Population Characteristics of the Upper Infralittoral Sea Urchin Arbacia lixula (Linnaeus, 1758) in Eastern Mediterranean (Central Greece): An Indicator Species for Coastal Water Quality
Abstract
:1. Introduction
2. Materials and Methods
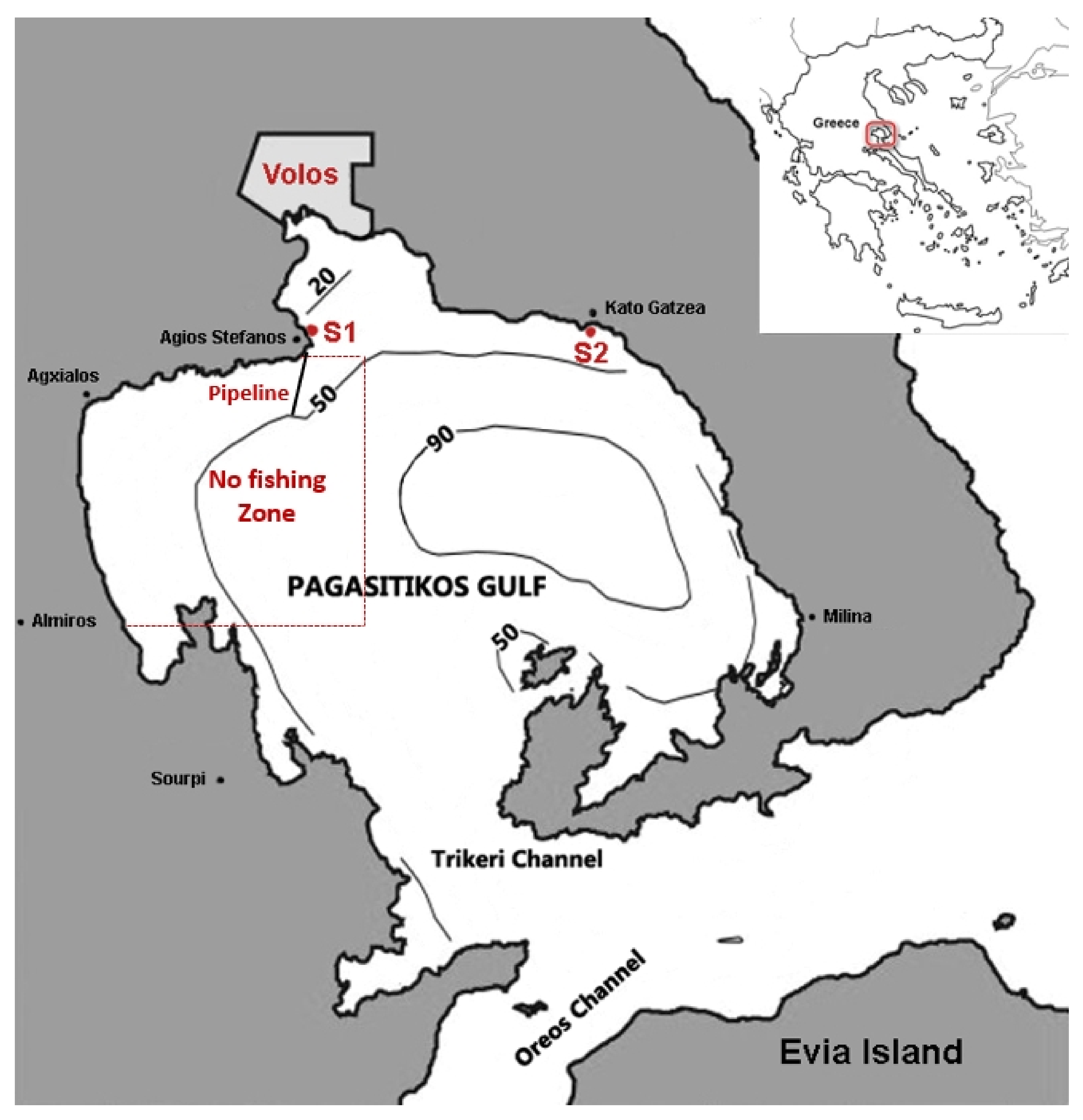
2.1. Study Area
2.2. Field Sampling
2.3. Data Analysis
3. Results
3.1. Physicochemical Measurements
3.2. Biometric Relationships
3.3. Allometric Relationships
3.4. Distribution Pattern
3.5. Age Composition
3.6. Growth
3.7. Reproduction
3.8. Sex Ratio
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alcoverro, T.; Mariani, S. Effects of sea urchin grazing on seagrass (Thalassodendron ciliatum) beds of a Kenyan lagoon. Mar. Ecol. Prog. Ser. 2002, 226, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, C.; Pasqualini, V.; Johnson, M.; Ferrat, L.; Caltagirone, A.; Boudouresque, C.F. Stock evaluation of the sea urchin Paracentrotus lividus in a lagoonal environment. Echinoderm Res. 2001, 319–323. [Google Scholar]
- Benedetti-Cecchi, L.; Pannacciulli, F.; Bulleri, F.; Moschella, P.S.; Airoldi, L.; Relini, G.; Cinelli, F. Predicting the consequences of anthropogenic disturbance: Large-scale effects of loss of canopy algae on rocky shores. Mar. Ecol. Prog. Ser. 2001, 214, 137–150. [Google Scholar] [CrossRef] [Green Version]
- Sala, E.; Zabala, M. Fish predation and the structure of the sea urchin Paracentrotus lividus populations in the NW Mediterranean. Mar. Ecol. Prog. Ser. 1996, 140, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Sala, E.; Boudouresque, C.F.; Harmelin-Vivien, M. Fishing, Trophic Cascades, and the Structure of Algal Assemblages: Evaluation of an Old but Untested Paradigm. Oikos 1998, 82, 425. [Google Scholar] [CrossRef]
- Chapman, A.R.O. Stability of sea urchin dominated barren grounds following destructive grazing of kelp in St. Margaret’s Bay, Eastern Canada. Mar. Biol. 1981, 62, 307–311. [Google Scholar] [CrossRef]
- Witman, J.D. Subtidal coexistence: Storms, grazing, mutualism, and the zonation of kelps and mussels. Ecol. Monogr. 1987, 57, 167–187. [Google Scholar] [CrossRef]
- Fletcher, W.J. Interactions among subtidal Australian sea urchins, gastropods, and algae: Effects of experimental removals. Ecol. Monogr. 1987, 57, 89–109. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Verlaque, M. Ecology of Paracentrotus lividus. Dev. Aquac. Fish. Sci. 2001, 32, 177–216. [Google Scholar] [CrossRef]
- Palacín, C.; Turon, X.; Ballesteros, M.; Giribet, G.; López, S. Stock evaluation of three littoral echinoid species on the Catalan coast (North-Western Mediterranean). Mar. Ecol. 1998, 19, 163–177. [Google Scholar] [CrossRef]
- Bulleri, F.; Benedetti-Cecchi, L.; Cinelli, F. Grazing by the sea urchins Arbacia lixula L. and Paracentrotus lividus Lam. in the Northwest Mediterranean. J. Exp. Mar. Bio. Ecol. 1999, 241, 81–95. [Google Scholar] [CrossRef]
- Privitera, D.; Chiantore, M.; Mangialajo, L.; Glavic, N.; Kozul, W.; Cattaneo-Vietti, R. Inter- and intra-specific competition between Paracentrotus lividus and Arbacia lixula in resource-limited barren areas. J. Sea Res. 2008, 60, 184–192. [Google Scholar] [CrossRef]
- Wangensteen, O.S.; Turon, X.; García-Cisneros, A.; Recasens, M.; Romero, J.; Palacín, C. A wolf in sheep’s clothing: Carnivory in dominant sea urchins in the Mediterranean. Mar. Ecol. Prog. Ser. 2011, 441, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Guidetti, P.; Fraschetti, S.; Terlizzi, A.; Boero, F. Distribution patterns of sea urchins and barrens in shallow Mediterranean rocky reefs impacted by the illegal fishery of the rock-boring mollusc Lithophaga lithophaga. Mar. Biol. 2003, 143, 1135–1142. [Google Scholar] [CrossRef]
- Bonaviri, C.; Fernández, T.V.; Fanelli, G.; Badalamenti, F.; Gianguzza, P. Leading role of the sea urchin Arbacia lixula in maintaining the barren state in southwestern Mediterranean. Mar. Biol. 2011, 158, 2505–2513. [Google Scholar] [CrossRef]
- Contreras, S.; Castilla, J. Feeding behavior and morphological adaptations in two sympatric sea urchin species in central Chile. Mar. Ecol. Prog. Ser. 1987, 38, 217–224. [Google Scholar] [CrossRef]
- Agnetta, D.; Badalamenti, F.; Ceccherelli, G.; Di Trapani, F.; Bonaviri, C.; Gianguzza, P. Role of two co-occurring Mediterranean sea urchins in the formation of barren from Cystoseira canopy. Estuar. Coast. Shelf Sci. 2015, 152, 73–77. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, J.M. Chapter 1 Edible sea urchins: Use and life-history strategies. In Edible Sea Urchins: Biology and Ecology; Lawrence; Elsevier: Amsterdam, The Netherlands, 2007; Volume 37, ISBN 0167-9309. [Google Scholar]
- Francour, P.; Boudouresque, C.F.; Harmelin, J.G.; Harmelin-Vivien, M.L.; Quignard, J.P. Are the Mediterranean waters becoming warmer? Information from biological indicators. Mar. Pollut. Bull. 1994, 28, 523–526. [Google Scholar] [CrossRef]
- Gianguzza, P.; Agnetta, D.; Bonaviri, C.; Di Trapani, F.; Visconti, G.; Gianguzza, F.; Riggio, S. The rise of thermophilic sea urchins and the expansion of barren grounds in the Mediterranean Sea. Chem. Ecol. 2011, 27, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Privitera, D.; Noli, M.; Falugi, C.; Chiantore, M. Benthic assemblages and temperature effects on Paracentrotus lividus and Arbacia lixula larvae and settlement. J. Exp. Mar. Bio. Ecol. 2011, 407, 6–11. [Google Scholar] [CrossRef]
- Petihakis, G.; Triantafyllou, G.; Korres, G.; Pollani, A.; Theodorou, A. Ecosystem modeling: Towards the development of a management tool for a marine coastal system. Part I: General circulation, hydrological and dynamical structure. J. Mar. Syst. 2012, 94, S34–S48. [Google Scholar] [CrossRef]
- Korres, G.; Triantafyllou, G.; Petihakis, G.; Raitsos, D.E.; Hoteit, I.; Pollani, A.; Colella, S.; Tsiaras, K. A data assimilation tool for the Pagasitikos Gulf ecosystem dynamics: Methods and benefits. J. Mar. Syst. 2012, 94, S102–S117. [Google Scholar] [CrossRef]
- Karageorgis, A.; Sioulas, A.; Anagnostou, C. Use of surface sediments in Pagassitikos Gulf, Greece, to detect anthropogenic influence. Geo-Mar. Lett. 2001, 21, 200–211. [Google Scholar] [CrossRef]
- Hatzikosti, M. Directive 2000/60—Common Ministerial Decree for the Treatment of Liquid Wastes and Biological Treatment of Volos City; University of Thessaly: Volos, Greece, 2011. [Google Scholar]
- Gotelli, N.J. Quantitative Ecology and Marine Biology; Bakus, G.J., Ed.; A.A. Balkema: Rotterdam, The Netherlands, 1992; Volume 67. [Google Scholar]
- Krishnamoorthy, K.; Mathew, T.; Ramachandran, G. Upper limits for exceedance probabilities under the one-way random effects model. Ann. Occup. Hyg. 2007, 51, 397–406. [Google Scholar] [PubMed] [Green Version]
- Morisita, M. Iσ-Index, a measure of dispersion of individuals. Res. Popul. Ecol. 1962, 4, 1–7. [Google Scholar] [CrossRef]
- Dale, M.R.T.; Dixon, P.; Fortin, M.J.; Legendre, P.; Myers, D.E.; Rosenberg, M.S. Conceptual and mathematical relationships among methods for spatial analysis. Ecography 2002, 25, 558–577. [Google Scholar] [CrossRef]
- Bhattacharya, C.G. A simple method of resolution of a distribution into Gaussian components. Biometrics 1967, 23, 115–135. [Google Scholar] [CrossRef]
- Gayanilo, F.; Sparre, P.; Pauly, D. FAO-ICLARM Stock Assessment Tools II (FiSAT II) User’s Guide; FAO: Rome, Italy, 2005. [Google Scholar]
- Lester, N.P.; Shuter, B.J.; Abrams, P.A. Interpreting the von Bertalanffy model of somatic growth in fishes: The cost of reproduction. Proc. R. Soc. B Biol. Sci. 2004, 271, 1625–1631. [Google Scholar] [CrossRef] [Green Version]
- Ouréns, R.; Flores, L.; Fernández, L.; Freire, J. Habitat and density-dependent growth of the sea urchin Paracentrotus lividus in Galicia (NW Spain). J. Sea Res. 2013, 76, 50–60. [Google Scholar] [CrossRef]
- RICKER, W.E. Growth rates and models. Fish Physiol. 1979, 8, 677–743. [Google Scholar]
- Elakkermi, M.; Mezali, K.; Soualili, D.L. Interpopulation variability of the reproductive cycle of Arbacia lixula (Echinodermata: Echinoidea) in the Mostaganem shallow-water area (south-western Mediterranean). Reg. Stud. Mar. Sci. 2021, 45, 101810. [Google Scholar] [CrossRef]
- Guillou, M.; Lumingas, L.J.L. Variation in the reproductive strategy of the sea urchin Sphaerechinus granularis (Echinodermata: Echinoidea) related to food availability. J. Mar. Biol. Assoc. UK 1999, 79, 131–136. [Google Scholar] [CrossRef]
- Rutherford, A. Introducing ANOVA And ANCOVA: A GLM Approach; Sage: New York, NY, USA, 2001; ISBN 9780761951612. [Google Scholar]
- The Jamovi Project. Jamovi. (Version 2.2) (Computer Software). 2021. Available online: https://www.jamovi.org (accessed on 15 October 2021).
- Mchugh, M.L. The Chi-square test of independence Lessons in biostatistics. Biochem. Med. 2013, 23, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Froese, R.; Binohlan, C. Empirical relationships to estimate asymptotic length, length at first maturity and length at maximum yield per recruit in fishes, with a simple method to evaluate length frequency data. J. Fish Biol. 2000, 56, 758–773. [Google Scholar] [CrossRef]
- Valentine, J.F.; Heck, K.L. The role of sea urchin grazing in regulating subtropical seagrass meadows: Evidence from field manipulations in the northern Gulf of Mexico. J. Exp. Mar. Bio. Ecol. 1991, 154, 215–230. [Google Scholar] [CrossRef]
- Alvarado, J.J. Seasonal occurrence and aggregation behavior of the sea urchin Astropyga pulvinata (Echinodermata: Echinoidea) in Bahía Culebra, Costa Rica. Pac. Sci. 2008, 62, 579–592. [Google Scholar] [CrossRef] [Green Version]
- Deli, T.; Mohamed, A.B.; Attia, M.H.B.; Zitari-Chatti, R.; Said, K.; Chatti, N. High genetic connectivity among morphologically differentiated populations of the black sea urchin Arbacia lixula (Echinoidea: Arbacioida) across the central African Mediterranean coast. Mar. Biodivers. 2019, 49, 603–620. [Google Scholar] [CrossRef]
- Bayed, A.; Quiniou, F.; Benrha, A.; Guilloi, M. The Paracentrotus lividus populations from the northern Moroccan Atlantic coast: Growth, reproduction and health condition. J. Mar. Biol. Assoc. UK 2005, 85, 999–1007. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, C.; Boudouresque, C.F. Phenotypic plasticity of Paracentrotus lividus (Echinodermata: Echinoidea) in a lagoonal environment. Mar. Ecol. Prog. Ser. 1997, 152, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Balisco, R.A.T. Notes on the Gracious Sea Urchin Tripneustes gratilla (Echinodermata: Echinoidea) in Pag-asa Island, Kalayaan, Palawan, Philippines. Palawan Sci. 2015, 7, 27–35. [Google Scholar]
- Cobb, J.; Lawrence, J.M. Diets and coexistence of the sea urchins Lytechinus variegatus and Arbacia punctulata (Echinodermata) along the central Florida gulf coast. Mar. Ecol. Prog. Ser. 2005, 295, 171–182. [Google Scholar] [CrossRef]
- Ebert, T.A. Longevity, Life History, and Relative Body Wall Size in Sea Urchins. Ecol. Monogr. 1982, 52, 353–394. [Google Scholar] [CrossRef]
- Ebert, T.A. Allometry, design and constraint of body components and of shape in sea urchins. J. Nat. Hist. 1988, 22, 1407–1425. [Google Scholar] [CrossRef]
- Gianguzza, P.; Chiantore, M.; Bonaviri, C.; Cattaneo-Vietti, R.; Vielmini, I.; Riggio, S. The effects of recreational Paracentrotus lividus fishing on distribution patterns of sea urchins at Ustica Island MPA (Western Mediterranean, Italy). Fish. Res. 2006, 81, 37–44. [Google Scholar] [CrossRef]
- Chiantore, M.; Vielmini, I.; Privitera, D.; Mangialajo, L.; Cattaneo-Vietti, R. Habitat effects on the population structure of Paracentrotus lividus and Arbacia lixula. Chem. Ecol. 2008, 24, 145–157. [Google Scholar] [CrossRef]
- Pearse, J.S.; Arch, S.W. The aggregation behavior of Diadema (Echinodermata, Echinoidea). Micronesia 1969, 5, 165–171. [Google Scholar]
- Tuya, F.; Cisneros-Aguirre, J.; Ortega-Borges, L.; Haroun, R.J. Bathymetric segregation of sea urchins on reefs of the Canarian Archipelago: Role of flow-induced forces. Estuar. Coast. Shelf Sci. 2007, 73, 481–488. [Google Scholar] [CrossRef]
- Rose, C.D.; Sharp, W.C.; Kenworthy, W.J.; Hunt, J.H.; Lyons, W.G.; Prager, E.J.; Valentine, J.F.; Hall, M.O.; Whitfield, P.E.; Fourqurean, J.W. Overgrazing of a large seagrass bed by the sea urchin Lytechinus variegatus in Outer Florida Bay. Mar. Ecol. Prog. Ser. 1999, 190, 211–222. [Google Scholar] [CrossRef]
- Benedetti-Cecchi, L.; Cinelli, F. Habitat heterogeneity, sea urchin grazing and the distribution of algae in littoral rock pools on the west coast of Italy (western Mediterranean). Mar. Ecol. Prog. Ser. 1995, 126, 219. [Google Scholar] [CrossRef] [Green Version]
- Sala, E.; Ribes, M.; Hereu, B.; Zabala, M.; Alvà, V.; Coma, R.; Garrabou, J. Temporal variability in abundance of the sea urchins Paracentrotus lividus and Arbacia lixula in the northwestern Mediterranean: Comparison between a marine reserve and an unprotected area. Mar. Ecol. Prog. Ser. 1998, 168, 135–145. [Google Scholar] [CrossRef]
- Sala, E. Fish predators and scavengers of the sea urchin Paracentrotus lividus in protected areas of the north-west Mediterranean sea. Mar. Biol. 1997, 129, 531–539. [Google Scholar] [CrossRef]
- López, S.; Turon, X.; Montero, E.; Palacín, C.; Duarte, C.M.; Tarjuelo, I. Larval abundance, recruitment and early mortality in Paracentrotus lividus (Echinoidea). Interannual variability and plankton-benthos coupling. Mar. Ecol. Prog. Ser. 1998, 172, 239–251. [Google Scholar] [CrossRef] [Green Version]
- Byrne, M. Annual reproductive cycles of the commercial sea urchin Paracentrotus lividus from an exposed intertidal and a sheltered subtidal habitat on the west coast of Ireland. Mar. Biol. 1990, 104, 275–289. [Google Scholar] [CrossRef]
- Hereu, B. Depletion of palatable algae by sea urchins and fishes in a Mediterranean subtidal community. Mar. Ecol. Prog. Ser. 2006, 313, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Russell, M.P.; Ebert, T.A.; Petraitis, P.S. Field estimates of growth and mortality of the green sea urchin, Strongylocentrotus droebachiensis. Ophelia 1998, 48, 137–153. [Google Scholar] [CrossRef]
- Rogers, L.; Bennett, D.R.; Bennett, W.; Ebert, T. Modeling Red Sea Urchin Growth Using Six Growth Models. Fish. Bull. 2003, 101, 614–626. [Google Scholar]
- Turon, X.; Giribet, G.; López, S.; Palacín, C. Growth and population structure of Paracentrotus lividus (Echinodermata: Echinoidea) in two contrasting habitats. Mar. Ecol. Prog. Ser. 1995, 122, 193–204. [Google Scholar] [CrossRef]
- Ebert, T.A.; Russell, M.P. Growth and mortality estimates for red sea urchin Strongylocentrotus franciscanus from San Nicolas Island, California. Mar. Ecol. Prog. Ser. Oldend. 1992, 81, 31–41. [Google Scholar] [CrossRef]
- Ebert, T.A.; Russell, M.P. Growth and mortality of subtidal red sea urchins (Strongylocentrotus franciscanus) at San Nicolas Island, California, USA: Problems with models. Mar. Biol. 1993, 117, 79–89. [Google Scholar] [CrossRef]
- Grosjean, P.; Spirlet, C.; Jangoux, M. Experimental study of growth in the echinoid Paracentrotus lividus (Lamarck, 1816) (Echinodermata). J. Exp. Mar. Bio. Ecol. 1996, 201, 173–184. [Google Scholar] [CrossRef]
- Conor, J.J. Gonad growth in the sea urchin, Strongylocentrotus purpuratus (Stimpson) (echinodermata: Echinoidea) and the assumptions of gonad index methods. J. Exp. Mar. Bio. Ecol. 1972, 10, 89–103. [Google Scholar] [CrossRef]
- Ebert, T.A. Negative growth and longevity in the purple sea urchin Strongylocentrotus purpuratus (Stimpson). Science 1967, 157, 557–558. [Google Scholar] [CrossRef] [PubMed]
- Ebert, T.A. Growth rates of the sea urchin Strongylocentrotus purpuratus related to food availability and spine abrasion. Ecology 1968, 49, 1075–1091. [Google Scholar] [CrossRef]
- Fuji, A. Ecological studies on the growth and food consumption of Japanese common littoral sea urchin, Strngylocentrotus Intermedius (A. Agassiz). In Memoirs of the Faculty of Fisheries Hokkaido University; Hokkaido University: Hokkaido, Japan, 1967; Volume 15 (2), pp. 83–160. [Google Scholar]
- Zachos, L.G. A new computational growth model for sea urchin skeletons. J. Theor. Biol. 2009, 259, 646–657. [Google Scholar] [CrossRef]
- Fernandez, C.; Pergent, G. Effect of different formulated diets and rearing conditions on growth parameters in the sea urchin Paracentrotus lividus. J. Shellfish Res. 1998, 17, 1571–1581. [Google Scholar]
- Meidel, S.K.; Scheibling, R.E. Effects of food type and ration on reproductive maturation and growth of the sea urchin Strongylocentrotus droebachiensis. Mar. Biol. 1999, 134, 155–166. [Google Scholar] [CrossRef]
- Shpigel, M.; McBride, S.C.; Marciano, S.; Lupatsch, I. The effect of photoperiod and temperature on the reproduction of European sea urchin Paracentrotus lividus. Aquaculture 2004, 232, 343–355. [Google Scholar] [CrossRef]
- Vadas, R.L. Preferential Feeding: An Optimization Strategy in Sea Urchins. Ecol. Monogr. 1977, 47, 337–371. [Google Scholar] [CrossRef]
- Tomšić, S.; Conides, A.; Radić, I.D.; Glamuzina, B. Growth, size class frequency and reproduction of purple sea urchin, Paracentrotus lividus (Lamarck, 1816) in Bistrina Bay (Adriatic Sea, Croatia). Acta Adriat. 2010, 51, 67–77. [Google Scholar]
- Wangensteen, O.S.; Turon, X.; Casso, M.; Palacín, C. The reproductive cycle of the sea urchin Arbacia lixula in northwest Mediterranean: Potential influence of temperature and photoperiod. Mar. Biol. 2013, 160, 3157–3168. [Google Scholar] [CrossRef] [Green Version]
- Pedrotti, M.L. Spatial and temporal distribution and recruitment of echinoderm larvae in the ligurian sea. J. Mar. Biol. Assoc. UK 1993, 73, 513–530. [Google Scholar] [CrossRef]
- Pearse, J.S.; McClary, D.J.; Sewell, M.A.; Austin, W.C.; Perez-Ruzafa, A.; Byrne, M. Simultaneous spawning of six species of echinoderms in barkley sound, british columbia. Int. J. Invertebr. Reprod. Dev. 1988, 14, 279–288. [Google Scholar] [CrossRef]
- Siikavuopio, S.I.; Christiansen, J.S.; Dale, T. Effects of temperature and season on gonad growth and feed intake in the green sea urchin (Strongylocentrotus droebachiensis). Aquaculture 2006, 255, 389–394. [Google Scholar] [CrossRef] [Green Version]
- Lares, M.T.; McClintock, J.B. The effects of food quality and temperature on the nutrition of the carnivorous sea urchin Eucidaris tribuloides (Lamarck). J. Exp. Mar. Bio. Ecol. 1991, 149, 279–286. [Google Scholar] [CrossRef]
- Lawrence, J.M.; Plank, L.R.; Lawrence, A.L. The effect of feeding frequency on consumption of food, absorption efficiency, and gonad production in the sea urchin Lytechinus variegatus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2003, 134, 69–75. [Google Scholar] [CrossRef]
- Baião, L.F.; Rocha, F.; Costa, M.; Sá, T.; Oliveira, A.; Maia, M.R.G.; Fonseca, A.J.M.; Pintado, M.; Valente, L.M.P. Effect of protein and lipid levels in diets for adult sea urchin Paracentrotus lividus (Lamarck, 1816). Aquaculture 2019, 506, 127–138. [Google Scholar] [CrossRef]
- Bernard, F.R. Fishery and Reproductive Cycle of the Red Sea Urchin, Strongylocentrotus franciscanus, in British Columbia. J. Fish. Res. Board Canada 1977, 34, 604–610. [Google Scholar] [CrossRef]
- GESAMP (IMO/FAO/UNESCO-IOC/WMO/WHO/IAEA/UN/UNEP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection) and Advisory Committee on Protection of the Sea. Protecting the Oceans from Land-Based Activities—Land-Based Sources and Activities Affecting the Quality and Uses of the Marine, Coastal and Associated Freshwater Environment; No. 71; Reports and Studies GESAMP: London, UK, 2001; p. 162. [Google Scholar]
- Sonico, M.G.I. Distribution of Sea Urchins in Punta Dumalag, Matina Aplaya, Davao City. J. Eng. Environ. Agric. Res. 2018, 1, 4. [Google Scholar] [CrossRef]
- Portocali, P.; Iliopoulou-Georgudaki, J.; Catsiki, V.A.; Papapetropoulou, M. The role of echinoderms as bioindicators of seawater pollution: A case study from Patraicos and Corinthiacos gulf, N. Peloponnesus, Greece. Toxicol. Environ. Chem. 1997, 59, 293–303. [Google Scholar] [CrossRef]
- Catsiki, V.A.; Papathanassiou, E.; Bei, F. Heavy metal levels in characteristic benthic flora and fauna in the central Aegean Sea. Mar. Pollut. Bull. 1991, 22, 566–569. [Google Scholar] [CrossRef]
- Savriama, Y.; Stige, L.C.; Gerber, S.; Pérez, T.; Alibert, P.; David, B. Impact of sewage pollution on two species of sea urchins in the Mediterranean Sea (Cortiou, France): Radial asymmetry as a bioindicator of stress. Ecol. Indic. 2015, 54, 39–47. [Google Scholar] [CrossRef]
- Carballeira, C.; De Orte, M.R.; Viana, I.G.; Carballeira, A. Implementation of a minimal set of biological tests to assess the ecotoxic effects of effluents from land-based marine fish farms. Ecotoxicol. Environ. Saf. 2012, 78, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Boán, M.; Fernández, L.; Freire, J. History and management strategies of the sea urchin Paracentrotus lividus fishery in Galicia (NW Spain). Ocean Coast. Manag. 2012, 69, 265–272. [Google Scholar] [CrossRef]
- Garrabou, J.; Sala, E.; Arcas, A.; Zabala, M. The impact of diving on rocky sublittoral communities: A case study of a bryozoan population. Conserv. Biol. 1998, 12, 302–312. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Sala, E. A Mediterranean rocky-bottom ecosystem fisheries model. Ecol. Modell. 1997, 104, 145–164. [Google Scholar] [CrossRef]
- Jackson, J.B.C.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Botsford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.; Erlandson, J.; Estes, J.A.; et al. Historical overfishing and the recent collapse of coastal ecosystems. Science 2001, 293, 629–637. [Google Scholar] [CrossRef] [Green Version]










| Sampling Period | Site 1 | ||||||
|---|---|---|---|---|---|---|---|
| Depth | T (°C) | S (psu) | O2 (mg L−1) | pH | Redox (mV) | Chla (mg m−3) | |
| December | 3.78 | 15.12 | 37.58 | 4.81 | 8.22 | 121.35 | 2.28 |
| January | 4.28 | 13.96 | 37.67 | 3.03 | 8.27 | 114.27 | 2.54 |
| February | 5.21 | 13.38 | 37.51 | 4.65 | 8.25 | 122.24 | 2.17 |
| March | 5.62 | 13.17 | 38.38 | 5.56 | 8.28 | 150.11 | 1.12 |
| April | 4.25 | 15.82 | 37.89 | 5.06 | 8.27 | 118.67 | 1.17 |
| May | 3.40 | 21.14 | 37.32 | 3.94 | 8.25 | 114.04 | 0.68 |
| June | 3.37 | 25.05 | 36.42 | 2.33 | 8.26 | 121.86 | 0.70 |
| July | 3.99 | 27.08 | 36.12 | 1.91 | 8.25 | 144.53 | 0.61 |
| August | 3.76 | 26.98 | 36.32 | 2.54 | 8.26 | 123.42 | 0.87 |
| September | 4.11 | 24.28 | 36.42 | 3.81 | 8.30 | 105.65 | 1.23 |
| October | 4.34 | 21.78 | 36.72 | 2.62 | 8.34 | 130.97 | 1.38 |
| November | 4.11 | 19.01 | 36.81 | 3.83 | 8.25 | 118.72 | 0.88 |
| Site 2 | |||||||
| December | 3.24 | 15.34 | 37.78 | 4.75 | 8.24 | 127.36 | 2.39 |
| January | 4.56 | 14.02 | 37.69 | 2.97 | 8.28 | 118.30 | 2.38 |
| February | 6.67 | 13.67 | 37.58 | 4.98 | 8.27 | 128.13 | 1.94 |
| March | 6.22 | 13.41 | 38.51 | 5.64 | 8.29 | 149.26 | 1.15 |
| April | 6.17 | 15.76 | 38.01 | 5.18 | 8.27 | 105.28 | 1.21 |
| May | 6.05 | 20.68 | 37.54 | 2.53 | 8.25 | 116.25 | 1.12 |
| June | 6.32 | 24.86 | 36.64 | 4.83 | 8.24 | 116.67 | 0.57 |
| July | 3.04 | 26.97 | 36.20 | 2.65 | 8.23 | 116.62 | 0.73 |
| August | 4.21 | 26.17 | 36.44 | 2.88 | 8.25 | 110.31 | 1.02 |
| September | 4.88 | 23.69 | 36.68 | 3.95 | 8.34 | 104.50 | 1.24 |
| October | 6.31 | 21.27 | 36.80 | 3.54 | 8.34 | 102.66 | 0.87 |
| November | 5.84 | 18.56 | 36.99 | 3.72 | 8.38 | 115.35 | 0.83 |
| Sampling Period | No. of Individuals | TD ± SE | TH ± SE | ToW ± SE | TW ± SE |
|---|---|---|---|---|---|
| Site | |||||
| Site 1 | 480 | 39.18 ± 0.29 | 19.26 ± 0.17 | 33.86 ± 0.60 | 20.96 ± 0.35 |
| Site 2 | 440 | 37.09 ± 0.27 | 17.48 ± 0.15 | 28.65 ± 0.52 | 17.99 ± 0.32 |
| Significance level | *** | *** | *** | *** | |
| Sex | |||||
| Male | 406 | 37.83 ± 0.30 | 18.20 ± 0.18 | 30.84 ± 0.61 | 19.54 ± 0.36 |
| Female | 470 | 38.64 ± 0.29 | 18.73 ± 0.16 | 32.27 ± 0.57 | 19.96 ± 0.33 |
| Significance level | ns | * | ns | ns | |
| Season | |||||
| Winter | 200 | 37.33 b,c ± 0.46 | 18.62 a,b ± 0.28 | 29.66 b ± 0.89 | 18.69 b ± 0.52 |
| Spring | 240 | 36.58 c ± 0.41 | 17.81 b ± 0.25 | 29.78 b ± 0.84 | 19.59 a,b ± 0.53 |
| Summer | 240 | 37.90 b ± 0.35 | 17.02 b ± 0.20 | 30.34 b ± 0.74 | 18.44 b ± 0.40 |
| Autumn | 240 | 40.79 a ± 0.35 | 19.22 a ±0.20 | 35.42 a ± 0.75 | 21.29 a ± 0.44 |
| Total | 920 | 38.18 ± 0.20 | 18.41 ± 0.12 | 31.37 ± 0.41 | 19.54 ± 0.24 |
| Morphometric Relationships | Equation Comparison | ||||||
|---|---|---|---|---|---|---|---|
| Factor | Equation | N | r | t-Test | Allometry | Slopes | Intercepts |
| Pooled | TW = 0.13816 × TH1.69304 | 920 | 0.87 | *** | −ve | ||
| Site 1 | TW = 0.14658 × TH1.67134 | 480 | 0.87 | *** | −ve | ns | ns |
| Site 2 | TW = 0.11209 × TH1.76758 | 440 | 0.85 | *** | −ve | ||
| Males | TW = 0.14812 × TH1.67550 | 406 | 0.89 | *** | −ve | ns | ns |
| Females | TW = 0.15026 × TH1.66260 | 470 | 0.85 | *** | −ve | ||
| Pooled | TW = 0.00627 × TD2.19896 | 920 | 0.90 | *** | −ve | ||
| Site 1 | TW = 0.00529 × TD2.24841 | 480 | 0.91 | *** | −ve | * | ns |
| Site 2 | TW = 0.00919 × TD2.09031 | 440 | 0.87 | *** | −ve | ||
| Males | TW = 0.00292 × TD2.53682 | 406 | 0.96 | *** | −ve | ns | ns |
| Females | TW = 0.00435 × TD2.42645 | 470 | 0.94 | *** | −ve | ||
| Pooled | TD = 4.58985 × TH0.728603 | 920 | 0.88 | *** | −ve | ||
| Site 1 | TD = 4.75275 × TH0.71449 | 480 | 0.86 | *** | −ve | * | * |
| Site 2 | TD = 4.01276 × TH0.77823 | 440 | 0.90 | *** | −ve | ||
| Males | TD = 4.86836 × TH0.70812 | 406 | 0.89 | *** | −ve | ns | ns |
| Females | TD = 4.35849 × TH0.74592 | 470 | 0.87 | *** | −ve | ||
| Pooled | ToW = 0.134454 × TH1.86251 | 920 | 0.91 | *** | −ve | ||
| Site 1 | ToW = 0.14616 × TH1.83239 | 480 | 0.90 | *** | −ve | * | ns |
| Site 2 | ToW = 0.10086 × TH1.96436 | 440 | 0.92 | *** | −ve | ||
| Males | ToW = 0.14283 × TH1.84274 | 406 | 0.92 | *** | −ve | ns | ns |
| Females | ToW = 0.13211 × TH1.86774 | 470 | 0.90 | *** | −ve | ||
| Pooled | ToW = 0.00366 × TD2.47347 | 920 | 0.94 | *** | −ve | ||
| Site 1 | ToW = 0.00304 × TD2.52638 | 480 | 0.95 | *** | −ve | * | ** |
| Site 2 | ToW = 0.00544 × TD2.36138 | 440 | 0.95 | *** | −ve | ||
| Males | ToW = 0.00292 × TD2.53682 | 406 | 0.96 | *** | −ve | ns | ns |
| Females | ToW = 0.00435 × TD2.42645 | 470 | 0.94 | *** | −ve | ||
| Sampling Season | Sites | Population Density (ind. m−2 ± SD) | Iδ | X2 | Dispersion Pattern | Significance Level | DF |
|---|---|---|---|---|---|---|---|
| Autumn | Site 1 | 0.8 ± 1.69 | 9.00 | 8.00 | clustered | ns | 9 |
| Site 2 | 1.2 ± 1.93 | 4.50 | 7.00 | clustered | ns | 9 | |
| Winter | Site 1 | 1.6 ± 2.80 | 4.67 | 11.00 | clustered | ns | 9 |
| Site 2 | 2.0 ± 3.40 | 4.25 | 13.00 | clustered | ns | 9 | |
| Spring | Site 1 | 2.4 ± 3.37 | 3.13 | 10.67 | clustered | ns | 9 |
| Site 2 | 2.8 ± 4.64 | 3.88 | 17.29 | clustered | * | 9 | |
| Summer | Site 1 | 3.2 ± 5.27 | 3.79 | 19.50 | clustered | * | 9 |
| Site 2 | 4.0 ± 5.96 | 3.22 | 20.00 | clustered | * | 9 |
| Age Group | Test Diameter (mm) | Standard Deviation | Population Size | Population% | Separation Index (SI) |
|---|---|---|---|---|---|
| 1 | 23.38 | 0.74 | 10.72 | 1.30 | |
| 2 | 28.80 | 1.59 | 76.76 | 9.28 | 2.26 |
| 3 | 34.34 | 1.29 | 182.43 | 22.06 | 2.18 |
| 4 | 38.25 | 1.44 | 258.00 | 31.20 | 2.07 |
| 5 | 42.98 | 1.79 | 248.92 | 30.10 | 2.08 |
| 6 | 49.83 | 1.35 | 50.10 | 6.06 | 2.17 |
| Source of Variation | MS | F | P |
|---|---|---|---|
| Site | 1545.44 | 575.10 | <0 .001 |
| Season | 124.00 | 46.14 | <0.001 |
| Sex | 22.51 | 8.38 | 0.004 |
| Test diameter | 21.70 | 8.08 | 0.005 |
| Site X Season | 4.60 | 1.71 | 0.163 |
| Site X Sex | 0.20 | 0.07 | 0.786 |
| Season X Sex | 2.89 | 1.08 | 0.358 |
| Site X Season X Sex | 0.39 | 0.15 | 0.933 |
| Residuals | 2.69 | ||
| Total | 6.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klaoudatos, D.; Tziantziou, L.; Lolas, A.; Neofitou, N.; Vafidis, D. Population Characteristics of the Upper Infralittoral Sea Urchin Arbacia lixula (Linnaeus, 1758) in Eastern Mediterranean (Central Greece): An Indicator Species for Coastal Water Quality. J. Mar. Sci. Eng. 2022, 10, 395. https://doi.org/10.3390/jmse10030395
Klaoudatos D, Tziantziou L, Lolas A, Neofitou N, Vafidis D. Population Characteristics of the Upper Infralittoral Sea Urchin Arbacia lixula (Linnaeus, 1758) in Eastern Mediterranean (Central Greece): An Indicator Species for Coastal Water Quality. Journal of Marine Science and Engineering. 2022; 10(3):395. https://doi.org/10.3390/jmse10030395
Chicago/Turabian StyleKlaoudatos, Dimitris, Labrini Tziantziou, Alexios Lolas, Nikos Neofitou, and Dimitris Vafidis. 2022. "Population Characteristics of the Upper Infralittoral Sea Urchin Arbacia lixula (Linnaeus, 1758) in Eastern Mediterranean (Central Greece): An Indicator Species for Coastal Water Quality" Journal of Marine Science and Engineering 10, no. 3: 395. https://doi.org/10.3390/jmse10030395
APA StyleKlaoudatos, D., Tziantziou, L., Lolas, A., Neofitou, N., & Vafidis, D. (2022). Population Characteristics of the Upper Infralittoral Sea Urchin Arbacia lixula (Linnaeus, 1758) in Eastern Mediterranean (Central Greece): An Indicator Species for Coastal Water Quality. Journal of Marine Science and Engineering, 10(3), 395. https://doi.org/10.3390/jmse10030395










