Initiating a DNA Barcoding Reference Library of Stony Corals from the Gulf of Eilat (Red Sea)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Traditional Taxonomy
2.3. DNA Extraction, PCR Amplification and Sequencing
2.4. Data Analysis
3. Results
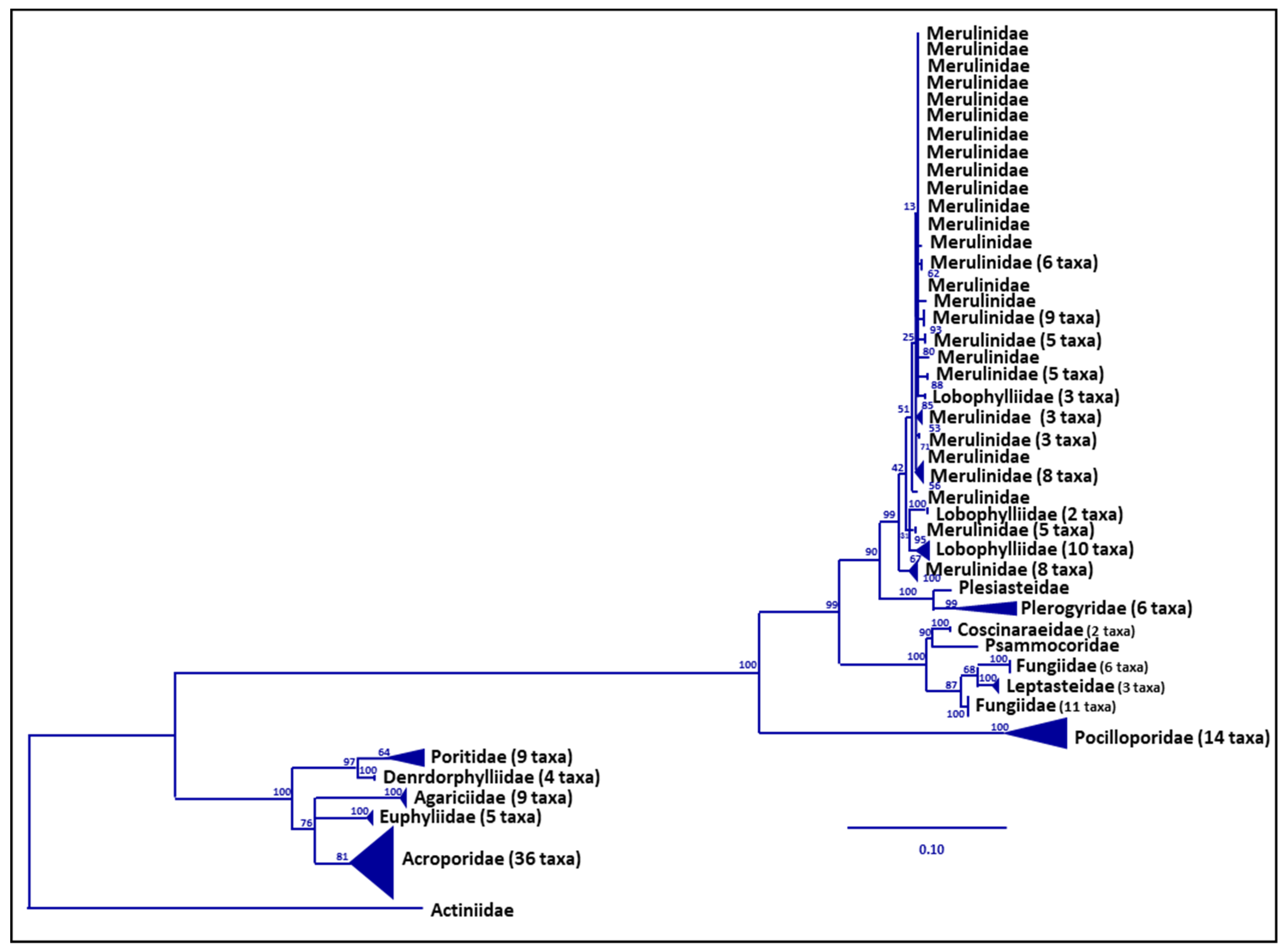
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dibattista, J.D.; Roberts, M.B.; Bouwmeester, J.; Bowen, B.W.; Coker, D.J.; Lozano-Cortés, D.F.; Howard Choat, J.; Gaither, M.R.; Hobbs, J.P.A.; Khalil, M.T.; et al. A Review of Contemporary Patterns of Endemism for Shallow Water Reef Fauna in the Red Sea. J. Biogeogr. 2016, 43, 423–439. [Google Scholar] [CrossRef] [Green Version]
- Richards, Z.T. A Comparison of Proxy Performance in Coral Biodiversity Monitoring. Coral Reefs 2012, 32, 287–292. [Google Scholar] [CrossRef]
- Guerrini, G.; Yerushalmy, M.; Shefy, D.; Shashar, N.; Rinkevich, B. Apparent Recruitment Failure for the Vast Majority of Coral Species at Eilat, Red Sea. Coral Reefs 2020, 39, 1715–1726. [Google Scholar] [CrossRef]
- Fisher, R.; O’Leary, R.A.; Low-Choy, S.; Mengersen, K.; Knowlton, N.; Brainard, R.E.; Caley, M.J. Species Richness on Coral Reefs and the Pursuit of Convergent Global Estimates. Curr. Biol. 2015, 25, 500–505. [Google Scholar] [CrossRef] [Green Version]
- Kusumoto, B.; Costello, M.J.; Kubota, Y.; Shiono, T.; Wei, C.L.; Yasuhara, M.; Chao, A. Global Distribution of Coral Diversity: Biodiversity Knowledge Gradients Related to Spatial Resolution. Ecol. Res. 2020, 35, 315–326. [Google Scholar] [CrossRef]
- Veron, J.E.N. Overview of the Taxonomy of Zooxanthellate Scleractinia. Zool. J. Linn. Soc. 2013, 169, 485–508. [Google Scholar] [CrossRef] [Green Version]
- Muir, P.R.; Pichon, M. Biodiversity of Reef-Building, Scleractinian Corals. In Mesophotic Coral, Ecosystems; Loya, Y., Puglise, K.A., Bridge, T.C.L., Eds.; Springer: Cham, Switzerland, 2019; pp. 589–620. ISBN 978-3-319-92735-0. [Google Scholar]
- Huang, D.; Meier, R.; Todd, P.A.; Chou, L.M. More Evidence for Pervasive Paraphyly in Scleractinian Corals: Systematic Study of Southeast Asian Faviidae (Cnidaria; Scleractinia) Based on Molecular and Morphological Data. Mol. Phylogenet. Evol. 2009, 50, 102–116. [Google Scholar] [CrossRef]
- Huang, D.; Licuanan, W.Y.; Baird, A.H.; Fukami, H. Cleaning up the “Bigmessidae”: Molecular Phylogeny of Scleractinian Corals from Faviidae, Merulinidae, Pectiniidae and Trachyphylliidae. BMC Evol. Biol. 2011, 11, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, D.; Benzoni, F.; Arrigoni, R.; Baird, A.H.; Berumen, M.L.; Bouwmeester, J.; Chou, L.M.; Fukami, H.; Licuanan, W.Y.; Lovell, E.R.; et al. Towards a Phylogenetic Classification of Reef Corals: The Indo-Pacific Genera Merulina, Goniastrea and Scapophyllia (Scleractinia, Merulinidae). Zool. Scr. 2014, 43, 531–548. [Google Scholar] [CrossRef]
- Huang, D.; Benzoni, F.; Fukami, H.; Knowlton, N.; Smith, N.D.; Budd, A.F. Taxonomic Classification of the Reef Coral Families Merulinidae, Montastraeidae, and Diploastraeidae (Cnidaria: Anthozoa: Scleractinia). Zool. J. Linn. Soc. 2014, 171, 277–355. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological Identifications through DNA Barcodes. Proc. R. Soc. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neigel, J.; Domingo, A.; Stake, J. DNA Barcoding as a Tool for Coral Reef Conservation. Coral Reefs 2007, 26, 487–499. [Google Scholar] [CrossRef]
- Ward, R.D.; Zemlak, T.S.; Innes, B.H.; Last, P.R.; Hebert, P.D.N. DNA Barcoding Australia’s Fish Species. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2005, 360, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, M.V.; Fukami, H.; Benzoni, F.; Huang, D. The New Systematics of Scleractinia: Integrating Molecular and Morphological Evidence. In The Cnidaria, Past, Present and Future; Pörtner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., et al., Eds.; Springer: Cambridge, UK; New York, NY, USA, 2016; pp. 41–59. ISBN 978-1-00-915796-4. [Google Scholar]
- Todd, P.A. Morphological Plasticity in Scleractinian Corals. Biol. Rev. 2008, 83, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Berumen, M.L.; Arrigoni, R.; Bouwmeester, J.; Terraneo, T.I.; Benzoni, F. Corals of the Red Sea—Coral Reefs of the RedSea. In Coral Reefs of the World; Voolstra, C.R., Berumen, M.L., Eds.; Springer International Publishing: Cham, Switzerland, 2019; Volume 11, pp. 123–155. ISBN 978-3-030-05802-9. [Google Scholar]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of Climate Change on the Future of Biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bindoff, N.L.; Cheung, W.W.L.; Kairo, J.G.; Arístegui, J.; Guinder, V.A.; Hallberg, R.; Hilmi, N.J.M.; Jiao, N.; Karim, M.S.; Levin, L.; et al. Changing Ocean, Marine Ecosystems, and Dependent Communities. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., et al., Eds.; Intergovernmental Panel on Climate Change: Geneve, Switzerland, 2019; pp. 477–587. [Google Scholar]
- Hebert, P.D.N.; Ratnasingham, S.; DeWaard, J.R. Barcoding Animal Life: Cytochrome c Oxidase Subunit 1 Divergences among Closely Related Species. Proc. R. Soc. B Biol. Sci. 2003, 270, 96–99. [Google Scholar] [CrossRef] [Green Version]
- Taylor, H.R.; Harris, W.E. An Emergent Science on the Brink of Irrelevance: A Review of the Past 8years of DNA Barcoding. Mol. Ecol. Resour. 2012, 12, 377–388. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The Barcode of Life Data System: Barcoding. Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Moura, C.J.; Lessios, H.; Cortés, J.; Nizinski, M.S.; Reed, J.; Santos, R.S.; Collins, A.G. Hundreds of Genetic Barcodes of the Species-Rich Hydroid Superfamily Plumularioidea (Cnidaria, Medusozoa) Provide a Guide toward More Reliable Taxonomy. Sci. Rep. 2018, 8, 17986. [Google Scholar] [CrossRef]
- Weigand, H.; Beermann, A.J.; Čiampor, F.; Costa, F.O.; Csabai, Z.; Duarte, S.; Geiger, M.F.; Grabowski, M.; Rimet, F.; Rulik, B.; et al. DNA Barcode Reference Libraries for the Monitoring of Aquatic Biota in Europe: Gap-Analysis and Recommendations for Future Work. Sci. Total Environ. 2019, 678, 499–524. [Google Scholar] [CrossRef]
- Paz, G.; Rinkevich, B. Gap Analysis of DNA Barcoding in ERMS Reference Libraries for Ascidians and Cnidarians. Environ. Sci. Eur. 2021, 33, 1–8. [Google Scholar] [CrossRef]
- Fukami, H.; Budd, A.F.; Paulay, G.; Solé-Cava, A.; Chen, C.A.; Iwao, K.; Knowlton, N. Conventional Taxonomy Obscures Deep Divergence between Pacific and Atlantic Corals. Nature 2004, 427, 832–835. [Google Scholar] [CrossRef] [PubMed]
- Fukami, H.; Chen, C.A.; Budd, A.F.; Collins, A.G.; Wallace, C.C.; Chuang, Y.Y.; Chen, C.; Dai, C.F.; Iwao, K.; Sheppard, C.; et al. Mitochondrial and Nuclear Genes Suggest That Stony Corals Are Monophyletic but Most Families of Stony Corals Are Not (Order Scleractinia, Class Anthozoa, Phylum Cnidaria). PLoS ONE 2008, 3, e3222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitahara, M.V.; Cairns, S.D.; Stolarski, J.; Blair, D.; Miller, D.J. A Comprehensive Phylogenetic Analysis of the Scleractinia (Cnidaria, Anthozoa) Based on Mitochondrial CO1 Sequence Data. PLoS ONE 2010, 5, e11490. [Google Scholar] [CrossRef]
- Arrigoni, R.; Stefani, F.; Pichon, M.; Galli, P.; Benzoni, F. Molecular Phylogeny of the Robust Clade (Faviidae, Mussidae, Merulinidae, and Pectiniidae): An Indian Ocean Perspective. Mol. Phylogenet. Evol. 2012, 65, 183–193. [Google Scholar] [CrossRef]
- Arrigoni, R.; Terraneo, T.I.; Galli, P.; Benzoni, F. Lobophylliidae (Cnidaria, Scleractinia) Reshuffled: Pervasive Non-Monophyly at Genus Level. Mol. Phylogenet. Evol. 2014, 73, 60–64. [Google Scholar] [CrossRef]
- Benzoni, F.; Arrigoni, R.; Stefani, F.; Stolarski, J. Systematics of the Coral Genus Craterastrea (Cnidaria, Anthozoa, Scleractinia) and Description of a New Family through Combined Morphological and Molecular Analyses. Syst. Biodivers. 2012, 10, 417–433. [Google Scholar] [CrossRef]
- Benzoni, F.; Arrigoni, R.; Stefani, F.; Reijnen, B.T.; Montano, S.; Hoeksema, B.W. Phylogenetic Position and Taxonomy of Cycloseris Explanulata and C. Wellsi (Scleractinia: Fungiidae): Lost Mushroom Corals Find Their Way Home. Contrib. Zool. 2012, 81, 125–146. [Google Scholar] [CrossRef] [Green Version]
- Keshavmurthy, S.; Yang, S.Y.; Alamaru, A.; Chuang, Y.Y.; Pichon, M.; Obura, D.; Fontana, S.; De Palmas, S.; Stefani, F.; Benzoni, F.; et al. DNA Barcoding Reveals the Coral “Laboratory-Rat”, Stylophora Pistillata Encompasses Multiple Identities. Sci. Rep. 2013, 3, 1520. [Google Scholar] [CrossRef] [Green Version]
- Budd, A.F.; Romano, S.L.; Smith, N.D.; Barbeitos, M.S. Rethinking the Phylogeny of Scleractinian Corals: A Review of Morphological and Molecular Data. Integr. Comp. Biol. 2010, 50, 411–427. [Google Scholar] [CrossRef]
- Budd, A.F.; Fukami, H.; Smith, N.D.; Knowlton, N. Taxonomic Classification of the Reef Coral Family Mussidae (Cnidaria: Anthozoa: Scleractinia). Zool. J. Linn. Soc. 2012, 166, 465–529. [Google Scholar] [CrossRef] [Green Version]
- Quek, R.Z.B.; Jain, S.S.; Neo, M.L.; Rouse, G.W.; Huang, D. Transcriptome-Based Target-Enrichment Baits for Stony Corals (Cnidaria: Anthozoa: Scleractinia). Mol. Ecol. Resour. 2020, 20, 807–818. [Google Scholar] [CrossRef] [Green Version]
- Shlesinger, T. Recruitment and Mortality of Corals in the Coral Reefs of Eilat. Master’s Thesis, Tel-Aviv University, Tel Aviv, Israel, 2014; p. 77. [Google Scholar]
- Loya, Y.; Slobodkin, L.B. The Coral Reefs of Eilat (Gulf of Eilat, Red Sea). Symp Zool SocLond 1971, 28, 117–139. [Google Scholar]
- Benayahu, Y.; Loya, Y. Space Partitioning by Stony Corals Soft Corals and Benthic Algae on the Coral Reefs of the Northern Gulf of Eilat (Red Sea). Helgoländer Wiss. Meeresunters. 1977, 30, 362–382. [Google Scholar] [CrossRef]
- Loya, Y. Community Structure and Species Diversity of Hermatypic Corals at Eilat, Red Sea. Mar. Biol. 1972, 13, 100–123. [Google Scholar] [CrossRef]
- Scheer, G. Coral Reefs and Coral Genera in the Red Sea and Indian Ocean. Symp Zool Soc Lond 1971, 28, 329–367. [Google Scholar]
- Wijsman Best, M. Indo-Pacific Coral Species Belonging to the Subfamily Montastreinae Vaughan and Wells, 1943 (Scleractinea-Coelenterata) Part I. the Genera Montastrea and Plesiastrea. Zool. Meded. 1977, 52, 81–97. [Google Scholar]
- Wijsman Best, M. Indo-Pacific Coral Species Belonging to the Subfamily Montastreinae Vaughan & Wells, 1943 (Scleractinea-Coelenterata) Part II. the Genera Cyphastrea, Leptastrea, Echinopora and Diploastrea. Zool. Meded. 1980, 55, 235–263. [Google Scholar]
- Head, S.M. An Undescribed Species of Merulina and a New Genus and Species of Siderastreid Coral from the Red Sea. J. Nat. Hist. 1983, 17, 419–435. [Google Scholar] [CrossRef]
- Head, S.M. A Cerioid Species of Blastomussa (Cnidaria, Scleractinia) from the Central Red Sea, with a Revision of the Genus. J. Nat. Hist. 1978, 12, 633–639. [Google Scholar] [CrossRef]
- Wallace, C.C. Staghorn Corals of the Worlds: A Revision of the Genus Acropora, Worldwide, with Emphasis on Morphology, Phylogeny and Biogeography; CSIRO Publishing: Collingwood, Australia, 1999. [Google Scholar]
- Veron, J.E.N. Corals of the World; Australian Institute of Marine Sciences and CRR Qld Pty Ltd.: Townsville, Australia, 2000. [Google Scholar]
- Scheer, G.; Pillai, C.S.G. Report on the Stony Corals from the Red Sea. Zoologica 1983, 45, 1–198. [Google Scholar]
- Mergner, H.; Schuhmacher, H. Morphology, Ecology and Zonation of Coral Reefs at Aqaba (Gulf of Aqaba, Red Sea). Helgoländer Wiss. Meeresunters. 1974, 26, 238–358. [Google Scholar] [CrossRef] [Green Version]
- Mergner, H.; Schuhmacher, H. Quantitative Analyse Der Korallenbesiedlung Eines Vorriffareals Bei Aqaba (Rotes Meer). Helgoländer Meeresunters. 1981, 34, 337–354. [Google Scholar] [CrossRef] [Green Version]
- Schuhmacher, H.; Mergner, H. Quantitative Analysis of Coral Communities of Sanganeb-Atoll (Central Red Sea). II. Comparison with a Reef Area near Aqaba (Northern Red Sea) at the Northern Margin of the Indopacific Reef-Belt. Helgoländer Meeresunters. 1985, 39, 419–440. [Google Scholar] [CrossRef] [Green Version]
- Ammar, M.S.A. Coral Diversity Indices along the Gulf of Aqaba and Ras Mohammed, Red Sea, Egypt. Biodiversitas J. Biol. Divers. 2011, 12, 92–98. [Google Scholar] [CrossRef]
- Antonius, A.; Scheer, G.; Bouchon, C. Corals of the Eastern Red Sea. Atoll Res. Bull. 1990, 330–338, 1–22. [Google Scholar] [CrossRef]
- Devantier, L.; Turak, E.; Al-Shaikh, K.; De’ath, G. Coral Communities of the Central-Northern Saudi Arabian Red Sea. Fauna Arab. 2000, 18, 23–66. [Google Scholar]
- Veron, J.E.N.; Devantier, L.M.; Turak, E.; Green, A.L.; Kininmonth, S.; Stafford-Smith, M.; Peterson, N. Delineating the Coral Triangle. Galaxea J. Coral Reef Stud. 2009, 11, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Epstein, N.; Vermeij, M.J.A.; Bak, R.P.M.; Rinkevich, B. Alleviating Impacts of Anthropogenic Activities by Traditional Conservation Measures: Can a Small Reef Reserve Be Sustainedly Managed? Biol. Conserv. 2005, 121, 243–255. [Google Scholar] [CrossRef]
- Rinkevich, B. Biodiversity and Active Conservation of Eilat Reef: Past and Future Considerations. In Aqaba-Eilat, the Improbable Gulf: Environment, Biodiversity and Preservation; Por, F.D., Ed.; Hebrew University Magnes Press: Jerusalem, Israel, 2008; pp. 299–319. ISBN 978-965-493-380-3. [Google Scholar]
- Epstein, N.; Bak, R.P.M.; Rinkevich, B. Implementation of a Small-Scale “no-Use Zone” Policy in a Reef Ecosystem: Eilat’s Reef-Lagoon Six Years Later. Coral Reefs 1999, 18, 327–332. [Google Scholar] [CrossRef]
- Rinkevich, B. What Do We Know about Eilat (Red Sea) Reef Degradation? A Critical Examination of the Published Literature. J. Exp. Mar. Biol. Ecol. 2005, 327, 183–200. [Google Scholar] [CrossRef]
- WoRMS Editorial board World Register of Marine Species (WoRMS). Available online: http://www.marinespecies.org (accessed on 15 September 2022).
- Veron, J.E.N.; Stafford-Smith, M.G.; Turak, E.; DeVantier, L.M. Corals of the World. Available online: http://www.coralsoftheworld.org/page/home/ (accessed on 15 September 2022).
- Graham, D.E. The Isolation of High Molecular Weight DNA from Whole Organisms or Large Tissue Masses. Anal. Biochem. 1978, 85, 609–613. [Google Scholar] [CrossRef]
- Douek, J.; Barki, Y.; Gateño, D.; Rinkevich, B. Possible Cryptic Speciation within the Sea Anemone Actinia Equina Complex Detected by AFLP Markers. Zool. J. Linn. Soc. 2002, 136, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA Primers for Amplification of Mitochondrial Cytochrome c Oxidase Subunit I from Diverse Metazoan Invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Arrigoni, R.; Berumen, M.L.; Huang, D.; Terraneo, T.I.; Benzoni, F. Cyphastrea (Cnidaria: Scleractinia:Merulinidae) in the Red Sea: Phylogeny and a New Reef Coral Species. Invertebr. Syst. 2017, 31, 141–156. [Google Scholar] [CrossRef]
- Kleemann, K.; Baal, C. Note on the Coral Blastomussa Loyae, a Valid Species from the Red Sea. J. Mar. Biol. Assoc. U. K. 2012, 92, 699–701. [Google Scholar] [CrossRef]
- Mergner, H. The Ecology Research on Coral Reef of the Red Sea. Deep Sea Res. Part Oceanogr. Res. Pap. 1984, 31, 855–884. [Google Scholar] [CrossRef]
- Arrigoni, R.; Berumen, M.L.; Terraneo, T.I.; Caragnano, A.; Bouwmeester, J.; Benzoni, F. Forgotten in the Taxonomic Literature: Resurrection of the Scleractinian Coral Genus Sclerophyllia (Scleractinia, Lobophylliidae) from the Arabian Peninsula and Its Phylogenetic Relationships. Syst. Biodivers. 2015, 13, 140–163. [Google Scholar] [CrossRef]
- Arrigoni, R.; Berumen, M.L.; Chen, C.A.; Terraneo, T.I.; Baird, A.H.; Payri, C.; Benzoni, F. Species Delimitation in the Reef Coral Genera Echinophyllia and Oxypora (Scleractinia, Lobophylliidae) with a Description of Two New Species. Mol. Phylogenet. Evol. 2016, 105, 146–159. [Google Scholar] [CrossRef]
- Benzoni, F.; Stefani, F.; Pichon, M.; Galli, P. The Name Game: Morpho-Molecular Species Boundaries in the Genus Psammocora (Cnidaria, Scleractinia). Zool. J. Linn. Soc. 2010, 160, 421–456. [Google Scholar] [CrossRef] [Green Version]
- Romano, S.L.; Palumbi, S.R. Evolution of Scleractinian Corals Inferred from Molecular Systematics. Science 1996, 271, 640–642. [Google Scholar] [CrossRef]
- Rogers, C.S. Coral Reef Resilience through Biodiversity. ISRN Oceanogr. 2013, 2013, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Kleypas, J.; Allemand, D.; Anthony, K.; Baker, A.C.; Beck, M.W.; Hale, L.Z.; Hilmi, N.; Hoegh-Guldberg, O.; Hughes, T.; Kaufman, L.; et al. Designing a Blueprint for Coral Reef Survival. Biol. Conserv. 2021, 257, 109107. [Google Scholar] [CrossRef]
- Vardi, T.; Hoot, W.C.; Levy, J.; Shaver, E.; Winters, R.S.; Banaszak, A.T.; Baums, I.B.; Chamberland, V.F.; Cook, N.; Gulko, D.; et al. Six Priorities to Advance the Science and Practice of Coral Reef Restoration Worldwide. Restor. Ecol. 2021, 29, e13498. [Google Scholar] [CrossRef]
- Hatcher, B.G. Coral Reef Ecosystems: How Much Greater Is the Whole than the Sum of the Parts? Coral Reefs 1997, 16, 77–91. [Google Scholar] [CrossRef]
- Roberts, C.M.; McClean, C.J.; Veron, J.E.N.; Hawkins, J.P.; Allen, G.R.; McAllister, D.E.; Mittermeier, C.G.; Schueler, F.W.; Spalding, M.; Wells, F.; et al. Marine Biodiversity Hotspots and Conservation Priorities for Tropical Reefs. Science 2002, 295, 1280–1284. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Hughes, T.P.; Folke, C.; Nyström, M. Confronting the Coral Reef Crisis. Nature 2004, 429, 827–833. [Google Scholar] [CrossRef]
- Folke, C.; Carpenter, S.; Walker, B.; Scheffer, M.; Elmqvist, T.; Gunderson, L.; Holling, C.S. Regime Shifts, Resilience, and Biodiversity in Ecosystem Management. Source Annu. Rev. Ecol. Evol. Syst. 2004, 35, 557–581. [Google Scholar] [CrossRef] [Green Version]
- Ayre, D.J.; Hughes, T.P. Climate Change, Genotypic Diversity and Gene Flow in Reef-Building Corals. Ecol. Lett. 2004, 7, 273–278. [Google Scholar] [CrossRef]
- Van Oppen, M.J.H.; Gates, R.D. Conservation Genetics and the Resilience of Reef-Building Corals. Mol. Ecol. 2006, 15, 3863–3883. [Google Scholar] [CrossRef]
- Rinkevich, B. Augmenting Coral Adaptation to Climate Change via Coral Gardening (the Nursery Phase). J. Environ. Manage. 2021, 291, 112727. [Google Scholar] [CrossRef]
- Casiraghi, M.; Labra, M.; Ferri, E.; Galimberti, A.; de Mattia, F. DNA Barcoding: A Six-Question Tour to Improve Users’ Awareness about the Method. Brief. Bioinform. 2010, 11, 440–453. [Google Scholar] [CrossRef]
- Krishna, K.P.; Francis, R.A. A Critical Review on the Utility of DNA Barcoding in Biodiversity Conservation. Biodivers. Conserv. 2012, 21, 1901–1919. [Google Scholar] [CrossRef]
- Shearer, T.L.; Coffroth, M.A. Genetic Identification of Caribbean Scleractinian Coral Recruits at the Flower Garden Banks and the Florida Keys. Mar. Ecol. Prog. Ser. 2006, 306, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Pawlowski, J.; Kelly-Quinn, M.; Altermatt, F.; Apothéloz-Perret-Gentil, L.; Beja, P.; Boggero, A.; Borja, A.; Bouchez, A.; Cordier, T.; Domaizon, I.; et al. The Future of Biotic Indices in the Ecogenomic Era: Integrating (e)DNA Metabarcoding in Biological Assessment of Aquatic Ecosystems. Sci. Total Environ. 2018, 637–638, 1295–1310. [Google Scholar] [CrossRef]
- Rinkevich, B. Management of Coral Reefs: We Have Gone Wrong When Neglecting Active Reef Restoration. Mar. Pollut. Bull. 2008, 56, 1821–1824. [Google Scholar] [CrossRef]
- Wielgus, J.; Glassom, D.; Fishelson, L. Long-Term Persistence of Low Coral Cover and Abundance on a Disturbed Coral Reef Flat in the Northern Red Sea. J. Exp. Mar. Biol. Ecol. 2003, 297, 31–41. [Google Scholar] [CrossRef]
- Huang, D.; Meier, R.; Todd, P.A.; Chou, L.M. Slow Mitochondrial COI Sequence Evolution at the Base of the Metazoan Tree and Its Implications for DNA Barcoding. J. Mol. Evol. 2008, 66, 167–174. [Google Scholar] [CrossRef]
- Shearer, T.L.; Coffroth, M.A. Barcoding Corals: Limited by Interspecific Divergence, Not Intraspecific Variation. Mol. Ecol. Resour. 2008, 8, 247–255. [Google Scholar] [CrossRef]
- Radulovici, A.E.; Archambault, P.; Dufresne, F. DNA Barcodes for Marine Biodiversity: Moving Fast Forward? Diversity 2010, 2, 450–472. [Google Scholar] [CrossRef] [Green Version]
- Shearer, T.L.; van Oppen, M.J.H.; Romano, S.L.; Wörheide, G. Slow Mitochondrial DNA Sequence Evolution in the Anthozoa (Cnidaria). Mol. Ecol. 2002, 11, 2475–2487. [Google Scholar] [CrossRef] [PubMed]
- Bucklin, A.; Steinke, D.; Blanco-Bercial, L. DNA Barcoding of Marine Metazoa. Annu. Rev. Mar. Sci. 2011, 3, 471–508. [Google Scholar] [CrossRef] [PubMed]
- Van Oppen, M.J.H.; Koolmees, E.M.; Veron, J.E.N. Patterns of Evolution in the Scleractinian Coral Genus Montipora (Acroporidae). Mar. Biol. 2004, 144, 9–18. [Google Scholar] [CrossRef]
- Van Oppen, M.J.H.; Willis, B.L.; van Vugt, H.W.J.A.; Miller, D.J. Examination of Species Boundaries in the Acropora Cervicornis Group (Scleractinia, Cnidaria) Using Nuclear DNA Sequence Analyses. Mol. Ecol. 2000, 9, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Van Oppen, M.J.H.; Mcdonald, B.J.; Willis, B.; Miller, D.J. The Evolutionary History of the Coral Genus Acropora (Scleractinia, Cnidaria) Based on a Mitochondrial and a Nuclear Marker: Reticulation, Incomplete Lineage Sorting, or Morphological Convergence? Mol. Biol. Evol. 2001, 18, 1315–1329. [Google Scholar] [CrossRef] [PubMed]

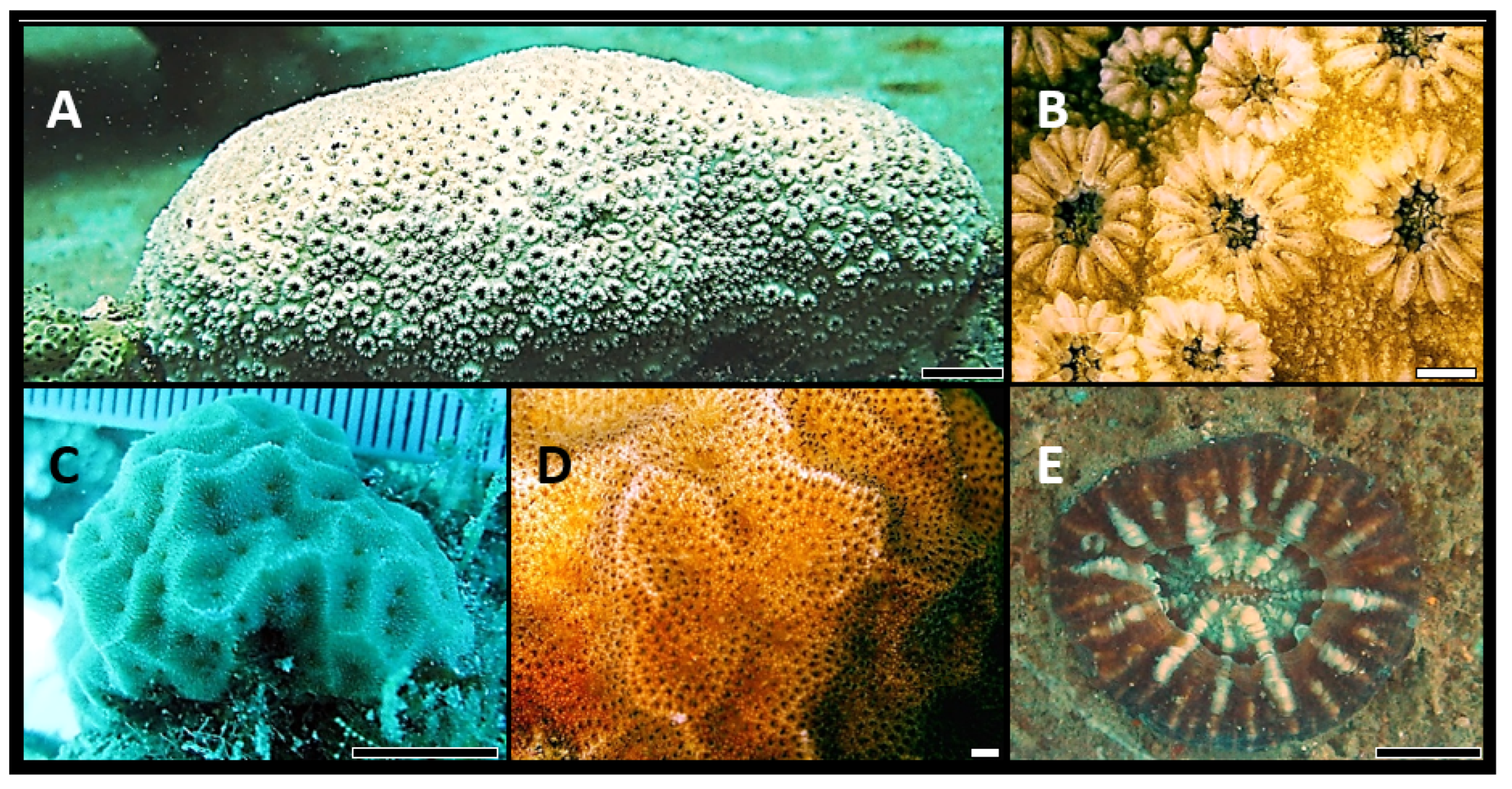
| Family/Genus | No of Vouchers | Subgroups | Species Details | Remarks | |
|---|---|---|---|---|---|
| No. | Types | ||||
| Acroporidae | 36 | 3 | |||
| Acropora | 21 | 11 | 5 a,3 b,4 c | A. plantaginea 4 b, A. squarrosa 3 b, A. tenuis 4 a + 1 b, A. cytherea 1 a, A. humilis 1 a, A. samoensis 1 a, A. valida 1 a, A. sp. 1–4: 1 c 1 c 1 c 2 c | COI N.I. |
| Alveopora | 4 | 3 | 3 a | A. daedalea 1 a, A. fenestrata 2 a, A. verrilliana 1 a | |
| Montipora | 11 | 7 | 3 a | M. cryptus 1 a, M. efflorescens 1 a, M. hemispherica 1 a, M. informis 2 a, M. maeandrina 3 a, M. tuberculosa 2 a, M. verrucosa 1 a | COI N.I. |
| Agariciidae | 9 | 2 | |||
| Leptoseris | 1 | 1 | 1 a | L. yabei 1 a | |
| Pavona | 8 | 4 | 3 a,1 c | P. danai 1 a, P. diffluens 3 a, P. varians 3 a, P. sp. 1 c | COI N.I. |
| Coscinaraeidae | 2 | 1 | |||
| Coscinaraea | 2 | 1 | 1 a | C. monile 2 a | |
| Dendrophylliidae | 4 | 1 | |||
| Turbinaria | 4 | 1 | 1 a | T. reniformis 4 a | |
| Euphylliidae | 5 | 1 | |||
| Galaxea | 5 | 1 | 1 a | G. fascicularis 5 a | |
| Fungiidae | 17 | 3 or 4 | Fungiidae #1–4: 1 1 1 1 | 4 vouchers are too small and young without adequate morphological characteristics and COI N.I. to assign a genus or a species | |
| Cycloseris | 10 | 3 | 3 a | C. cyclolites 3 a, C. fragilis 1 a, C. vaughani 6 a | COI N.I. |
| Danafungia | 2 | 2 | 2 a | D. horrida 1 a, D. scruposa 1 a | COI N.I. |
| Fungia | 1 | 1 | 1 a | F. fungites 1 a | COI N.I. |
| Leptastreidae | 3 | 1 | |||
| Leptastrea | 3 | 3 | 3 a | L. inaequalis 1 a, L. purpurea 1 a, L. transversa 1 a | |
| Lobophylliidae | 15 | 5 | |||
| Acanthastrea | 2 | 1 | 1 a | A. brevis 2 a | |
| Echinophyllia | 3 | 2 | 1 a,1 c | E. aspera 2 a, E. sp. 1 c | |
| Lobophyllia | 5 | 2 | 2 a | L. corymbosa 4 a, L. hemprichii 1 a | |
| Oxypora | 3 | 2 | 1 a,2 b | O. crassispinosa 1 b, O. lacera 1 a + 1 b | |
| Sclerophyllia | 2 | 1 | 1 a | S. margariticola 2 a | |
| Merulinidae | 69 | 12 | COI is partly informative in this group | ||
| Astraeosmilia | 1 | 1 | 1 a | A. maxima 1 a | |
| Coelastrea | 1 | 1 | 1 a | C. aspera 1 a | |
| Cyphastrea | 5 | 3 | 3 a | C. magna 1 a, C. microphthalma 2 a, C. serailia 2 a | |
| Dipsastraea | 27 | 9 | 7 a,2 b,1 c | D. amicorum 4 a, D. matthaii 2 b, D. speciosa 2 a + 1 b, D. danai 7 a, D. faviaformis 5 a, D. lacuna 1 a, D. laxa 3 a, D. veroni 1 a, D. sp. 1 c | |
| Echinopora | 6 | 3 | 2 a,1 c | E. fruticulosa 2 a, E. irregularis 3 a, E. sp. 1 c | COI N.I. |
| Favites | 4 | 2 | 1 a,1 b | F. paraflexuosus 1 b, F. pentagona 3 a | COI N.I. |
| Hydnophora | 5 | 1 | 1 a | H. exesa 5 a | |
| Merulina | 1 | 1 | 1 a | M. ampliata 1 a | |
| Mycedium | 4 | 1 | 1 a | M. umbra 4 a | |
| Paragoniastrea | 1 | 1 | 1 c | P. sp. 1 c | |
| Paramontastraea | 5 | 1 | 1 a | P. peresi 5 a | |
| Platygyra | 9 | 5 | 5 a | P. acuta 1 a, P. carnosa 2 a, P. crosslandi 2 a, P. daedalea 1 a, P. lamellina 3 a | COI N.I. |
| Plerogyridae | 6 | 2 | |||
| Blastomussa | 2 | 2 | 2 a | B. loyai 1 a. B. merleti 1 a | |
| Plerogyra | 4 | 1 | 1 a | P. sinuosa 4 a | |
| Plesiastreidae | 1 | 1 | |||
| Plesiastrea | 1 | 1 | 1 a | P. versipora 1 a | |
| Pocilloporidae | 14 | 3 | |||
| Pocillopora | 5 | 1 | 1 a | P. damicornis 5 a | |
| Seriatopora | 4 | 1 | 1 a | S. hystrix 4 a | |
| Stylophora | 5 | 2 | 2 a | S. kuehlmanni 1 a, S. pistillata 4 a | |
| Poritidae | 9 | 2 | |||
| Goniopora | 3 | 2 | 2 a | G. pearsoni 2 a, G. tenuidens 1 a | COI N.I. |
| Porites | 6 | 4 | 4 a | P. harrisoni 1 a, P. lutea 2 a, P. nodifera 1 a, P. rus 2 a | COI N.I. |
| Psammocoridae | 1 | 1 | |||
| Psammocora | 1 | 1 | 1 a | P. profundacella 1 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rachmilovitz, E.N.; Shabbat, O.; Yerushalmy, M.; Rinkevich, B. Initiating a DNA Barcoding Reference Library of Stony Corals from the Gulf of Eilat (Red Sea). J. Mar. Sci. Eng. 2022, 10, 1917. https://doi.org/10.3390/jmse10121917
Rachmilovitz EN, Shabbat O, Yerushalmy M, Rinkevich B. Initiating a DNA Barcoding Reference Library of Stony Corals from the Gulf of Eilat (Red Sea). Journal of Marine Science and Engineering. 2022; 10(12):1917. https://doi.org/10.3390/jmse10121917
Chicago/Turabian StyleRachmilovitz, Elad Nehoray, Omri Shabbat, Maayan Yerushalmy, and Baruch Rinkevich. 2022. "Initiating a DNA Barcoding Reference Library of Stony Corals from the Gulf of Eilat (Red Sea)" Journal of Marine Science and Engineering 10, no. 12: 1917. https://doi.org/10.3390/jmse10121917






