Lateral Export and Sources of Subsurface Dissolved Carbon and Alkalinity in Mangroves: Revising the Blue Carbon Budget
Abstract
:1. Introduction
2. The ‘Missing Carbon’ Problem
3. Rates of DOC, DIC, and Alkalinity Flux in Mangrove Ecosystems
4. Blue Carbon Cycling
5. Reconciling Organic Matter Sources and Subsurface Mineralization
5.1. Quality and Origin of SOC
5.2. Are Deep, Old Soil Deposits a Source of Dissolved Carbon?
5.3. A Revised Mangrove Blue Carbon Mass Balance
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alongi, D.M. Coastal Ecosystem Processes; CRC Press: Roca Raton, FL, USA, 1998; pp. 183–253. [Google Scholar] [CrossRef]
- Teal, J.M. Energy flow in the salt marsh ecosystem of Georgia. Ecology 1962, 43, 614–624. [Google Scholar] [CrossRef]
- Odum, E.P.; de la Cruz, A.A. Detritus as a major component of ecosystems. AIBS Bull. 1963, 13, 39–40. [Google Scholar] [CrossRef]
- Odum, E.P. A research challenge: Evaluating the productivity of coastal and estuarine water. In Proceedings of the 2nd Sea Grant Conference, Newport, RI, USA, 17–18 October 1968; pp. 63–64. [Google Scholar]
- Nixon, S.W. Between salt marshes and coastal waters—A review of twenty years of speculation and research on the role of salt marshes in estuarine productivity and water chemistry. In Estuarine and Wetland Processes with Emphasis on Modeling. Marine Science; Hamilton, P., MacDonald, K.B., Eds.; Plenum Press: New York, NY, USA, 1980; Volume 11, pp. 437–525. [Google Scholar] [CrossRef]
- Childers, D.L.; Day, J.W.; Mckellar, H.N. Twenty More Years of Marsh and Estuarine Flux Studies: Revisiting Nixon (1980). In Concepts and Controversies in Tidal Marsh Ecology; Weinstein, M.P., Kreeger, D.A., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 391–423. [Google Scholar] [CrossRef]
- Golley, F.B.; Odum, H.T.; Wilson, R.F. The structure and metabolism of a Puerto Rican red mangrove forest in May. Ecology 1962, 43, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Heald, E.J. The Production of Organic Detritus in a South Florida Estuary. Ph.D. Thesis, University of Miami, Coral Gables, FL, USA, 1969. [Google Scholar]
- Boto, K.G.; Bunt, J.S. Tidal export of particulate organic matter from a northern Australian mangrove system. Estuar. Coast. Shelf Sci. 1981, 13, 247–255. [Google Scholar] [CrossRef]
- Twilley, R.R. The exchange of organic carbon in basin mangrove forests in a southwest Florida estuary. Estuar. Coast. Shelf Sci. 1985, 20, 543–557. [Google Scholar] [CrossRef]
- Boto, K.G.; Wellington, J.H. Seasonal variations in concentrations and fluxes of dissolved organic and inorganic materials in a tropical, tidally dominated, mangrove waterway. Mar. Ecol. Prog. Ser. 1988, 50, 151–160. [Google Scholar] [CrossRef]
- Lee, S.Y. Mangrove outwelling: A review. Hydrobiologia 1995, 295, 203–212. [Google Scholar] [CrossRef]
- Mazda, Y.; Ikeda, Y. Behavior of the groundwater in a riverine-type mangrove forest. Wetl. Ecol. Manag. 2006, 14, 477–488. [Google Scholar] [CrossRef]
- Ridd, P.V. Flow through animal burrows in mangrove creeks. Estuar. Coast. Shelf Sci. 1996, 43, 617–625. [Google Scholar] [CrossRef]
- Arnaud, M.; Baird, A.J.; Morris, P.J.; Taylor, A.; Huyen Dang, T.; Tran Hong, H.; Dinh Quang, T.; Tue Nguyen, T.; Polsenaere, P. The effect of crab burrows on soil-water dynamics in mangroves. Hydrol. Process. 2022, 36, e14522. [Google Scholar] [CrossRef]
- Kitheka, J.U.; Mwashote, B.M.; Ohowa, B.O.; Kamau, J. Water circulation, groundwater flow and nutrient dynamics in Mida Creek, Kenya. Mangroves Salt Marshes 1999, 3, 135–146. [Google Scholar] [CrossRef]
- Bouillon, S.; Borges, A.V.; Castañeda-Moya, E.; Diele, K.; Dittmar, T.; Duke, N.C.; Kristensen, E.; Lee, S.Y.; Marchand, C.; Middelburg, J.J.; et al. Mangrove production and carbon sinks: A revision of global budget estimates. Glob. Biogeochem. Cycles 2008, 22, GB2013. [Google Scholar] [CrossRef] [Green Version]
- Alongi, D.M. The Energetics of Mangrove Forests; Springer: Dordrecht, The Netherlands, 2009; pp. 163–167. [Google Scholar] [CrossRef]
- Alongi, D.M. The influence of mangrove biomass and production on biogeochemical processes in contrasting tropical coastal settings. In Organism-Sediment Interactions; Aller, J., Woodin, S., Aller, R.C., Eds.; University of South Carolina Press: Columbia, SC, USA, 2001; pp. 223–241. [Google Scholar]
- Alongi, D.M.; de Carvalho, N.A.; Amaral, A.L.; Da Costa, A.; Trott, L.A.; Tirendi, F. Uncoupling between surface and belowground soil metabolism in mangrove forests of Timor-Leste. Biogeochemistry 2012, 109, 151–162. [Google Scholar] [CrossRef]
- Bouillon, S.; Middelburg, J.J.; Dehairs, F.; Borges, A.V.; Abril, G.; Flindt, M.R.; Ulomi, S.; Kristensen, E. Importance of intertidal sediment processes and porewater exchange on the water column biogeochemistry in a pristine mangrove creek (Ras Dege, Tanzania). Biogeosciences 2007, 4, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Koné, Y.J.-M.; Borges, A.V. Dissolved inorganic carbon dynamics in the waters surrounding forested mangroves of the Ca Mau Province (Vietnam). Estuar. Coast. Shelf Sci. 2008, 77, 409–421. [Google Scholar] [CrossRef] [Green Version]
- Sippo, J.Z.; Maher, D.T.; Tait, D.R.; Holloway, C.; Santos, I.R. Are mangrove drivers or buffers of coastal acidification? Insights from alkalinity and dissolved inorganic carbon export estimates across a latitudinal transect. Glob. Biogeochem. Cycles 2016, 30, 753–766. [Google Scholar] [CrossRef]
- Lugo, A.E.; Snedaker, S.C. The ecology of mangroves. Annu. Rev. Ecol. Syst. 1974, 5, 39–64. [Google Scholar] [CrossRef]
- Davis, S.E.; Childers, D.L.; Day, J.W.J.; Rudnick, D.T.; Sklar, F.H. Factors affecting the concentration and flux of materials in two southern Everglades mangrove wetlands. Mar. Ecol. Prog. Ser. 2003, 253, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Quasim, S.Z.; Sen Gupta, R. Environmental characteristics of the Mandovi-Zuari estuarine system in Goa. Estuar. Coast. Shelf Sci. 1981, 13, 557–578. [Google Scholar] [CrossRef]
- Wafar, S.; Untawale, G.; Wafar, M. Litter fall and energy flux in a mangrove ecosystem. Estuar. Coast. Shelf Sci. 1997, 44, 111–124. [Google Scholar] [CrossRef]
- Davis, S.E.; Childers, D.L.; Day, J.W.J.; Rudnick, D.; Sklar, F. Nutrient dynamics in vegetated and unvegetated areas of a southern Everglades mangrove creek. Estuar. Coast. Shelf Sci. 2001, 52, 753–768. [Google Scholar] [CrossRef]
- Davis, S.E.; Childers, D.L.; Day, J.W.J.; Rudnick, D.; Sklar, F. Wetland-water column exchanges of carbon, nitrogen, and phosphorus dynamics in a southern Everglades dwarf mangrove. Estuaries 2001, 24, 610–622. [Google Scholar] [CrossRef]
- Twilley, R.R.; Lugo, A.L.; Patterson-Zuca, C. Litter production and turnover in basin mangrove forests in southwest Florida. Ecology 1986, 67, 670–683. [Google Scholar] [CrossRef]
- Rezende, C.; Lacerda, L.; Ovalle, A.; Silva, L. Diel organic carbon fluctuations in a mangrove tidal creek in Sepetiba Bay, southeast Brazil. Braz. J. Biol. 2007, 67, 673–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayukai, T.; Miller, D.; Wolanski, E.; Spagnol, S. Fluxes of nutrients and dissolved and particulate organic carbon in two mangrove creeks in northeastern Australia. Mangr. Salt Marsh. 1998, 2, 223–230. [Google Scholar] [CrossRef]
- Alongi, D.M.; Wattayakorn, G.; Ayukai, T.; Clough, B.F.; Wolanski, E.; Brunskill, G.J. An organic carbon budget for mangrove-fringed Sawi Bay, southern Thailand. Phuket Mar. Biol. Cent. Res. Bull. 2000, 22, 79–85. [Google Scholar]
- Dittmar, T.; Lara, R.J. Do mangroves rather than rivers provide nutrients to coastal environments south of the Amazon River? Evidence from long-term flux measurements. Mar. Ecol. Prog. Ser. 2001, 213, 67–77. [Google Scholar] [CrossRef]
- Young, M.; Eagle Gonneea, M.; Herrera-Silveira, J.; Paytan, A. Export of dissolved and particulate carbon and nitrogen from a mangrove-dominated lagoon, Yucantan Peninsula, Mexico. Int. J. Ecol. Environ. Sci. 2005, 31, 189–202. [Google Scholar]
- Dittmar, T.; Hertkorn, G.; Kattner, G.; Lara, R.J. Mangroves, a major source of dissolved organic carbon to the oceans. Glob. Biogeochem. Cycles 2006, 20, 1–7. [Google Scholar] [CrossRef]
- Romigh, M.M.; Davis, S.E.; Rivera-Monroy, V.H.; Twilley, R.R. Flux of organic carbon in a riverine mangrove wetland in the Florida coastal Everglades. Hydrobiologia 2006, 569, 505–516. [Google Scholar] [CrossRef] [Green Version]
- Rajkaran, A.; Adams, J.B. Mangrove litter production and organic carbon pools in the Mngazana Estuary, South Africa. Afr. J. Aquat. Sci. 2007, 32, 17–25. [Google Scholar] [CrossRef]
- Sánchez-Carrillo, S.; Sánchez-Andrés, R.; Alatoore, L.C.; Angeler, D.G.; Álvarez-Cobelas, A.-L. Nutrient fluxes in a semi-arid microtidal mangrove wetland in the Gulf of California. Estuar. Coast. Shelf Sci. 2009, 82, 654–662. [Google Scholar] [CrossRef]
- Bergamaschi, B.A.; Krabbenhoft, D.P.; Aiken, G.R.; Patino, E.; Rumbold, D.G.; Orem, W.H. Tidally driven export of dissolved organic carbon, total mercury, and methylmercury from a mangrove-dominated estuary. Environ. Sci. Technol. 2012, 46, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Maher, D.T.; Santos, I.R.; Golsby-Smith, L.; Gleeson, J.; Eyre, B.D. Groundwater-derived dissolved inorganic and organic carbon exports from a mangrove tidal creek: The missing mangrove carbon sink? Limnol. Oceanogr. 2013, 58, 475–488. [Google Scholar] [CrossRef]
- Faber, P.A.; Evrard, V.; Woodland, R.J.; Cartwright, I.C.; Cook, P.L.M. Pore-water exchange driven by tidal pumping causes alkalinity export in two intertidal inlets. Limnol. Oceanogr. 2014, 59, 1749–1763. [Google Scholar] [CrossRef]
- Stewart, B.T.; Santos, I.R.; Tait, D.R.; Macklin, P.A.; Maher, D.T. Submarine groundwater discharge and associated fluxes of alkalinity and dissolved carbon into Moreton Bay (Australia) estimated via radium isotopes. Mar. Chem. 2015, 174, 1–12. [Google Scholar] [CrossRef]
- Sadat-Noori, M.; Maher, D.T.; Santos, I.R. Groundwater discharge as a source of dissolved carbon and greenhouse gases in a subtropical estuary. Estuaries Coast. 2016, 39, 639–656. [Google Scholar] [CrossRef]
- Ho, D.T.; Ferrón, S.; Engel, V.C.; Anderson, W.T.; Swart, P.K.; Price, R.M.; Barbero, L. Dissolved carbon biogeochemistry and export in mangrove-dominated rivers of the Florida Everglades. Biogeosciences 2017, 14, 2543–2559. [Google Scholar] [CrossRef]
- Ray, R.; Baum, A.; Rixen, T.; Gleixner, G.; Jana, T.K. Exportation of dissolved (inorganic and organic) and particulate carbon from mangroves and its implications to the carbon budget in the Indian Sundarbans. Sci. Total Environ. 2018, 621, 535–547. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, F.; Lao, Y.; Wang, X.; Du, J.; Santos, I.R. Submarine groundwater discharge-derived carbon fluxes in mangroves: An important component of blue carbon budgets? J. Geophys. Res. Oceans 2018, 123, 6962–6979. [Google Scholar] [CrossRef]
- Maher, D.T.; Call, M.; Santos, I.R.; Sanders, C.J. Beyond burial: Lateral exchange is a significant atmospheric carbon sink in mangrove forests. Biol. Lett. 2018, 14, 20180200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Call, M.; Sanders, C.J.; Macklin, P.A.; Santos, I.R.; Maher, D.T. Carbon outwelling and emissions from two contrasting mangrove creeks during the monsoon storm season in Palau, Micronesia. Estuar. Coast. Shelf Sci. 2019, 218, 340–348. [Google Scholar] [CrossRef]
- Sadat-Noori, M.; Glamore, W. Porewater exchange drives trace metal, dissolved organic carbon and total dissolved nitrogen export from a temperate mangrove wetland. J. Environ. Manag. 2019, 248, 109264. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.R.; Maher, D.T.; Larkin, R.; Webb, J.R.; Sanders, C.J. Carbon outwelling and outgassing vs. burial in an estuarine tidal creek surrounded by mangrove and salt marsh wetlands. Limnol. Oceanogr. 2019, 64, 996–1013. [Google Scholar] [CrossRef]
- Sippo, J.Z.; Maher, D.T.; Schulz, K.G.; Sanders, C.J.; McMahon, A.; Tucker, J.; Santos, I.R. Carbon outwelling across the shelf following a massive mangrove dieback in Australia: Insights from radium isotopes. Geochim. Cosmochim. Acta 2019, 253, 142–158. [Google Scholar] [CrossRef]
- Volta, C.; Ho, D.T.; Maher, D.T.; Wanninkhof, R.; Friedrich, G.; Del Castillo, C.; Dulai, H. Seasonal variations in dissolved carbon inventory and fluxes in a mangrove-dominated estuary. Glob. Biogeochem. Cycles 2020, 34, e2019GB006515. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Onishi, T.; Yoshitake, S.; Tomotsune, M.; Kida, M.; Iimura, Y.; Kondo, M.; Suchewaboripont, V.; Cao, R.; Kinjo, K.; et al. Lateral export of dissolved inorganic and organic carbon from a small mangrove estuary with tidal fluctuation. Forests 2020, 11, 1041. [Google Scholar] [CrossRef]
- Ray, R.; Thouzeau, G.; Walcker, R.; Vanrepotte, V.; Gleixner, G.; Morvan, S.; Devesa, J.; Michaud, E. Mangrove-derived organic and inorganic carbon exchanges between the Sinnamary estuarine system (French Guiana, South America) and Atlantic Ocean. J. Geophys. Res. Biogeosci. 2020, 125, e2020JG005739. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, F.; Chen, X. Influence of submarine groundwater discharge in the blue carbon budget of a typical mangrove: A case study from the Zhenzhu Bay, Guangxi. Haiyang Xuebao 2020, 42, 37–46. [Google Scholar] [CrossRef]
- Saderne, V.; Fusi, M.; Thomson, T.; Dunne, A.; Mahmud, F.; Roth, F.; Carvalho, S.; Duarte, C.M. Total alkalinity production in a mangrove ecosystem reveals an overlooked blue carbon component. Limnol. Oceanogr. Lett. 2021, 6, 61–67. [Google Scholar] [CrossRef]
- Reithmaier, G.M.S.; Ho, D.T.; Johnston, S.G.; Maher, D.T. Mangroves as a source of greenhouse gases to the atmosphere and alkalinity and dissolved carbon to the coastal ocean: A case study from the Everglades National Park, Florida. J. Geophys. Res. Biogeosci. 2020, 125, e2020JG005812. [Google Scholar] [CrossRef]
- Akhand, A.; Watanabe, K.; Chanda, A.; Tokoro, T.; Chakraborty, K.; Moki, H.; Tanaya, T.; Ghosh, J.; Kuwae, T. Lateral carbon fluxes and CO2 evasion from a subtropical mangrove-seagrass-coral continuum. Sci. Total Environ. 2021, 752, 142190. [Google Scholar] [CrossRef] [PubMed]
- Cabral, A.; Dittmar, T.; Call, M.; Scholten, J.; de Rezenda, C.E.; Asp, N.; Gledhill, M.; Seidel, M.; Santos, I.R. Carbon and alkalinity outwelling across the groundwater-creek-shelf continuum off Amazonian mangroves. Limnol. Oceanogr. Lett. 2021, 6, 369–378. [Google Scholar] [CrossRef]
- Ray, R.; Miyajima, T.; Watanabe, A.; Yoshikai, M.; Ferrera, C.M.; Orizar, I.; Nakamura, T.; San Diego-McGlone, M.L.; Herrera, E.C.; Nadaoka, K. Dissolved and particulate carbon export from a tropical mangrove-dominated riverine system. Limnol. Oceangr. 2021, 66, 3944–3962. [Google Scholar] [CrossRef]
- Reithmaier, G.M.S.; Chen, X.; Santos, I.R.; Drexl, M.J.; Holloway, C.; Call, M.; Álvarez, P.G.; Euler, S.; Maher, D.T. Rainfall drives rapid shifts in carbon and nutrient source-sink dynamics of an urbanized, mangrove-fringed estuary. Estuar. Coast. Shelf Sci. 2021, 249, 107064. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, H.; Tang, D.; Wang, Y.; Zidan, A.; Cui, Z. Submarine groundwater discharge as a significant export of dissolved inorganic carbon from a mangrove tidal creek to Qinglan Bay (Hainan Island, China). Cont. Shelf Res. 2021, 223, 104451. [Google Scholar] [CrossRef]
- Belliard, J.-P.; Hernandez, S.; Temmerman, S.; Suello, R.H.; Dominguez-Granda, E.; Rosado-Moncayo, A.M.; Ramos-Veliz, J.A.; Parra-Narera, R.N.; Pollete-Ramirez, K.; Govers, G.; et al. Carbon dynamics and CO2 and CH4 exchange in the mangrove dominated Guayas River delta, Ecuador. Estuar. Coast. Shelf Sci. 2022, 267, 107766. [Google Scholar] [CrossRef]
- Leal, M.; Spalding, M.D. (Eds.) The State of the World’s Mangrove Forests; Global Mangrove Alliance: Wageningen, The Netherlands, 2022; pp. 1–91. [Google Scholar]
- Borges, A.V.; Djenidi, S.; Lacroix, G.; Théate, J.; Delille, B.; Frankignoulle, M. Atmospheric CO2 flux from mangrove surrounding waters. Geophys. Res. Lett. 2003, 30, 1558. [Google Scholar] [CrossRef]
- Kristensen, E.; Connolly, R.M.; Otero, X.L.; Marchand, C.; Ferreira, T.O.; Rivera-Monroy, V.H. Biogeochemical cycles: Global approaches and perspectives. In Mangrove Ecosystems: A Global Biogeographic Perspective; Rivera-Monroy, V.H., Lee, S.Y., Kristensen, E., Twilley, R.R., Eds.; Springer: Cham, Switzerland, 2017; pp. 163–209. [Google Scholar] [CrossRef]
- Reithmaier, G.M.S.; Johnston, S.G.; Junginger, T.; Goddard, M.M.; Sanders, C.J.; Hutley, L.B.; Ho, D.T.; Maher, D.T. Alkalinity production coupled to pyrite formation represents an unaccounted blue carbon sink. Glob. Biogeochem. Cycles 2021, 35, e2020GB0006785. [Google Scholar] [CrossRef]
- Fakhraee, M.; Planavsky, N.; Reinhard, C. Ocean alkalinity enhancement through blue carbon ecosystem restoration. Nat. Sustain. 2022, 5, 1–10. [Google Scholar] [CrossRef]
- Huang, T.-H.; Fu, Y.-H.; Pan, P.-Y.; Chen, C.-T.A. Fluvial carbon fluxes in tropical rivers. Curr. Opin. Environ. Sustain. 2012, 4, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Barron, C.; Duarte, C.M. Dissolved organic carbon pools and fluxes from the coastal ocean. Glob. Biogeochem. Cycles 2015, 29, 1725–1738. [Google Scholar] [CrossRef] [Green Version]
- Bauer, J.E.; Cai, W.-J.; Raymond, P.A.; Bianchi, T.S.; Hopkinson, C.S.; Regnier, P.A.G. The changing carbon cycle of the coastal ocean. Nature 2013, 504, 610–670. [Google Scholar] [CrossRef] [PubMed]
- Middelburg, J.J.; Soetaert, K.; Hagan, M. Ocean alkalinity, buffering and biogeochemical processes. Rev. Geophys. 2020, 58, e2019RG000681. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon cycling and storage in mangrove forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef]
- de Oliveira Gomes, L.E.; Sanders, C.J.; Nobrega, G.N.; Vescovi, L.C.; Quiroz, H.M.; Kauffman, J.B.; Ferreira, T.O.; Bernardino, A.F. Ecosystem carbon losses following a climate -induced mangrove mortality in Brazil. J. Environ. Manag. 2021, 297, 113381. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon cycling in the world’s mangrove ecosystems revisited: Significance of non-steady state diagenesis and subsurface linkages between the forest floor and the coastal ocean. Forests 2020, 11, 977. [Google Scholar] [CrossRef]
- Suwa, R.; Hagihara, A. Seasonal changes in canopy photosynthesis and foliage respiration in a Rhizophoa stylosa stand at the northern limit of its natural distribution. Wetlands Ecol. Manag. 2008, 16, 313–321. [Google Scholar] [CrossRef]
- Barr, J.G.; Engel, V.; Fuentes, J.D.; Zieman, J.C.; O’Halloran, T.; Smith, T.J., III; Anderson, G.H. Controls on mangrove forest-atmosphere carbon dioxide exchanges in western Everglades National Park. J. Geophys. Res. 2010, 115, G02020. [Google Scholar] [CrossRef] [Green Version]
- Hoque, A.T.M.R.; Sharma, S.; Suwa, R.; Mori, S.; Hagihara, A. Seasonal variation in the size-dependent respiration of mangroves Kandelia obovata. Mar. Ecol. Prog. Ser. 2010, 404, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Lu, W.; Yan, G.; Yang, S.; Lin, G. Typhoons exert significant but differential impacts on net ecosystem carbon exchange of subtropical mangrove forests in China. Biogeosciences 2014, 11, 5323–5333. [Google Scholar] [CrossRef] [Green Version]
- Lovelock, C.E.; Simpson, L.T.; Duckett, L.J.; Feller, I.C. Carbon budgets for Caribbean mangrove forests of varying structure and with phosphorus enrichment. Forests 2015, 6, 3528–3546. [Google Scholar] [CrossRef] [Green Version]
- Rodda, S.R.; Thumaty, K.C.; Jha, C.S.; Dadhwal, V.K. Seasonal variations in carbon dioxide, water vapor and energy fluxes in tropical Indian mangroves. Forests 2016, 7, 35. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Liang, J.; Lu, W.; Chen, H.; Liu, F.; Lin, G.; Xu, F.; Luo, Y.; Lin, G. Stronger ecosystem carbon sequestration potential of mangrove wetlands with respect to terrestrial forests in subtropical China. Agric. For. Meteorol. 2018, 249, 71–80. [Google Scholar] [CrossRef]
- Liu, J.; Lai, D.Y. Subtropical mangrove wetland is a stronger carbon dioxide sink in the dry than in the wet season. Agric. For. Meteorol. 2019, 278, 107644. [Google Scholar] [CrossRef]
- Gnanamoorthy, P.; Selvam, V.; Burman, P.K.D.; Chakraborty, S.; Karipot, A.; Nagarajan, R.; Ramasubramian, R.; Song, Q.; Zhang, Y.; Grace, J. Seasonal variations of net ecosystem (CO2) exchange in the Indian tropical mangrove forest of Pichavarum. Estuar. Coast. Shelf Sci. 2020, 243, 106828. [Google Scholar] [CrossRef]
- Lele, N.; Kripa, M.K.; Panda, M.; Das, S.K.; Nivas, A.H.; Divakaran, N.; Naik-Gaonkar, S.; Sawant, A.; Pattnaik, A.K.; Samal, R.N.; et al. Seasonal variation in photosynthetic rates and satellite-based GPP estimation over mangrove forest. Environ. Monit. Assess. 2021, 193, 1–20. [Google Scholar] [CrossRef]
- Zheng, Y.; Takeuchi, W. Estimating mangrove forest gross primary production by quantifying environmental stressors in the coastal area. Sci. Rep. 2022, 12, 2238. [Google Scholar] [CrossRef]
- Burkholder, P.R.; Almodóvar, L.R. Studies on mangrove algal communities in Puerto Rico. Fla. Sci. 1973, 36, 66–74. [Google Scholar]
- Hoffman, W.E.; Dawes, C.J. Photosynthetic rates and primary production by two Florida benthic algal species from a salt marsh and a mangrove community. Bull. Mar. Sci. 1980, 30, 358–364. [Google Scholar]
- Salamanca, E.J. Physiological Ecology of Mangrove-Associated Macroalgae in a Tropical Estuary; University of South Carolina: Columbia, SC, USA, 1998. [Google Scholar]
- Dawes, C.J. Mangrove structure, litter and macroalgal productivity in a northern-most forest of Florida. Mangrove Salt Marsh 1999, 3, 259–267. [Google Scholar] [CrossRef]
- Laursen, W.J.; King, R.J. The distribution and abundance of mangrove macroalgae in Woolooware Bay, New South Wales, Australia. Bot. Mar. 2000, 43, 377–384. [Google Scholar] [CrossRef]
- Koch, M.S.; Madden, C.J. Patterns of primary production and nutrient availability in a Bahamas lagoon with fringing mangroves. Mar. Ecol. Prog. Ser. 2001, 219, 109–119. [Google Scholar] [CrossRef]
- Kristensen, E.; Bouillon, S.; Dittmar, T.; Marchand, C. Organic carbon dynamics in mangrove ecosystems: A review. Aquat. Bot. 2008, 89, 201–219. [Google Scholar] [CrossRef] [Green Version]
- Robertson, A.I.; Alongi, D.M. Massive turnover rates of fine root detrital carbon in tropical Australian mangroves. Oecologia 2016, 180, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Alongi, D.M.; Tirendi, F.; Dixon, P.; Trott, L.A.; Brunskill, G.J. Mineralization of organic matter in intertidal sediments of a tropical semi-enclosed delta. Estuar. Coast. Shelf Sci. 1999, 48, 451–467. [Google Scholar] [CrossRef]
- Alongi, D.M.; Wattayakorn, G.; Tirendi, F.; Zagorskis, I.; Brunskill, G.J.; Davidson, A.; Clough, B.F. Organic carbon accumulation and metabolic rates in sediments of mangrove forests in southern Thailand. Mar. Geol. 2001, 179, 85–103. [Google Scholar] [CrossRef]
- Alongi, D.M.; Sasekumar, A.; Tirendi, F.; Trott, L.; Pfitzner, J.; Dixon, P.; Brunskill, G.J. Sediment accumulation and rates of carbon and nitrogen flow in mangrove forests of different age: Estimates of land-ocean-atmosphere exchange in peninsular Malaysia. Mar. Geol. 2004, 208, 383–402. [Google Scholar] [CrossRef]
- Alongi, D.M.; Pfitzner, J.; Trott, L.; Tirendi, F.; Dixon, P.; Klumpp, D.K. Rapid sedimentation and microbial mineralization in mangrove forests of the Jiulongjiang estuary, China. Estuar. Coast. Shelf Sci. 2005, 63, 605–618. [Google Scholar] [CrossRef]
- Kuramoto, T.; Minagawa, M. Stable carbon and nitrogen isotopic characterization of organic matter in a mangrove ecosystem on the southwestern coast of Thailand. J. Oceanogr. 2001, 57, 421–431. [Google Scholar] [CrossRef]
- Marchand, C.; Lallier-Vergès, E.; Baltzer, F. The composition of sedimentary organic matter in relation to the dynamic features of a mangrove-fringed coast in French Guiana. Estuar. Coast. Shelf Sci. 2003, 56, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Marchand, C.; Disnar, J.R.; Lallier-Vergés, E.; Lottier, N. Early diagenesis of carbohydrates and lignin in mangrove sediments subject to variable redox conditions (French Guiana). Geochim. Cosmochim. Acta 2005, 69, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Ray, R.; Michaud, E.; Aller, R.C.; Vantrepotte, V.; Gleixer, G.; Walcker, R.; Devasa, J.; Le Goff, M.; Morvan, S.; Thouzeau, G. The sources and distribution of carbon (DOC, POC, DIC) in a mangrove dominated estuary (French Guiana, South America). Biogeochemistry 2018, 138, 297–321. [Google Scholar] [CrossRef]
- Bouillon, S.; Dahdouh-Guebas, F.; Rao, A.V.V.S.; Koedam, N.; Dehairs, F. Sources of organic carbon in mangrove sediments: Variability and possible ecological implications. Hydrobiologia 2003, 495, 33–39. [Google Scholar] [CrossRef]
- Bouillon, S.; Moens, T.; Dehairs, F. Carbon sources supporting benthic mineralization in mangrove and adjacent seagrass sediments (Gazi Bay, Kenya). Biogeosciences 2004, 1, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Ralison, O.H.; Borges, A.V.; Dehairs, F.; Middelburg, J.J.; Bouillon, S. Carbon biogeochemistry of the Betsiboka estuary (north-western Madagascar). Org. Geochem. 2008, 39, 1649–1658. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Gao, M.; Pang, B.; Chen, S.; Ye, Y. Top-meter soil organic carbon stocks and sources in restored mangrove forests for different ages. For. Ecol. Manag. 2018, 422, 87–94. [Google Scholar] [CrossRef]
- Kida, M.; Kondo, M.; Tomotsune, M.; Kinjo, K.; Ohtsuka, T.; Fujitake, N. Molecular composition and decomposition stages of organic matter in a mangrove mineral soil with time. Estuar. Coast. Shelf Sci. 2019, 231, 106478. [Google Scholar] [CrossRef]
- Suello, R.H.; Hernandez, S.L.; Bouillon, S.; Belliard, J.-P.; Dominguez-Granda, L.; Van de Broek, M.; Moncayo, A.M.R.; Veliz, J.R.; Ramirez, K.P.; Govers, G.; et al. Mangrove sediment organic carbon storage and sources in relation to forest age and position along a deltaic salinity gradient. Biogeosciences 2022, 19, 1571–1585. [Google Scholar] [CrossRef]
- Kida, M.; Watanabe, I.; Kinjo, K.; Kondo, M.; Yoshitake, S.; Tomotsune, M.; Iimura, Y.; Umnouysin, S.; Suchewaboripont, V.; Poungparn, S.; et al. Organic carbon stock and composition in 3.5-m core mangrove soils (Trat, Thailand). Sci. Total Environ. 2021, 801, 149682. [Google Scholar] [CrossRef]
- Cheriyan, A.S.; Moushmi, K.S.; Cheriyan, E.; Baby, L.; Chandramohanakumar, N. Sources and degradation of organic matter and its relation to trophic status in the core sediments of a tropical mangrove ecosystem along the southwest coast of India. Mar. Chem. 2022, 244, 104137. [Google Scholar] [CrossRef]
- Sreelekshmi, S.; Harikrishnan, M.; Nandan, S.B.; Kaimal, V.S.; Hershey, N.R. Ecosystem carbon stock and stable isotope signatures of soil organic carbon sources across the mangrove ecosystems of Kerala, southern India. Wetlands 2022, 42, 29. [Google Scholar] [CrossRef]
- Dai, Z.; Trettin, C.C.; Mangora, M.M.; Tang, W. Soil carbon within the mangrove landscape in Rufiji River Delta, Tanzania. Wetlands 2022, 42, 89. [Google Scholar] [CrossRef]
- Krishna Prasad, M.B.; Ramanathan, A.L. Organic matter characteristics in a tropical estuarine-mangrove ecosystem of India: Preliminary assessment by using stable isotope and lignin phenols. Estuar. Coast. Shelf Sci. 2009, 84, 617–624. [Google Scholar] [CrossRef]
- Ranjan, R.K.; Routh, J.; Ramanathan, A.L.; Val Klump, J. Elemental and stable isotope records of organic matter input and its fate in the Pichavaram mangrove-estuarine sediments (Tamil Nadu, India). Mar. Chem. 2011, 126, 163–172. [Google Scholar] [CrossRef]
- Stringer, C.E.; Trettin, C.C.; Zarnoch, S.J. Soil properties of mangroves in contrasting geomorphic settings within the Zambesi River Delta, Mozambique. Wetlands Ecol. Manage. 2016, 24, 139–152. [Google Scholar] [CrossRef]
- Matsui, N.; Meepol, W.; Chukwamdee, J. Soil organic carbon in mangrove ecosystems with different vegetation and sedimentological conditions. J. Mar. Sci. Eng. 2015, 3, 1404–1424. [Google Scholar] [CrossRef] [Green Version]
- Mfilinge, P.L.; Meziane, T.; Bachok, Z.; Tsuchiya, M. Litter dynamics and particulate organic matter outwelling from a subtropical mangrove in Okinawa Island, South Japan. Estuar. Coast. Shelf Sci. 2005, 63, 301–313. [Google Scholar] [CrossRef]
- Tue, N.T.; Ngoc, N.T.; Quy, T.D.; Hamaoka, H.; Nhuan, M.T.; Omori, K. A cross-system analysis of sedimentary organic carbon in the mangrove ecosystems of Xuan Thuy National Park, Vietnam. J. Sea Res. 2012, 67, 69–76. [Google Scholar] [CrossRef]
- Iimura, Y.; Kinjo, K.; Kondo, M.; Ohtsuka, T. Soil carbon stocks and their primary origin at mature mangrove ecosystems in the estuary of Fukido River, Ishigaki Island, southwestern Japan. Soil Sci. Plant Nutr. 2019, 65, 435–443. [Google Scholar] [CrossRef]
- Costa, M.T.; Ezcurra, E.; Aburto-Oropeza, O.; Maltz, M.; Arogyaswamy, K.; Botthoff, J.; Aronson, E. Baja California mangrove deep peat microbial communities cycle nitrogen but do not affect old carbon pool. Mar. Ecol. Prog. Ser. 2022, 695, 15–31. [Google Scholar] [CrossRef]
- Ezcurra, P.; Ezcurra, E.; Garcillán, P.G.; Costa, M.T.; Aburto-Oropeza, O. Coastal landforms and accumulation of mangrove peat increase carbon sequestration and storage. Proc. Nat. Acad. Sci. USA 2016, 113, 4404–4409. [Google Scholar] [CrossRef] [Green Version]
- Thomas, N.; Lee, S.K.; Simard, M.; Ebanega, M.O.; Stoval, A.; Fatoyinbo, T.E. Mangrove carbon stocks in Pongara National Park, Gabon. Estuar. Coast. Shelf Sci. 2021, 259, 107432. [Google Scholar] [CrossRef]
- Cohen, M.C.L.; Pessenda, L.C.R.; Behling, H.; de Fátima Rossetti, D.; França, M.C.; Guimarães, J.T.F.; Friaes, Y.; Smith, C.B. Holocene paleoenvironmental history of the Amazonian mangrove belt. Quatern. Sci. Rev. 2012, 55, 50–58. [Google Scholar] [CrossRef]
- Punwong, P.; Marchant, R.; Selby, K. Holocene mangrove dynamics in Makoba Bay, Zanzibar. Paleogeogr. Paleoclim. Paleoecol. 2013, 379–380, 54–67. [Google Scholar] [CrossRef]
- Andreetta, A.; Fusi, M.; Cameldi, I.; Cimò, F.; Carnicelli, S.; Cannicci, S. Mangrove carbon sink. Do burrowing crabs contribute to sediment carbon storage? Evidence from a Kenyan mangrove system. J. Sea Res. 2014, 85, 524–533. [Google Scholar] [CrossRef] [Green Version]
- Gleeson, J.; Santos, I.R.; Maher, D.T.; Golsby-Smith, L. Groundwater-surface water exchange in a mangrove tidal creek: Evidence from natural geochemical tracers and implications for nutrient budgets. Mar. Chem. 2013, 156, 27–37. [Google Scholar] [CrossRef]
- Maher, D.T.; Santos, I.R.; Schulz, K.G.; Call, M.; Jacobsen, G.E.; Sanders, C.J. Blue carbon oxidation revealed by radiogenic and stable isotopes in a mangrove system. Geophys. Res. Lett. 2017, 44, 4889–4896. [Google Scholar] [CrossRef]
- Choi, Y.; Wang, Y. Dynamics of carbon sequestration in a coastal wetland using radiocarbon measurements. Glob. Biogeochem. Cycles 2004, 18, GB4016. [Google Scholar] [CrossRef]
- Aller, R.C.; Blair, N.E. Carbon remineralization in the Amazon–Guianas tropical mobile mudbelt: A sedimentary incinerator. Contin Shelf Res. 2006, 26, 2241–2259. [Google Scholar] [CrossRef]
- Alongi, D.M.; Wirasantosa, S.; Wagey, T.; Trott, L.A. Early diagenetic processes in relation to river discharge and coastal upwelling in the Aru Sea, Indonesia. Mar. Chem. 2012, 140, 10–23. [Google Scholar] [CrossRef]
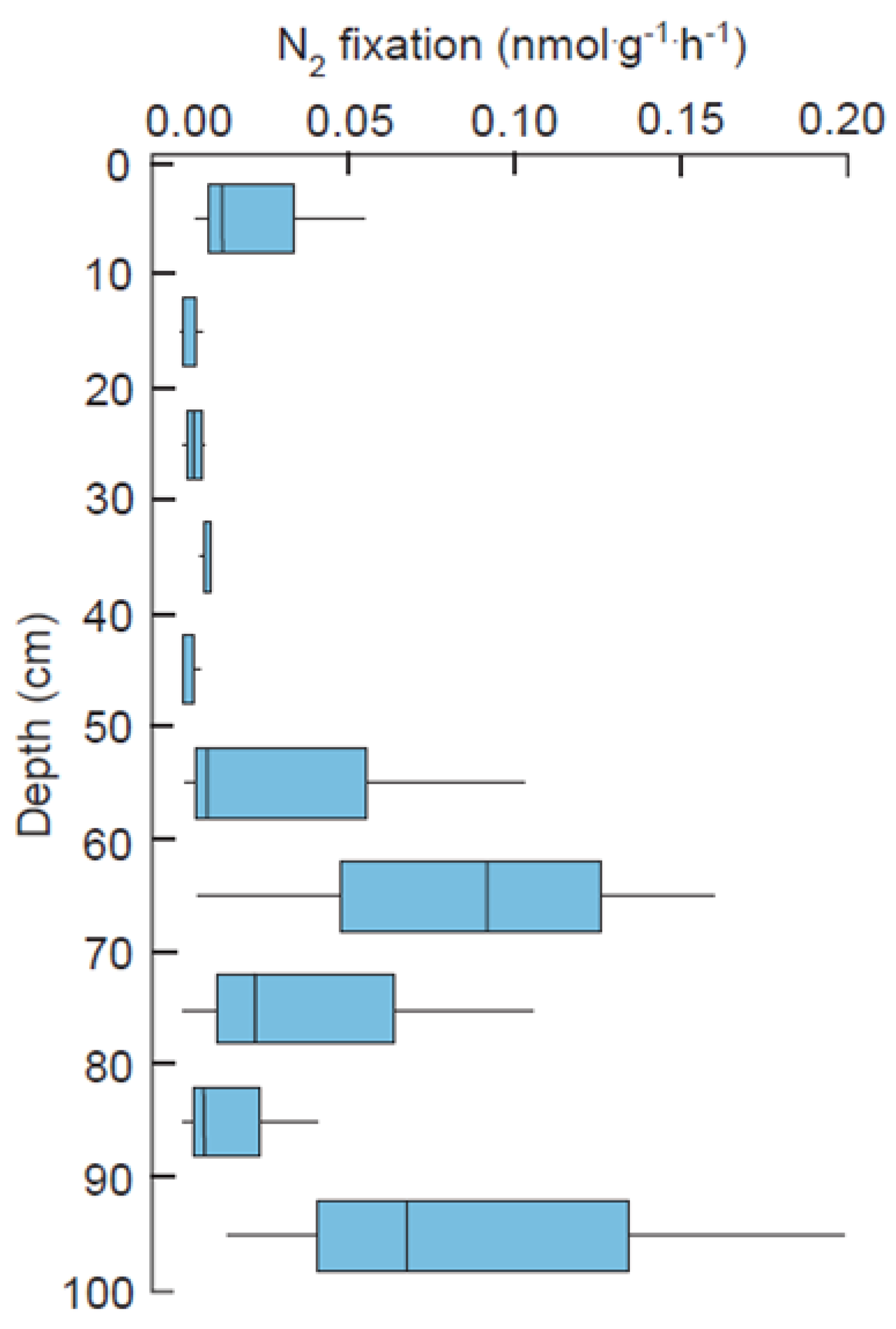
- Luo, Z.; Zhong, Q.; Han, X.; Hu, R.; Liu, X.; Xu, W.; Wu, Y.; Huang, W.; Zhou, Z.; Zhuang, W.; et al. Depth-dependent variability of biological nitrogen fixation and diazotrophic communities in mangrove sediments. Microbiome 2021, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Nie, S.; Sang, Y.; Mo, S.; Li, J.; Kashif, M.; Su, G.; Yan, B.; Jiang, C. Effects of Spartina alterniflora invasion on nitrogen fixation and phosphorus solubilization in a subtropical marine mangrove ecosystem. Microbiol. Spectr. 2022, 10, e0068221. [Google Scholar] [CrossRef] [PubMed]
- Pülmanns, N.; Diele, K.; Mehlig, U.; Nordhaus, I. Burrows of the semi-terrestrial crab Ucides cordatus enhance CO2 release in a North Brazilian mangrove forest. PLoS ONE 2014, 9, e109532. [Google Scholar] [CrossRef]
- Karniati, R.; Sulistiyono, N.; Amelia, R.; Slamet, B.; Bimantara, Y.; Basyuni, M. Mangrove ecosystem in North Sumatran (Indonesia) forests serves as a suitable habitat for mud crabs (Scylla serrata and S. olivacea). Biodiversitas 2021, 22, 1489–1496. [Google Scholar] [CrossRef]
- Luyssaert, S.; Inglima, I.; Jung, M.; Richardson, A.D.; Reichstein, M.; Papale, D.; Piao, S.L.; Schulze, E.D.; Wingate, L.; Matteucci, G.; et al. CO2 balance of boreal, temperate, and tropical forests. Glob. Chang. Biol. 2007, 13, 2509–2537. [Google Scholar] [CrossRef] [Green Version]
- Malhi, Y.; Doughty, C.; Galbraith, D. The allocation of ecosystem net primary productivity in tropical forests. Philos. Trans. R. Soc. B 2011, 366, 3225–3245. [Google Scholar] [CrossRef] [Green Version]
- Twilley, R.R.; Castañeda-Moya, E.; Rivera-Monroy, V.H.; Rovai, A. Mangrove Ecosystems: A Global Biogeographic Perspective; Rivera-Monroy, V.H., Lee, S.Y., Kristensen, E., Twilley, R.R., Eds.; Springer: Cham, Switzerland, 2017; pp. 113–162. [Google Scholar] [CrossRef]
- Fernandez-Martinez, M.; Vicca, S.; Janssens, I.A.; Sardan, J.; Luyddaert, S.; Campioli, M.; Chapin, F.S., III; Ciais, P.; Malhi, Y.; Obersteiner, M.; et al. Nutrient availability as the key regular of global forest carbon balance. Nat. Clim. Chang. 2014, 4, 471–476. [Google Scholar] [CrossRef]

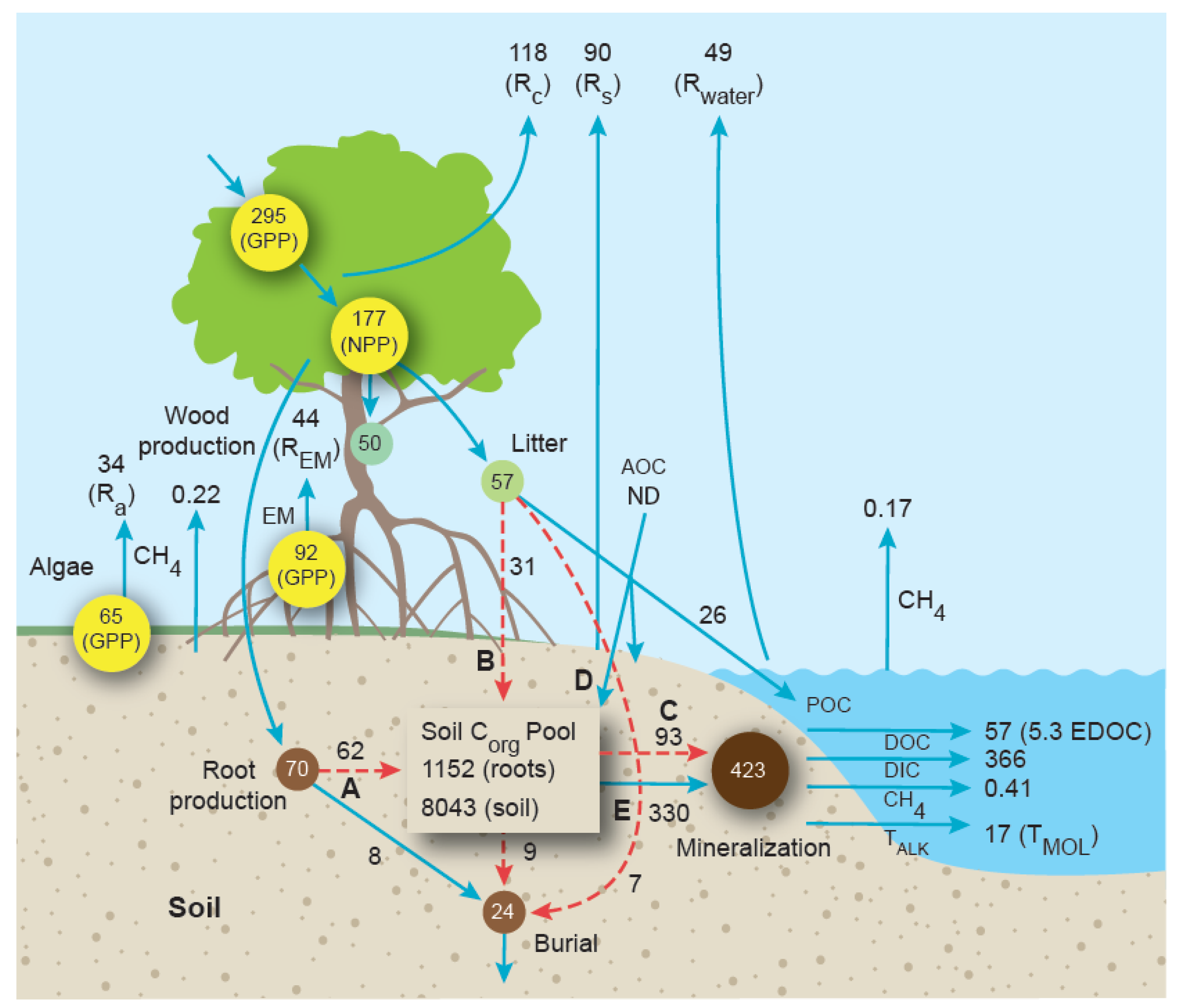

| Location | Mangrove Area (km2) | DOC | DIC | Alkalinity | Reference |
|---|---|---|---|---|---|
| Taylor R, USA | 2 | −0.46 | - | - | [24,25] |
| Mandovi–Zuari, India | 16 | 0.1 | - | - | [26,27] |
| Taylor R, USA | 2 | −67.3 | - | - | [28,29] |
| Rookery Bay, USA | 16 | 36.7 | - | - | [10] |
| Rookery Bay, USA | 180 | 44.3 | - | - | [30] |
| Itacuruca, Brazil | 38 | 13.1 | - | - | [31] |
| Coral Creek, Australia | 5 | −13 | - | - | [9,11] |
| Conn Creek, Australia | 6 | 20.9 | - | - | [32] |
| Sawi Bay, Thailand | 33 | 32 | - | - | [33] |
| Furo de Meio, Brazil | 6 | 48 | - | - | [34] |
| Celestun, Mexico | 22 | 108.1 | - | - | [35] |
| Caete Coast, Brazil | 10,000 | 138.1 | - | - | [36] |
| Shark R, SW Florida, USA | 1400 | 56 | - | - | [37] |
| Mngazana, S. Africa | 1 | 0 | - | - | [38] |
| Lobos Bay, Mexico | 5.4 | −0.4 | - | - | [39] |
| Shark R, SW Florida, USA | 1400 | 180 | - | - | [40] |
| Moreton Bay, Qld, Australia | 2 | 110 | 1095 | - | [41] |
| Watson Inlet, SE Australia | 1.8 | 109.5 | 2014.8 | 113.15 | [42] |
| Chinaman Inlet, SE Australia | 0.56 | 0 | 613.2 | 16.79 | [42] |
| Moreton Bay, Qld, Australia | 140 | 157.7 | 683.3 | 58.77 | [43] |
| Darwin Harbour, N Australia | 0.73 | - | 372.3 | 42.34 | [23] |
| Hinchinbrook Is, NE Australia | 3.6 | - | 96.4 | 7.67 | [23] |
| Seventeen Seventy, E Australia | 1.75 | - | −424.9 | 29.57 | [23] |
| Jacobs Well, E Australia | 0.4 | - | 363.5 | 4.38 | [23] |
| Newcastle, E Australia | 1.26 | - | 337.3 | 42.34 | [23] |
| Barwon Heads, SE Australia | 0.38 | - | −13.1 | −0.37 | [23] |
| Korogoro Creek, SE Australia | 2 | 5768.5 | 13,494.8 | 1300.86 | [44] |
| Shark R, SW Florida, USA | 1400 | 51.7 | 603.5 | - | [45] |
| Harney R, SW Florida, USA | 17.4 | 47.8 | 223 | - | [45] |
| Sundarbans, India | 4000 | 757.5 | 922.5 | - | [46] |
| Maowei Sea, China | 23 | 1226.4 | 2080.5 | - | [47] |
| Moreton Bay, Qld, Australia | 140 | 258.4 | 928.6 | [48] | |
| Creek 1, Airai Bay, Palau | 2 | 153.3 | 346 | 28.83 | [49] |
| Creek 2, Airai Bay, Palau | 2 | 35 | 43.8 | 16.79 | [49] |
| Kooragang Island, SE Australia | 6.3 | 865.9 | −122 | 8.39 | [50] |
| Evans Head, SE Australia | 0.08 | 8.8 | 52.56 | 4.38 | [51] |
| Gulf of Carpentaria. N. Australia | 3.43 | 635.1 | 6802.1 | 566.12 | [52] |
| Shark Bay, SW Florida, USA | 1400 | 368.2 | 1867.2 | 147.43 | [53] |
| Fukido R, SW Japan | 0.19 | 193.5 | 859.6 | - | [54] |
| Sinnamary estuary, French Guiana | 11.34 | 2971.1 | −328.5 | - | [55] |
| Zhenzhu Bay, SW China | 17.33 | 496.5 | 6101.6 | - | [56] |
| central Red Sea, Saudi Arabia | 0.5 | - | - | 147.09 | [57] |
| Shark Bay, SW Florida, USA | 1400 | 170.8 | 622 | 35.41 | [58] |
| Iriomote Is, Japan | 2 | 0.007 | 0.015 | 0.0025 | [59] |
| Caete Estuary, N Brazil | 7210 | 1.2 | 87.6 | 5.55 | [60] |
| Panay Is, Philippines | 0.39 | 75.3 | 613.2 | - | [61] |
| Coffs Creek, E Australia | 0.2 | −4 | 24.1 | 2.08 | [62] |
| Qinglan Bay, S China | 0.59 | 795.7 | 39,055 | - | [63] |
| Guayas R, Ecuador | 1121 | - | 7.7 | - | [64] |
| DOC (g C m−2 a−1) | DIC (g C m−2 a−1) | TALK (mol m−2 a−1) | |
|---|---|---|---|
| Mean | 386.6 | 2482 | 117.5 |
| ±1SE | 159 | 1274 | 62 |
| N | 41 | 32 | 22 |
| Mean (Tg C/Tmol a−1) | 56.9 | 365.9 | 17.3 |
| Location | Regression Slope | Metabolic Process | Reference |
|---|---|---|---|
| Nagada Creek, Papua New Guinea | 0.99 | sulfate reduction | [66] |
| Gaderu Creek, India | 0.61 | aerobic respiration + sulfate reduction | [66] |
| Darwin Harbour, N Australia | 0.82 | sulfate reduction + aerobic respiration | [23] |
| Hinchinbrook Island, E Australia | 0.44 | aerobic respiration = sulfate reduction | [23] |
| Seventeen Seventy, E Australia | 0.78 | sulfate reduction + aerobic respiration | [23] |
| Jacobs Well, E Australia | 0.65 | sulfate reduction + aerobic respiration | [23] |
| Barwon Heads, SE Australia | 0.85 | sulfate reduction | [23] |
| Shark, Harney R, Florida, USA | 0.82, 0.84, 0.92,0.93, 0.95 | sulfate reduction, aerobic respiration, CaCO3 dissolution | [45,53,58] |
| Creek 1, Palau, Micronesia | 0.7 | sulfate reduction + aerobic respiration | [49] |
| Creek 2, Palau, Micronesia | 1.1 | sulfate reduction + CaCO3 dissolution | [49] |
| Evans Head, E Australia | 0.68 | sulfate reduction + aerobic respiration + denitrification | [51] |
| Central Red Sea, Saudi Arabia | 0.44 | aerobic respiration + denitrification+ CaCO3 dissolution | [57] |
| Caete Estuary, N Brazil | 0.75, 0.95, 0.96 | sulfate reduction + aerobic respiration | [60] |
| Upper Coffs Harbour, SE Australia | 0.63, 0.66 | Aerobic respiration + sulfate reduction | [63] |
| Lower Coffs Harbour, SE Australia | 0.93, 1.09 | Denitrification, iron reduction | [63] |
| Location | Forest Type | C:N (Molar) and δ13C (‰) | SOC Sources | Reference |
|---|---|---|---|---|
| Ko Muk, SW Thailand | Nypa fruticans, Rhizophora apiculata, Avicennia alba | δ13C = −18‰ to −23.8‰ | 42% seagrass, 23% mangrove, 13% coastal POC | [100] |
| Ko Talibang, SW Thailand | riverine Nypa fruticans, Rhizophora apiculata, Avicennia alba | δ13C = −19.6 to −25.4‰ | 36% seagrass, 23% mangrove, 19% POC | [100] |
| Sinnamary R, French Guiana | Pioneering A. germinans | δ13C = −24‰ to −27‰ C:N = 7–19 | Amazonian riverine OC, algal OC (surface soils) | [101,102,103] |
| Sinnamary R, French Guiana | Senescent A. germinans | δ13C = −25‰ to −29‰ C:N = 30–82 | Mangrove root and lignocellulosic material | [101,102,103] |
| Godavari delta, India | A. officinalis–Excoecaria agallocha | δ13C = −20‰ to −25‰ C:N = 8–10 | Pelagic suspended matter and phytoplankton-derived matter | [104] |
| Galle and Pambala, Sri Lanka | R. apiculata, R. mucronata, A. officinalis, E. agallocha | δ13C = −27.5‰ to −30.5‰ C:N = 30–43 | Mangrove-derived material | [104] |
| Gazi Bay, Kenya |
|
|
| [105] |
| Betsiboka estuary, NW Madagascar | R. mucronata, C. tagal, B. gymnorrhiza | δ13C = −18.8‰ to −21.4‰ C:N = 9.1–11.0 | Riverine suspended matter (mostly C4 derived plant material) plus algal sources | [106] |
| Jiulongjiang estuary, China | Kandel obovata | δ13C = −23‰(12 year old stand) δ13C = −24‰(24 year old stand) δ13C = −23.3‰ to −25.9‰ (48 year old stand) | Mangrove contribution to soil OC: <20% (12 year old stand) <40% (24 year old stand) <80% (48 year old stand) | [107] |
| Okinawa, Japan | R. mucronata, B. gymnorrhiza | δ13C = 27.8‰ to −28.6‰ C:N = 27.6–33.6 | Mangrove root matter | [108] |
| Guayas delta, Ecuador | Rhizophora mangle, A. germinans | Young forest: δ13C = −26.6‰; C:N = 14.1 Old forest: δ13C = −27.0‰, C:N = 20.7 | Young forest ≈ 80% allochthonous (POC water) Old forests ≈ 60% autochthonous (vegetation) | [109] |
| Trat, Thailand | A. marina, R. apiculata-R. mucronata, X. granatum | Soil Fluorescence components | Rhizophora = 27% mangrove X. granatum = 27% mangrove | [110] |
| Iriomote Is, Japan | R. stylosa, B. gymnorrhiza, A. marina | δ13C = −28.2‰ C:N = 20.5 | 88% = mangrove + riverine POM 4% = seagrass + coral+ seaweed + benthic microalgae 8% = oceanic OM | [59] |
| Cochin estuary, SW India |
| C:N = 1.86–13.11; amino acid composition | 0–10 cm = phytoplankton + algae 20–25 cm = new bacterial biomass production Deeper soil = mostly higher land plants | [111] |
| Kerala, SW India | (Site 1) Munroe Island: E. agallocha, A. officinalis, R. mucronata (Site 2) Kayamkulam estuary: A. officinalis, A. marina, A. alba, Bruguiera cylindrica (Site 3) Vypin/Valappu Island: B. gymnnorhiza, B. cylindrica, A. officinalis, E. agallocha | δ13C = −26.81‰ (site 1), −23.09‰ (site 2), −28.47‰ (site 3) C:N = 14.34 (site 1), 7.07 (site 2), 14.98 (site 3) | Site 1 = mangrove litter Site 2 = marine phytoplankton Site 3 = mangrove litter | [112] |
| Rufiji delta, Tanzania | R. mucronata, C. tagal, B. gymnorrhiza, X. granatum, A. marina, S. alba, Heritiera littoralis | Interior mangroves: C:N = 22.6; δ13C ≈ −24‰ Riverine mangroves: C:N = 20.1; δ13C ≈ −24.6‰ Seaward mangroves: C:N = 21.2 δ13C ≈ −24.8‰ | Mangrove and upstream terrestrial plant matter sources. | [113] |
| Pichavarum, SE India | A. marina, R. apiculata | C:N = 14.2–16.6 δ13C = −24.3‰ to −27.2‰ | Mangrove and upstream terrestrial plant material | [114,115] |
| Zambesi delta, Mozambique | A. marina, B. gymnorrhiza, C. tagal, H. littoralis, Lumnitzera racemosa, S. alba, X. granatum | δ13C = −21‰ to −26‰ | Mangrove contribution = 42–58% | [116] |
| Chumphon, SE Thailand | R. apiculata, A. marina, S. alba | δ13C = −24‰ to −26‰ | Mangrove, algae and riverine POM | [117] |
| Okinawa, Japan | R. mucronata, B. gymnorrhiza | Fatty acid analysis | Green macroalgae (Ulva pertusa, Enteromorpha intestinalis), diatoms, bacteria, mangrove POM | [118] |
| Xuan Thuy National Park, Vietnam | S. caseolaris, K. obovata, A. corniculatum, A. marina | C:N = 4.5–19.5 (mean = 11.0) δ13C = −20.4‰ to −27.7‰ (mean = −24.1) | Mixture of mangrove litter and marine phytoplankton | [119] |
| Ishigaki Is, SW Japan | B. gymnorrhiza, R. stylosa | δ13C = −27.0‰ to −29.3‰ | Below-ground dead roots | [120] |
| Baja California, Mexico | R. mangle | C:N = 35–58 δ13C = −24.6‰ to −25.6‰ | Mangrove peat, algal biomass, other coastal plants | [121] |
| Inputs | Outputs | ||
|---|---|---|---|
| Mangrove GPP | 20.0 | RCANOPY | 8.0 |
| Macroalgal GPP | 6.2 | RMACROALGAE | 2.9 |
| Microalgal GPP | 4.4 | RMICROALGAE | 2.3 |
| Soil DIC production | 22.4 | RTIDAL WATER | 3.3 |
| Groundwater (land-derived) | ND | RSOIL | 6.1 |
| DIC export | 24.8 | ||
| DOC export | 3.8 | ||
| POC export | 1.9 | ||
| Burial | 1.6 | ||
| CH4 export | 0.03 | ||
| CH4 (air–soil release) | 0.015 | ||
| CH4 (air–water efflux) | 0.012 | ||
| Total | 53.0 | Total | 54.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alongi, D.M. Lateral Export and Sources of Subsurface Dissolved Carbon and Alkalinity in Mangroves: Revising the Blue Carbon Budget. J. Mar. Sci. Eng. 2022, 10, 1916. https://doi.org/10.3390/jmse10121916
Alongi DM. Lateral Export and Sources of Subsurface Dissolved Carbon and Alkalinity in Mangroves: Revising the Blue Carbon Budget. Journal of Marine Science and Engineering. 2022; 10(12):1916. https://doi.org/10.3390/jmse10121916
Chicago/Turabian StyleAlongi, Daniel M. 2022. "Lateral Export and Sources of Subsurface Dissolved Carbon and Alkalinity in Mangroves: Revising the Blue Carbon Budget" Journal of Marine Science and Engineering 10, no. 12: 1916. https://doi.org/10.3390/jmse10121916






