4% |
| East Sea | 50.0% | 41.7% | 16.7% |
| West Sea | 66.7% | 41.7% | 33.3% |
| South Sea | 90% | 90% | 0% |
| East China sea | 66.7% | 50% | 0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.J.; Oh, H.J.; Youn, S.-H.; Park, Y.; Kim, E.; Joo, H.T.; Hwang, J.D. Estimation of Phytoplankton Size Classes in the Littoral Sea of Korea Using a New Algorithm Based on Deep Learning. J. Mar. Sci. Eng. 2022, 10, 1450. https://doi.org/10.3390/jmse10101450
Kang JJ, Oh HJ, Youn S-H, Park Y, Kim E, Joo HT, Hwang JD. Estimation of Phytoplankton Size Classes in the Littoral Sea of Korea Using a New Algorithm Based on Deep Learning. Journal of Marine Science and Engineering. 2022; 10(10):1450. https://doi.org/10.3390/jmse10101450
Chicago/Turabian StyleKang, Jae Joong, Hyun Ju Oh, Seok-Hyun Youn, Youngmin Park, Euihyun Kim, Hui Tae Joo, and Jae Dong Hwang. 2022. "Estimation of Phytoplankton Size Classes in the Littoral Sea of Korea Using a New Algorithm Based on Deep Learning" Journal of Marine Science and Engineering 10, no. 10: 1450. https://doi.org/10.3390/jmse10101450
APA StyleKang, J. J., Oh, H. J., Youn, S.-H., Park, Y., Kim, E., Joo, H. T., & Hwang, J. D. (2022). Estimation of Phytoplankton Size Classes in the Littoral Sea of Korea Using a New Algorithm Based on Deep Learning. Journal of Marine Science and Engineering, 10(10), 1450. https://doi.org/10.3390/jmse10101450







