Seasonal Compositions of Size-Fractionated Surface Phytoplankton Communities in the Yellow Sea
Abstract
1. Introduction
2. Materials and Methods
2.1. Water Sampling and Study Area
2.2. Dissolved Inorganic Nutrients
2.3. Chlorophyll-a Concentration
2.4. High-Performance Liquid Chromatography Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics in the Environment
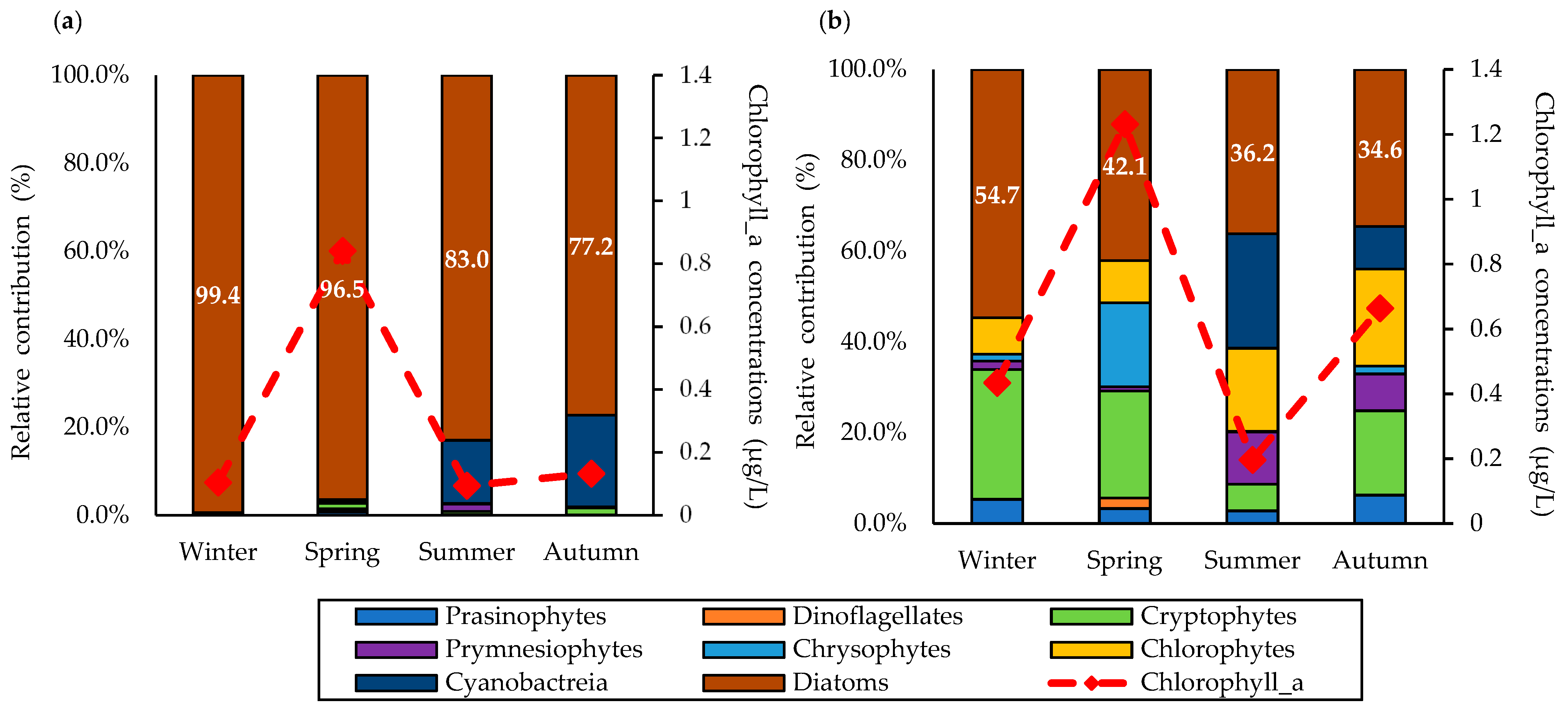
3.2. Total Chlorophyll-a Concentrations and Total Phytoplankton Community
3.3. Size-Fractionated Chlorophyll-a Concentration and Phytoplankton Community Structure
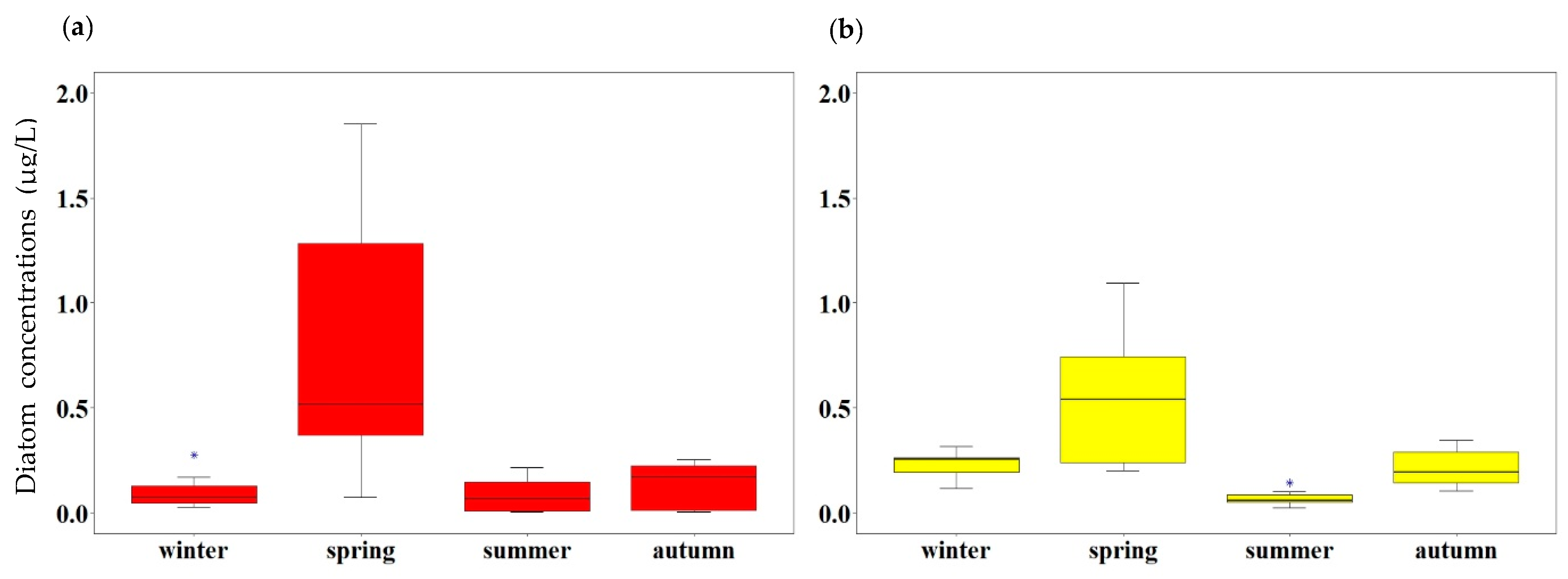
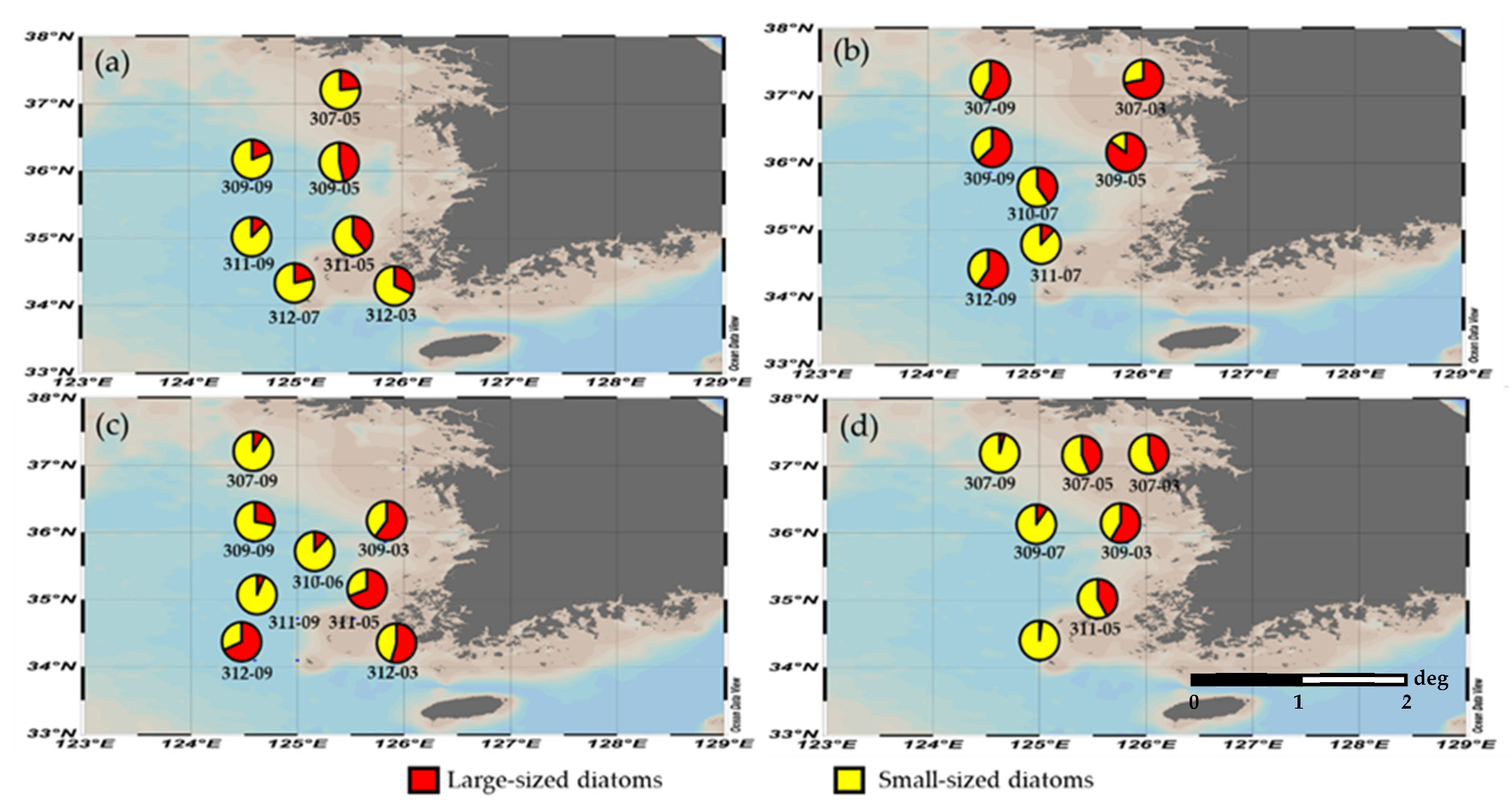
3.4. Seasonal Variation in the Size Structure of Diatoms
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falkowski, P.G.; Barber, R.T.; Smetacek, V. Biogeochemical controls and feedbacks on ocean primary production. Science 1998, 281, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Behrenfeld, M.J.; Randerson, J.T.; McClain, C.R.; Feldman, G.C.; Los, S.O.; Tucker, C.J.; Falkowski, P.G.; Field, C.B.; Frouin, R.; Esaias, W.E. Biospheric primary production during an enso transition. Science 2001, 291, 2594–2597. [Google Scholar] [CrossRef] [PubMed]
- Winder, M.; Sommer, U. Phytoplankton response to a changing climate. Hydrobiologia 2012, 698, 5–16. [Google Scholar] [CrossRef]
- Froneman, P.; Pakhomov, E.; Balarin, M.G. Size-fractionated phytoplankton biomass, production and biogenic carbon flux in the eastern Atlantic sector of the Southern Ocean in late austral summer 1997–1998. Deep-Sea Res. II: Top. Stud. Oceanogr. 2004, 51, 2715–2729. [Google Scholar] [CrossRef][Green Version]
- Chisholm, S.W. Phytoplankton Size. In Primary Productivity and Biogeochemical Cycles in the Sea; Falkowski, P.G., Woodhead, A.D., Eds.; Springer: New York, NY, USA, 1992. [Google Scholar]
- Agustí, S.; González-Gordillo, J.I.; Vaqué, D.; Estrada, M.; Cerezo, M.I.; Salazar, G.; Gasol, J.M.; Duarte, C.M. Ubiquitous healthy diatoms in the deep sea confirm deep carbon injection by the biological pump. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Laws, E.A.; Barber, R.T.; Murray, J.W. Phytoplankton and Their Role in Primary, New, and Export Production. In Ocean Biogeochemistry; Fasham, M.J.R., Ed.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 99–121. [Google Scholar]
- Peter, K.H.; Sommer, U.J.P.o. Phytoplankton cell size reduction in response to warming mediated by nutrient limitation. PLoS ONE 2013, 8, e71528K. [Google Scholar] [CrossRef]
- Morán, X.A.G.; López-Urrutia, Á.; Calvo-Díaz, A.; LI, W.K.W. Increasing importance of small phytoplankton in a warmer ocean. Glob. Chang. Biol. 2010, 16, 1137–1144. [Google Scholar] [CrossRef]
- Jang, H.K.; Kang, J.J.; Lee, J.H.; Kim, M.; Ahn, S.H.; Jeong, J.Y.; Yun, M.S.; Han, I.S.; Lee, S.H. Recent Primary Production and Small Phytoplankton Contribution in the Yellow Sea during the Summer in 2016. Ocean Sci. J. 2018, 53, 509–519. [Google Scholar] [CrossRef]
- Finkel, Z.V. Does phytoplankton cell size matter? The evolution of modern marine food webs. In Evolution of Primary Producers in the Sea; Falkowski, P.G., Knoll, A.H., Eds.; Academic Press: New York, NY, USA, 2007; pp. 333–350. [Google Scholar]
- F Finkel, Z.V.; Beardall, J.; Flynn, K.J.; Quigg, A.; Rees, T.A.V.; Raven, J.A. Phytoplankton in a changing world: Cell size and elemental stoichiometry. J. Plankton Res. 2010, 32, 119–137. [Google Scholar] [CrossRef]
- G Gin, K.Y.-H.; Lin, X.; Zhang, S. Dynamics and size structure of phytoplankton in the coastal waters of Singapore. J. Plankton Res. 2000, 22, 1465–1484. [Google Scholar] [CrossRef]
- Yoo, S.; An, Y.-R.; Bae, S.; Choi, S.; Ishizaka, J.; Kang, Y.-S.; Kim, Z.G.; Lee, C.; Lee, J.B.; Li, R.; et al. Status and trends in the Yellow Sea and East China Sea region. In Marine Ecosystems of the North Pacific Ocean, 2003–2008; McKinnell, S.M., Dagg, M.J., Eds.; PICES Special Publication: Sidney, BC, Canada, 2010; Volume 4, pp. 360–393. [Google Scholar]
- B Belkin, I.M.; Cornillon, P.C.; Sherman, K. Fronts in Large Marine Ecosystems. Prog. Oceanogr. 2009, 81, 223–236. [Google Scholar] [CrossRef]
- Bian, C.W.; Jiang, W.S.; Greatbatch, R.J. An exploratory model study of sediment transport sources and deposits in the Bohai Sea, Yellow Sea, and East China Sea. J. Geophys. Res. Ocean. 2013, 118, 5908–5923. [Google Scholar] [CrossRef]
- Jiang, Z.; Gao, Y.; Zhai, H.; Jin, H.; Zhou, F.; Shou, L.; Yan, X.; Chen, Q. Regulation of spatial changes in phytoplankton community by water column stability and nutrients in the southern Yellow Sea. J. Geophys. Res. Biogeosci. 2019, 124, 2610–2627. [Google Scholar] [CrossRef]
- Wright, S.; Jeffrey, S.; Mantoura, R. Phytoplankton Pigments in Oceanography: Guidelines to Modern Methods; Jeffrey, S., Mantoura, R., Wright, S., Eds.; Unesco Pub.: Paris, France, 2005. [Google Scholar]
- Agirbas, E.; Feyzioglu, A.M.; Kopuz, U.; Llewellyn, C.A. Phytoplankton community composition in the south-eastern Black Sea determined with pigments measured by HPLC-CHEMTAX analyses and microscopy cell counts. J. Mar. Biol. Assoc. UK 2015, 95, 35–52. [Google Scholar] [CrossRef]
- Schlüter, L.; Lauridsen, T.; Krogh, G.; Jørgensen, T. Identification and quantification of phytoplankton groups in lakes using new pigment ratios—A comparison between pigment analysis by HPLC and microscopy. Freshw. Biol. 2006, 51, 1474–1485. [Google Scholar] [CrossRef]
- Luan, Q.; Kang, Y.; Wang, J. Long-term changes within the phytoplankton community in the Yellow Sea (1985–2015). J. Fish. Sci. China 2020, 27, 1–11. [Google Scholar]
- Gao, Y.; Jiang, Z.; Liu, J.; Chen, Q.; Zeng, J.; Huang, W. Seasonal variations of net-phytoplankton community structure in the southern Yellow Sea. J. Ocean Univ. China 2013, 12, 557–567. [Google Scholar] [CrossRef]
- Liu, H.J.; Huang, Y.J.; Zhai, W.D.; Jin, H.; Sun, J. Phytoplankton communities and its controlling factors in summer and autumn in the southern Yellow Sea, China. Acta Oceanol. Sin. 2015, 34, 114–123. (In Chinese) [Google Scholar] [CrossRef]
- Kang, J.-J.; Min, J.-O.; Kim, Y.; Lee, C.-H.; Yoo, H.; Jang, H.-K.; Kim, M.-J.; Oh, H.-J.; Lee, S.-H. Vertical Distribution of Phytoplankton Community and Pigment Production in the Yellow Sea and the East China Sea during the Late Summer Season. Water 2021, 13, 3321. [Google Scholar] [CrossRef]
- Fu, M.Z.; Wang, Z.L.; Li, Y.; Li, R.X.; Sun, P.; Wei, X.H.; Lin, X.Z.; Guo, J.S. Phytoplankton biomass size structure and its regulation in the Southern Yellow Sea (China): Seasonal variability. Cont. Shelf Res. 2009, 29, 2178–2194. [Google Scholar] [CrossRef]
- Ming, F.; Ping, S.; Zong, W.; Yan, L.; Rui, L. Seasonal variations of phytoplankton community size structures in the Huanghai (Yellow) Sea Cold Water Mass area. Haiyang Xuebao 2010, 32, 120–129. [Google Scholar]
- Cho, B.C.; Park, M.G.; Shim, J.H.; Choi, D.H. Sea-surface temperature and f-ratio explain large variability in the ratio of bacterial production to primary production in the Yellow Sea. Mar. Ecol. Prog. Ser. 2001, 216, 31–41. [Google Scholar] [CrossRef]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Biological and Chemical Methods for Seawater Analysis; Pergamon Press: Oxford, UK, 1984. [Google Scholar]
- Kang, J.J.; Lee, J.H.; Kim, H.C.; Lee, W.C.; Lee, D.; Jo, N.; Min, J.-O.; Lee, S.H. Monthly Variations of Phytoplankton Community in Geoje-Hansan Bay of the Southern Part of Korea Based on HPLC Pigment Analysis. J. Coast. Res. 2018, 85, 356–360. [Google Scholar] [CrossRef]
- Lee, Y.-W.; Park, M.-O.; Im, Y.-S.; Kim, S.-S.; Kang, C.-K. Application of photosynthetic pigment analysis using a HPLC and CHEMTAX program to studies of phytoplankton community composition. Sea 2011, 16, 117–124. [Google Scholar] [CrossRef]
- Mackey, M.D.; Mackey, D.J.; Higgins, H.W.; Wright, S.W. CHEMTAX—A program for estimating class abundances from chemical markers: Application to HPLC measurements of phytoplankton. Mar. Ecol. Prog. Ser. 1996, 144, 265–283. [Google Scholar] [CrossRef]
- Liu, X.; Huang, B.Q.; Huang, Q.; Wang, L.; Ni, X.B.; Tang, Q.S.; Sun, S.; Wei, H.; Liu, S.M.; Li, C.L.; et al. Seasonal phytoplankton response to physical processes in the southern Yellow Sea. J. Sea Res. 2015, 95, 45–55. [Google Scholar] [CrossRef]
- Claustre, H. The trophic status of various oceanic provinces as revealed by phytoplankton pigment signatures. Limnol. Oceanogr. 1994, 39, 1206–1210. [Google Scholar] [CrossRef]
- Vidussi, F.; Claustre, H.; Manca, B.B.; Luchetta, A.; Marty, J.-C. Phytoplankton Pigment Distribution in Relation to Upper Thermocline Circulation in the Eastern Mediterranean Sea during Winter. J. Geophys. Res. 2001, 106, 19939–19956. [Google Scholar] [CrossRef]
- Uitz, J.; Claustre, H.; Morel, A.; Hooker, S.B. Vertical Distribution of Phytoplankton Communities in Open Ocean: An Assessment Based on Surface Chlorophyll. J. Geophys. Res. 2006, 111, C08005. [Google Scholar] [CrossRef]
- Sun, X.; Shen, F.; Brewin, R.J.; Liu, D.; Tang, R. Twenty-year variations in satellite-derived chlorophyll-a and phytoplankton size in the Bohai Sea and Yellow Sea. J. Geophys. Res. Ocean. 2019, 124, 8887–8912. [Google Scholar] [CrossRef]
- Sun, D.; Huan, Y.; Wang, S.; Qiu, Z.; Ling, Z.; Mao, Z.; He, Y. Remote sensing of spatial and temporal patterns of phytoplankton assemblages in the Bohai Sea, Yellow Sea, and east China sea. Water Res. 2019, 157, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.K.; Youn, S.H.; Joo, H.; Kim, Y.; Kang, J.J.; Lee, D.; Jo, N.; Kim, K.; Kim, M.-J.; Kim, S.; et al. First Concurrent Measurement of Primary Production in the Yellow Sea, the South Sea of Korea, and the East/Japan Sea, 2018. J. Mar. Sci. Eng. 2021, 9, 1237. [Google Scholar] [CrossRef]
- Tian, T.; Hao, W.; Jian, S.; Changsoo, C.; Wenxin, S. Study on cycle and budgets of nutrients in the Yellow Sea. Adv. Mar. Biol. 2003, 21, 1–11. [Google Scholar]
- Goldman, C.R.; Elser, J.J.; Richards, R.C.; Reuter, J.E.; Priscu, J.C.; Levin, A.L. Thermal stratification, nutrient dynamics and phytoplankton productivity during the onset of spring phytoplankton growth in Lake Baikal, Russia. Hydrobiologia 1996, 331, 9–24. [Google Scholar] [CrossRef]
- Tang, Q.; Su, J.; Zhang, J.; Tong, L. Spring blooms and the ecosystem processes: The case study of the Yellow Sea. Deep-Sea Res. II Top. Stud. Oceanogr. 2013, 97, 1–3. [Google Scholar] [CrossRef]
- Lu, L.; Jiang, T.; Xu, Y.; Zheng, Y.; Chen, B.; Cui, Z.; Qu, K. Succession of phytoplankton functional groups from spring to early summer in the central Bohai Sea using HPLC–CHEMTAX approaches. J. Oceanogr. 2018, 74, 381–392. [Google Scholar] [CrossRef]
- Wang, L.; Ou, L.; Huang, K.; Chai, C.; Wang, Z.; Wang, X.; Jiang, T. Determination of the spatial and temporal variability of phytoplankton community structure in Daya Bay via HPLC-CHEMTAX pigment analysis. Chin. J. Oceanol. Limnol. 2018, 36, 750–760. [Google Scholar] [CrossRef]
- Lin, C.; Ning, X.; Su, J.; Lin, Y.; Xu, B. Environmental changes and the responses of the ecosystems of the Yellow Sea during 1976–2000. J. Mar. Syst. 2005, 55, 223–234. [Google Scholar] [CrossRef]
- Wei, Q.; Yao, Q.; Wang, B.; Wang, H.; Yu, Z. Long-term variation of nutrients in the southern Yellow Sea. Cont. Shelf Res. 2015, 111, 184–196. [Google Scholar] [CrossRef]
- Jin, J.; Liu, S.M.; Ren, J.L.; Liu, C.G.; Zhang, J.; Zhang, G.L.; Huang, D.J. Nutrient dynamics and coupling with phytoplankton species composition during the spring blooms in the Yellow Sea. Deep. Res. Part II Top. Stud. Oceanogr. 2013, 97, 16–32. [Google Scholar] [CrossRef]
- Egge, J.K. Are diatoms poor competitors at low phosphate concentrations? J. Mar. Syst. 1998, 16, 191–198. [Google Scholar] [CrossRef]
- Cerino, F.; Zingone, A. A survey of cryptomonad diversity and seasonality at a coastal Mediterranean site. Eur. J. Phycol. 2006, 41, 363–378. [Google Scholar] [CrossRef]
- Kang, Y.; Moon, C.-H.; Kim, H.-J.; Yoon, Y.H.; Kang, C.-K. Water Quality Improvement Shifts the Dominant Phytoplankton Group From Cryptophytes to Diatoms in a Coastal Ecosystem. Front. Mar. Sci. 2021, 1125. [Google Scholar] [CrossRef]
- Liu, X.; Huang, B.; Liu, Z.; Wang, L.; Wei, H.; Li, C.; Huang, Q. High-resolution phytoplankton diel variations in the summer stratified central Yellow Sea. J. Oceanogr. 2012, 68, 913–927. [Google Scholar] [CrossRef]
- Tilman, D. Resource Competition and Community Structure; Princeton University Press: Princeton, NJ, USA, 1982. [Google Scholar]
- Tilman, D. Resource competition between plankton algae: An experimental and theoretical approach. Ecology 1977, 58, 338–348. [Google Scholar] [CrossRef]
- Smith, R.E.; Kalff, J. Size dependence of growth rate, respiratory electron transport system activity and chemical composition in marine diatoms in the laboratory. J. Phycol. 1982, 18, 275–284. [Google Scholar] [CrossRef]
- Raven, J. The twelfth Tansley Lecture. Small is beautiful: The picophytoplankton. Funct. Ecol. 1998, 12, 503–513. [Google Scholar] [CrossRef]
- Mur, R.; Skulberg, O.; Utkilen, H. Cyanobacteria in the environment. In Toxic Cyanobacteria in Water; Chorus, I., Batram, J., Eds.; E&FN Spon: London, UK, 1999; pp. 15–40. [Google Scholar]
- Peperzak, L. Climate change and harmful algal blooms in the North Sea. Acta Oecol. 2003, 24, S139–S144. [Google Scholar] [CrossRef]
- Choi, J. Phytoplankton Ecology in the Yellow Sea; Bumwoo Publishing Company: Seoul, Korea, 2002; pp. 311–330. [Google Scholar]
- Choi, J.K.; Noh, J.H.; Shin, K.S.; Hong, K.H. The early autumn distribution of chlorophyll-a and primary productivity in the Yellow Sea, 1992. Yellow Sea 1995, 1, 68–80. [Google Scholar]
- Lee, M.; Kim, Y.-B.; Park, C.-H.; Baek, S.-H. Characterization of Seasonal Phytoplankton Pigments and Functional Types around Offshore Island in the East/Japan Sea, Based on HPLC Pigment Analysis. Sustainability 2022, 14, 5306. [Google Scholar] [CrossRef]
- Leblanc, K.; Queguiner, B.; Diaz, F.; Cornet, V.; Michel-Rodriguez, M.; de Madron, X.; Bowler, C.; Malviya, S.; Thyssen, M.; Gregori, G.; et al. Nanoplanktonic diatoms are globally overlooked but play a role in spring blooms and carbon export. Nat. Commun. 2018, 9, 953. [Google Scholar] [CrossRef]
- December, P. Uncoupling of bacteria and phytoplankton during a spring diatom bloom in the mouth of the Yellow Sea. Mar. Ecol. Prog Ser. 1994, 115, 181–190. [Google Scholar]
- Abonyi, A.; Kiss, K.T.; Hidas, A.; Borics, G.; Várbíró, G.; Ács, É. Cell Size Decrease and Altered Size Structure of Phytoplankton Constrain Ecosystem Functioning in the Middle Danube River Over Multiple Decades. Ecosystems 2020, 23, 1254–1264. [Google Scholar] [CrossRef]
- Montagnes, D.J.S.; Franklin, D.J. Effect of temperature on diatom volume, growth rate, and carbon and nitrogen content: Reconsidering some paradigms. Limnol. Oceanogr. 2001, 46, 2008–2018. [Google Scholar] [CrossRef]
- Jørgensen, E.G. The adaptation of plankton algae:II. Aspects of the temperature adaptation of Skeletonema costatum. Physiol. Plant 1968, 21, 423–427. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, J.; Meng, J.; Liu, Y. Sea surface temperature characteristics and trends in China offshore seas from 1982 to 2017. J. Coast. Res. 2019, 90, 27–34. [Google Scholar] [CrossRef]
- Finkel, Z.V.; Vaillancourt, C.J.; Irwin, A.J.; Reavie, E.D.; Smol, J.P. Environmental control of diatom community size structure varies across aquatic ecosystems. Proc. R. Soc. B: Biol. Sci. 2009, 276, 1627–16344. [Google Scholar] [CrossRef]
- Lee, S.H.; Yun, M.S.; Kim, B.K.; Saitoh, S.I.; Kang, C.K.; Kang, S.H.; Whitledge, T. Latitudinal carbon productivity in the Bering and Chukchi Seas during the summer in 2007. Cont. Shelf Res. 2013, 59, 28–36. [Google Scholar]
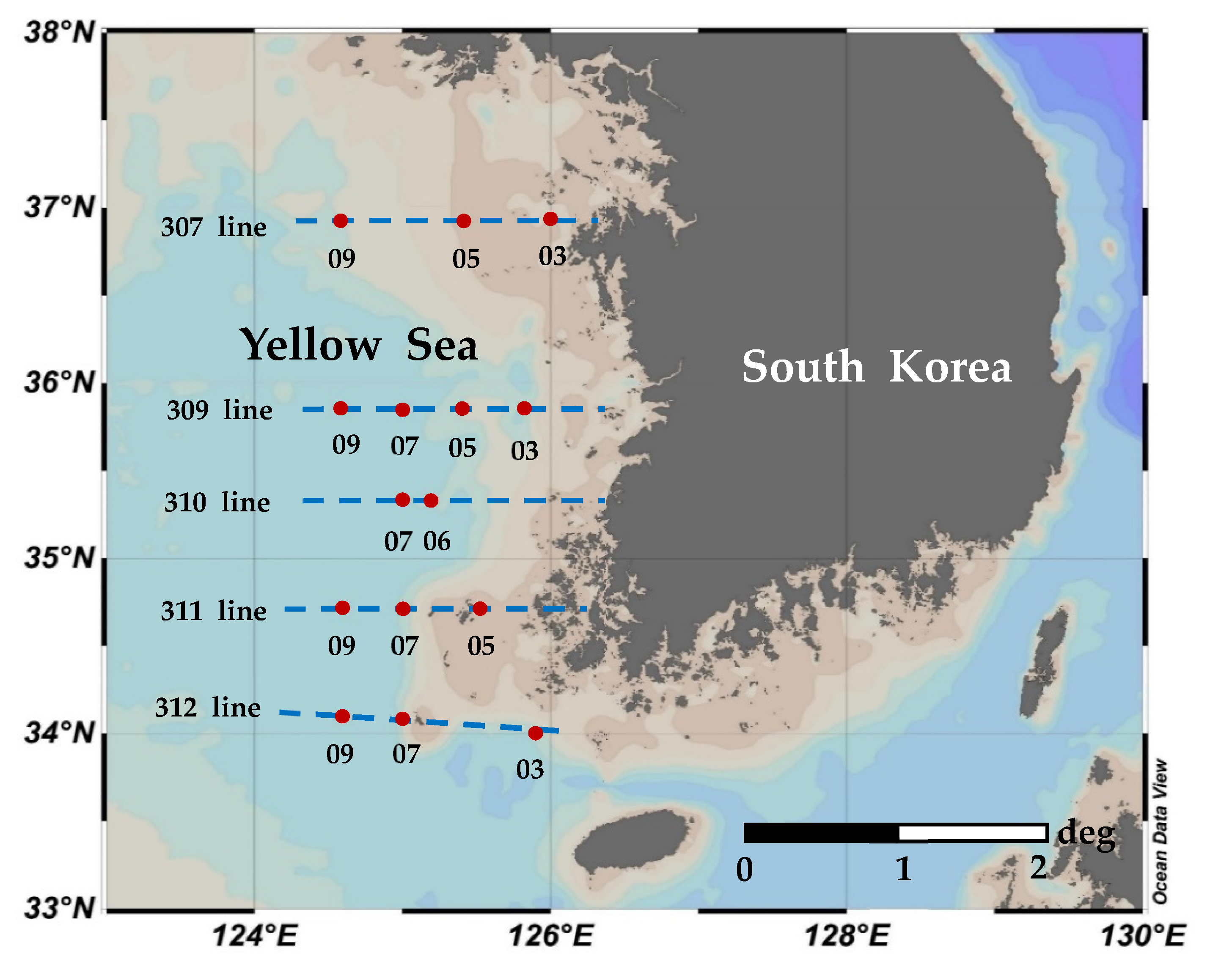
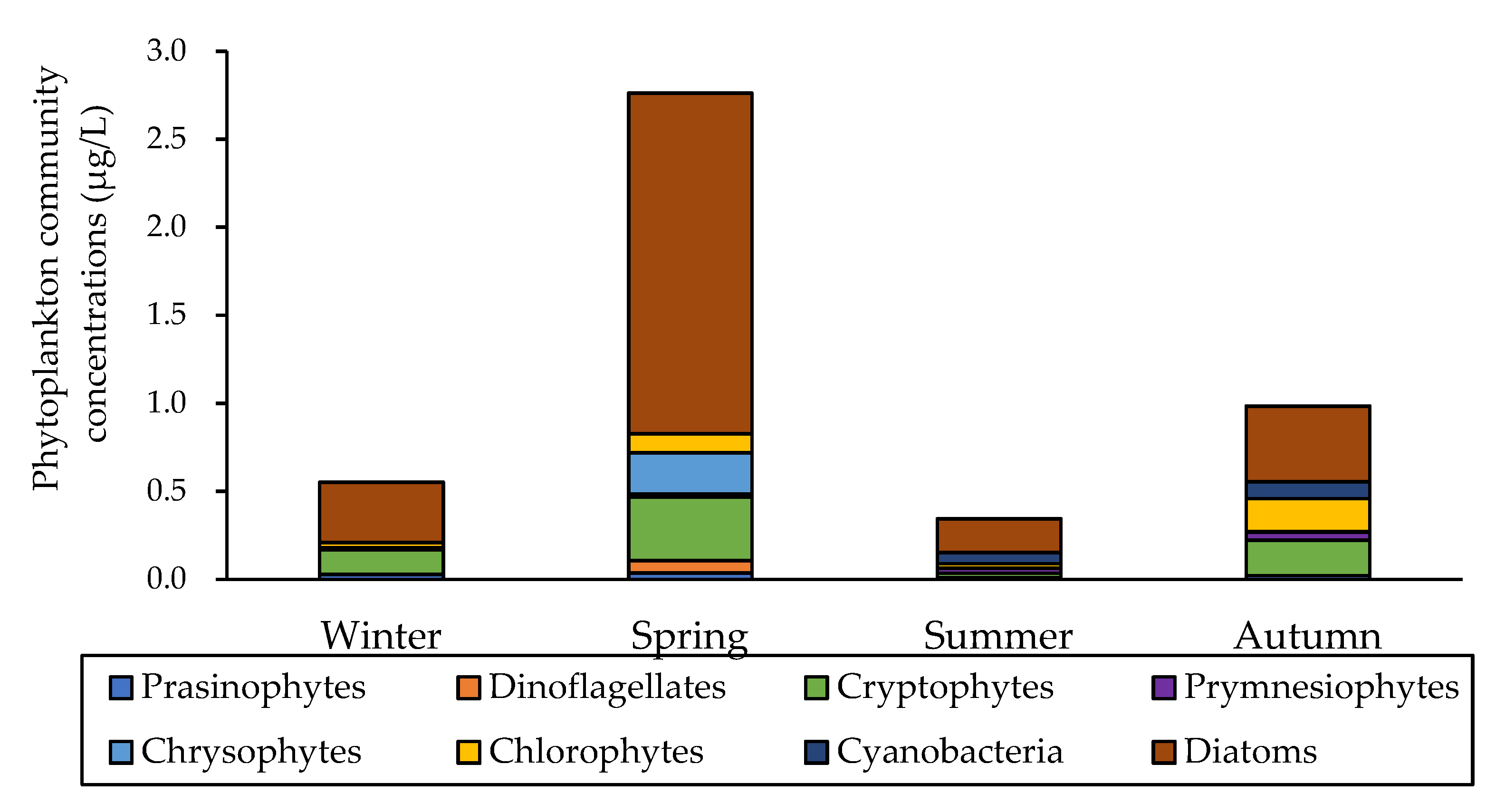
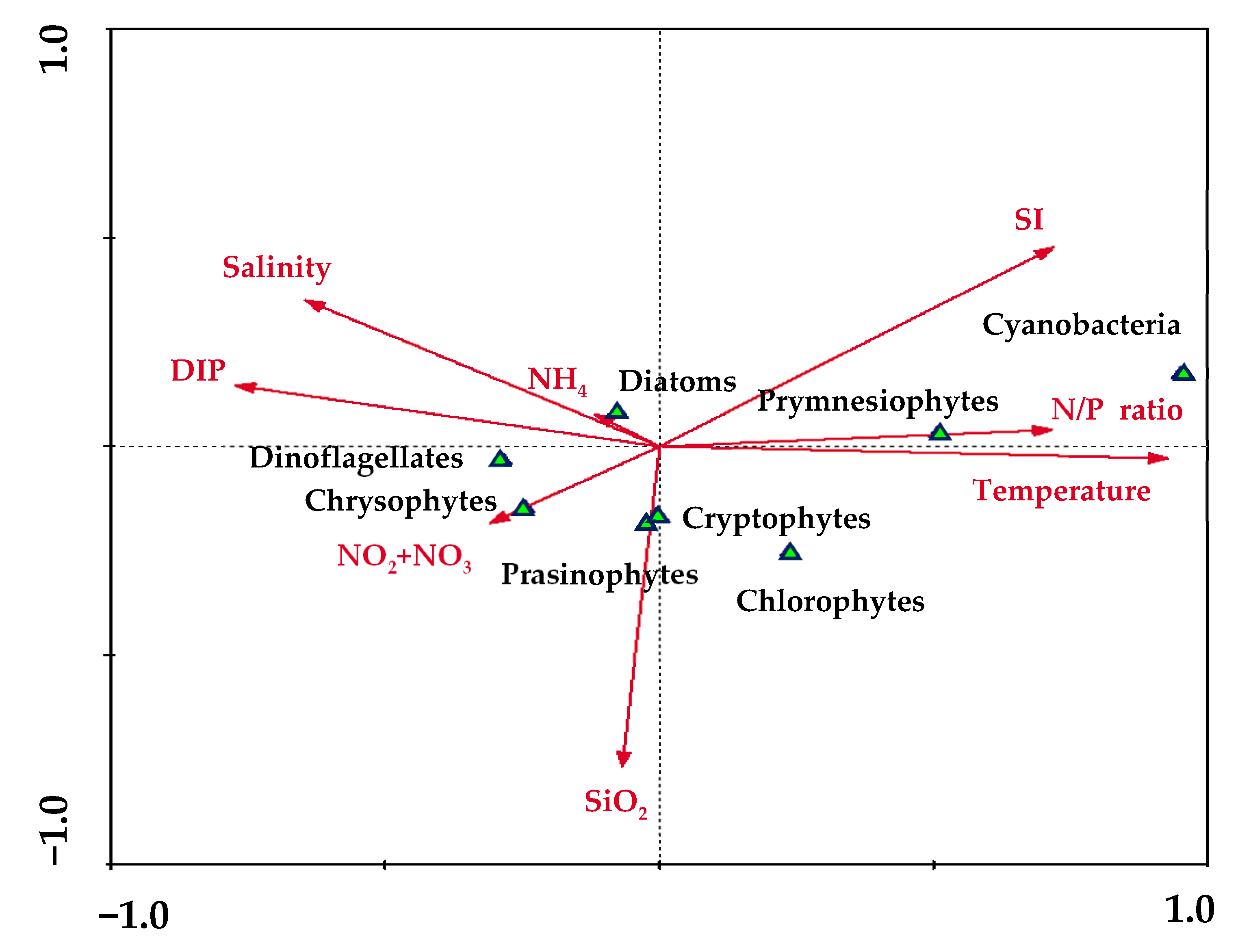
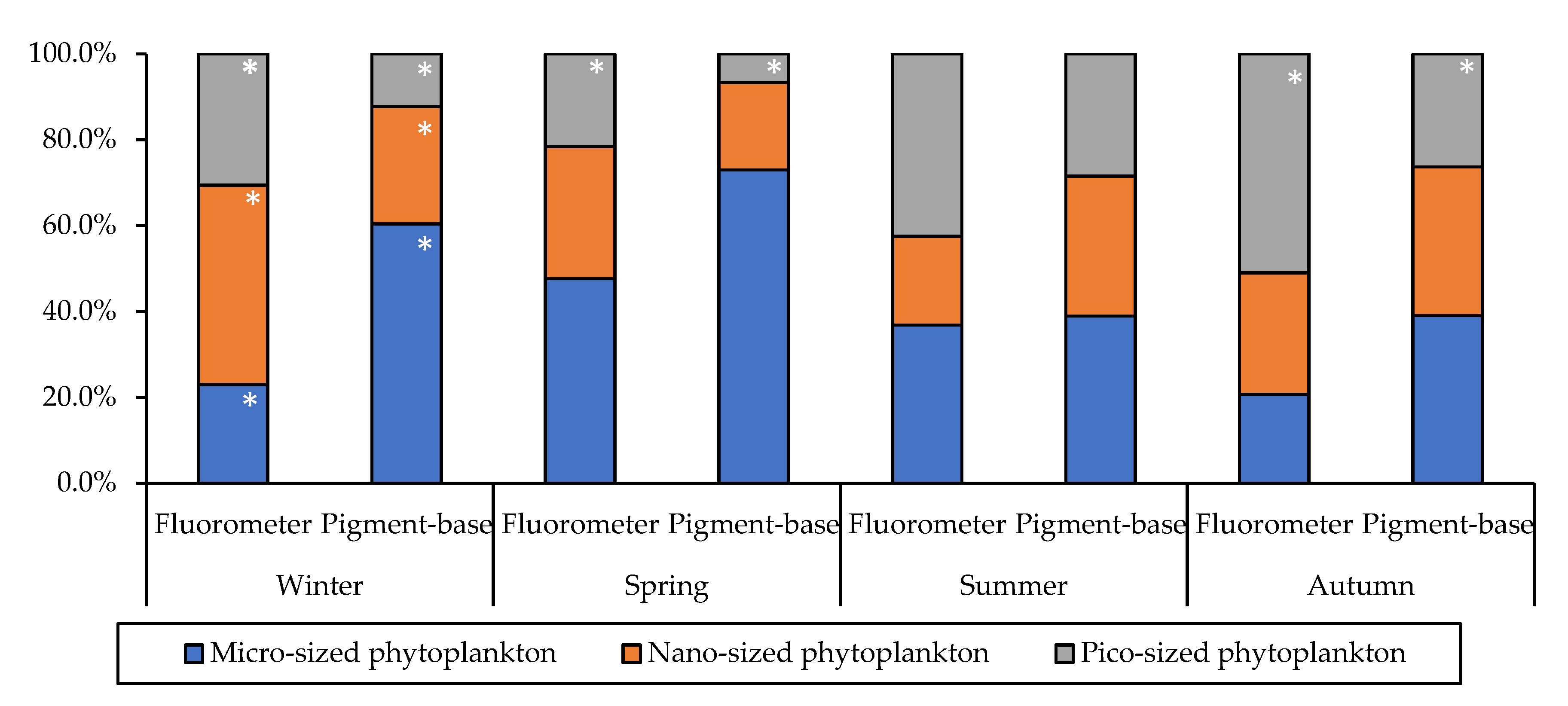
| Month | Station | Latitude (°N) | Longitude (°E) | Bottom Depth | Euphotic Depth | Stability Index | T (°C) | S (psu) | NO2 + NO3 (µM) | NH4 (µM) | PO4 (μM) | SiO2 (μM) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Winter | 307-05 | 36.92 | 125.42 | 54 | 11 | 0.00 | 4.88 | 31.34 | 10.04 | 0.33 | 0.68 | 8.27 |
| 309-05 | 35.85 | 125.40 | 69 | 11 | 0.01 | 6.67 | 32.39 | 6.42 | 0.35 | 0.52 | 7.41 | |
| 309-09 | 35.85 | 124.59 | 82 | 11 | 0.00 | 7.60 | 32.36 | 5.52 | 0.38 | 0.40 | 8.23 | |
| 311-05 | 34.72 | 125.52 | 75 | 5 | 0.00 | 5.88 | 32.32 | 6.88 | 0.81 | 0.58 | 7.70 | |
| 311-09 | 34.72 | 124.59 | 89 | 14 | 0.01 | 8.27 | 32.35 | 6.44 | 0.41 | 0.41 | 7.67 | |
| 312-03 | 34.00 | 125.90 | 85 | 22 | 0.01 | 6.91 | 32.48 | 6.29 | 0.53 | 0.51 | 7.95 | |
| 312-07 | 34.10 | 125.00 | 92 | 5 | 0.00 | 8.14 | 32.50 | 4.65 | 0.39 | 0.41 | 5.60 | |
| Spring | 307-03 | 36.92 | 126.00 | 37 | 8 | 0.01 | 5.76 | 31.60 | 11.95 | 0.59 | 0.63 | 8.32 |
| 307-09 | 36.92 | 124.57 | 67 | 22 | 0.01 | 7.77 | 32.32 | 4.82 | 0.43 | 0.40 | 6.74 | |
| 309-03 | 35.85 | 125.82 | 54 | 16 | 0.00 | 7.22 | 32.12 | 5.37 | 1.06 | 0.73 | 3.74 | |
| 309-09 | 35.85 | 124.59 | 82 | 22 | 0.01 | 8.71 | 32.36 | 1.26 | 0.34 | 0.56 | 2.25 | |
| 310-07 | 35.34 | 125.00 | 82 | 22 | 0.00 | 8.66 | 32.37 | 3.25 | 0.32 | 0.34 | 7.56 | |
| 311-07 | 34.72 | 125.00 | 90 | 22 | 0.00 | 8.42 | 32.34 | 6.16 | 0.30 | 0.37 | 8.86 | |
| 312-09 | 34.09 | 124.60 | 89 | 22 | 0.00 | 8.81 | 32.50 | 5.51 | 0.32 | 0.33 | 6.51 | |
| Summer | 307-09 | 36.92 | 124.57 | 67 | 24 | 0.13 | 26.81 | 31.93 | 1.35 | 0.17 | 0.05 | 2.82 |
| 309-03 | 35.85 | 125.82 | 54 | 24 | 0.13 | 26.27 | 31.79 | 6.18 | 0.33 | 0.11 | 3.63 | |
| 309-09 | 35.85 | 124.59 | 82 | 46 | 0.11 | 27.65 | 32.07 | 1.10 | 0.30 | 0.06 | 3.29 | |
| 310-06 | 35.34 | 125.20 | 72 | 43 | 0.10 | 27.52 | 32.40 | 0.68 | 0.30 | 0.04 | 2.25 | |
| 311-05 | 34.72 | 125.52 | 75 | 27 | 0.03 | 21.99 | 32.17 | 0.92 | 0.30 | 0.09 | 4.29 | |
| 311-07 | 34.72 | 125.00 | 90 | 27 | 0.10 | 27.52 | 32.17 | 0.76 | 0.41 | 0.08 | 2.18 | |
| 312-03 | 34.00 | 125.90 | 85 | 19 | 0.09 | 24.07 | 31.72 | 0.51 | 0.55 | 0.11 | 3.58 | |
| 312-09 | 34.09 | 124.60 | 89 | 30 | 0.10 | 27.47 | 31.49 | 1.31 | 0.37 | 0.05 | 2.19 | |
| Autumn | 307-03 | 36.92 | 126.00 | 37 | 11 | 0.00 | 21.43 | 31.88 | 3.57 | 0.59 | 0.57 | 6.22 |
| 307-05 | 36.92 | 125.42 | 54 | 16 | 0.00 | 20.24 | 32.06 | 0.90 | 0.50 | 0.29 | 5.95 | |
| 307-09 | 36.92 | 124.57 | 67 | 16 | 0.00 | 20.23 | 31.83 | 1.07 | 0.47 | 0.08 | 5.56 | |
| 309-03 | 35.85 | 125.82 | 54 | 38 | 0.00 | 19.42 | 31.72 | 3.25 | 0.35 | 0.39 | 7.65 | |
| 309-07 | 35.86 | 125.00 | 66 | 22 | 0.01 | 20.54 | 31.51 | 1.16 | 0.38 | 0.04 | 6.58 | |
| 311-05 | 34.72 | 125.52 | 75 | 19 | 0.02 | 20.49 | 31.81 | 5.56 | 0.34 | 0.29 | 9.96 | |
| 312-07 | 34.10 | 125.00 | 92 | 38 | 0.03 | 21.28 | 32.01 | 0.92 | 0.29 | 0.04 | 3.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Youn, S.-H.; Oh, H.-J.; Joo, H.; Jang, H.-K.; Kang, J.-J.; Lee, D.; Jo, N.; Kim, K.; Park, S.; et al. Seasonal Compositions of Size-Fractionated Surface Phytoplankton Communities in the Yellow Sea. J. Mar. Sci. Eng. 2022, 10, 1087. https://doi.org/10.3390/jmse10081087
Kim Y, Youn S-H, Oh H-J, Joo H, Jang H-K, Kang J-J, Lee D, Jo N, Kim K, Park S, et al. Seasonal Compositions of Size-Fractionated Surface Phytoplankton Communities in the Yellow Sea. Journal of Marine Science and Engineering. 2022; 10(8):1087. https://doi.org/10.3390/jmse10081087
Chicago/Turabian StyleKim, Yejin, Seok-Hyun Youn, Hyun-Ju Oh, Huitae Joo, Hyo-Keun Jang, Jae-Joong Kang, Dabin Lee, Naeun Jo, Kwanwoo Kim, Sanghoon Park, and et al. 2022. "Seasonal Compositions of Size-Fractionated Surface Phytoplankton Communities in the Yellow Sea" Journal of Marine Science and Engineering 10, no. 8: 1087. https://doi.org/10.3390/jmse10081087
APA StyleKim, Y., Youn, S.-H., Oh, H.-J., Joo, H., Jang, H.-K., Kang, J.-J., Lee, D., Jo, N., Kim, K., Park, S., Kim, J., & Lee, S.-H. (2022). Seasonal Compositions of Size-Fractionated Surface Phytoplankton Communities in the Yellow Sea. Journal of Marine Science and Engineering, 10(8), 1087. https://doi.org/10.3390/jmse10081087









