Sea Turtles Employ Drag-Reducing Techniques to Conserve Energy
Abstract
:1. Introduction
2. Methods
2.1. Overview of Methods
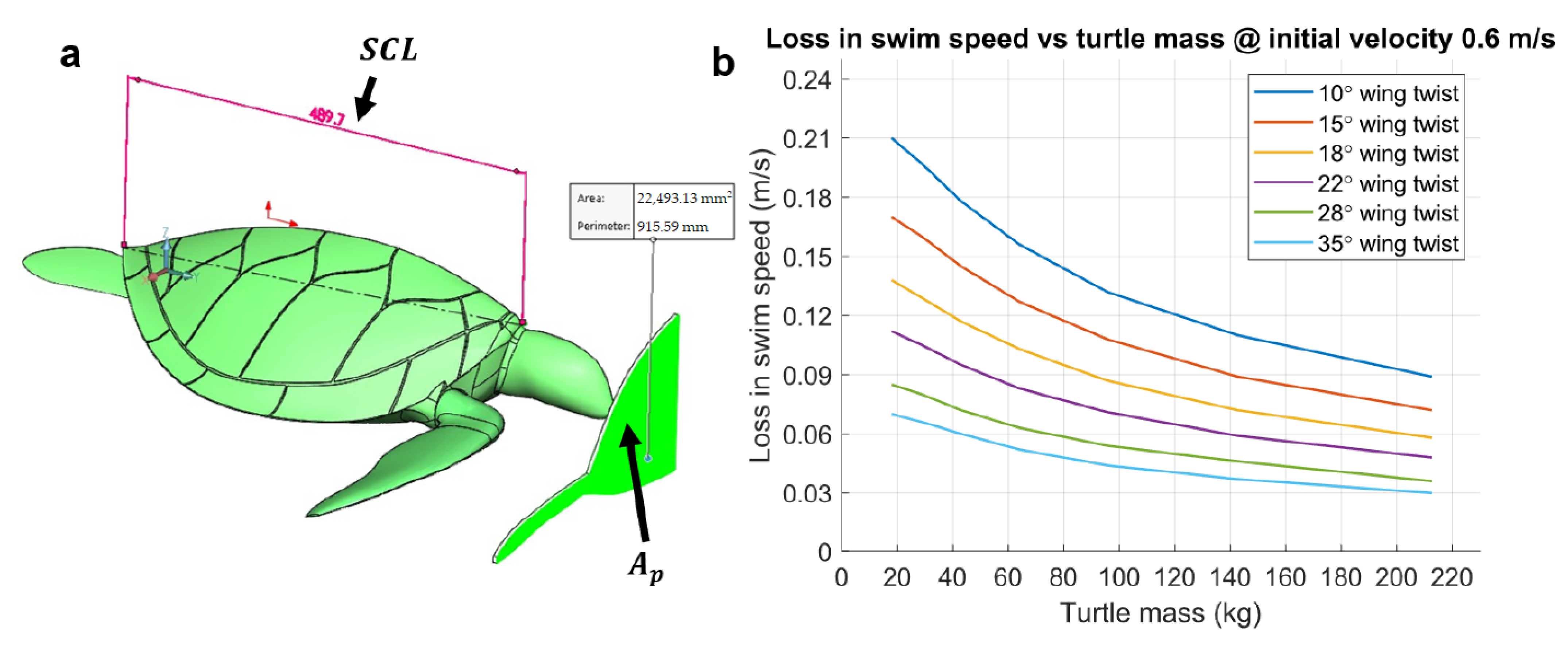
2.2. Sea Turtle Geometry
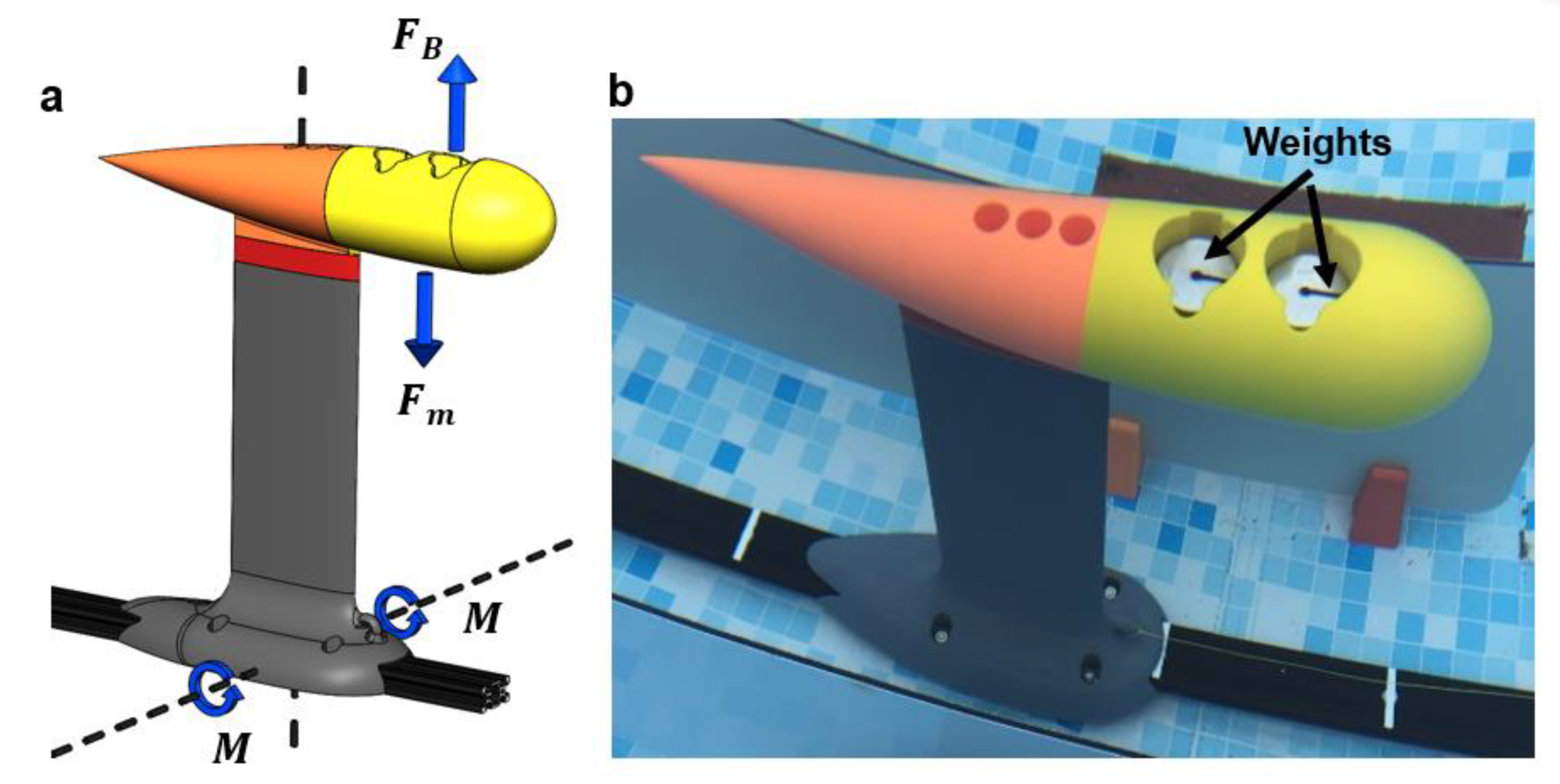
2.3. Test Rig Design and Manufacture
2.4. Testing Procedures
- -
- Pylon only (no attachments);
- -
- Pylon with force sensitivity device (to measure system sensitivity to lift forces);
- -
- Pylon with sea turtle model and rigidly mounted wings (wings could not rotate);
- -
- Pylon with sea turtle model and passive wings (wings are free to rotate);
- -
- Pylon with sea turtle model with wings removed (no wings, turtle carapace only).
2.5. Flow Pattern Generation
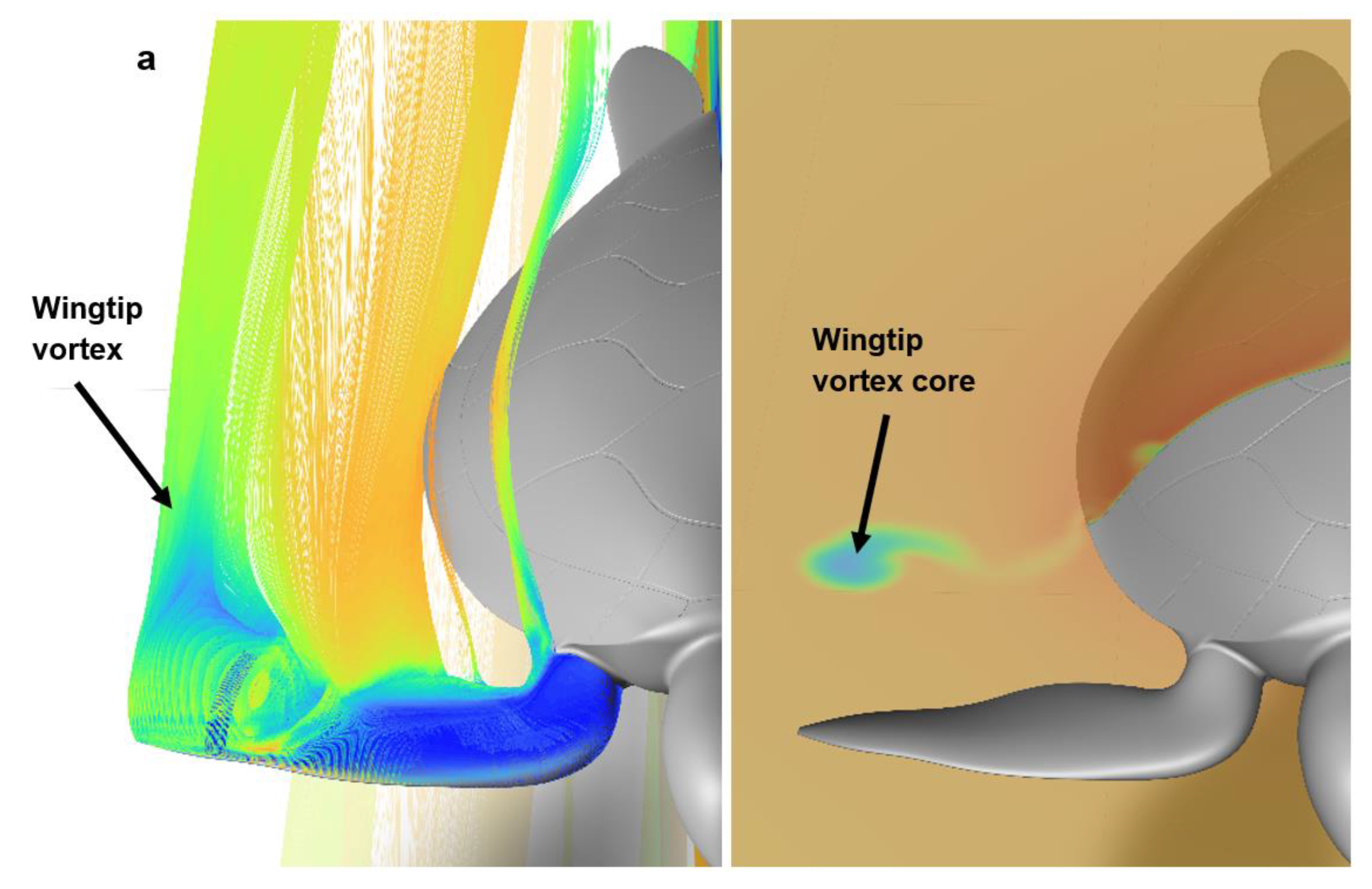
2.6. C.F.D. Analysis
3. Results and Discussion
3.1. Effect of Passive Wings on the Turtle’s Drag Coefficient
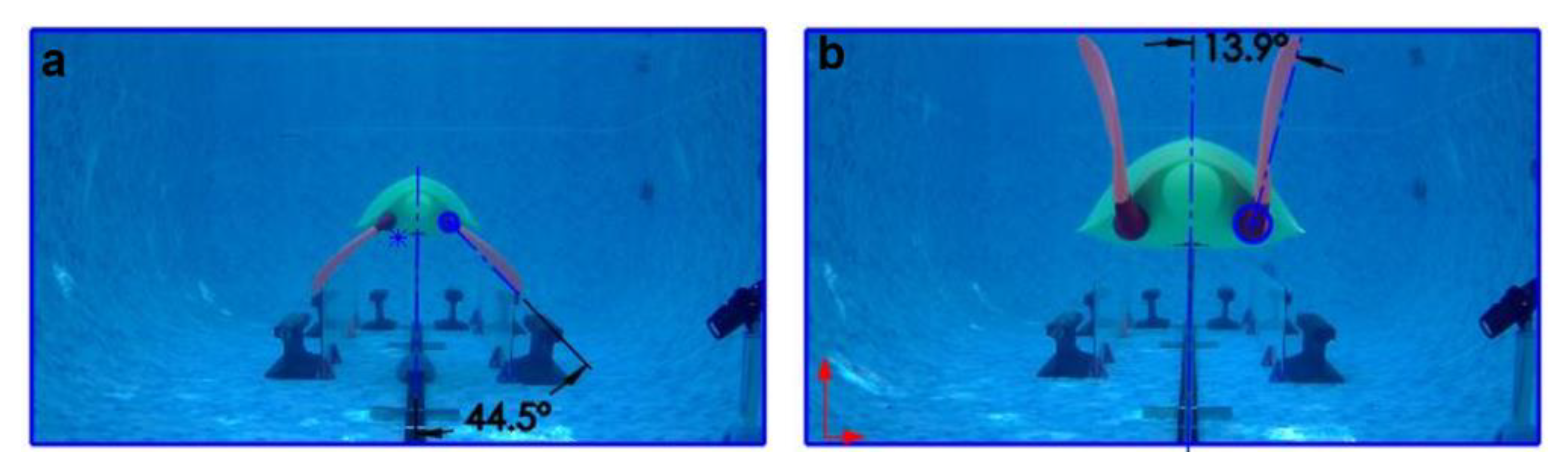
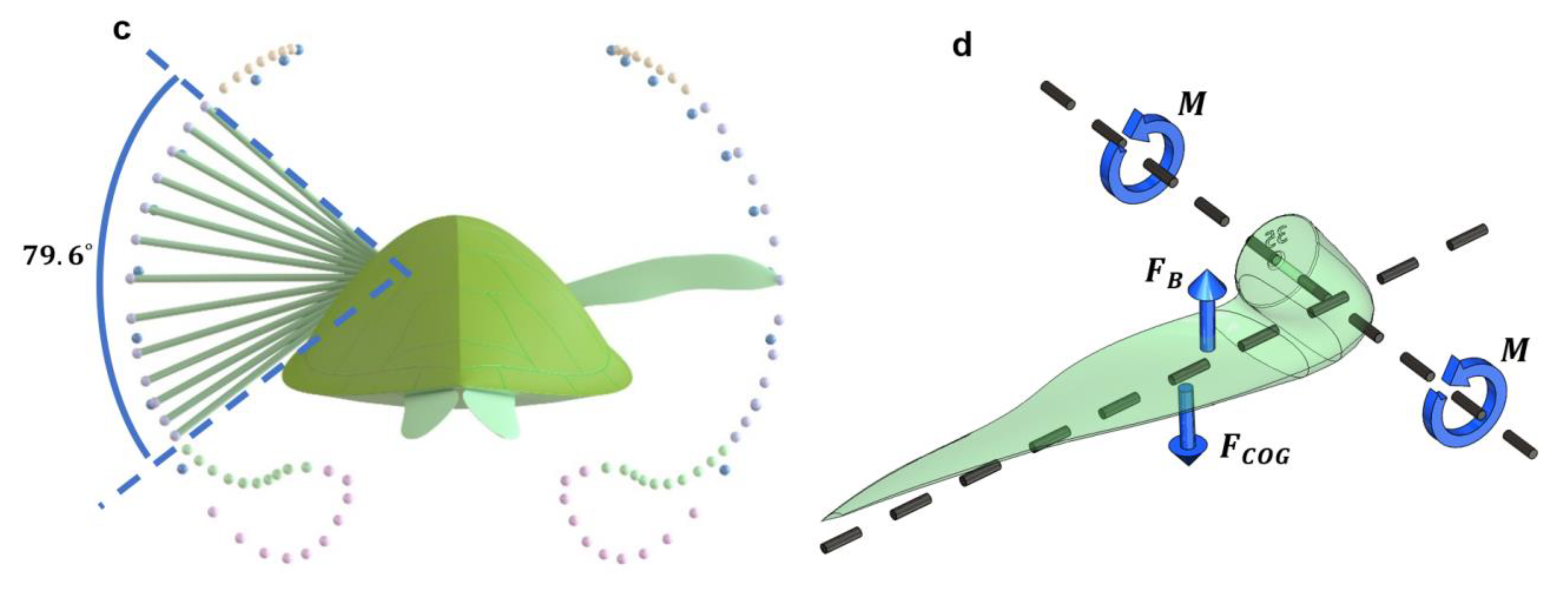
3.2. Passive Wing Rotation Speed Based on Wing Twist
3.3. Velocity Drop during the Nonpropulsive Upstroke
4. Conclusions and Future Work
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hays, G.C.; Scott, R. Global patterns for upper ceilings on migration distance in sea turtles and comparisons with fish, birds and mammals. Funct. Ecol. 2013, 27, 748–756. [Google Scholar] [CrossRef]
- Luschi, P.; Hays, G.C.; Del Seppia, C.; Marsh, R.; Papi, F. The navigational feats of green sea turtles migrating from Ascension Island investigated by satellite telemetry. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1998, 265, 2279–2284. [Google Scholar] [CrossRef]
- Wilme, L.; Waeber, P.O.; Ganzhorn, J.U. Marine turtles used to assist Austronesian sailors reaching new islands. C. R. Biol. 2016, 339, 78–82. [Google Scholar] [CrossRef]
- Díaz-Abad, L.; Bacco-Mannina, N.; Madeira, F.M.; Neiva, J.; Aires, T.; Serrao, E.A.; Regalla, A.; Patrício, A.R.; Frade, P.R. eDNA metabarcoding for diet analyses of green sea turtles (Chelonia mydas). Mar. Biol. 2021, 169, 1–12. [Google Scholar] [CrossRef]
- Howell, L.N.; Shaver, D.J. Foraging Habits of Green Sea Turtles (Chelonia mydas) in the Northwestern Gulf of Mexico. Front. Mar. Sci. 2021. Available online: https://www.frontiersin.org/articles/10.3389/fmars.2021.658368/full (accessed on 1 October 2022). [CrossRef]
- Bjorndal, K.A. Nutritional Ecology of Sea Turtles. Copeia 1985, 1985, 736–751. [Google Scholar] [CrossRef]
- McDermid, K.; Stuercke, B.; Balazs, G. Nutritional composition of marine plants in the diet of the green sea turtle (Chelonia mydas) in the Hawaiian Islands. Bull. Mar. Sci. 2007, 81, 55–71. [Google Scholar]
- Kinoshita, C.; Fukuoka, T.; Narazaki, T.; Niizuma, Y.; Sato, K. Analysis of why sea turtles swim slowly: A metabolic and mechanical approach. J. Exp. Biol. 2021, 224, jeb236216. [Google Scholar] [CrossRef]
- Chin, D.D.; Lentink, D. Birds repurpose the role of drag and lift to take off and land. Nat. Commun 2019, 10, 5354. [Google Scholar] [CrossRef] [Green Version]
- Henningsson, P.; Hedenstrom, A.; Bomphrey, R.J. Efficiency of lift production in flapping and gliding flight of swifts. PLoS ONE 2014, 9, e90170. [Google Scholar] [CrossRef] [Green Version]
- Iosilevskii, G. Centre-of-mass and minimal speed limits of the great hammerhead. R. Soc. Open Sci. 2020, 7, 200864. [Google Scholar] [CrossRef]
- Hochscheid, S.; Bentivegna, F.; Speakman, J.R. The dual function of the lung in chelonian sea turtles: Buoyancy control and oxygen storage. J. Exp. Mar. Biol. Ecol. 2003, 297, 123–140. [Google Scholar] [CrossRef]
- Davari, A.R. Wake structure and similar behavior of wake profiles downstream of a plunging airfoil. Chin. J. Aeronaut. 2017, 30, 1281–1293. [Google Scholar] [CrossRef]
- Davenport, J.; Munks, S.A.; Oxford, P.J. A Comparison of the swimming of Marine and Freshwater Turtles. R. Soc. London. Ser. B Biol. Sci. 1984, 220, 447–475. [Google Scholar] [CrossRef]
- Walker, W.F. Swimming in Sea Turtles of the Family Cheloniidae. Copeia 1971, 1971, 229. [Google Scholar] [CrossRef]
- Van der Geest, N.; Garcia, L.; Nates, R.; Godoy, D.A. New insights into the swimming kinematics of wild Green sea turtles (Chelonia mydas). Sci. Rep. 2022, 12, 1–12. [Google Scholar] [CrossRef]
- Izraelevitz, J.S.; Triantafyllou, M.S. Adding in-line motion and model-based optimisation offers exceptional force control authority in flapping foils. J. Fluid Mech. 2014, 742, 5–34. [Google Scholar] [CrossRef]
- Licht, S.C.; Wibawa, M.S.; Hover, F.S.; Triantafyllou, M.S. In-line motion causes high thrust and efficiency in flapping foils that use power downstroke. J. Exp. Biol. 2010, 213, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Liu, J.-k.; Chen, W.-s. Numerical and experimental studies of hydrodynamics of flapping foils. J. Hydrodyn. 2018, 30, 258–266. [Google Scholar] [CrossRef]
- Rivera, A.R.V.; Wyneken, J.; Blob, R.W. Forelimb kinematics and motor patterns of swimming loggerhead sea turtles (Caretta caretta): Are motor patterns conserved in the evolution of new locomotor strategies? J. Exp. Biol. 2011, 214, 3314–3323. [Google Scholar] [CrossRef] [Green Version]
- Booth, D.T. Kinematics of swimming and thrust production during powerstroking bouts of the swim frenzy in green turtle hatchlings. Biol. Open 2014, 3, 887–894. [Google Scholar] [CrossRef]
- Watanabe, Y.Y.; Sato, K.; Watanuki, Y.; Takahashi, A.; Mitani, Y.; Amano, M.; Aoki, K.; Narazaki, T.; Iwata, T.; Minamikawa, S.; et al. Scaling of swim speed in breath-hold divers. J. Anim. Ecol. 2011, 80, 57–68. [Google Scholar] [CrossRef]
- Narazaki, T.; Sato, K.; Abernathy, K.J.; Marshall, G.J.; Miyazaki, N. Sea turtles compensate deflection of heading at the sea surface during directional travel. J. Exp. Biol. 2009, 212, 4019–4026. [Google Scholar] [CrossRef] [Green Version]
- Bandyopadhyay, P.R.; Beal, D.N.; Hrubes, J.D.; Mangalam, A. Relationship of roll and pitch oscillations in a fin flapping at transitional to high Reynolds numbers. J. Fluid Mech. 2012, 702, 298–331. [Google Scholar] [CrossRef] [Green Version]
- Carr, Z.R.; Chen, C.; Ringuette, M.J. Finite-span rotating wings: Three-dimensional vortex formation and variations with aspect ratio. Exp. Fluids 2013, 54, 1–26. [Google Scholar] [CrossRef]
- Firat, E.; Ozkan, G.M.; Akilli, H. Flow past a hollow cylinder with two spanwise rows of holes. Exp. Fluids 2019, 60, 1–17. [Google Scholar] [CrossRef]
- Kartheeswaran, A.; Jothi, T.J.S.; Arackal, R.S. Visualisation studies on plane jets at low Reynolds numbers. Sādhanā 2021, 46, 1–12. [Google Scholar] [CrossRef]
- Todd Jones, T.; Van Houtan, K.S.; Bostrom, B.L.; Ostafichuk, P.; Mikkelsen, J.; Tezcan, E.; Carey, M.; Imlach, B.; Seminoff, J.A.; Rands, S. Calculating the ecological impacts of animal-borne instruments on aquatic organisms. Methods Ecol. Evol. 2013, 4, 1178–1186. [Google Scholar] [CrossRef]
- Sato, K. Body temperature stability achieved by the large body mass of sea turtles. J. Exp. Biol. 2014, 217, 3607–3614. [Google Scholar] [CrossRef] [Green Version]
- Eguchi, T.; Seminoff, J.A.; LeRoux, R.A.; Prosperi, D.; Dutton, D.L.; Dutton, P.H. Morphology and Growth Rates of the Green Sea Turtle (Chelonia mydas) in a Northern-most Temperate Foraging Ground. Herpetologica 2012, 68, 76–87. [Google Scholar] [CrossRef]
- Chaloupka, M.; Limpus, C. Estimates of sex- and age-class-specific survival probabilities for a southern Great Barrier Reef green sea turtle population. Mar. Biol. 2004, 146, 1251–1261. [Google Scholar] [CrossRef]
- Goshe, L.R.; Avens, L.; Scharf, F.S.; Southwood, A.L. Estimation of age at maturation and growth of Atlantic green turtles (Chelonia mydas) using skeletochronology. Mar. Biol. 2010, 157, 1725–1740. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Geest, N.; Garcia, L.; Nates, R.; Gonzalez-Vazquez, A. Sea Turtles Employ Drag-Reducing Techniques to Conserve Energy. J. Mar. Sci. Eng. 2022, 10, 1770. https://doi.org/10.3390/jmse10111770
van der Geest N, Garcia L, Nates R, Gonzalez-Vazquez A. Sea Turtles Employ Drag-Reducing Techniques to Conserve Energy. Journal of Marine Science and Engineering. 2022; 10(11):1770. https://doi.org/10.3390/jmse10111770
Chicago/Turabian Stylevan der Geest, Nick, Lorenzo Garcia, Roy Nates, and Alberto Gonzalez-Vazquez. 2022. "Sea Turtles Employ Drag-Reducing Techniques to Conserve Energy" Journal of Marine Science and Engineering 10, no. 11: 1770. https://doi.org/10.3390/jmse10111770






