Effects of Irradiance and Temperature on the Photosynthesis of the Crustose Coralline Algae Pneophyllum fragile (Corallinales, Rhodophyta) in the Coastal Waters of Korea
Abstract
1. Introduction
2. Materials and Methods
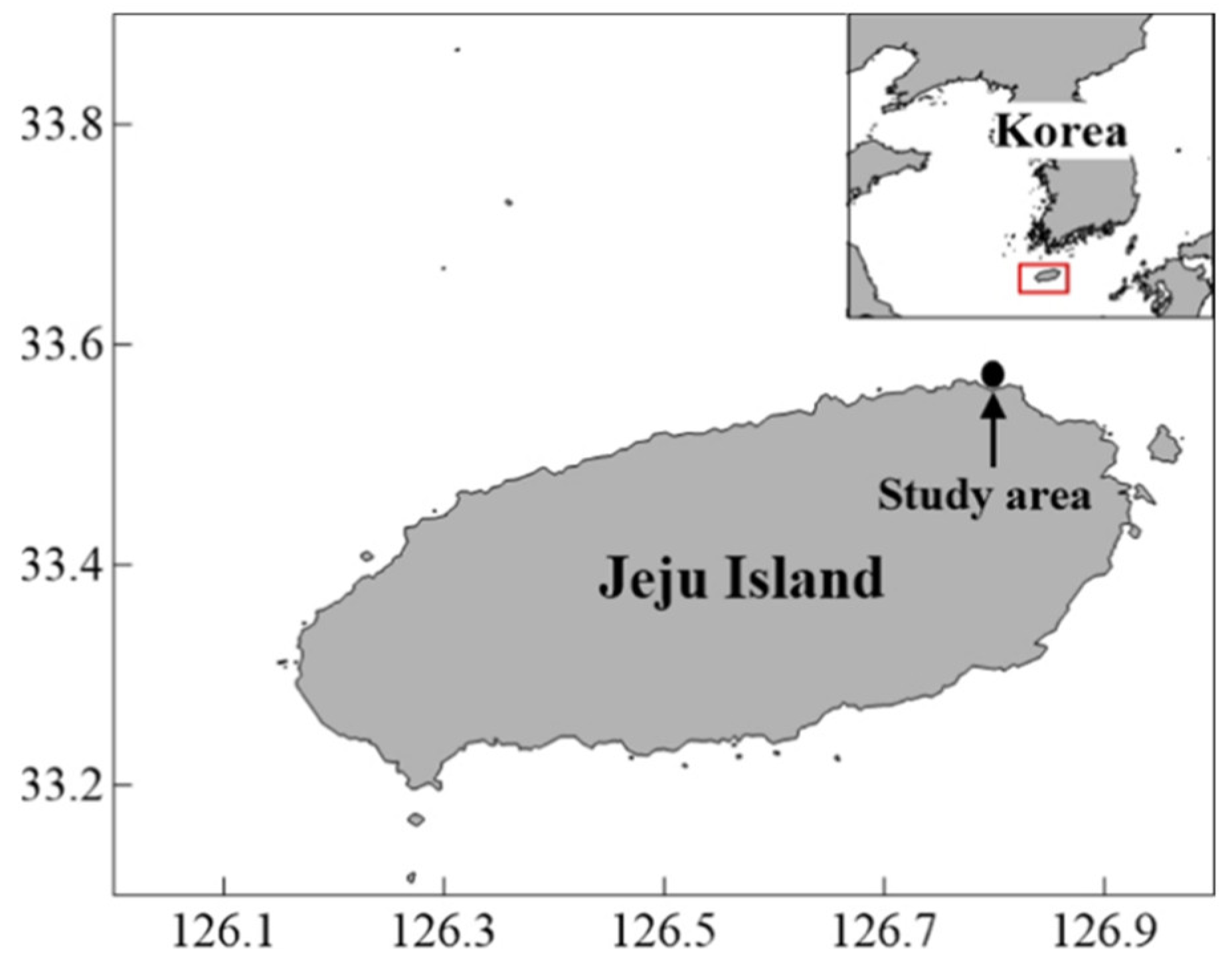
2.1. Study Area
2.2. Sample Collection
2.3. Experimental Setup
2.4. Photosynthesis and Respiration Measurement
2.5. Calculation of Net Photosynthesis and Respiration
3. Results
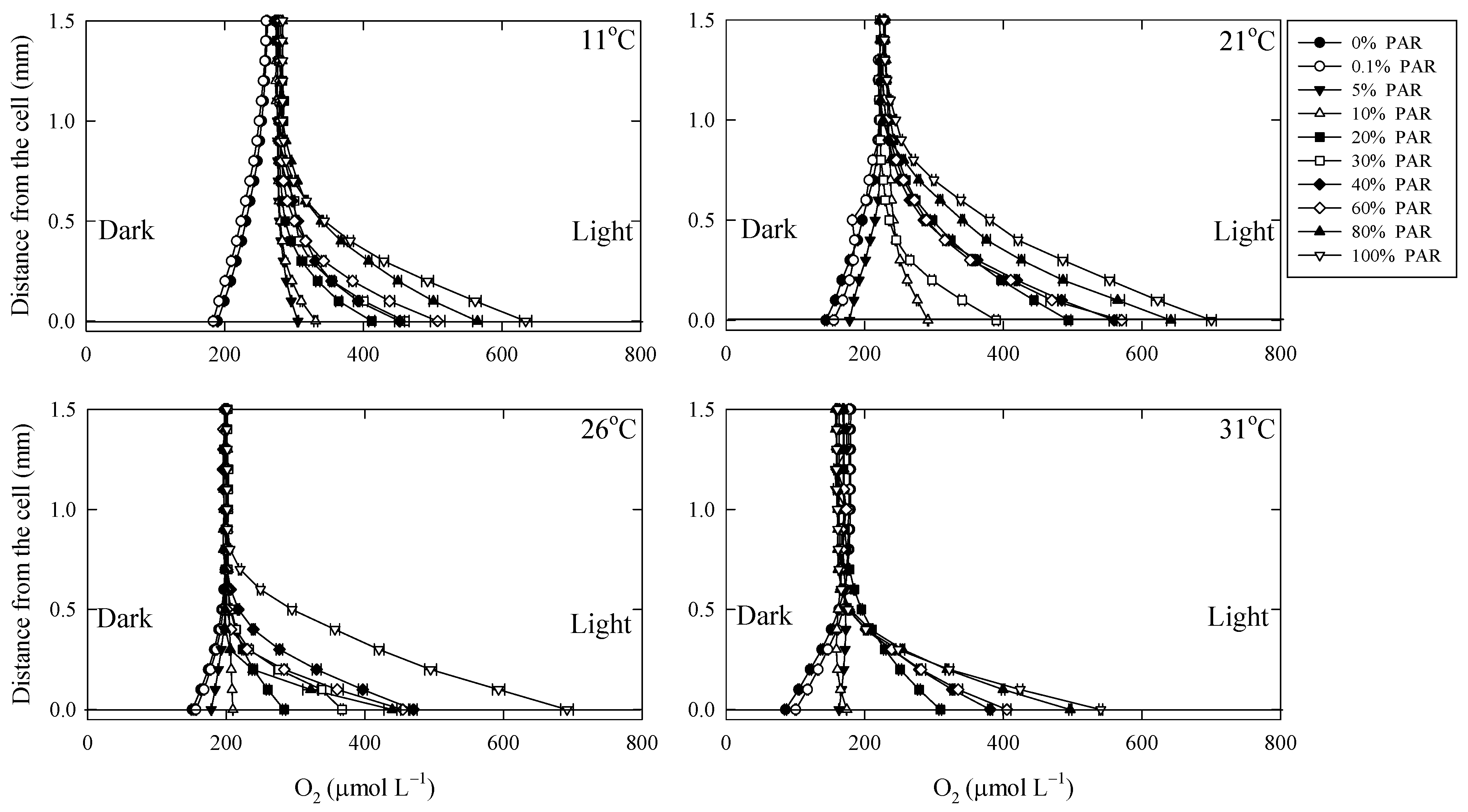
3.1. O2 in the DBL
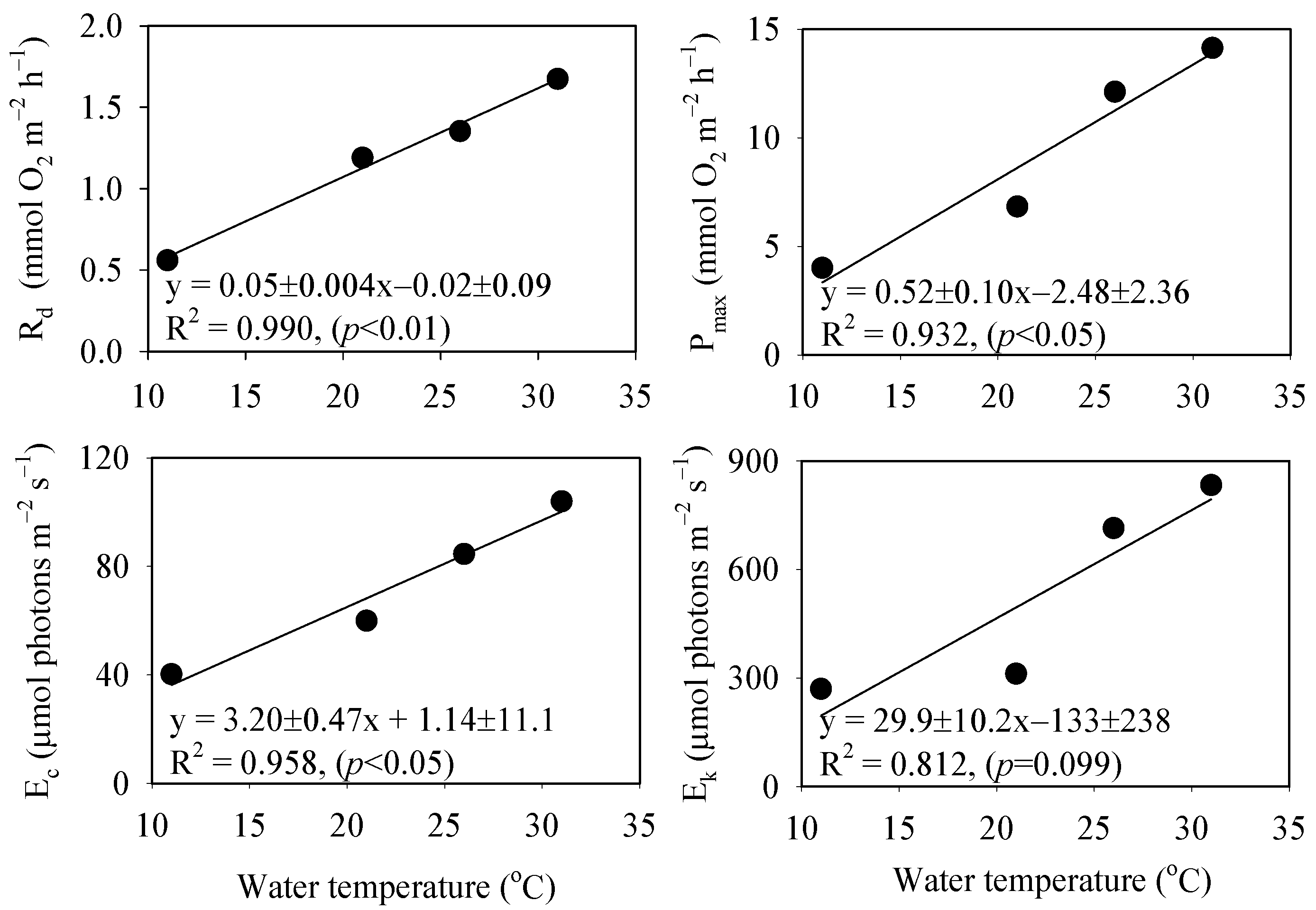
3.2. Effects of Irradiance and Temperature on Respiration and Photosynthesis
4. Discussion
4.1. O2 Dynamics in the DBL
4.2. Rates of Respiration and Photosynthesis of P. fragile
4.3. Environmental Implications for the Coastal Ecosystem
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NIFS. A Study of Restoration of Barren Ground in Jeju Island; NIFS: Busan, Korea, 2007. [Google Scholar]
- Kim, M.-G.; Sin, J.-G.; Cha, J.-H. Variation of species composition of benthic algae and whitening in the coast of Dokdo Island during summer. Algae 2004, 19, 69–78. [Google Scholar] [CrossRef]
- Kim, Y.-D.; Park, M.-S.; Yoo, H.-I.; Min, B.-H.; Moon, T.-S.; Choi, H.-G. Seasonal variation in subtidal seaweed community structure at Hajung, on the southeast coast of Korea. Korean J. Fish. Aquat. Sci. 2011, 44, 740–746. [Google Scholar] [CrossRef][Green Version]
- Hwang, S.-I.; Kim, D.-K.; Sung, B.-J.; Jun, S.-K.; Bae, J.-I.; Jeon, B.-H. Effects of climate change on whitening event proliferation the coast of Jeju. Korean J. Environ. Ecol. 2017, 31, 529–536. [Google Scholar] [CrossRef]
- Roberts, R.D.; Kühl, M.; Glud, R.N.; Rysgaard, S. Primary production of crustose coralline red algae in a high Arctic fjord. J. Phycol. 2002, 38, 273–283. [Google Scholar] [CrossRef]
- Chisholm, J.R. Primary productivity of reef-building crustose coralline algae. Limnol. Oceanogr. 2003, 48, 1376–1387. [Google Scholar]
- Martin, S.; Charnoz, A.; Gattuso, J.-P. Photosynthesis, respiration and calcification in the Mediterranean crustose coralline alga Lithophyllum cabiochae (Corallinales, Rhodophyta). Eur. J. Phycol. 2013, 48, 163–172. [Google Scholar] [CrossRef]
- Johansen, H.W. Coralline Algae, a First Synthesis; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Gattuso, J.-P.; Allemand, D.; Frankingnoulle, M. Photosynthesis and calcification at cellular, organismal and community levels in coral reefs: A review on interactions and control by carbonate chemistry. Am. Zool. 1999, 39, 160–183. [Google Scholar] [CrossRef]
- King, R.J.; Schramm, W. Calcification in the maerl coralline alga Phymatolithon calcareum: Effects of salinity and temperature. Mar. Biol. 1982, 70, 197–204. [Google Scholar] [CrossRef]
- Wilson, S.; Blake, C.; Berges, J.A.; Maggs, C.A. Environmental tolerances of free-living coralline algae (maerl): Implications for European marine conservation. Biol. Conserv. 2004, 120, 279–289. [Google Scholar] [CrossRef]
- Martin, S.; Castets, M.-D.; Clavier, J. Primary production, respiration and calcification of the temperate free-living coralline alga Lithothamnion corallioides. Aquat. Bot. 2006, 85, 121–128. [Google Scholar] [CrossRef]
- Egilsdottir, H.; Olafsson, J.; Martin, S. Photosynthesis and calcification in the articulated coralline alga Ellisolandia elongata (Corallinales, Rhodophyta) from intertidal rock pools. Eur. J. Phycol. 2015, 51, 59–70. [Google Scholar] [CrossRef]
- Vasquez-Elizondo, R.M.; Enriquez, S. Coralline algal physiology is more adversely affected by elevated temperature than reduced pH. Sci. Rep. 2016, 6, 19030. [Google Scholar] [CrossRef] [PubMed]
- Sordo, L.; Santos, R.; Barrote, I.; Freitas, C.; Silva, J. Seasonal photosynthesis, respiration, and calcification of a temperate maërl Bed in Southern Portugal. Front. Mar. Sci. 2020, 7, 136. [Google Scholar] [CrossRef]
- Yoshioka, S.; Kato, A.; Koike, K.; Murase, N.; Baba, M.; Liao, L.M. Effects of water temperature, light and nitrate on the growth of sporelings of the non-geniculate coralline alga Lithophyllum okamurae (Corallinales, Rhodophyta). J. Appl. Phycol. 2020, 32, 1923–1931. [Google Scholar] [CrossRef]
- Martin, S.; Clavier, J.; Chauvaud, L.; Thouzeau, G. Community metabolism in temperate maerl beds. I. Carbon and carbonate fluxes. Mar. Ecol. Prog. Ser. 2007, 335, 19–29. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, N.; Moon, H.; Lee, S.; Jeong, S.Y.; Diaz-Pulido, G.; Edwards, M.S.; Kang, J.H.; Kang, E.J.; Oh, H.J. Global warming offsets the ecophysiological stress of ocean acidification on temperate crustose coralline algae. Mar. Pollut. Bull. 2020, 157, 111324. [Google Scholar] [CrossRef]
- Hofmann, L.C.; Koch, M.; de Beer, D. Biotic control of surface pH and evidence of light-induced H+ pumping and Ca2+-H+ exchange in a tropical crustose coralline alga. PLoS ONE 2016, 11, e0159057. [Google Scholar] [CrossRef]
- Revsbech, N.P.; JØrgensen, B.B.; Brix, O. Primary production of microalgae in sediments measured by oxygen microprofile, H14CO3-fixation, and oxygen exchange methods. Limnol. Oceanogr. 1981, 26, 717–730. [Google Scholar] [CrossRef]
- Revsbech, N.P.; Jorgensen, B.B. Photosynthesis of benthic microflora measured with high spatial resolution by the oxygen microprofile method: Capabilities and limitations of the method. Limnol. Oceanogr. 1983, 28, 749–756. [Google Scholar] [CrossRef]
- Kwon, H.K.; Yang, H.S.; Yoon, Y.H.; Choi, O.I.; Choi, I.H.; Oh, S.J. Characteristics of marine environment and primary productivity of phytoplankton in the seaweed bed of northwestern coast of Jeju Island during autumn 2014. Sea 2015, 20, 180–191. [Google Scholar] [CrossRef]
- Larkum, A.W.D.; Koch, E.M.W.; Kühl, M. Diffusive boundary layers and photosynthesis of the epilithic algal community of coral reefs. Mar. Biol. 2003, 142, 1073–1082. [Google Scholar] [CrossRef]
- Song, J.N.; Park, S.K.; Heo, J.S.; Oh, J.C.; Kim, Y.S.; Choi, H.G.; Nam, K.W. Effects of temperature on the spore release and growth of Lithophyllum yessoense and Hildenbrandia rubra. Korean J. Fish. Aquat. Sci. 2013, 46, 296–302. [Google Scholar] [CrossRef][Green Version]
- Song, J.N.; Park, S.K.; Oh, J.C.; Yoo, H.I.; Kim, Y.S.; Choi, H.G.; Nam, K.W. The effects of environmental factors on the growth of Lithophyllum yessoense and Hildenbrandia rubra sporelings in laboratory culture. Korean J. Fish. Aquat. Sci. 2013, 46, 827–834. [Google Scholar] [CrossRef][Green Version]
- Penrose, D.; Woelkerling, W.J. Pneophyllum fragile in southern Australia: Implications for generic concepts in the Mastophoroideae (Corallinaceae, Rhodophyta). Phycologia 1991, 30, 495–506. [Google Scholar] [CrossRef]
- Lorenzen, J.; Glud, R.N.; Revsbech, N.P. Impact of microsensor-caused changes in diffusive boundary layer thickness on O2 profiles and photosynthetic rates in benthic communities of microorganisms. Mar. Ecol. Prog. Ser. 1995, 119, 237–241. [Google Scholar] [CrossRef]
- Revsbech, N.P. An oxygen microsensor with a guard cathode. Limnol. Oceanogr. 1989, 34, 474–478. [Google Scholar] [CrossRef]
- Kühl, M.; Cohen, Y.; Dalsgaard, T.; Jørgensen, B.B.; Revsbech, N.P. Microenvironment and photosynthesis of zooxanthellae in scleractinian corals studied with microsensors for O2, pH and light. Mar. Ecol. Prog. Ser. 1995, 117, 159–172. [Google Scholar] [CrossRef]
- Webb, W.L.; Newton, M.; Duane, S. Carbon dioxide exchange of Alnus rubra. A mathematical model. Oecologia 1974, 17, 281–291. [Google Scholar] [CrossRef]
- Jørgensen, B.B.; Revsbech, N.P. Diffusive boundary layers and the oxygen uptake of sediments and detritus. Limnol. Oceanogr. 1985, 30, 111–122. [Google Scholar] [CrossRef]
- Jørgensen, B.B.; Des Marais, D.J. The diffusive boundary layer of sediments: Oxygen microgradients over a microbial mat. Limnol. Oceanogr. 1990, 35, 1343–1355. [Google Scholar] [CrossRef]
- Schubert, N.; Hofmann, L.C.; Almeida Saa, A.C.; Moreira, A.C.; Arenhart, R.G.; Fernandes, C.P.; de Beer, D.; Horta, P.A.; Silva, J. Calcification in free-living coralline algae is strongly influenced by morphology: Implications for susceptibility to ocean acidification. Sci. Rep. 2021, 11, 11232. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberg, M.; Nørregaard, R.D.; Kühl, M. Diffusion or advection? Mass transfer and complex boundary layer landscapes of the brown alga Fucus vesiculosus. J. R. Soc. Interface 2017, 14, 20161015. [Google Scholar] [CrossRef] [PubMed]
- Guy-Haim, T.; Silverman, J.; Wahl, M.; Aguirre, J.; Noisette, F.; Rilov, G. Epiphytes provide micro-scale refuge from ocean acidification. Mar. Environ. Res. 2020, 161, 105093. [Google Scholar] [CrossRef] [PubMed]
- Ichiki, S.; Mizuta, H.; Yasui, H.; Yamamoto, H. Effects of irradiance and water temperature on the photosynthesis and growth of the crustose coralline alga Lithophyllum yessoense Foslie (Corallinales, Rhodophyceae). Bull. Fish. Sci. 2001, 52, 103–109. [Google Scholar]
- Martin, S.; Gattuso, J.-P. Response of mediterranean coralline algae to ocean acidification and elevated temperature. Glob. Chang. Biol. 2009, 15, 2089–2100. [Google Scholar] [CrossRef]
- Burdett, H.L.; Keddie, V.; MacArthur, N.; McDowall, L.; McLeish, J.; Spielvogel, E.; Hatton, A.D.; Kamenos, N.A. Dynamic photoinhibition exhibited by red coralline algae in the Red Sea. BMC Plant Biol. 2014, 14, 139. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Pritchard, D.W.; Desmond, M.J.; Hepburn, C.D. Coralline photosynthetic physiology across a steep light gradient. Photosynth. Res. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Kirk, J.T. Light and Photosynthesis in Aquatic Ecosystems; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Kim, W.; Kim, S.H.; Baek, S.-I.; Kim, H.; Oh, J. A Preliminary Study on Benthic Mapping of Uljin Coast Based on Airborne Hyperspectral Imagery Towards the Whitening Detection. J. Coast. Res. 2021, 114, 464–468. [Google Scholar] [CrossRef]
- Fujita, D. Current status and problems of isoyake in Japan. Bull. Fish. Res. Agen. 2010, 32, 33–42. [Google Scholar]
- NIFS. Study on the Status of Whitening Occurrence in the Waters of Korea; NIFS: Busan, Korea, 2009. [Google Scholar]
- KMA. Korea Climate Change Assessment Report 2020: Climate Change Impact and Adaptation; Ministry of Environment: Seoul, Korea, 2020.
- Ju, S.-J.; Kim, S. Assessment of the Impact of Climate Change on Marine Ecosystem in the South Sea of Korea II. Ocean. Polar Res. 2013, 35, 123–125. [Google Scholar] [CrossRef]
- Kim, H.-S.; Jung, M.-M.; Lee, J.-B. The Korean Peninsula Warming Based on Appearance Trend of Tropical Dinoflagellate Species, Genus Ornithocercus. Sea 2008, 13, 303–307. [Google Scholar]
- Myoung, J.G.; Kim, N.-G.; Myoung, S.-H.; Kim, J.-K.; Lee, Y.-U.; Kim, B.-I. Comparison of the species composition of tropical and subtropical fish observed at the coast of Dokdo and Jejudo by scuba diving. Underw. Sci. Technol. 2017, 16–17, 1–11. [Google Scholar]


| Parameter/Temperature | 11 °C | 21 °C | 26 °C | 31 °C |
|---|---|---|---|---|
| Rd (mmol m−2 h−1) | −0.56 ± 0.18 | −1.19 ± 0.37 | −1.35 ± 0.37 | −1.67 ± 0.26 |
| Pmax (mmol m−2 h−1) | 4.01 ± 0.23 | 6.83 ± 0.51 | 12.1 ± 1.46 | 14.1 ± 1.16 |
| Ec (μmol photon m−2 s−1) | 40.3 | 60.0 | 84.6 | 104 |
| Ek (μmol photon m−2 s−1) | 270 | 312 | 714 | 833 |
| Species | Region | Temperature (°C) | Reference |
|---|---|---|---|
| Phymatolithon lusitanicum | Portugal | 24 | [15] |
| Lithophyllum yessoense | Japan | 15 | [36] |
| Lithophyllum yessoense | Korea | 20 | [24] |
| Hildenbrandia rubra | 25 | ||
| Neogoniolithon sp. (rhodolith) | Mexico | 30 | [14] |
| Amphiroa tribulus | |||
| Lithothamnion sp. (CCA) | |||
| Pneophyllum fragile | Jeju | ≥31 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, J.-W.; Lee, J.S.; Kim, S.-H.; Lee, T.; Jung, S.W.; Lee, W.-C.; Kim, K.-T.; An, S.-U. Effects of Irradiance and Temperature on the Photosynthesis of the Crustose Coralline Algae Pneophyllum fragile (Corallinales, Rhodophyta) in the Coastal Waters of Korea. J. Mar. Sci. Eng. 2022, 10, 851. https://doi.org/10.3390/jmse10070851
Baek J-W, Lee JS, Kim S-H, Lee T, Jung SW, Lee W-C, Kim K-T, An S-U. Effects of Irradiance and Temperature on the Photosynthesis of the Crustose Coralline Algae Pneophyllum fragile (Corallinales, Rhodophyta) in the Coastal Waters of Korea. Journal of Marine Science and Engineering. 2022; 10(7):851. https://doi.org/10.3390/jmse10070851
Chicago/Turabian StyleBaek, Ju-Wook, Jae Seong Lee, Sung-Han Kim, Taehee Lee, Seung Won Jung, Won-Chan Lee, Kyung-Tae Kim, and Sung-Uk An. 2022. "Effects of Irradiance and Temperature on the Photosynthesis of the Crustose Coralline Algae Pneophyllum fragile (Corallinales, Rhodophyta) in the Coastal Waters of Korea" Journal of Marine Science and Engineering 10, no. 7: 851. https://doi.org/10.3390/jmse10070851
APA StyleBaek, J.-W., Lee, J. S., Kim, S.-H., Lee, T., Jung, S. W., Lee, W.-C., Kim, K.-T., & An, S.-U. (2022). Effects of Irradiance and Temperature on the Photosynthesis of the Crustose Coralline Algae Pneophyllum fragile (Corallinales, Rhodophyta) in the Coastal Waters of Korea. Journal of Marine Science and Engineering, 10(7), 851. https://doi.org/10.3390/jmse10070851







