Abstract
The giant freshwater prawn, Macrobrachium rosenbergii, is a prawn that has economic significance throughout the world. It exhibits sex-related growth dimorphism, whereby the males grow significantly more rapidly than the females. Therefore, a study on the molecular regulatory mechanism, which underlies the sexual differentiation of M. rosenbergii, is of both scientific and commercial importance. However, a scarcity of genomic and transcriptomic resources severely limits our knowledge of the sexual differentiation mechanisms in M. rosenbergii. Here, transcriptome sequencing of several gonadic samples of males and females in M. rosenbergii was performed to investigate the molecular basis underlying gonadal development. Our results showed that 2149 unigenes presented as differentially expressed genes (DEGs) in the ovaries of females compared to the testes of males, which contained 484 down-regulated and 1665 up-regulated genes. Enrichment analysis of DEGs revealed many of these genes to be related to sexual differentiation and gonadal development. From our transcriptome analyses, and as confirmed by quantitative real-time PCR, male-related genes (Mrr, MRPINK, IR, IAGBP, TESK1, and dsx) in the testes were significantly up-regulated, and female-related genes (ERR, Sxl3, cyclinB, Dmrt99B, PPP2A, and ADCY9) in the ovaries were also significantly up-regulated. This indicates the potential role these genes play in the gonadal development of M. rosenbergii. Furthermore, multiple signal transduction pathways relating to gonadal maturation and spermatogenesis, including MAPK, were identified herein. Our data also supports previous ideas that IAG and IAGBP-IR signaling schemes could help in the regulation of testis’ development in M. rosenbergii and the ERR gene could regulate ovarian development by affecting the expression of cyclinB, PPP2A, and ADCY9. The data from this study provides incredibly usefully genomic resources for future research on the sexual differentiation and practical aquaculture of M. rosenbergii.
1. Introduction
The giant freshwater prawn (Macrobrachium rosenbergii) is a crustacean of great economic importance in the aquaculture of many countries and regions throughout the world due to its low disease rate, rapid growth, and high nutritional value [1]. It exhibits a sex-related growth dimorphism, whereby the males grow significantly faster than the females [2,3]. In order to facilitate the increased production of M. rosenbergii, artificial breeding or selection is performed. Of the methods used for this, controlling the sex is regarded as an important technique. Therefore, understanding the detailed mechanism that is involved in sexual development is of great importance.
Related research on the sex determination of crustaceans has shown that many crustaceans have the ZZ/ZW sex determination system [4,5,6]. In M. rosenbergii, researchers have discovered a few sex marker genes, including the two genes of zinc knuckle domain (ZKD) and ANCDUO, which further confirms that M. rosenbergii contained the ZZ/ZW sex-determination system [7], and that the males have ZZ-type sex chromosomes, while the females have ZW-type sex chromosomes [8]. In addition, the discovery of some male-related genes (male reproductive-related gene (Mrr), male reproduction-related peptidase inhibitor kazal-type (MRPINK), insulin-like receptor (IR), and insulin-like androgenic gland hormone binding protein gene (IAGBP)) [9,10,11,12,13] associated with the maintenance of male development and female-related genes (estrogen-related receptor (ERR), cyclinB, protein phosphatase 2A (PPP2A), and adenylate cyclase 9 (ADCY9)) [14,15] related to ovarian tissues has further deepened our understanding of the mechanism of sex determination in M. rosenbergii. Moreover, some cell signaling pathways related to sexual differentiation have been reported, and one of the most important signaling pathways is the insulin signaling pathway, as the insulin-like hormone gene, IAG, appears quite frequently in the androgenic gland (AG) of M. rosenbergii [16,17,18]. Previously, we found that the IAG and IAGBP-insulin-like receptor (IR) signaling schemes might play a vital role in the sexual differentiation process of M. rosenbergii [10].
Although relatively few sex-related genes have been reported, the sexual regulatory mechanisms of M. rosenbergii remain unclear. A scarcity of genomic and transcriptomic resources hinders our understanding of M. rosenbergii’s sexual regulatory mechanisms. The identification of more sex-related genes and the investigation of their expression profiles are necessary for understanding these processes in this species of prawn. In this study, high-throughput sequencing was used to sequence the mRNA of the testes and ovaries of M. rosenbergii. The aim was to detect related differences in the gene transcription profiles of the testes and ovaries of M. rosenbergii and investigate the genetic basis of gonadal development. This data can be used as a resource for further studies for elucidating the molecular basis of reproductive system development and reproduction in prawns.
2. Materials and Methods
2.1. Sample Preparation
Five males and five females of M. rosenbergii with a late stage of gonads maturation were sourced from the Jin Yang Aquaculture Co., Ltd. in Guangzhou, China. Their genders were identified by determining the external morphology of the reproductive season, combined with the verification of dissected gonads. According to the previous studies, the ovary development of M. rosenbergii was divided into early, middle, and late maturation stages [19], and the testicular development of M. rosenbergii was divided into early, middle, and late maturation stages [20]. Liquid nitrogen was used for the immediate freezing of the gonads, and the gonads were stored at −80 °C until RNA extraction. All the healthy experimental individuals used in this study were five months of age and were maintained in aerated freshwater at 26 ± 2 °C on the previously mentioned farm. The growth performance of the sample individuals is listed in Table S1.
2.2. RNA Extraction and Transcriptome Sequencing
Based on the producer’s guidelines, the total RNA from ten gonads samples was separated using RNAiso plus (TaKaRa, Dalian, China). An Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) was used for the evaluation of RNA quality. A similar quantity of the total RNA Integrity Number (RIN) ≥ 7.0 and the RNA with a 28S/18S ratio ≥ 1.0 from the five with the same gender was pooled together in order to construct a consensus cDNA library. As there are two genders, two different libraries were made, which were sequenced on an Illumina HiSeq2500 platform (Illumina, San Diego, CA, USA) at OE Biotech Shanghai (Shanghai, China). Then, 10 GB of clean data were formed from each of the two pooled samples. The raw sequences were deposited into the NCBI Sequence Read Archive (SRA) database (Accessions PRJNA728824).
2.3. De novo Assembly and Gene Function Annotation
Raw data, which were obtained from sequencing, were processed in order to obtain high-quality sequencing data. At the same time, the GC content and the Q30 and Q20 sequence levels were calculated. High-quality data were spliced, filtrated, and assembled using Trinity software to obtain a high-quality unigene [21]. The unigene sequence was aligned with the following database using BLAST software in order to obtain functional annotation information for the genes [22]. The databases contained the non-redundant protein (Nr) [23], Swissprot [24], clusters of orthologous groups for eukaryotic complete genomes (KOG) [25], Gene Ontology (GO) [26], and the Kyoto Encyclopedia of Genes and Genomes (KEGG) [27] databases. The cutoff E-value was fixed at 1e-5, and only the top hit was used for annotating each unigene. By using the Blast2GO, the GO annotation was completed [28] based on the Nr BLAST results. KOBAS was utilized for performing KEGG pathway analysis [29].
2.4. Analysis of DEGs and Functional Enrichment
By using RSEM software (RNASeq by Expectation Maximization) (http://deweylab.biostat.wisc.edu/rsem, accessed on 3 September 2019) [30], each unigene’s expression level was calculated from the two libraries, based on the FPKM (Fragment Per Kilo bases per Million Reads) value. Using the R package [31], the DEGs were shown through default parameters, followed by multiple testing to correct the p-value. This study set the absolute value of the p-value at < 0.05 and the Fold-Change to ≥2 as the threshold for important differential expression. The two gene expression profiles were compared, and then all DEGs performed KEGG pathway enrichment and GO functional analysis through the KEGG and GO databases using Goseq [32] and KOBAS [29]. As demonstrated by the DEGs, only KEGG and GO terms with an accurate p-value of < 0.01 were determined.
2.5. Quantitative Real-Time PCR Validation
For validation of the sequencing data, 12 DEGs were chosen for quantitative real-time PCR (qRT-PCR) analysis. The total RNA of qRT-PCR was obtained from residual gonad samples used in RNA sequencing (RNA-seq) experiments, and then cDNA was synthesized from RNA (n = 5), which was used for qRT-PCR quantification, with the support of GoScriptTM Reverse Transcription System (Promega, Madison, WI, USA). Primer Premier 6.0 software was used for designing the gene-specific primers (Table S2). In addition, endogenous control acted as a β-actin gene [10]. With the support of SYBR® Premix Ex TaqTM II (TaKaRa) and LightCycler 480 real-time PCR instrument (Roche, Basel, Switzerland), qRT-PCR was completed. The qRT-PCR was performed under the following conditions: denaturation at 95 °C for 5 min, followed by 45 cycles of 95 °C for 10 s, 60 °C for 25 s, 95 °C for 15 s, and finally 60 °C for 1 min. Further melting curve analysis was then performed to confirm whether only one PCR product was amplified. All samples were analyzed with three biological replicates, and the relative expression levels of target genes were normalized to β-actin and calculated by the 2−ΔΔCT method [33].
2.6. Screening of Genes Relating to Gonadal Development
Based on the functional annotation information of DEGs in male and female samples, in combination with the relevant literature reports, functional genes relating to the development of the gonads of M. rosenbergii were screened and identified.
2.7. Statistical Analysis
Through the means ± SE, all data were presented. SPSS 22.0 software (SPSS Inc., Chicago, IL, USA) was utilized to assess the statistics, and one-way ANOVA was used to provide statistical importance before an independent t-test was performed. The significance was set at p < 0.05.
3. Results
3.1. Transcriptome Sequencing and De Novo Assembly
At OE Biotech Shanghai, cDNA libraries for the two gonads (ovaries and testes) were individually formed and subjected to transcriptome sequencing to make the transcriptome profiling of the gonads available. Table 1 shows the RNA-seq outcomes. A total of 78,074,612 and 89,598,146 raw reads were created from the ovary and testis samples. Following the removal of the low-quality reads, 20 Gb of clean data were retained for subsequent de novo assembly. All of the transcriptome assemblies were combined into a single file, which contained 75,887 unigenes with an N50 length of 2063 bp and an average length of 1132.93 bp (see Table 2). Figure S1 shows the length of the distribution of the assembled unigenes. A significant proportion (29.28%) of the unigenes were in the range of 301–400 bp; 11,410 unigenes (15.04%) were longer than 2000 bp, and 11,242 unigenes (14.81%) were in the range of 401–500 bp.

Table 1.
Statistical assessment of transcriptome sequencing data.

Table 2.
The sequencing results from the testes and the ovary of M. rosenbergii.
3.2. Functional Annotation and Classification of the Transcriptome
Of the 75,887 unigenes, 18,654 (24.58%), 13,526 (17.82%), 11,825 (15.58%), 5342 (7.04%), and 12,622 (16.63%) were annotated in the Nr, SwissProt, KOG, KEGG, and GO databases (Figure S2). The greatest number of unigenes was annotated in the Nr database. A species distribution analysis of 18,654 unigenes was conducted, and the results showed the unigenes to have a high matching degree with Daphnia magna (965, 5.17%), Limulus polyphemus (1025, 5.49%), and Zootermopsis nevadensis (1619, 8.68%) (Figure S3). In addition, a smaller portion of the unigenes was similar to crustacean species, such as Daphnia pulex (559, 3%). The main reason for the low proportion of unigenes being annotated as crustaceans is the limited crustacean gene information in the databases.
A GO analysis divided the 12,622 unigenes into 64 subcategories within the three major categories of “biological processes,” “cellular components,” and “molecular functions” (Figure S4). The major subcategories were “cellular process,” “cell,“ and “cell part.“ The unigenes were annotated according to the KEGG database, and 5342 unigenes were categorized into 360 pathways, including the six main groups of organismal systems, metabolism, human disease, genetic information processing, environmental information processing, and cellular processes (Table S3). Of the 360 predicted pathways, “Huntington’s disease” was the major group, which contained 234 unigenes. In addition, 222 and 225 unigenes were grouped into “pathway in cancer” and “ribosome.” Using the KOG database for the annotation of the unigenes, a total of 11,825 unigenes were annotated. These 11,825 unigenes were divided into 25 KOG functional groups. The largest functional group was “general function prediction only” (3013, 25.48%), followed by “signal transduction mechanisms” (1804, 15.26%) (Figure S5).
3.3. Analysis and Functional Enrichment of DEGs
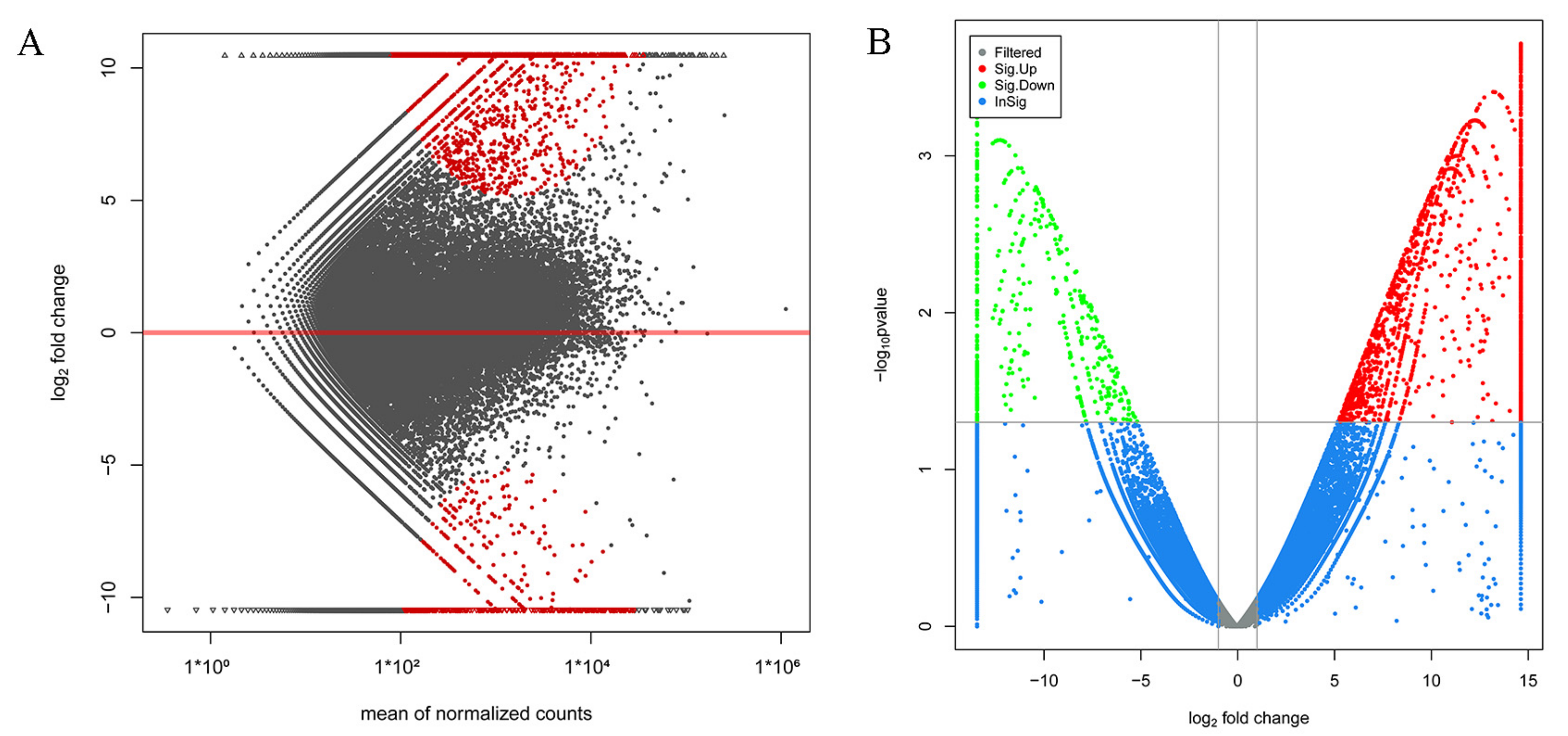
The assembled transcriptome was utilized as a reference for gene expression analysis in the testes and ovaries of M. rosenbergii, and many of the genes were searched to become DEGs. Of these, 484 DEGs (down-regulated) were significantly highly expressed in the testis of M. rosenbergii, while 1665 DEGs (up-regulated) were significantly highly expressed in the ovary of M. rosenbergii. Figure 1A,B shows the distribution of these DEGs, among which 699 DEGs were expressed only in the ovaries of M. rosenbergii, and 191 DEGs were only expressed in the testes of M. rosenbergii. These DEGs could help to clarify the molecular mechanism of sexual differentiation in M. rosenbergii. Table S4 shows the annotation details of all the DEGs.
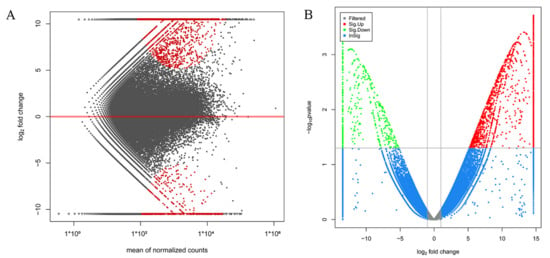
Figure 1.
MA map and volcano map of DEGs. (A) is the MA map of the DEGs’ distribution in the testis and in the ovary of M. rosenbergii; the horizontal x-axis is the normalized average, and the y-axis is a log2 FoldChange. Each dot represents one gene. The red dots represent the DEGs. (B) is a volcano plot of the DEGs’ distribution in the testis and the ovary of M. rosenbergii; the horizontal axis is a log2 FoldChange, and the vertical axis is a log10 p-value. Each dot represents one gene. The DEGs are marked in red and green.
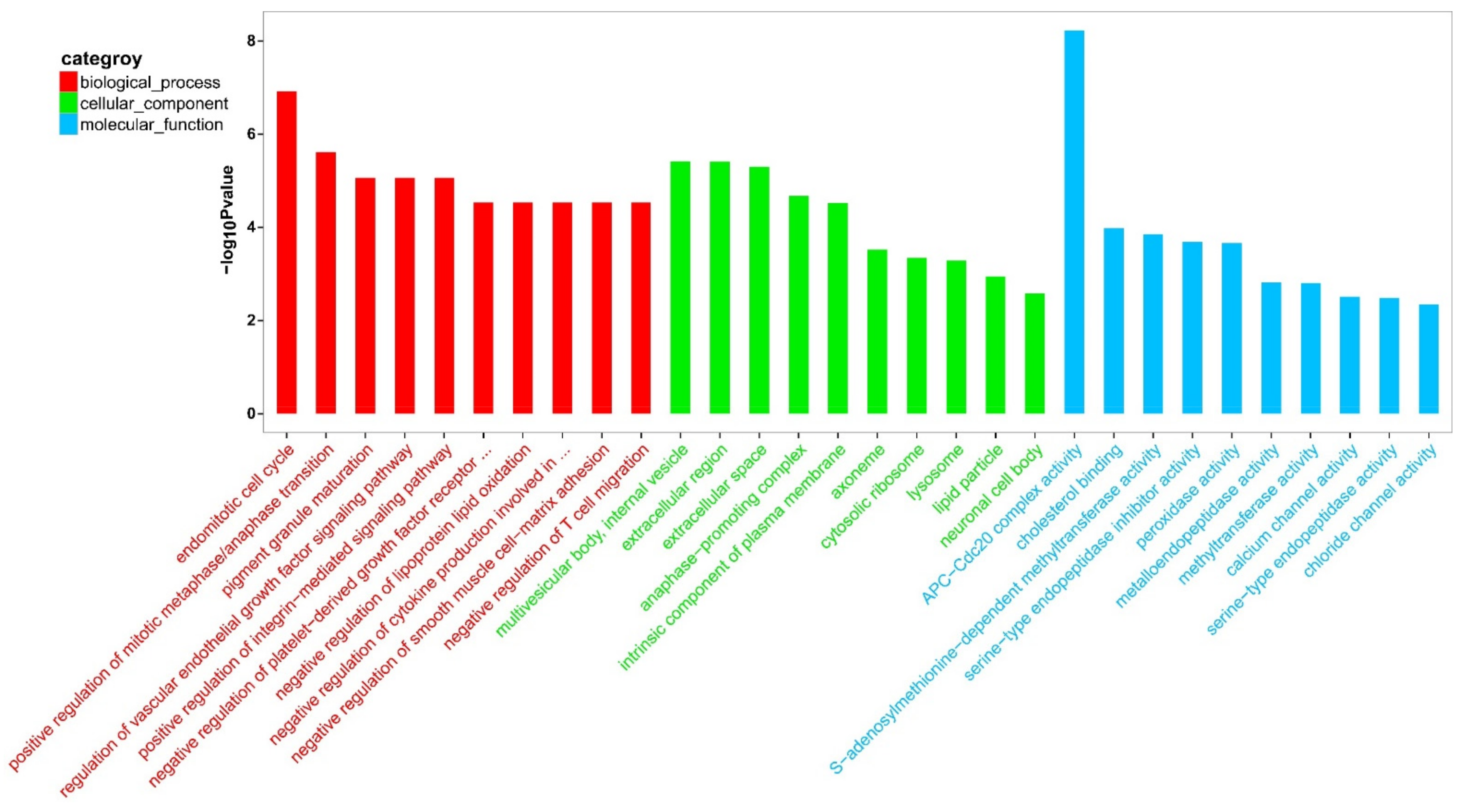
To better understand the biological functions of the DEGs, a GO functional enrichment analysis was conducted on M. rosenbergii’s ovaries and testes. The GO functional enrichment analysis of the DEGs showed many GO terms to be expressively enriched with a corrected p-value cutoff of 0.01. The top 30 GO terms are shown in Figure 2. Between the testis and the ovary, “APC-Cdc20 complex activity” (GO:0090302) and “endomitotic cell cycle” (GO:0007113) were the predominant subclasses in the molecular function and biological process. Regarding the cellular component category, “multivesicular body,” “internal vesicle” (GO:0097487), “extracellular region” (GO:0005576), and “extracellular space” (GO:0005615) were the most predominant subclasses. In addition, some gonadal development and sexual differentiation-related GO terms, including “post-embryonic root development” (GO:0048528), “uterus development” (GO:0060065), “hatching behavior” (GO:0035187), “sperm-egg recognition” (GO:0035036), “embryonic process involved in female pregnancy” (GO:0060136), “embryonic body morphogenesis” (GO:0010172), “embryonic camera-type eye morphogenesis” (GO:0048596), “post-embryonic development” (GO:0009791), “blastoderm segmentation” (GO:0007350), “sperm midpiece” (GO:0097225), “embryonic hemopoiesis” (GO:0035162), “male gonad development” (GO:0008584), “male courtship behavior” (GO:0008049), “germ cell development” (GO:0007281), “spermatogenesis” (GO:0007283), and “oogenesis” (GO:0048477) were also significantly enriched (Table S5).
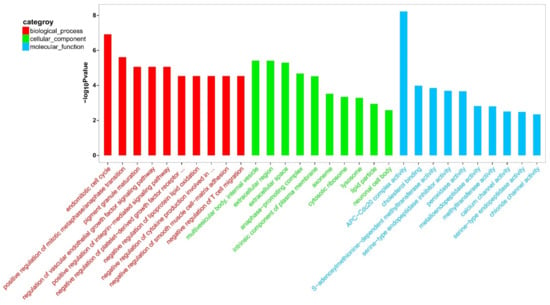
Figure 2.
GO category analysis of top30 entry map. The horizontal axis in the figure is the name of the GO entry, and the vertical axis is a −log10 p-value.
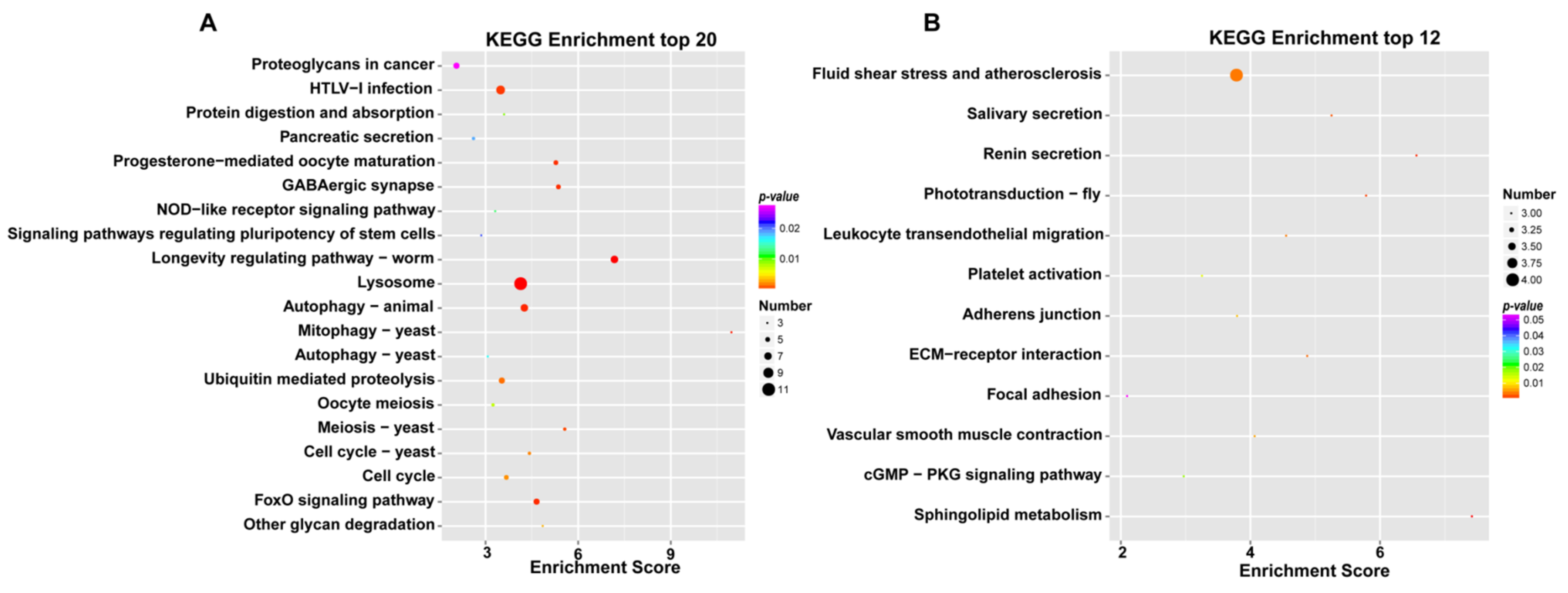
In addition, the KEGG pathway analysis further determined the sets of the DEGs, which were included in certain biological functions. The KEGG analysis of the female and male M. rosenbergii transcriptome revealed the vast majority of the KEGG pathways of female M. rosenbergii to be involved in the “longevity regulating pathway” (ko04212), “lysosome” (ko04142), “progesterone-mediated oocyte maturation” (ko04914), “oocyte meiosis” (ko04114), “meiosis-yeast” (ko04113), “autophagy-animal” (ko04140), and “HTLV-I infection” (ko05166), which suggests that these metabolic pathways are actively expressed in female M. rosenbergii (Figure 3A). The seven metabolic pathways had the highest enrichment scores in the ovaries of M. rosenbergii. The KEGG analysis of the female and male M. rosenbergii transcriptome revealed the vast majority of the KEGG pathways of male M. rosenbergii to be involved in the “sphingolipid metabolism” (ko00600), “renin secretion” (ko04924), “phototransduction-fly” (ko04745), “salivary secretion” (ko04970), “ECM-receptor interaction” (ko04512), “fluid shear stress and atherosclerosis” (ko05418), “leukocyte transendothelial migration” (ko04670), “vascular smooth muscle contraction” (ko04270), “adherens junction” (ko04520), and “platelet activation” (ko04611), which suggests that these metabolic pathways are actively expressed in male M. rosenbergii (Figure 3B). These 10 metabolic pathways had the highest enrichment scores in the testis of male M. rosenbergii.
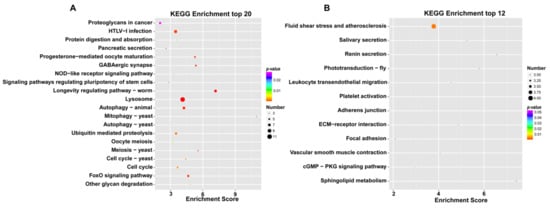
Figure 3.
Bubble chart of KEGG enrichment of DEGs highly in the ovary (A) and testis (B). The x-axis in the figure is the enrichment score. The larger the bubble, the more differential the genes, the red-blue-green-yellow changes, and the enriched p-value increases.
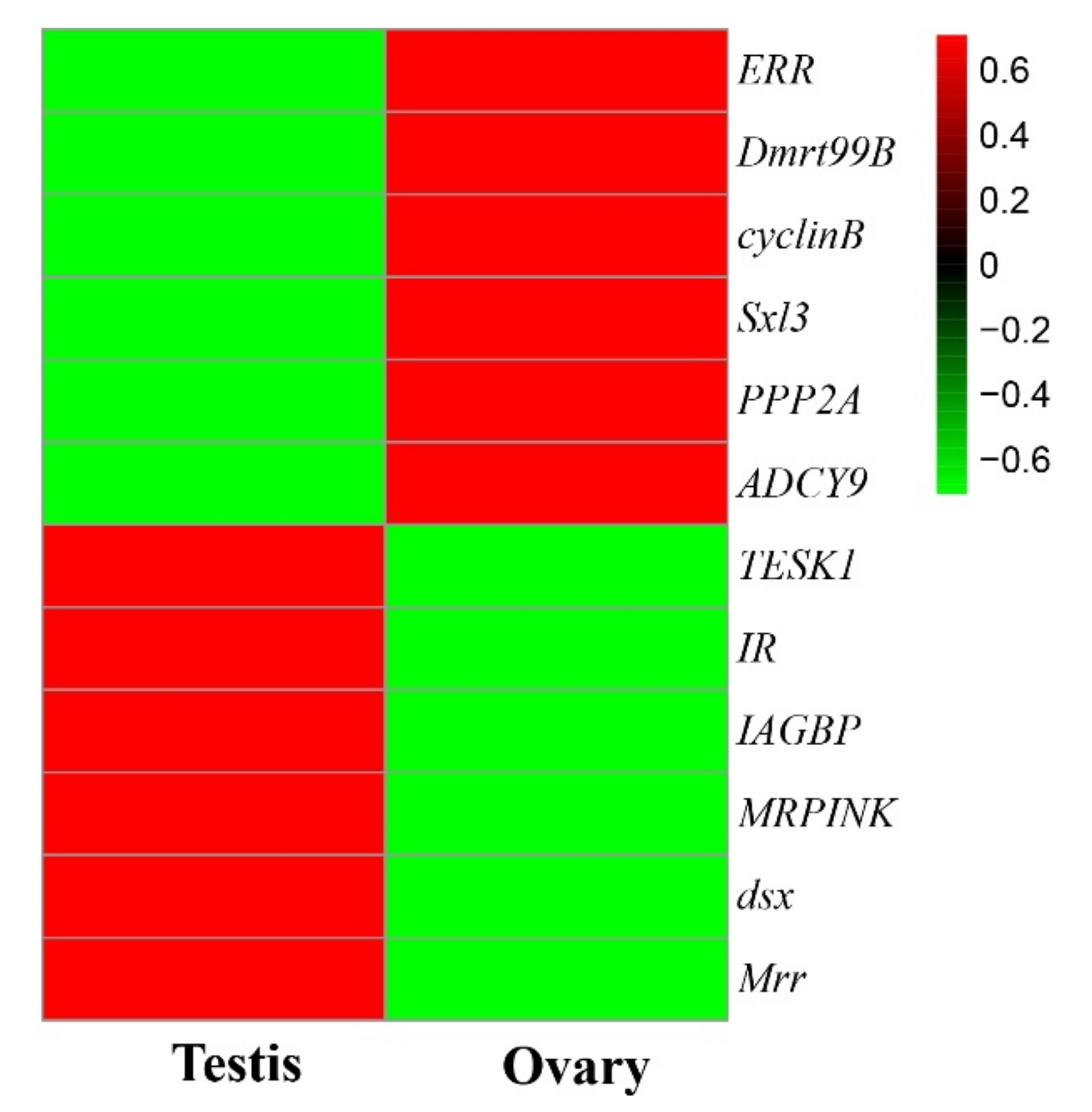
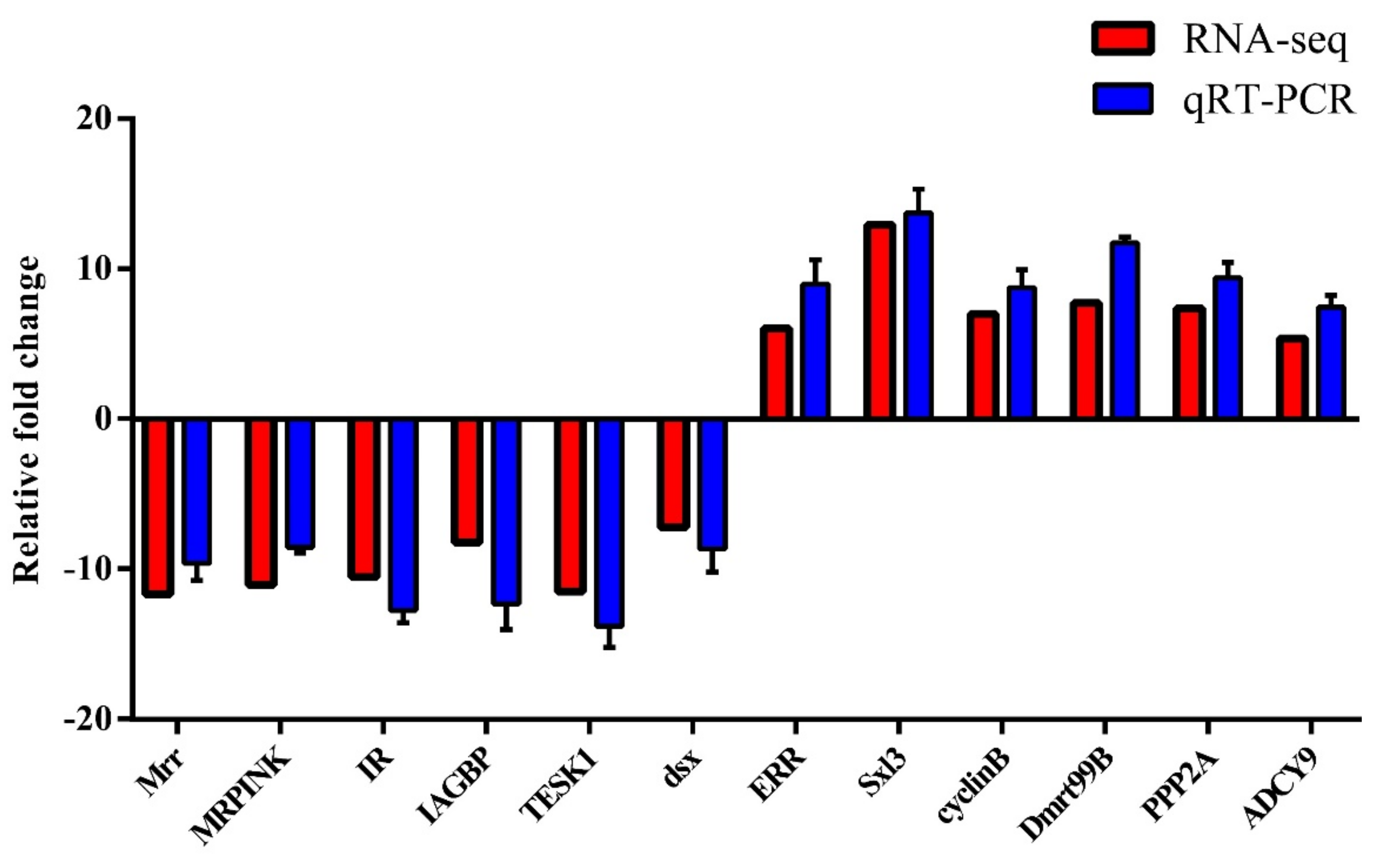
The DEGs were further examined to search for evidence of any potential relationship with sexual differentiation in M. rosenbergii. The previous studies found some genes relating to the development of the testes [9,10,12,13,34,35], including Mrr, MRPINK, IR, IAGBP, testis-specific protein kinase 1 (TESK1), and doublesex gene (dsx) to be up-regulated in the testes, compared to ovary development. At the same time, the transcription of some genes relating to the ovaries ((ERR, Sex-lethal 3 (Sxl3), cyclinB, Doublesex-mab3-related transcription factor 99B (Dmrt99B), PPP2A, and ADCY9)) was found to be significantly upregulated in the ovaries compared to the testes. These candidate genes’ expression levels in the testes and ovaries of M. rosenbergii are significantly different (Figure 4, Table S6), thereby indicating that they are related to the sexual determination and differentiation of M. rosenbergii.
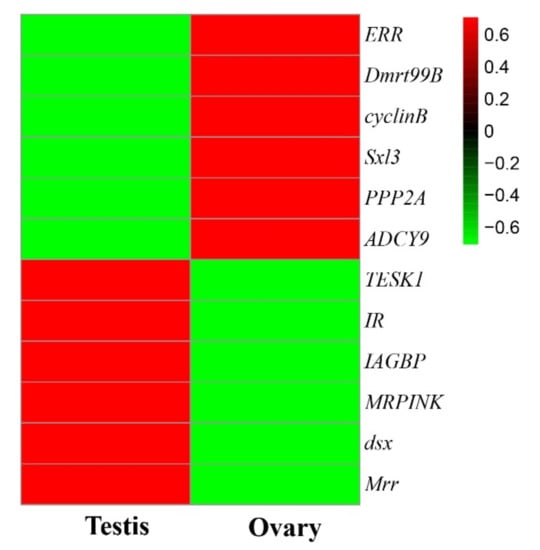
Figure 4.
The hierarchical cluster analysis of the gene relationship with sexual differentiation. Male-related genes (Mrr, MRPINK, IR, IAGBP, TESK1, and dsx) were highly transcribed in the testis, and the transcription levels of female-related genes (ERR, Sxl3, cyclinB, Dmrt99B, PPP2A, and ADCY9) were highly transcribed in the ovary. The upper color key represents the FPKM-normalized z-score with the formula of log10 (FPKM + 1).
3.4. qRT-PCR Validation of DEGs
In order to validate the reliability of the transcriptome data, 12 sex-related DEGs significant to the regulation of gonadal development and sexual differentiation were chosen for the qRT-PCR analysis. These DEGs were Mrr, MRPINK, IR, IAGBP, TESK1, dsx, ERR, Sxl3, cyclinB, Dmrt99B, PPP2A, and ADCY9. RNA-seq expression profiles were compared with the fold changes identified by qRT-PCR. All 12 DEGs showed uniformly consistent results in the transcriptome sequencing data and qRT-PCR (Figure 5), thereby indicating the reliability of the transcriptome sequencing data. These findings serve as a useful genomic resource for validating candidate genes for gonadal development and sexual differentiation in M. rosenbergii.
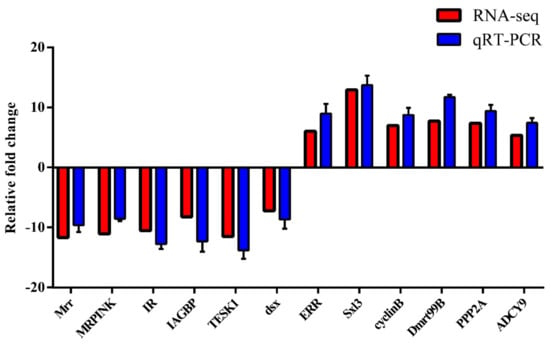
Figure 5.
The qRT-PCR validation of DEGs was analyzed compared with RNA-seq data; the qRT-PCR analysis of 12 selected DEGs: The x-axis is the 12 DEGs, and the y-axis shows the relative mRNA expression levels are based on qRT-PCR, and the log2 FC is based on DGEs’ analysis. The values are shown as the means ± SE.
4. Discussion
The gonads play a vital role in crustacean development and are involved in several important physiological activities, including hormone secretion, fertilization, and gametogenesis [36]. So far, some genes related to the sex differentiation and gonadal development of M. rosenbergii have been studied and reported, but their number is limited, and there are no classification studies on the genes related to males and females [37,38,39]. In addition, there are few reports on signal transduction pathways related to gonad maturation, spermatogenesis, and the oogenesis of M. rosenbergii. Due to the lack of knowledge of the molecular mechanisms of sexual differentiation in M. rosenbergii, high throughput RNA sequencing was performed in this study in order to expand the limited information.
The previous studies have suggested that certain genes are predominantly or exclusively expressed in one sex and drive the phenotypic differences in males and females [40,41]. These sex-biased genes are potentially responsible for phenotypic sexual dimorphism in some aquatic animals [42]. In our current work with M. rosenbergii, the number of DEGs between the testis and ovary has been determined to be 2149. These DEGs possibly contribute to gonadal development, gametogenesis, and even sex determination and differentiation. A similar male-biased phenomenon has also been reported in other crustaceans, including the swimming crab (Portunus trituberculatus) [43].
Multiple candidate genes, which are potentially involved in testis determination and differentiation of M. rosenbergii, were identified (Table S6), which contained Mrr, MRPINK, IR, IAGBP, TESK1, and dsx, all of which are highly expressed in the testes of male M. rosenbergii. These results are consistent with the findings of the previous studies, thereby revealing that the Mrr and MRPINK genes are only specifically and highly expressed in M. rosenbergii testes and that the two genes may be involved in physiological processes relating to male reproduction [9,13]. Furthermore, MRPINK has a regulatory effect on M. rosenbergii fertilization [13,44,45].
The insulin receptor (IR) is a member of the tyrosine kinase receptor superfamily. As an important protein in the signaling pathway of the insulin family, it plays a vital role in the regulation of the homeostasis of the intracellular and intercellular environment [46]. The previous studies have shown that the insulin signaling pathway plays an important role in the gender development process [47]. Sharabi et al. confirmed that Mr-IR silencing significantly affects the androgenic gland (AG), which promotes the up-regulation of the IAG gene expression and a substantial increase in the quantity of immature sperm cells in the distal vas deferens [12]. They suggested that Mr-IR regulates the sexual differentiation of crustaceans by acting on the AG. The previous findings also suggest that Mr-IAG combined with Mr-IAGBP could help the regulation of AG development in M. rosenbergii [10]. In this study, IAGBP was found to exhibit a high expression in M. rosenbergii testes, which indicates that the gene is associated with testis development. The data support the hypothesis that IAG and IAGBP-IR signaling programs exist in M. rosenbergii and play vital roles in gonadal development. TESK1 is a protein serine-threonine kinase with structural features which consist of an N-terminal kinase domain and a C-terminal proline-rich domain. The previous findings have shown the TESK1 gene to be mainly expressed in the testicular germ cells of mice and the expression of the TESK1 gene to be modulated by the CREM transcription activity, which indicates that this gene plays a role in mouse spermatogenesis [35]. Furthermore, a study showed that dsx is essential for the sexual determination of Drosophila melanogaster [34]. The sxl gene controls the sex of Drosophila melanogaster through the regulation of the alternative splicing of the mRNA precursors of the tra and dsx gene [48]. The exact function the TESK1 and dsx genes play in M. rosenbergii remains unknown but is worthy of investigation.
Some well-documented ovary markers (ERR, Sxl3, cyclinB, Dmrt99B, PPP2A, and ADCY9) have been identified as female-biased genes herein, and they are involved in ovarian development and differentiation [14,15]. ERR is regarded as the third subfamily of the nuclear receptor superfamily, and it is involved in the estrogen receptor signaling pathway [49]. As a eukaryotic transcription factor, ERR is essential for ovarian development and the production of sperm [15]. Furthermore, due to the high expression levels in the ovaries of female-biased genes ERR, cyclinB, PPP2A, and ADCY9, the idea is that the ERR may regulate the ovarian development of M. rosenbergii by affecting the expression of cyclinB, PPP2A, and ADCY9 is supported [14].
The Sxl gene is a key factor in the sexual determination of the drosophilid [50]. Yu et al. obtained four Sxl (Sxl1-Sxl4) of M. rosenbergii by degenerating the PCR and cDNA library construction [51]. Among them, Sxl1 is found in spermatogonia, which is suspected to be involved in spermatogenesis; Sxl3 and Sxl4 are specifically and highly expressed in the ovary, which suggests their involvement in ovarian development. These results indicate that the Sxl gene family’s role in the sexual differentiation of M. rosenbergii is one that is complicated. Dmrt (Doublesex and mab3 related transcription factor) is a gene family which plays a vital role in the sexual determination and sexual differentiation [52,53]. The results of this study are in agreement with those of a previous study that discovered the Dmrt99B gene to be specifically and highly expressed in the ovaries. In addition, as M. rosenbergii embryos developed, the expression of the Dmrt99B gene increased gradually [51].
Signal transduction pathways relating to gonadal maturation, spermatogenesis, and oogenesis, including mitogen-activated protein kinase (MAPK), have been identified herein (Table S7). It is believed that the extracellular signal-regulated kinase 2 (Erk2) plays an important role in the regulation of the ERK signal transduction pathway, a member of the MAPK family, during Scylla paramamosain ovarian development [54]. Ubiquitin-related genes exist in the ubiquitin-mediated proteolysis signal pathways, which are involved in the process of crustacean gametogenesis [55]. In eukaryotes, ubiquitin can be fused with ribosomal polypeptide L40 or S27, and it acts as a molecular chaperone in ribosomal biogenesis, participating in cell division and growth [56,57]. The in situ hybridization of ubiquitin-L40 and ubiquitin-S27 found in the ovaries and testes of Eriocheir sinensis reflects their involvement in Eriocheir sinensis gametogenesis [58]. In addition to the signal transduction pathway, which was previously mentioned, we have also reported various genes which play regulatory roles in the crustacean reproduction process, including myosin va and phosphorylatin. The previous studies have indicated that myosin va plays an important role in spermatogenesis in Eriocheir sinensis [59], while the phosphorylated protein plays a vital role in the ovarian development of Penaeus monodon [60]. In addition, we also found some reproductive hormone-related DEGs in the testis and ovary of M. rosenbergii, such as the androgenic gland hormone-like protein (MAL) (NCBI accession number: FJ595507), crustacean female sex hormones (CFSH), vitellogenin receptor (VgR), and neuroparsin (NP). The previous studies have indicated that MAL distributed in the terminal ampulla and sperm of M. rosenbergii participates in regulating sperm proteolytic activity and performs a crucial role in sperm maturation and degradation of the vitelline coat during fertilization in M. rosenbergii [61]. Recent studies have shown that CFSH is involved in the development of a reproductive phenotype in crustaceans, such as the regulation of sex differentiation of early juvenile in Scylla paramamosain [62], suggesting that CFSH may also play an important role in the sex differentiation of M. rosenbergii. Furthermore, the previous studies have shown that the VgR expression is elevated in oocytes during the yolk formation in M. rosenbergii [63]. This is consistent with our report that the expression of VgR in the ovary of M. rosenbergii is much higher than that in the testis, which implies that VgR plays an important role in the ovarian development of M. rosenbergii. In addition, some studies have shown that NP3 is related to ovarian maturation in Macrobrachium japonicus and may be involved in vitellogenesis [64], suggesting that it may be closely related to the development of the ovary in M. rosenbergii.
5. Conclusions
In this study, the transcriptome of the testes and ovaries of M. rosenbergii were sequenced. A total of 75,887 unigenes were collected, and some of the genes were potentially included in signal transduction, gametogenesis, and gonadal development. DEG analysis showed that some genes play a significant part in signal transduction, gametogenesis, and gonadal development. The results of this study suggest that male-related genes (Mrr, MRPINK, IR, IAGBP, TESK1, and dsx) are highly transcribed in the testes of M. rosenbergii. Furthermore, multiple female-related marker genes (ERR, Sxl3, cyclinB, PPP2A, ADCY9, and Dmrt99B) in the ovaries were distinctly up-regulated, which may be related to ovarian development. Our results are in agreement with the previous findings, which suggest that IAG and IAGBP-IAG receptor signaling schemes play important roles in the testis’ development and that ERR is involved in ovarian development through the regulation of the expressions of cyclinB, PPP2A, and ADCY9 in M. rosenbergii. Identifying the potential candidate genes and regulatory pathways relating to gonadal development in M. rosenbergii will provide a better understanding of the basic molecular mechanisms of this important process. It is anticipated that the results will be important for a comparative study of the genomics of other prawns and relevant crustacean species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jmse10060737/s1. Table S1, the growth performance of the sample individuals is listed. Table S2, primers used in the present study. Table S3, the unigenes were also annotated against the KEGG database. Table S4, the analysis and functional enrichment of differentially expressed genes (DEGs). Table S5, the GO functional enrichment analysis of the DEGs. Table S6, some important genes related to sexual differentiation. Table S7, some KEGG signaling pathway of the DEGs. Figure S1, length distribution of unigenes of testis and ovarian transcriptome of M. rosenbergii. Figure S2, number of unigenes annotated to different databases. Figure S3, distribution of the top 10 species with high sequence similarity to M. rosenbergii unigenes. Figure S4, GO classification of the assembled unigenes of M. rosenbergii of testis and ovary. Figure S5, KOG annotation analysis of the unigenes.
Author Contributions
K.Z. and L.L. conceived and designed the project. G.Y. and K.Z. participated in the data analysis and figures preparation. K.Z. and G.Y. prepared the manuscript. K.Z. and L.L. revised the manuscript. Z.L., Z.Q., L.Z., R.L. and G.P. participated in discussions and provided suggestions. All authors have read and agreed to the published version of the manuscript.
Funding
This work was jointly supported by the “Innovation and Strong Universities” special fund from the Department of Education of Guangdong Province (KA2001960); the National Natural Science Foundation of China (31902409, 31872606, 31572657, U1701233); Foundation of Guangdong Provincial Marine and Fisheries Bureau (GDME-2018C006, D21822202); Foundation of China-ASEAN Maritime Cooperation (CAMC-2018F); Guangdong Provincial Special Fund For Modern Agriculture Industry Technology Innovation Teams (2019KJ141).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw sequences supporting the conclusions of this article were deposited into the NCBI Sequence Read Archive (SRA) database under the accession number PRJNA728824.
Acknowledgments
We acknowledge the support received from Jin Yang Aquaculture Co. Ltd., Guangzhou, China.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- New, M. Freshwater prawn farming: Global status, recent research and a glance at the future. Aquac. Res. 2005, 36, 210–230. [Google Scholar] [CrossRef]
- Sagi, A.; Aflalo, E. The androgenic gland and monosex culture of freshwater prawn Macrobrachium rosenbergii (De Man): A biotechnological perspective. Aquac. Res. 2005, 36, 231–237. [Google Scholar] [CrossRef]
- Nair, C.M.; Salin, K.R.; Raju, M.; Sebastian, M. Economic analysis of monosex culture of giant freshwater prawn (Macrobrachium rosenbergii De Man): A case study. Aquac. Res. 2006, 37, 949–954. [Google Scholar] [CrossRef]
- Waiho, K.; Shi, X.; Fazhan, H.; Li, S.; Zhang, Y.; Zheng, H.; Liu, W.; Fang, S.; Ikhwanuddin, M.; Ma, H. High-Density Genetic Linkage Maps Provide Novel Insights Into ZW/ZZ Sex Determination System and Growth Performance in Mud Crab (Scylla paramamosain). Front. Genet. 2019, 10, 298. [Google Scholar] [CrossRef]
- Benzie, J.; Kenway, M.; Ballment, E. Growth of Penaeus monodon X Penaeus esculentus tiger prawn hybrids relative to the parental species. Aquaculture 2001, 193, 227–237. [Google Scholar] [CrossRef]
- Coman, F.; Sellars, M.; Norris, B.; Coman, G.; Preston, N. The effects of triploidy on Penaeus (Marsupenaeus) japonicus (Bate) survival, growth and gender when compared to diploid siblings. Aquaculture 2008, 276, 50–59. [Google Scholar] [CrossRef]
- Ma, K.Y.; Yu, S.H.; Du, Y.X.; Feng, S.Q.; Qiu, L.J.; Ke, D.Y.; Luo, M.Z.; Qiu, G.F. Construction of a Genomic Bacterial Artificial Chromosome (BAC) Library for the Prawn Macrobrachium rosenbergii and Initial Analysis of ZW Chromosome-Derived BAC Inserts. Mar. Biotechnol. 2019, 21, 206–216. [Google Scholar] [CrossRef]
- Aflalo, E.; Hoang, T.T.T.; Nguyen, V.H.; Lam, Q.; Nguyen, D.M.; Trinh, Q.S.; Raviv, S.; Sagi, A. A novel two-step procedure for mass production of all-male populations of the giant freshwater prawn Macrobrachium rosenbergii. Aquaculture 2006, 256, 468–478. [Google Scholar] [CrossRef]
- Phoungpetchara, I.; Tinikul, Y.; Poljaroen, J.; Changklungmoa, N.; Siangcham, T.; Sroyraya, M.; Chotwiwatthanakun, C.; Vanichviriyakit, R.; Hanna, P.J.; Sobhon, P. Expression of the male reproduction-related gene (Mar-Mrr) in the spermatic duct of the giant freshwater prawn, Macrobrachium rosenbergii. Cell Tissue Res. 2012, 348, 609–623. [Google Scholar] [CrossRef]
- Yang, G.; Lu, Z.; Qin, Z.; Zhao, L.; Pan, G.; Shen, H.; Zhang, M.; Liang, R.; Lin, L.; Zhang, K. Insight into the Regulatory Relationships between the Insulin-Like Androgenic Gland Hormone Gene and the Insulin-Like Androgenic Gland Hormone-binding Protein Gene in Giant Freshwater Prawns (Macrobrachium rosenbergii). Int. J. Mol. Sci. 2020, 21, 4207. [Google Scholar] [CrossRef]
- Cao, J.X.; Yin, G.L.; Yang, W.J. Identification of a novel male reproduction-related gene and its regulated expression patterns in the prawn, Macrobrachium rosenbergii. Peptides 2006, 27, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, O.; Manor, R.; Weil, S.; Aflalo, E.D.; Lezer, Y.; Levy, T.; Aizen, J.; Ventura, T.; Mather, P.B.; Khalaila, I.; et al. Identification and Characterization of an Insulin-Like Receptor Involved in Crustacean Reproduction. Endocrinology 2016, 157, 928–941. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, W.M.; Dai, J.Q.; Feng, C.Z.; Yang, F.; Ohira, T.; Nagasawa, H.; Yang, W.J. Inhibition of a novel sperm gelatinase in prawn sperm by the male reproduction-related Kazal-type peptidase inhibitor. Mol. Reprod. Dev. 2008, 75, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Deng, S.P.; Jiang, D.N.; Chen, H.P.; Guang-Li, L.I.; Tian-Li, W.U.; Tian, C.X.; Zhu, C.H.; University, G.O. Screening of Ovarian Genes Associated with Reproduction in Macrobrachium rosenbergii and Their Changes in Expression Pattern in Different Development Stages after ERR Interference. J. Guangdong Ocean Univ. 2018, 3, 8–16. [Google Scholar] [CrossRef]
- Zhao, M.X.; Chen, H.P.; Liu, J.L.; Deng, S.P.; Guang-Li, L.I.; Zhu, C.H.; Hong, Y.C. Prokaryotic Expression and Purification of Estrogen Related Receptor(ERR) Gene from Macrobrachium rosenbergii. J. Guangdong Ocean Univ. 2017, 1, 108–112. [Google Scholar] [CrossRef]
- Ventura, T.; Manor, R.; Aflalo, E.D.; Weil, S.; Raviv, S.; Glazer, L.; Sagi, A. Temporal silencing of an androgenic gland-specific insulin-like gene affecting phenotypical gender differences and spermatogenesis. Endocrinology 2009, 150, 1278–1286. [Google Scholar] [CrossRef]
- Ventura, T.; Manor, R.; Aflalo, E.D.; Weil, S.; Rosen, O.; Sagi, A. Timing sexual differentiation: Full functional sex reversal achieved through silencing of a single insulin-like gene in the prawn, Macrobrachium rosenbergii. Biol. Reprod. 2012, 86, 90. [Google Scholar] [CrossRef]
- Ventura, T.; Rosen, O.; Sagi, A. From the discovery of the crustacean androgenic gland to the insulin-like hormone in six decades. Gen. Comp. Endocrinol. 2011, 173, 381–388. [Google Scholar] [CrossRef]
- Meeratana, P.; Sobhon, P. Classification of differentiating oocytes during ovarian cycle in the giant freshwater prawn, Macrobrachium rosenbergii De Man. Aquaculture 2007, 270, 249–258. [Google Scholar] [CrossRef]
- Chen, J.; Liu, P.; Li, Z.; Chen, Y.; Qiu, G.-F. The cloning of the cdk2 transcript and the localization of its expression during gametogenesis in the freshwater giant prawn, Macrobrachium rosenbergii. Mol. Biol. Rep. 2013, 40, 4781–4790. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Deng, Y.Y.; Li, J.Q.; Wu, S.F.; Zhu, Y.; Chen, Y.W.; He, F.C. Integrated nr Database in Protein Annotation System and Its Localization. Comput. Eng. 2006, 32, 71–72. [Google Scholar]
- Apweiler, R.; Bairoch, A.; Wu, C.H.; Barker, W.C.; Boeckmann, B.; Ferro, S.; Gasteiger, E.; Huang, H.; Lopez, R.; Magrane, M.; et al. UniProt: The Universal Protein knowledgebase. Nucleic Acids Res. 2004, 32, D115–D119. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Fedorova, N.D.; Jackson, J.D.; Jacobs, A.R.; Krylov, D.M.; Makarova, K.S.; Mazumder, R.; Mekhedov, S.L.; Nikolskaya, A.N.; Rao, B.S.; et al. A comprehensive evolutionary classification of proteins encoded in complete eukaryotic genomes. Genome Biol. 2004, 5, R7. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Kobayashi, K.; Watanabe, H.; Iguchi, T. Environmental sex determination in the branchiopod crustacean Daphnia magna: Deep conservation of a Doublesex gene in the sex-determining pathway. PLoS Genet. 2011, 7, e1001345. [Google Scholar] [CrossRef] [PubMed]
- Toshima, J.; Nakagawara, K.; Mori, M.; Noda, T.; Mizuno, K. Structural organization and chromosomal localization of the mouse tesk1 (testis-specific protein kinase 1) gene. Gene 1998, 206, 237–245. [Google Scholar] [CrossRef]
- Nagaraju, G.P. Reproductive regulators in decapod crustaceans: An overview. J. Exp. Biol. 2011, 214, 3–16. [Google Scholar] [CrossRef]
- Guo, H.; Chen, L.L.; Li, G.L.; Deng, S.P.; Zhu, C.H. Accumulation and Depuration of Nonylphenol and Its Effect on the Expressions of Vitellogenin and Vitellogenin Receptor in Freshwater Prawn Macrobrachium rosenbergii. Bull. Environ. Contam. Toxicol. 2019, 103, 729–733. [Google Scholar] [CrossRef]
- Jiang, J.; Yuan, X.; Qiu, Q.; Huang, G.; Jiang, Q.; Fu, P.; Zhang, Y.; Jia, Y.; Yang, X.; Jiang, H. Comparative Transcriptome Analysis of Gonads for the Identification of Sex-Related Genes in Giant Freshwater Prawns (Macrobrachium rosenbergii) Using RNA Sequencing. Genes 2019, 10, 1035. [Google Scholar] [CrossRef]
- Peranandam, R.; Palanisamy, I.; Lourdaraj, A.V.; Natesan, M.; Vimalananthan, A.P.; Thangaiyan, S.; Perumal, A.; Muthukalingan, K. TBT effects on the development of intersex (ovotestis) in female fresh water prawn Macrobrachium rosenbergii. BioMed Res. Int. 2014, 2014, 412619. [Google Scholar] [CrossRef]
- Assis, R.; Zhou, Q.; Bachtrog, D. Sex-biased transcriptome evolution in Drosophila. Genome Biol. Evol. 2012, 4, 1189–1200. [Google Scholar] [CrossRef]
- Ellegren, H.; Parsch, J. The evolution of sex-biased genes and sex-biased gene expression. Nat. Rev. Genet. 2007, 8, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Small, C.M.; Carney, G.E.; Mo, Q.; Vannucci, M.; Jones, A.G. A microarray analysis of sex- and gonad-biased gene expression in the zebrafish: Evidence for masculinization of the transcriptome. BMC Genom. 2009, 10, 579. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, L.; Guan, W.; Zhou, C.; Tang, B.; Cheng, Y.; Huang, J.; Xuan, F. De novo transcriptome sequencing and analysis of male and female swimming crab (Portunus trituberculatus) reproductive systems during mating embrace (stage II). BMC Genet. 2018, 19, 3. [Google Scholar] [CrossRef]
- Cao, J.X.; Dai, J.Q.; Dai, Z.M.; Yin, G.L.; Yang, W.J. A male reproduction-related Kazal-type peptidase inhibitor gene in the prawn, Macrobrachium rosenbergii: Molecular characterization and expression patterns. Mar. Biotechnol. 2007, 9, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qian, Y.Q.; Ma, W.M.; Yang, W.J. Inhibition mechanism and the effects of structure on activity of male reproduction-related peptidase inhibitor Kazal-type (MRPINK) of Macrobrachium rosenbergii. Mar. Biotechnol. 2009, 11, 252–259. [Google Scholar] [CrossRef]
- Fafalios, A.; Ma, J.; Tan, X.; Stoops, J.; Luo, J.; Defrances, M.C.; Zarnegar, R. A hepatocyte growth factor receptor (Met)-insulin receptor hybrid governs hepatic glucose metabolism. Nat. Med. 2011, 17, 1577–1584. [Google Scholar] [CrossRef]
- Nef, S.; Verma-Kurvari, S.; Merenmies, J.; Vassalli, J.D.; Efstratiadis, A.; Accili, D.; Parada, L.F. Testis determination requires insulin receptor family function in mice. Nature 2003, 426, 291–295. [Google Scholar] [CrossRef]
- Toyota, K.; Kato, Y.; Sato, M.; Sugiura, N.; Miyagawa, S.; Miyakawa, H.; Watanabe, H.; Oda, S.; Ogino, Y.; Hiruta, C.; et al. Molecular cloning of doublesex genes of four cladocera (water flea) species. BMC Genom. 2013, 14, 239. [Google Scholar] [CrossRef]
- Escriva, H.; Delaunay, F.; Laudet, V. Ligand binding and nuclear receptor evolution. BioEssays News Rev. Mol. Cell. Dev. Biol. 2000, 22, 717–727. [Google Scholar] [CrossRef]
- Song, Y. Cloning and Prokaryotic Expression of Bmand-Sxl Gene In Bombyx mandarina. Jiangsu Seric. 2009, 31, 14–18. [Google Scholar] [CrossRef]
- Fei, J.M.; Shi, L.L.; Li, Y.H. Cloning and expression analysis of the sex-lethal gene of Procterus cruzi. J. Huazhong Agric Univ 2021, 40, 120–128. [Google Scholar] [CrossRef]
- Kopp, A. Dmrt genes in the development and evolution of sexual dimorphism. Trends Genet. TIG 2012, 28, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wilken, J.; Phillips, N.B.; Narendra, U.; Chan, G.; Stratton, S.M.; Kent, S.B.; Weiss, M.A. Sexual dimorphism in diverse metazoans is regulated by a novel class of intertwined zinc fingers. Genes Dev. 2000, 14, 1750–1764. [Google Scholar] [CrossRef]
- Ma, A.; Wang, Y.; Zou, Z.; Fu, M.; Lin, P.; Zhang, Z. Erk2 in ovarian development of green mud crab Scylla paramamosain. DNA Cell Biol. 2012, 31, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Zhang, Z.; Wang, Y.; Wang, G.; Chen, Y.; Lin, P.; Wang, S.; Zou, Z. Differential expression of ubiquitin-conjugating enzyme E2r in the developing ovary and testis of penaeid shrimp Marsupenaeus japonicus. Mol. Biol. Rep. 2009, 36, 1149–1157. [Google Scholar] [CrossRef]
- Archibald, J.M.; Teh, E.M.; Keeling, P.J. Novel ubiquitin fusion proteins: Ribosomal protein P1 and actin. J. Mol. Biol. 2003, 328, 771–778. [Google Scholar] [CrossRef]
- Finley, D.; Bartel, B.; Varshavsky, A. The tails of ubiquitin precursors are ribosomal proteins whose fusion to ubiquitin facilitates ribosome biogenesis. Nature 1989, 338, 394–401. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, L.; Wang, Y.; Li, W.; He, L.; Jiang, H. Expression characteristics of two ubiquitin/ribosomal fusion protein genes in the developing testis, accessory gonad and ovary of Chinese mitten crab, Eriocheir sinensis. Mol. Biol. Rep. 2012, 39, 6683–6692. [Google Scholar] [CrossRef]
- Sun, X.; He, Y.; Hou, L.; Yang, W.X. Myosin Va participates in acrosomal formation and nuclear morphogenesis during spermatogenesis of Chinese mitten crab Eriocheir sinensis. PLoS ONE 2010, 5, e12738. [Google Scholar] [CrossRef]
- Zhou, J.; Qiu, L.; Jiang, S.; Zhou, F.; Huang, J.; Yang, L.; Su, T.; Zhang, D. Molecular cloning and mRNA expression of M-phase phosphoprotein 6 gene in black tiger shrimp (Penaeus monodon). Mol. Biol. Rep. 2013, 40, 1301–1306. [Google Scholar] [CrossRef]
- Ma, W.M.; Qian, Y.Q.; Wang, M.R.; Yang, F.; Yang, W.J. A novel terminal ampullae peptide is involved in the proteolytic activity of sperm in the prawn, Macrobrachium rosenbergii. Reproduction 2010, 140, 235–245. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiang, Q.; Lu, B.; Lin, D.; Huang, H.; Chen, X.; Ye, H. Role of crustacean female sex hormone (CFSH) in sex differentiation in early juvenile mud crabs, Scylla paramamosain. Gen. Comp. Endocrinol. 2020, 289, 113383. [Google Scholar] [CrossRef] [PubMed]
- Roth, Z.; Khalaila, I. Identification and characterization of the vitellogenin receptor in Macrobrachium rosenbergii and its expression during vitellogenesis. Mol. Reprod. Dev. 2012, 79, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Xiong, Y.; Jiang, S.; Zhang, W.; Xu, L.; Jin, S.; Gong, Y.; Wu, Y.; Fu, H. Three neuroparsin genes from oriental river prawn, Macrobrachium nipponense, involved in ovary maturation. 3 Biotech 2020, 10, 537. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).