DNA Barcoding Is a Useful Tool for the Identification of the Family Scaridae in Hainan
Abstract
:1. Introduction
2. Material and Methods
2.1. Sample Collection
2.2. DNA Extraction
2.3. PCR and DNA Sequencing
2.4. Analysis of the Utility of the BOLD as an Identification Tool for Parrotfish
2.5. Data Analysis
2.6. Ethics Statement
3. Results
3.1. Sequence Characteristics of CO I Gene Fragment
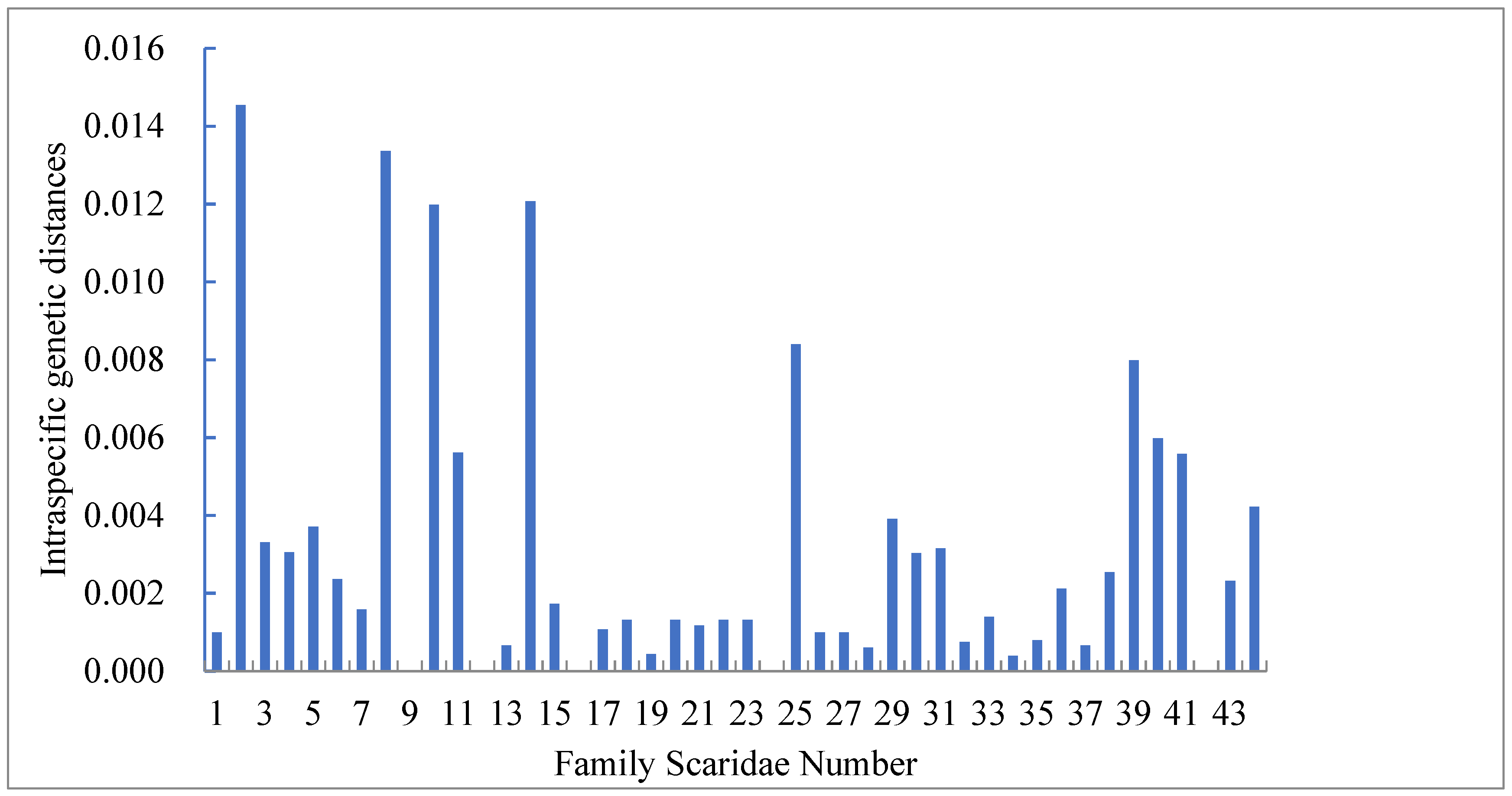
3.2. Genetic Distance between Species and within Species
3.3. Molecular Phylogenetic Tree
3.4. New Country Records
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Francini-Filho, R.B.; Moura, R.L. Evidence for spillover of reef fishes from a no-take marine reserve: An evaluation using the before-after control-impact (BACI) approach. Fish. Res. 2008, 93, 346–356. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Hoey, A.S.; Choat, J.H. Limited functional redundancy in high diversity systems: Resilience and ecosystem function on coral reefs. Ecol. Lett. 2003, 6, 281–285. [Google Scholar] [CrossRef]
- Carlon, D.B.; LippÉ, C. Isolation and characterization of 17 new microsatellite markers for the ember parrotfish Scarus rubroviolaceus, and cross-amplification in four other parrotfish species. Mol. Ecol. Notes 2007, 7, 613–616. [Google Scholar] [CrossRef]
- Mumby, P.J.; Dahlgren, C.P.; Harbome, A.R.; Kappel, C.V.; Micheli, F.; Brumbaugh, D.R.; Holmes, K.E.; Mendes, J.M.; Broad, K.; Sanchirico, J.N.; et al. Fishing, Trophic Cascades, and the Process of Grazing on Coral Reefs. Science 2006, 311, 98. [Google Scholar] [CrossRef] [Green Version]
- Goatley, C.; Bellwood, D. Biologically mediated sediment fluxes on coral reefs: Sediment removal and off-reef transportation by the surgeonfish Ctenochaetus striatus. Mar. Ecol. Prog. Ser. 2010, 415, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Glynn, P.; Manzello, D. Bioerosion and coral reef growth: A dynamic balance. In Coral Reefs in the Anthropocene; Springer: Dordrecht, The Netherlands, 2015; pp. 67–97. [Google Scholar] [CrossRef]
- Bellwood, D.R. Production and reworking of sediment by Parrotfish (family Scaridae) on the Great Barrier Reef, Australia. Mar. Biol. 1996, 125, 795–800. [Google Scholar] [CrossRef]
- Perry, C.T.; Kench, P.S.; O’Leary, M.J.; Morgan, K.; Januchowski-Hartley, F.A. Linking reef ecology to island building: Parrotfish identified as major producers of island-building sediment in the Maldives. Geology 2015, 43, 503–506. [Google Scholar] [CrossRef] [Green Version]
- Bellwood, D.R.; Hughes, T.P.; Folke, C.; Nystrom, M. Confronting the coral reef crisis. Nature 2004, 429, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Morgan, K.M.; Kench, P.S. Parrotfish erosion underpins reef growth, sand talus development and island building in the Maldives. Sediment. Geol. 2016, 341, 50–57. [Google Scholar] [CrossRef]
- Vallès, H.; Oxenford, H.A. Simple family-level parrotfish indicators are robust to survey method. Ecol. Indic. 2018, 85, 244–252. [Google Scholar] [CrossRef]
- Ebisawa, A.; Ohta, I.; Uehara, M.; Nakamura, H.; Kanashiro, K.; Yasui, R. Life history variables, annual change in sex ratios with age, and total mortality observed on commercial catch on Pacific steephead parrotfish, Chlorurus microrhinos in waters off the Okinawa Island, southwestern Japan. Reg. Stud. Mar. Sci. 2016, 8, 65–76. [Google Scholar] [CrossRef]
- Edwards, C.B.; Friedlander, A.M.; Green, A.G.; Hardt, M.J.; Sala, E.; Sweatman, H.P.; Williams, I.D.; Zgliczynski, B.; Sandin, S.A.; Smith, J.E. Global assessment of the status of coral reef herbivorous fishes: Evidence for fishing effects. Proc. Biol. Sci. 2014, 281, 20131835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, K.E.; Niem, V.H. (Eds.) FAO Species Identification Guide for Fishery Purposes. Bony Fishes Part 4 (Labridae to Latimeriidae), Estuarine Crocodiles, Sea Turtles, Sea Snakes and Marine Mammals. In The Living Marine Resources of the Western Central Pacific; FAO: Rome, Italy, 2001; Volume 6, pp. 468–3492. Available online: https://ci.nii.ac.jp/ncid/BA67023968 (accessed on 1 June 2022).
- Nelson, J.S.; Grande, T.; Wilson, M.V.H. Fishes of the World; John Wiley & Sons: Hoboken, NJ, USA, 2016; Volume 15, pp. 429–430. [Google Scholar] [CrossRef]
- Wu, H.L.; Shao, G.Z.; Lai, C.F.; Chong, D.H.; Lin, P.L. Latin-Chinese Dictionary of Fish Names by Classification System; China Ocean University Press: Qingdao, China, 2017; pp. 319–320. Available online: http://cpfd.cnki.com.cn/Article/CPFDTOTAL-ZGHI201209001002.htm (accessed on 2 June 2022).
- Arai, T.; Amalina, R.; Bachok, Z. Similarity in the feeding ecology of parrotfish (Scaridae) in coral reef habitats of the Malaysian South China Sea, as revealed by fatty acid signatures. Biochem. Syst. Ecol. 2015, 59, 85–90. [Google Scholar] [CrossRef]
- Rotjan, R.D.; Dimond, J.L. Discriminating causes from consequences of persistent parrotfish corallivory. J. Exp. Mar. Biol. Ecol. 2010, 390, 188–195. [Google Scholar] [CrossRef]
- Francini-Filho, R.; Moura, R.; Ferreira, C.M.; Coni, E.O.C. Live coral predation by Parrotfish (Perciformes: Scaridae) in the Abrolhos Bank, eastern Brazil, with comments on the classification of species into functional groups. Neotrop. Ichthyol. 2008, 6, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Adam, T.C.; Deron, E.B.; Ruttenberg, B.I.; Paddack, M.J. Herbivory and the Resilience of Caribbean Coral Reefs: Knowledge Gaps and Implications for Management. Mar. Ecol. Prog. Ser. 2021, 520, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Roos, N.C.; Carvalho, A.R.; Lopes, P.F.M.; Pennino, M.G. Modeling sensitive parrotfish (Labridae: Scarini) habitats along the Brazilian coast. Mar. Environ. Res. 2015, 110, 92–100. [Google Scholar] [CrossRef]
- Jawad, L.A. A comparative morphological investigation of otoliths of six parrotfish species (Scaridae) from the Solomon Islands. J. Fish Biol. 2018, 93, 1046–1058. [Google Scholar] [CrossRef]
- Barman, A.S.; Singh, M.; Pandey, P.K. DNA barcoding and genetic diversity analyses of fishes of Kaladan River of Indo-Myanmar biodiversity hotspot. Mitochondrial DNA A 2018, 29, 367–378. [Google Scholar] [CrossRef]
- Ritchie, P.; Bargelloni, L.; Meyer, A.; Taylor, J.A.; Macdonald, J.A.; Lambert, D.M. Mitochondrial Phylogeny of Trematomid Fishes (Nototheniidae, Perciformes) and the Evolution of Antarctic Fish. Mol. Phylogenet. Evol. 1996, 5, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Kochzius, M.; Seidel, C.; Antoniou, A.; Botla, S.K.; Campo, D.; Cariani, A.; Vazquez, E.G.; Hauschild, J.; Hervet, C.; Hjorleifsdottir, S.; et al. Identifying Fishes through DNA Barcodes and Microarrays. PLoS ONE 2010, 5, e12620. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.T.; Ma, X.H.; Shen, Y.J.; Mao, Y.T.; He, S.P. The fish diversity in the upper reaches of the Salween River, Nujiang River, revealed by DNA barcoding. Sci. Rep. 2015, 5, 17437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.Y.; Ma, C.Y.; Ma, L.B. Molecular identification of genus Scylla (Decapoda: Portunidae) based on DNA barcoding and polymerase chain reaction. Biochem. Syst. Ecol. 2012, 41, 41–47. [Google Scholar] [CrossRef]
- Khedkar, G.D.; Jamdade, R.; Naik, S.; David, L.; Haymer, D. DNA barcodes for the fishes of the Narmada, one of India’s longest rivers. PLoS ONE 2014, 9, e105490. [Google Scholar] [CrossRef] [Green Version]
- Dhar, B.; Ghosh, S.K. Genetic assessment of ornamental fish species from North East India. Gene 2015, 555, 382–392. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; de Waard, J.R. Biological identification through DNA barcodes. Proc. R. Soc. Lond. 2003, 270, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Stein, E.D.; Martinez, M.C.; Stiles, S.; Miller, P.E.; Zakharov, E.V. Is DNA Barcoding Actually Cheaper and Faster than Traditional Morphological Methods: Results from a Survey of Freshwater Bioassessment Efforts in the United States. PLoS ONE 2014, 9, e95525. [Google Scholar] [CrossRef] [PubMed]
- Sethusa, M.T.; Yessoufou, K.; der Bank, M.V.; der Bank, H.V.; Sethusa, M.T.; Millar, I.M.; Jacobs, A. DNA barcode efficacy for the identification of economically important scale insects (Hemiptera: Coccoidea) in South Africa. Afr. Entomol. 2014, 22, 257–266. [Google Scholar] [CrossRef]
- Decru, E.; Moelants, T.; De Gelas, K.; Vreven, E.; Verheyen, E.; Snoeks, J. Taxonomic challenges in freshwater fishes: A mismatch between morphology and DNA barcoding in fish of the north-eastern part of the Congo basin. Mol. Ecol. Resour. 2016, 16, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Nigro, L.M.; Angel, M.V.; Blachowiak-Samolyk, K.; Hopcroft, R.R.; Bucklin, A. Identification, Discrimination, and Discovery of Species of Marine Planktonic Ostracods Using DNA Barcodes. PLoS ONE 2016, 11, e0146327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Yang, J.W.; Liu, B.S.; Zhang, N.; Guo, L.; Guo, H.Y.; Zhang, D.C. Detection and identification of marine fish mislabeling in Guangzhou’s supermarkets and sushi restaurants using DNA barcoding. J. Food Sci. 2022, 87, 2440–2449. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Ratnasingham, S.; de Waard, J.R. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proc. Biol. Sci. 2003, 270, 96–99. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.H.; Qin, G.; Zhang, H.X.; Wang, X.; Lin, Q. DNA barcoding reflects the diversity and variety of brooding traits of fish species in the family Syngnathidae along China’s coast. Fish. Res. 2017, 185, 137–144. [Google Scholar] [CrossRef]
- Chambers, E.A.; Hebert, P.D. Assessing DNA Barcodes for Species Identification in North American Reptiles and Amphibians in Natural History Collections. PLoS ONE 2016, 11, e0154363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.Y.; Yang, T.Y.; Meng, W.; Yang, B.Q.; Zhang, T. Analysis of the mitochondrial genome characteristics and phylogenetic relationships of eight sciaenid fishes. Mar. Sci. 2017, 41, 48–54. Available online: https://oversea.cnki.net/HYKX201703007 (accessed on 2 June 2022).
- Smith, L.L.; Fessler, J.L.; Alfaro, M.E.; Streelman, J.T.; Westneat, M.W. Phylogenetic relationships and the evolution of regulatory gene sequences in the Parrotfish. Mol. Phylogenet. Evol. 2008, 49, 136–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellwood, D.R. A phylogenetic study of the parrotfish family Scaridae (Pisces: Labroidea), with a revision of genera. Rec. Aust. Mus. 1994, 20, 1–86. [Google Scholar] [CrossRef]
- Choat, J.H.; Klanten, O.S.; Herwerden, L.V.; Robertson, D.R.; Clements, K.D. Patterns and processes in the evolutionary history of Parrotfish (Family Labridae). Biol. J. Linn. Soc. 2012, 107, 529–557. [Google Scholar] [CrossRef] [Green Version]
- Ma, K.P. DNA barcode: From species to biome. Biodivers. Sci. 2015, 23, 279–280. [Google Scholar] [CrossRef]
- Barco, A.; Raupach, M.J.; Laakmann, S.; Neumnn, H.; Knebelsberger, T. Identification of North Sea molluscs with DNA barcoding. Mol. Ecol. Resour. 2016, 16, 288–297. [Google Scholar] [CrossRef]
- Zhang, J.B.; Hanner, R. DNA barcoding is a useful tool for the identification of marine fishes from Japan. Biochem. Syst. Ecol. 2011, 39, 31–42. [Google Scholar] [CrossRef]

| Genus | Species Studied | BOLD | Voucher ID | Process ID | Location |
|---|---|---|---|---|---|
| Calotomus | Calotomus carolinus | Calotomus carolinus | ss-18 | MK765061 | Sansha |
| Chlorurus | Chlorurus japanensis | Chlorurus japanensis | ss-67 | MK765062 | Sansha |
| Chlorurus microrhinos | Chlorurus microrhinos | ss-53 | MK765063 | Sansha | |
| Chlorurus microrhinos | Chlorurus microrhinos | ss-54 | MK765064 | Sansha | |
| Chlorurus sordidus | Chlorurus sordidus | Ssfri-F0165-01 | MK765065 | Hainan | |
| Chlorurus sordidus | Chlorurus sordidus | Ssfri-F0165-02 | MK765066 | Hainan | |
| Chlorurus sordidus | Chlorurus sordidus | Ssfri-F0165-03 | MK765067 | Hainan | |
| Chlorurus sordidus | Chlorurus sordidus | Ssfri-F0165-04 | MK765068 | Hainan | |
| Chlorurus sordidus | Chlorurus spilurus | ss-16 | MK765069 | Sansha | |
| Chlorurus sordidus | Chlorurus spilurus | ss-44 | MK765070 | Sansha | |
| Chlorurus sordidus | Chlorurus spilurus | ss-45 | MK765071 | Sansha | |
| Hipposcarus | Hipposcarus longiceps | Hipposcarus longiceps | ss-17 | MK765072 | Sansha |
| Scarus | Scarus dimidiatus | Scarus dimidiatus | ss-65 | MK765073 | Sansha |
| Scarus chameleon | Scarus chameleon | Ssfri-F0158-02 | MK765074 | Hainan | |
| Scarus forsteni | Scarus forsteni | ss-48 | MK765075 | Sansha | |
| Scarus forsteni | Scarus forsteni | ss-57 | MK765076 | Sansha | |
| Scarus forsteni | Scarus forsteni | ss-66 | MK765077 | Sansha | |
| Scarus forsteni | Scarus forsteni | Ssfri-F0164-01 | MK765078 | Hainan | |
| Scarus forsteni | Scarus forsteni | Ssfri-F0164-02 | MK765079 | Hainan | |
| Scarus forsteni | Scarus forsteni | Ssfri-F0164-03 | MK765080 | Hainan | |
| Scarus forsteni | Scarus forsteni | Ssfri-F0164-04 | MK765081 | Hainan | |
| Scarus forsteni | Scarus forsteni | Ssfri-F0164-05 | MK765082 | Hainan | |
| Scarus forsteni | Scarus forsteni | Ssfri-F0164-06 | MK765083 | Hainan | |
| Scarus ghobban | Scarus ghobban | ss-14 | MK765084 | Sansha | |
| Scarus ghobban | Scarus ghobban | ss-15 | MK765085 | Sansha | |
| Scarus ghobban | Scarus ghobban | Ssfri-F0347-01 | MK765086 | Hainan | |
| Scarus ghobban | Scarus ghobban | Ssfri-F0347-02 | MK765087 | Hainan | |
| Scarus ghobban | Scarus ghobban | Ssfri-F0347-03 | MK765088 | Hainan | |
| Scarus ghobban | Scarus ghobban | Ssfri-F0347-04 | MK765089 | Hainan | |
| Scarus ghobban | Scarus ghobban | Ssfri-F0347-05 | MK765090 | Hainan | |
| Scarus niger | Scarus niger | ss-7 | MK765091 | Sansha | |
| Scarus niger | Scarus niger | ss-41 | MK765092 | Sansha | |
| Scarus niger | Scarus niger | ss-42 | MK765093 | Sansha | |
| Scarus oviceps | Scarus oviceps | ss-33 | MK765094 | Sansha | |
| Scarus oviceps | Scarus oviceps | ss-46 | MK765095 | Sansha | |
| Scarus psittacus | Scarus psittacus | Ssfri-F0171-01 | MK765096 | Hainan | |
| Scarus psittacus | Scarus psittacus | Ssfri-F0171-02 | MK765097 | Hainan | |
| Scarus globiceps | Scarus rivulatus | ss-93 | MK765098 | Sansha | |
| Scarus globiceps | Scarus rivulatus | Ssfri-F0161-03 | MK765099 | Hainan | |
| Scarus globiceps | Scarus rivulatus | Ssfri-F0161-04 | MK7650100 | Hainan | |
| Scarus globiceps | Scarus rivulatus | Ssfri-F0161-05 | MK7650101 | Hainan | |
| Scarus rivulatus | Scarus rivulatus | Ssfri-F0161-06 | MK7650102 | Hainan | |
| Scarus rivulatus | Scarus rivulatus | Ssfri-F0161-07 | MK7650103 | Hainan | |
| Scarus rivulatus | Scarus rivulatus | Ssfri-F0161-08 | MK7650104 | Hainan | |
| Scarus rivulatus | Scarus rivulatus | Ssfri-F0161-09 | MK7650105 | Hainan | |
| Scarus rivulatus | Scarus rivulatus | Ssfri-F0161-10 | MK7650106 | Hainan | |
| Scarus schlegeli | Scarus schlegeli | ss-20 | MK7650107 | Sansha | |
| Scarus schlegeli | Scarus schlegeli | ss-47 | MK7650108 | Sansha | |
| Scarus schlegeli | Scarus schlegeli | ss-51 | MK7650109 | Sansha | |
| Scarus schlegeli | Scarus schlegeli | ss-72 | MK7650110 | Sansha | |
| Scarus spinus | Scarus spinus | Ssfri-F0166-01 | MK7650111 | Hainan |
| Domain | ii | si | sv | R | T | C | A | G | Total |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 458.00 | 52.00 | 21.00 | 29.5 | 29.5 | 22.7 | 18.2 | 530.9 | |
| 1st | 174.00 | 4.00 | 0.00 | 9.40 | 19.0 | 27.5 | 25.3 | 28.2 | 178.0 |
| 2nd | 175.00 | 2.00 | 0.00 | 5.89 | 42.1 | 28.8 | 15.0 | 14.1 | 178.0 |
| 3rd | 109.00 | 46.00 | 20.00 | 2.23 | 27.5 | 32.2 | 27.9 | 12.3 | 175.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Yan, Y.; Zhang, N.; Guo, H.; Liu, B.; Yang, J.; Zhu, K.; Zhang, D. DNA Barcoding Is a Useful Tool for the Identification of the Family Scaridae in Hainan. J. Mar. Sci. Eng. 2022, 10, 1915. https://doi.org/10.3390/jmse10121915
Liu B, Yan Y, Zhang N, Guo H, Liu B, Yang J, Zhu K, Zhang D. DNA Barcoding Is a Useful Tool for the Identification of the Family Scaridae in Hainan. Journal of Marine Science and Engineering. 2022; 10(12):1915. https://doi.org/10.3390/jmse10121915
Chicago/Turabian StyleLiu, Bo, Yali Yan, Nan Zhang, Huayang Guo, Baosuo Liu, Jingwen Yang, Kecheng Zhu, and Dianchang Zhang. 2022. "DNA Barcoding Is a Useful Tool for the Identification of the Family Scaridae in Hainan" Journal of Marine Science and Engineering 10, no. 12: 1915. https://doi.org/10.3390/jmse10121915
APA StyleLiu, B., Yan, Y., Zhang, N., Guo, H., Liu, B., Yang, J., Zhu, K., & Zhang, D. (2022). DNA Barcoding Is a Useful Tool for the Identification of the Family Scaridae in Hainan. Journal of Marine Science and Engineering, 10(12), 1915. https://doi.org/10.3390/jmse10121915








