Artificial Seaweed Reefs That Support the Establishment of Submerged Aquatic Vegetation Beds and Facilitate Ocean Macroalgal Afforestation: A Review
Abstract
1. Introduction
2. Materials and Methods
3. Benefits of Macroalgal Forests
3.1. CO2 Reduction
3.2. Creation of Marine Habitats
3.3. Products That Aid Human Well-Being and Serve as Functional Foods
3.4. Other Useful Materials
3.5. Sources of Pure Bioenergy
4. Threats to Macroalgae
4.1. Ocean Warming and Marine Heatwaves
4.2. El Niño Events
4.3. Grazing
4.4. Commercial Kelp Harvesting
4.5. Increased Sediment Load
4.6. Pollution
4.7. High-Energy Storms or Swells
4.8. Multiple Factors
5. Restoration of Macroalgae
5.1. Spore Transplantation
5.2. Vegetative Transplantation
5.3. Green Gravel
6. Artificial Seaweed Reefs
6.1. The Pendleton Artificial Reef in Southern California
6.2. Artificial Seaweed Reefs in Japan
6.3. Artificial Seaweed Reefs of South Korea
7. Marine Forest Projects in South Korea
7.1. Marine Forest Formation
7.2. Seed Banks
7.3. Marine Gardening Day
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Falkowski, P. Ocean science: The power of plankton. Nature 2012, 483, S17–S20. [Google Scholar] [CrossRef] [PubMed]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Sigman, D.M.; Hain, M.P. The biological productivity of the ocean. Nat. Educ. Knowl. 2012, 3, 21. [Google Scholar]
- Mora, C.; Wei, C.L.; Rollo, A.; Amaro, T.; Baco, A.R.; Billett, D.; Bopp, L.; Chen, Q.; Collier, M.; Danovaro, R.; et al. Biotic and human vulnerability to projected changes in ocean biogeochemistry over the 21st century. PLoS Biol. 2013, 11, e1001682. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.E.; Benedetti-Cecchi, L.; Trinanes, J.; Muller-Karger, F.E.; Ambo-Rappe, R.; Boström, C.; Buschmann, A.H.; Byrnes, J.; Coles, R.G.; Creed, J.; et al. Toward a coordinated global observing system for seagrasses and marine macroalgae. Front. Mar. Sci. 2019, 6, 317. [Google Scholar] [CrossRef]
- Wernberg, T.; Filbee-Dexter, K. Missing the marine forest for the trees. Mar. Ecol. Prog. Ser. 2019, 612, 209–215. [Google Scholar] [CrossRef]
- Lee, I.C.; Kim, D.; Jung, S.; Na, W.B. Prediction of primary physical measures for cost-effective management of artificial seaweed reefs. Mar. Technol. Soc. J. 2020, 54, 25–43. [Google Scholar] [CrossRef]
- N‘Yeurt, A.R.; Chynoweth, D.P.; Capron, M.E.; Stewart, J.R.; Hasan, M.A. Negative carbon via ocean afforestation. Process Saf. Environ. Protect. 2012, 90, 467–474. [Google Scholar] [CrossRef]
- Bach, L.T.; Tamsitt, V.; Gower, J.; Hurd, C.L.; Raven, J.A.; Boyd, P.W. Testing the climate intervention potential of ocean afforestation using the Great Atlantic Sargassum Belt. Nat. Commun. 2021, 12, 2556. [Google Scholar] [CrossRef]
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse applications of marine macroalgae. Mar. Drugs 2020, 18, 17. [Google Scholar] [CrossRef]
- Pereira, L. Macroalgae. Encyclopedia 2021, 1, 177–188. [Google Scholar] [CrossRef]
- Duffy, J.E.; Hay, M.E. Seaweed adaptations to herbivory. BioScience 1990, 40, 368–375. [Google Scholar] [CrossRef]
- Underwood, A.J.; Jernakoff, P. The effect of tidal height, wave-exposure, seasonality and rock-pools on grazing and the distribution of intertidal macroalgae in New South Wales. J. Exp. Mar. Biol. Ecol. 1984, 75, 71–96. [Google Scholar] [CrossRef]
- Koh, C.H.; Oh, S.H. Distribution pattern of macroalgae in the Eastern Yellow Sea, Korea. Algae 1992, 7, 139–146. [Google Scholar]
- Jonsson, P.R.; Granhag, L.; Moschella, P.S.; Åberg, P.; Hawkins, S.J.; Thompson, R.C. Interactions between wave action and grazing control the distribution of intertidal macroalgae. Ecology 2006, 87, 1169–1178. [Google Scholar] [CrossRef]
- Duran, A.; Collado-Vides, L.; Burkepile, D.E. Seasonal regulation of herbivory and nutrient effects on macroalgal recruitment and succession in a Florida coral reef. PeerJ 2016, 4, e2643. [Google Scholar] [CrossRef]
- Graham, M.H.; Kinlan, B.P.; Druehl, L.D.; Garske, L.E.; Banks, S. Deep-water kelp refugia as potential hotspots of tropical marine diversity and productivity. Proc. Natl. Acad. Sci. USA 2007, 104, 16576–16580. [Google Scholar] [CrossRef]
- Fulton, C.J.; Depczynski, M.; Holmes, T.H.; Noble, M.M.; Radford, B.; Wernberg, T.; Wilson, S.K. Sea temperature shapes seasonal fluctuations in seaweed biomass within the Ningaloo coral reef ecosystem. Limnol. Oceanogr. 2014, 59, 156–166. [Google Scholar] [CrossRef]
- Steneck, R.S.; Johnson, C.R. Kelp forests: Dynamic patterns, processes and feedbacks. In Marine Community Ecology and Conservation; Bertness, M.D., Bruno, J.F., Silliman, B.R., Stachowicz, J.J., Eds.; Sinaur Associates Inc.: Sunderland, MA, USA, 2013; pp. 315–336. ISBN 978-1-6053-5228-2. [Google Scholar]
- Wernberg, T.; Krumhansl, K.; Filbee-Dexter, K.; Pedersen, M.F. Chapter 3—Status and trends of the world’s kelp forests. In World Seas: An Environmental Evaluation. Volume III: Ecological Issue and Environmental Impacts, 2nd ed.; Sheppard, C., Ed.; Academic Press: London, UK, 2019; pp. 57–78. ISBN 978-0-12-805052-1. [Google Scholar]
- Krause-Jensen, D.; Duarte, C.M. Substantial role of macroalgae in marine carbon sequestration. Nat. Geosci. 2016, 9, 737–742. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Anton, A.; Raven, J.A.; Beaumont, N.; Connolly, R.M.; Friess, D.A.; Kelleway, J.J.; Kennedy, H.; Kuwae, T.; Lavery, P.S.; et al. The future of blue carbon science. Nat. Commun. 2019, 10, 3998. [Google Scholar] [CrossRef]
- Mcleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef]
- Hill, R.; Bellgrove, A.; Macreadie, P.I.; Petrou, K.; Beardall, J.; Steven, A.; Ralph, P.J. Can macroalgae contribute to blue carbon? An Australian perspective. Limnol. Oceanogr. 2015, 60, 1689–1706. [Google Scholar] [CrossRef]
- Trevathan-Tackett, S.M.; Kelleway, J.; Macreadie, P.I.; Beardall, J.; Ralph, P.; Bellgrove, A. Comparison of marine macrophytes for their contributions to blue carbon sequestration. Ecology 2015, 96, 3043–3057. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. The possible roles of algae in restricting the increase in atmospheric CO2 and global temperature. Eur. J. Phycol. 2017, 52, 506–522. [Google Scholar] [CrossRef]
- Valiela, I.; Bowen, J.L.; York, J.K. Mangrove forests: One of the world’s threatened major tropical environments: At least 35% of the area of mangrove forests has been lost in the past two decades, losses that exceed those for tropical rain forests and coral reefs, two other well-known threatened environments. BioScience 2001, 51, 807–815. [Google Scholar] [CrossRef]
- Lotze, H.K.; Lenihan, H.S.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.G.; Kay, M.C.; Kidwell, S.M.; Kirby, M.X.; Peterson, C.H.; Jackson, J.B.C. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 2006, 312, 1806–1809. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.W.; Brumbaugh, R.D.; Airoldi, L.; Carranza, A.; Coen, L.D.; Crawford, C.; Defeo, O.; Edgar, G.J.; Hancock, B.; Kay, M.C.; et al. Oyster reefs at risk and recommendations for conservation, restoration, and management. BioScience 2011, 61, 107–116. [Google Scholar] [CrossRef]
- Wernberg, T.; Bennett, S.; Babcock, R.C.; De Bettignies, T.; Cure, K.; Depczynski, M.; Dufois, F.; Fromont, J.; Fulton, C.J.; Hovey, R.K.; et al. Climate-driven regime shift of a temperate marine ecosystem. Science 2016, 353, 169–172. [Google Scholar] [CrossRef]
- Saunders, M.I.; Doropoulos, C.; Bayraktarov, E.; Babcock, R.C.; Gorman, D.; Eger, A.M.; Vozzo, M.L.; Gillies, C.L.; Vanderklift, M.A.; Steven, A.D.L.; et al. Bright spots in coastal marine ecosystem restoration. Curr. Biol. 2020, 30, R1500–R1510. [Google Scholar] [CrossRef]
- Filbee-Dexter, K. Ocean forests hold unique solutions to our current environmental crisis. One Earth 2020, 2, 398–401. [Google Scholar] [CrossRef]
- Waltham, N.J.; Elliott, M.; Lee, S.Y.; Lovelock, C.; Duarte, C.M.; Buelow, C.; Simenstad, C.; Nagelkerken, I.; Claassens, L.; Wen, C.K.C.; et al. UN decade on ecosystem restoration 2021–2030—What chance for success in restoring coastal ecosystems? Front. Mar. Sci. 2020, 7, 71. [Google Scholar] [CrossRef]
- Visch, W.; Kononets, M.; Hall, P.O.J.; Nylund, G.M.; Pavia, H. Environmental impact of kelp (Saccharina latissima) aquaculture. Mar. Pollut. Bull. 2020, 155, 110962. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.S. A review of destruction of seaweed habitats along the coast of the Korean Peninsula and its consequences. Bull. Fish. Res. Agen. 2010, 32, 25–31. [Google Scholar]
- Kim, Y.D.; Shim, J.M.; Park, M.S.; Hong, J.P.; Yoo, H.I.; Min, B.H.; Jin, H.J.; Yarish, C.; Kim, J.K. Size determination of Ecklonia cava for successful transplantation onto artificial seaweed reef. Algae 2013, 28, 365–369. [Google Scholar] [CrossRef][Green Version]
- Kim, D.; Jung, S.; Na, W.B. Efficiency index diagram for wake region evaluation of artificial reefs facilitated for marine forest creation. J. Adv. Res. Ocean. Eng. 2016, 2, 169–178. [Google Scholar] [CrossRef]
- Jung, S.; Na, W.B. Placement models of marine forest artificial reefs to increase wake region efficiency. J. Fish. Mar. Sci. Educ. 2018, 30, 132–143. [Google Scholar] [CrossRef]
- Kim, D.; Jung, S.; Kim, J.; Na, W.B. Efficiency and unit propagation indices to characterize wake volumes of marine forest artificial reefs established by flatly distributed placement models. Ocean. Eng. 2019, 175, 138–148. [Google Scholar] [CrossRef]
- Korea Fisheries Resources Agency (FIRA). Marine Forest Furtherance. Available online: http://www.fira.or.kr/english/english_tap_010304.jsp (accessed on 6 May 2022).
- Nellemann, C.; Corcoran, E.; Duarte, C.M.; Valdés, L.; De Young, C.; Fonseca, L.; Grimsditch, G. Blue Carbon. A Rapid Response Assessment; United Nations Environment Programme: GRID-Arendal, Norway, 2009; pp. 5–78. ISBN 978-8-2770-1060-1. [Google Scholar]
- Hilmi, N.; Chami, R.; Sutherland, M.D.; Hall-Spencer, J.M.; Lebleu, L.; Benitez, M.B.; Levin, L.A. The role of blue carbon in climate change mitigation and carbon stock conservation. Front. Clim. 2021, 3, 710546. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Katz, M.E.; Knoll, A.H.; Quigg, A.; Raven, J.A.; Schofield, O.; Taylor, F.J.R. The evolution of modern eukaryotic phytoplankton. Science 2004, 305, 354–360. [Google Scholar] [CrossRef]
- Arrigo, K.R. Marine micro-organisms and global nutrient cycles. Nature 2005, 437, 349–355. [Google Scholar] [CrossRef]
- Bowler, C.; Karl, D.M.; Colwell, R.R. Microbial oceanography in a sea of opportunity. Nature 2009, 459, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Simon, N.; Cras, A.L.; Foulon, E.; Lemée, R. Diversity and evolution of marine phytoplankton. C. R. Biol. 2009, 332, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Houghton, R.A. Balancing the global carbon budget. Annu. Rev. Earth Planet. Sci. 2007, 35, 313–347. [Google Scholar] [CrossRef]
- Bouillon, S.; Borges, A.V.; Castañeda-Moya, E.; Diele, K.; Dittmar, T.; Duke, N.C.; Kristensen, E.; Lee, S.Y.; Marchand, C.; Middelburg, J.J.; et al. Mangrove production and carbon sinks: A revision of global budget estimates. Glob. Biogeochem. Cycle 2008, 22, GB20134. [Google Scholar] [CrossRef]
- Krause-Jensen, D.; Lavery, P.; Serrano, O.; Marbà, N.; Masque, P.; Duarte, C.M. Sequestration of macroalgal carbon: The elephant in the blue carbon room. Biol. Lett. 2018, 14, 20180236. [Google Scholar] [CrossRef]
- Duarte, C.M.; Losada, I.J.; Hendriks, I.E.; Mazarrasa, I.; Marbà, N. The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Chang. 2013, 3, 961–968. [Google Scholar] [CrossRef]
- Duarte, C.M.; Wu, J.; Xiao, X.; Bruhn, A.; Krause-Jensen, D. Can seaweed farming play a role in climate change mitigation and adaptation? Front. Mar. Sci. 2017, 4, 100. [Google Scholar] [CrossRef]
- Chung, I.K.; Beardall, J.; Mehta, S.; Sahoo, D.; Stojkovic, S. Using marine macroalgae for carbon sequestration: A critical appraisal. J. Appl. Phycol. 2011, 23, 877–886. [Google Scholar] [CrossRef]
- Chung, I.K.; Sondak, C.F.A.; Beardall, J. The future of seaweed aquaculture in a rapidly changing world. Eur. J. Phycol. 2017, 52, 495–505. [Google Scholar] [CrossRef]
- Moreira, D.; Pires, J.C.M. Atmospheric CO2 capture by algae: Negative carbon dioxide emission path. Bioresour. Technol. 2016, 215, 371–379. [Google Scholar] [CrossRef]
- Filbee-Dexter, K.; Wernberg, T. Substantial blue carbon in overlooked Australian kelp forests. Sci. Rep. 2020, 10, 12341. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.M.; Cebrián, J. The fate of marine autotrophic production. Limnol. Oceanogr. 1996, 41, 1758–1766. [Google Scholar] [CrossRef]
- Barrón, C.; Duarte, C.M. Dissolved organic carbon pools and export from the coastal ocean. Glob. Biogeochem. Cycle 2015, 29, 1725–1738. [Google Scholar] [CrossRef]
- Kennedy, H.; Beggins, J.; Duarte, C.M.; Fourqurean, J.W.; Holmer, M.; Marbà, N.; Middelburg, J.J. Seagrass sediments as a global carbon sink: Isotopic constraints. Glob. Biogeochem. Cycle 2010, 24, GB4026. [Google Scholar] [CrossRef]
- Thormar, J.; Hasler-Sheetal, H.; Baden, S.; Boström, C.; Clausen, K.K.; Krause-Jensen, D.; Olesen, B.; Rasmussen, J.R.; Svensson, C.J.; Holmer, M. Eelgrass (Zostera marina) food web structure in different environmental settings. PLoS ONE 2016, 11, e0146479. [Google Scholar] [CrossRef]
- Almahasheer, H.; Serrano, O.; Duarte, C.M.; Arias-Ortiz, A.; Masque, P.; Irigoien, X. Low carbon sink capacity of Red Sea mangroves. Sci. Rep. 2017, 7, 9700. [Google Scholar] [CrossRef]
- Barrón, C.; Apostolaki, E.T.; Duarte, C.M. Dissolved organic carbon fluxes by seagrass meadows and macroalgal beds. Front. Mar. Sci. 2014, 1, 42. [Google Scholar] [CrossRef]
- Maher, D.T.; Eyre, B.D. Benthic fluxes of dissolved organic carbon in three temperate Australian estuaries: Implications for global estimates of benthic DOC fluxes. J. Geophys. Res. 2010, 115, G04039. [Google Scholar] [CrossRef]
- Hughes, A.D.; Black, K.D.; Campbell, I.; Davidson, K.; Kelly, M.S.; Stanley, M.S. Does seaweed offer a solution for bioenergy with biological carbon capture and storage? Greenh. Gases Sci. Technol. 2012, 2, 402–407. [Google Scholar] [CrossRef]
- Sabine, C.L.; Feely, R.A.; Gruber, N.; Key, R.M.; Lee, K.; Bullister, J.L.; Wanninkhof, R.; Wong, C.S.; Wallace, D.W.R.; Tilbrook, B.; et al. The oceanic sink for anthropogenic CO2. Science 2004, 305, 367–371. [Google Scholar] [CrossRef]
- Cornwall, C.E.; Diaz-Pulido, G.; Comeau, S. Impacts of ocean warming on coralline algal calcification: Meta-analysis, knowledge gaps, and key recommendations for future research. Front. Mar. Sci. 2019, 6, 186. [Google Scholar] [CrossRef]
- Barton, A.; Hales, B.; Waldbusser, G.G.; Langdon, C.; Feely, R.A. The Pacific oyster, Crassostrea gigas, shows negative correlation to naturally elevated carbon dioxide levels: Implications for near-term ocean acidification effects. Limnol. Oceanogr. 2012, 57, 698–710. [Google Scholar] [CrossRef]
- Miles, N.L.; Richardson, S.J.; Davis, K.J.; Lauvaux, T.; Andrews, A.E.; West, T.O.; Bandaru, V.; Crosson, E.R. Large amplitude spatial and temporal gradients in atmospheric boundary layer CO2 mole fractions detected with a tower-based network in the U.S. upper Midwest. J. Geophys. Res. 2012, 117, G01019. [Google Scholar] [CrossRef]
- Noisette, F.; Hurd, C. Abiotic and biotic interactions in the diffusive boundary layer of kelp blades create a potential refuge from ocean acidification. Funct. Ecol. 2018, 32, 1329–1342. [Google Scholar] [CrossRef]
- Mann, K.H.; Lazier, J.R.N. Vertical Structure in Coastal Waters: Coastal Upwelling Regions. In Dynamics of Marine Ecosystems: Biological-Physical Interactions in the Oceans, 3rd ed.; Mann, K.H., Lazier, J.R.N., Eds.; Blackwell Science: Cambridge, MA, USA, 2005; pp. 163–215. ISBN 978-1-4051-1118-8. [Google Scholar]
- Denny, M.; Wethey, D. Physical Processes that Generate Patterns in Marine Communities. In Marine Community Ecology; Bertness, M.D., Gaines, S.D., Hay, M.E., Eds.; Sinauer Associates: Sunderland, MA, USA, 2001; pp. 3–37. ISBN 978-08-7893-057-9. [Google Scholar]
- Tokeshi, M. Species Coexistence: Ecological and Evolutionary Perspectives; Blackwell Science: Oxford, UK, 1999; pp. 1–214. ISBN 978-0-865-42744-0. [Google Scholar]
- Bruno, J.F.; Bertness, M.D. Habitat Modification and Facilitation in Benthic Marine Communities. In Marine Community Ecology; Berness, M.D., Gaines, S.D., Hay, M.E., Eds.; Sinauer Associates: Sunderland, MA, USA, 2001; pp. 201–208. ISBN 978-08-7893-057-9. [Google Scholar]
- Kawai, T.; Tokeshi, M. Variable modes of facilitation in the upper intertidal: Goose barnacles and mussels. Mar. Ecol. Prog. Ser. 2004, 272, 203–213. [Google Scholar] [CrossRef]
- Duffy, J.E. Biodiversity and the functioning of seagrass ecosystems. Mar. Ecol. Prog. Ser. 2006, 311, 233–250. [Google Scholar] [CrossRef]
- Thomaz, S.M.; da Cunha, E.R. The role of macrophytes in habitat structuring in aquatic ecosystems: Methods of measurement, causes and consequences on animal assemblages’ composition and biodiversity. Acta Limnol. Bras. 2010, 22, 218–236. [Google Scholar] [CrossRef]
- Matias, M.G.; Arenas, F.; Rubal, M.; Pinto, I.S. Macroalgal composition determines the structure of benthic assemblages colonizing fragmented habitats. PLoS ONE 2015, 10, e0142289. [Google Scholar] [CrossRef][Green Version]
- Tokeshi, M.; Arakaki, S. Habitat complexity in aquatic systems: Fractals and beyond. Hydrobiologia 2012, 685, 27–47. [Google Scholar] [CrossRef]
- Lefcheck, J.S.; Hughes, B.B.; Johnson, A.J.; Pfirrmann, B.W.; Rasher, D.B.; Smyth, A.R.; Williams, B.L.; Beck, M.W.; Orth, R.J. Are coastal habitats important nurseries? A meta-analysis. Conserv. Lett. 2019, 12, e12645. [Google Scholar] [CrossRef]
- Layton, C.; Shelamoff, V.; Cameron, M.J.; Tatsumi, M.; Wright, J.T.; Johnson, C.R. Resilience and stability of kelp forests: The importance of patch dynamics and environment-engineer feedbacks. PLoS ONE 2019, 14, e0210220. [Google Scholar] [CrossRef]
- Teagle, H.; Hawkins, S.J.; Moore, P.J.; Smale, D.A. The role of kelp species as biogenic habitat formers in coastal marine ecosystems. J. Exp. Mar. Biol. Ecol. 2017, 492, 81–98. [Google Scholar] [CrossRef]
- Mann, K.H. Seaweeds: Their productivity and strategy for growth. Science 1973, 182, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Steneck, R.S.; Graham, M.H.; Bourque, B.J.; Corbett, D.; Erlandson, J.M.; Estes, J.A.; Tegner, M.J. Kelp forest ecosystems: Biodiversity, stability, resilience and future. Environ. Conserv. 2002, 29, 436–459. [Google Scholar] [CrossRef]
- D’Archino, R.; Piazzi, L. Macroalgal assemblages as indicators of the ecological status of marine coastal systems: A review. Ecol. Indic. 2021, 129, 107835. [Google Scholar] [CrossRef]
- Fulton, C.J.; Berkström, C.; Wilson, S.K.; Abesamis, R.A.; Bradley, M.; Åkerlund, C.; Barrett, L.T.; Bucol, A.A.; Chacin, D.H.; Chong-Seng, K.M.; et al. Macroalgal meadow habitats support fish and fisheries in diverse tropical seascapes. Fish Fish. 2020, 21, 700–717. [Google Scholar] [CrossRef]
- Tano, S.; Eggertsen, M.; Wikström, S.A.; Berkström, C.; Buriyo, A.S.; Halling, C. Tropical seaweed beds are important habitats for mobile invertebrate epifauna. Estuar. Coast. Shelf Sci. 2016, 183, 1–12. [Google Scholar] [CrossRef]
- Krumhansl, K.A.; Okamoto, D.K.; Rassweiler, A.; Novak, M.; Bolton, J.J.; Cavanaugh, K.C.; Connell, S.D.; Johnson, C.R.; Konar, B.; Ling, S.D.; et al. Global patterns of kelp forest change over the past half-century. Proc. Nat. Acad. Sci. USA 2016, 113, 13785–13790. [Google Scholar] [CrossRef]
- Edwards, M.; Konar, B.; Kim, J.H.; Gabara, S.; Sullaway, G.; McHugh, T.; Spector, M.; Small, S. Marine deforestation leads to widespread loss of ecosystem function. PLoS ONE 2020, 15, e0226173. [Google Scholar] [CrossRef]
- Coleman, M.A.; Wood, G.; Filbee-Dexter, K.; Minne, A.J.P.; Goold, H.D.; Vergés, A.; Marzinelli, E.M.; Steinberg, P.D.; Wernberg, T. Restore or redefine: Future trajectories for restoration. Front. Mar. Sci. 2020, 7, 237. [Google Scholar] [CrossRef]
- Kharkwal, H.; Joshi, D.D.; Panthari, P.; Pant, M.K.; Kharkwal, A.C. Algae as future drugs. Asian J. Pharm. Clin. Res. 2012, 5, 1–4. [Google Scholar]
- Øverland, M.; Mydland, L.T.; Skrede, A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J. Sci. Food Agric. 2019, 99, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, B.; Chauhan, O.P.; Mishra, A. Edible seaweeds: A potential novel source of bioactive metabolites and nutraceuticals with human health benefits. Front. Mar. Sci. 2021, 8, 740054. [Google Scholar] [CrossRef]
- Kılınç, B.; Cirik, S.; Turan, G.; Tekogul, H.; Koru, E. Seaweeds for food and industrial applications. In Food Industry; Muzzalupo, I., Ed.; IntechOpen: London, UK, 2013; ISBN 978-953-51-0911-2. [Google Scholar]
- Aryee, A.N.A.; Agyei, D.; Akanbi, T.O. Recovery and utilization of seaweed pigments in food processing. Curr. Opin. Food Sci. 2018, 19, 113–119. [Google Scholar] [CrossRef]
- Manivasagan, P.; Bharathiraja, S.; Moorthy, M.S.; Mondal, S.; Seo, H.; Lee, K.D.; Oh, J. Marine natural pigments as potential sources for therapeutic applications. Crit. Rev. Biotechnol. 2018, 38, 745–761. [Google Scholar] [CrossRef]
- McLachlan, J. Macroalgae (seaweeds): Industrial resources and their utilization. Plant Soil 1985, 89, 137–157. [Google Scholar] [CrossRef]
- Häder, D.P. Chapter 9—Phycocolloids from macroalgae. In Natural Bioactive Compounds, 1st ed.; Shiha, R.P., Häder, D.P., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 187–201. ISBN 978-0-1282-0655-3. [Google Scholar]
- Güven, K.C.; Percot, A.; Sezik, E. Alkaloids in marine algae. Mar. Drugs 2010, 8, 269–284. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Pal, A.; Kamthania, M.C.; Kumar, A. Bioactive compounds and properties of seaweeds—A review. Open Access Libr. J. 2014, 1, e752. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Rengasamy, K.R.R.; Mahomoodally, M.F.; Aumeeruddy, M.Z.; Zengin, G.; Xiao, J.; Kim, D.H. Bioactive compounds in seaweeds: An overview of their biological properties and safety. Food Chem. Toxicol. 2020, 135, 111013. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.R.M.; Bezerra, W.P.; Souto, J.T. Marine alkaloids with anti-inflammatory activity: Current knowledge and future perspectives. Mar. Drugs 2020, 18, 147. [Google Scholar] [CrossRef]
- Tanna, B.; Mishra, A. Metabolites unravel nutraceutical potential of edible seaweeds: An emerging source of functional food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- Losada-Lopez, C.; Dopico, D.C.; Faína-Medín, J.A. Neophobia and seaweed consumption: Effects on consumer attitude and willingness to consume seaweed. Int. J. Gastron. Food Sci. 2021, 24, 100338. [Google Scholar] [CrossRef]
- Chan, J.C.C.; Cheung, P.C.K.; Ang, P.O. Comparative studies on the effect of three drying methods on the nutritional composition of seaweed Sargassum hemiphyllum (Turn.) C. Ag. J. Agric. Food Chem. 1997, 45, 3056–3059. [Google Scholar] [CrossRef]
- Bartle, W.R.; Madorin, P.; Ferland, G. Seaweed, vitamin K, and warfarin. Am. J. Health Syst. Pharm. 2001, 58, 2300. [Google Scholar] [CrossRef]
- Škrovánková, S. Seaweed vitamins as nutraceuticals. Adv. Food Nutr. Res. 2011, 64, 357–369. [Google Scholar] [CrossRef]
- Watanabe, F.; Yabuta, Y.; Bito, T.; Teng, F. Vitamin B12-containing plant food sources for vegetarians. Nutrients 2014, 6, 1861–1873. [Google Scholar] [CrossRef]
- Hughes, L.J.; Black, L.J.; Sherriff, J.L.; Dunlop, E.; Strobel, N.; Lucas, R.M.; Bornman, J.F. Vitamin D content of Australian native food plants and Australian-grown edible seaweed. Nutrients 2018, 10, 876. [Google Scholar] [CrossRef]
- Nielsen, C.W.; Rustad, T.; Holdt, S.L. Vitamin C from seaweed: A review assessing seaweed as contributor to daily intake. Foods 2021, 10, 198. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, C.I.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, I.L.; Díaz, N.F. Minerals in edible seaweed: Health benefits and food safety issues. Crit. Rev. Food Sci. Nutr. 2022, 62, 1592–1607. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, A.R.J.; Maia, M.R.G.; Oliveira, H.M.; Sousa-Pinto, I.; Almeida, A.A.; Pinto, E.; Fonseca, A.J.M. Tracing seaweeds as mineral sources for farm-animals. J. Appl. Phycol. 2016, 28, 3135–3150. [Google Scholar] [CrossRef]
- Circuncisão, A.R.; Catarion, M.D.; Cardoso, S.M.; Silva, A.M.S. Minerals from macroalgae origin: Health benefits and risks for consumers. Mar. Drugs 2018, 16, 400. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Miranda, J.M.; Mondragon, A.D.C.; Lamas, A.; Cardelle-Cobas, A.; Franco, C.M.; Cepeda, A. Potential use of marine seaweeds as prebiotics: A review. Molecules 2020, 25, 1004. [Google Scholar] [CrossRef]
- Chudasama, N.A.; Sequeira, R.A.; Moradiya, K.; Prasad, K. Seaweed polysaccharide based products and materials: An assessment on their production from a sustainability point of view. Molecules 2021, 26, 2608. [Google Scholar] [CrossRef]
- Charoensiddhi, S.; Lorbeer, A.J.; Lahnstein, J.; Bulone, V.; Franco, C.M.; Zhang, W. Enzyme-assisted extraction of carbohydrates from the brown alga Ecklonia radiata: Effect of enzyme type, pH and buffer on sugar yield and molecular weight profiles. Process Biochem. 2016, 51, 1503–1510. [Google Scholar] [CrossRef]
- Afonso, N.C.; Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Brown macroalgae as valuable food ingredients. Antioxidants 2019, 8, 365. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on α-amylase, α-glucosidase, pancreatic lipase and tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef]
- López-Hortas, L.; Domínguez, H.; Torres, M.D. Valorisation of edible brown seaweeds by the recovery of bioactive compounds from aqueous phase using MHG to develop innovative hydrogels. Process Biochem. 2019, 78, 100–107. [Google Scholar] [CrossRef]
- Silva, A.; Rodrigues, C.; Garcia-Oliveira, P.; Lourenço-Lopes, C.; Silva, S.A.; Garcia-Perez, P.; Carvalho, A.P.; Domingues, V.F.; Barroso, M.F.; Delerue-Matos, C.; et al. Screening of bioactive properties in brown algae from the Northwest Iberian Peninsula. Foods 2021, 10, 1915. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Mu, T.; Marco, C.V. Chapter 10—Seaweeds and microalgal biomass: The future of food and nutraceuticals. In Future Foods; Bhat, R., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 183–201. ISBN 978-0-3239-1001-9. [Google Scholar]
- Negreanu-Pîrjol, B.; Negreanu-Pîrjol, T.; Paraschiv, G.; Bratu, M.; Sîrbu, R.; Roncea, F.; Meghea, A. Physical-chemical characterization of some green and red macrophyte algae from the Romanian Black Sea littoral. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2011, 12, 173–184. [Google Scholar]
- Indira, K.; Balakrishnan, S.; Srinivasan, M.; Bragadeeswaran, S.; Balasubramanian, T. Evaluation of in vitro antimicrobial property of seaweed (Halimeda tuna) from Tuticorin coast, Tamil Nadu, Southeast coast of India. Afr. J. Biotechnol. 2013, 12, 284–289. [Google Scholar] [CrossRef]
- Bulota, M.; Budtova, T. Valorisation of macroalgae industrial by-product as filler in thermoplastic composites. Compos. Pt. A Appl. Sci. Manuf. 2016, 90, 271–277. [Google Scholar] [CrossRef]
- Ganesan, A.; Tiwari, U.; Rajauria, G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci. Human Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Biris-Dorhoi, E.S.; Michiu, D.; Pop, C.R.; Rotar, A.M.; Tofana, M.; Pop, O.L.; Socaci, S.A.; Farcas, A.C. Macroalgae—A sustainable source of chemical compounds with biological activities. Nutrients 2020, 12, 3085. [Google Scholar] [CrossRef]
- El Boukhari, M.E.M.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in seaweed extract based biostimulants: Manufacturing process and beneficial effect on soil-plant systems. Plants 2020, 9, 359. [Google Scholar] [CrossRef]
- Carina, D.; Sharma, S.; Jaiswal, A.K.; Jaiswal, S. Seaweeds polysaccharides in active food packaging: A review of recent progress. Trends Food Sci. Technol. 2021, 110, 559–572. [Google Scholar] [CrossRef]
- Arthur, G.D.; Stirk, W.A.; van Staden, J.; Scott, P. Effect of a seaweed concentrate on the growth and yield of three varieties of Capsicum annuum. S. Afr. J. Bot. 2003, 69, 207–211. [Google Scholar] [CrossRef]
- Zodape, S.T.; Kawarkhe, V.J.; Patolia, J.S.; Warade, A.D. Effect of liquid seaweed fertilizer on yield and quality of okra (Abelmoschus esculentus L.). J. Sci. Ind. Res. 2008, 67, 1115–1117. [Google Scholar]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants 2021, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, R.K.; Nandwani, D.; Nwosisi, S. Assessing seaweed extract as a biostimulant on the yield of organic leafy greens in Tennessee. J. Agric. Univ. Puerto Rico 2018, 102, 53–64. [Google Scholar] [CrossRef]
- Kauffman, G.L.; Kneivel, D.P.; Watschke, T.L. Effects of a biostimulant on the heat tolerance associated with photosynthetic capacity, membrane thermostability, and polyphenol production of perennial ryegrass. Crop Sci. 2007, 47, 261–267. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Lim, C.; Yusoff, S.; Ng, C.G.; Lim, P.E.; Ching, Y.C. Bioplastic made from seaweed polysaccharides with green production methods. J. Environ. Chem. Eng. 2021, 9, 105895. [Google Scholar] [CrossRef]
- Lomartire, S.; Marques, J.C.; Gonçalves, A.M.M. An overview of the alternative use of seaweeds to produce safe and sustainable bio-packaging. Appl. Sci. 2022, 12, 3123. [Google Scholar] [CrossRef]
- Rosenboom, J.G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef]
- Hassan, M.M.; Mueller, M.; Wagners, M.H. Exploratory study on seaweed as novel filler in polypropylene composite. J. Appl. Polym. Sci. 2008, 109, 1242–1247. [Google Scholar] [CrossRef]
- Thiruchelvi, R.; Das, A.; Sikdar, E. Bioplastics as better alternative to petro plastic. Mater. Today Proc. 2021, 37, 1634–1639. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Lai, T.K.; Tye, Y.Y.; Rizal, S.; Chong, E.W.N.; Yap, S.W.; Hamzah, A.A.; Nurul Fazita, M.R.; Paridah, M.T. A review of extractions of seaweed hydrocolloids: Properties and applications. Express Polym. Lett. 2018, 12, 296–317. [Google Scholar] [CrossRef]
- Skrede, A.; Mydland, L.T.; Ahlstrøm, Ø.; Reitan, K.I.; Gislerød, H.R.; Øverland, M. Evaluation of microalgae as sources of digestible nutrients for monogastric animals. J. Anim. Feed Sci. 2011, 20, 131–142. [Google Scholar] [CrossRef]
- Saadaoui, I.; Rasheed, R.; Aguilar, A.; Cherif, M.; Jabri, H.A.; Sayadi, S.; Manning, S.R. Microalgal-based feed: Promising alternative feedstock for livestock and poultry production. J. Anim. Sci. Biotechnol. 2021, 12, 76. [Google Scholar] [CrossRef]
- Gülzari, Ş.Ö.; Lund, V.; Aasen, I.M.; Steinshamn, H. Effect of supplementing sheep diets with macroalgae species on in vivo nutrient digestibility, rumen fermentation and blood amino acid profile. Animal 2019, 13, 2792–2801. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Chojnacka, K.; Korniewicz, D. Effect of marine macroalga Enteromorpha sp. enriched with Zn(II) and Cu(II) ions on the digestibility, meat quality and carcass characteristics of growing pigs. J. Mar. Sci. Eng. 2020, 8, 347. [Google Scholar] [CrossRef]
- Morais, T.; Inácio, A.; Coutinho, T.; Ministro, M.; Cotas, J.; Pereira, L.; Bahcevandziev, K. Seaweed potential in the animal feed: A review. J. Mar. Sci. Eng. 2020, 8, 559. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Jaramillo-Torres, A.; Ahlstrøm, Ø.; Chikwati, E.; Aasen, I.M.; Kortner, T.M. Protein value and health aspects of the seaweeds Saccharina latissima and Palmaria palmata evaluated with mink as model for monogastric animals. Anim. Feed Sci. Technol. 2021, 276, 114902. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Hayes, M. Red and green macroalgae for fish and animal feed and human functional food development. Food Rev. Int. 2016, 32, 15–45. [Google Scholar] [CrossRef]
- Biancarosa, I.; Belghit, I.; Bruckner, C.G.; Liland, N.S.; Waagbø, R.; Amlund, H.; Heesch, S.; Lock, E.J. Chemical characterization of 21 species of marine macroalgae common in Norwegian waters: Benefits of and limitations to their potential use in food and feed. J. Sci. Food Agric. 2018, 98, 2035–2042. [Google Scholar] [CrossRef]
- Pirian, K.; Piri, K.; Sohrabipour, J.; Blomster, J. Three species of Ulva (Ulvophyceae) from the Persian Gulf as potential sources of protein, essential amino acids and fatty acids. Phycol. Res. 2018, 66, 149–154. [Google Scholar] [CrossRef]
- Milledge, J.J.; Heaven, S. Methods of energy extraction from microalgal biomass: A review. Rev. Environ. Sci. Biotechnol. 2014, 13, 301–320. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, D.; Luo, G.; Zhang, S.; Chen, J. Macroalgae for biofuels production: Progress and perspectives. Renew. Sustain. Energy Rev. 2015, 47, 427–437. [Google Scholar] [CrossRef]
- Rajkumar, R.; Yaakob, Z.; Takriff, M.S. Potential of the micro and macro algae for biofuel production: A brief review. BioResources 2014, 9, 1606–1633. [Google Scholar] [CrossRef]
- Benedetti, M.; Vecchi, V.; Barera, S.; Dall’Osto, L. Biomass from microalgae: The potential of domestication towards sustainable biofactories. Microb. Cell. Fact. 2018, 17, 173. [Google Scholar] [CrossRef] [PubMed]
- Kraan, S. Mass-cultivation of carbohydrate rich macroalgae, a possible solution for sustainable biofuel production. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 27–46. [Google Scholar] [CrossRef]
- Cheng, T.H. Production of kelp—A major aspect of China’s exploitation of the sea. Econ. Bot. 1969, 23, 215–236. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). The State of World Fisheries and Aquaculture 2016. Contributing to Food Security and Nutrition for All; FAO: Rome, Italy, 2016; pp. 1–200. ISBN 978-9-2510-9185-2. [Google Scholar]
- Hu, Z.M.; Shan, T.F.; Zhang, J.; Zhang, Q.S.; Critchley, A.T.; Choi, H.G.; Yotsukura, N.; Liu, F.L.; Duan, D.L. Kelp aquaculture in China: A retrospective and future prospects. Rev. Aquac. 2021, 13, 1324–1351. [Google Scholar] [CrossRef]
- Mac Monagail, M.; Cornish, L.; Morrison, L.; Araújo, R.; Critchley, A.T. Sustainable harvesting of wild seaweed resources. Eur. J. Phycol. 2017, 52, 371–390. [Google Scholar] [CrossRef]
- Araújo, R.; Calderón, F.V.; López, J.S.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Tasende, M.G.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current status of the algae production industry in Europe: An emerging sector of the blue bioeconomy. Front. Mar. Sci. 2021, 7, 626389. [Google Scholar] [CrossRef]
- Grebe, G.S.; Byron, C.J.; Gelais, A.S.; Kotowicz, D.M.; Olson, T.K. An ecosystem approach to kelp aquaculture in the Americas and Europe. Aquacult. Rep. 2019, 15, 100215. [Google Scholar] [CrossRef]
- Kim, J.K.; Stekoll, M.; Yarish, C. Opportunities, challenges and future directions of open-water seaweed aquaculture in the United States. Phycologia 2019, 58, 446–461. [Google Scholar] [CrossRef]
- Kain, J.M.; Holt, T.J.; Dawes, C.P. European Laminariales and their cultivation. In Economically Important Marine Plants of the Atlantic: Their Biology and Cultivation; Yarish, C., Penniman, C.A., Van Patten, P., Eds.; Connecticut Sea Grant College Program: Groton, MA, USA, 1990; pp. 95–111. ISBN 978-1-8783-0101-7. [Google Scholar]
- Kraan, S.; Verges Tramullas, A.; Guiry, M.D. The edible brown seaweed Alaria esculenta (Phaeophyceae, Laminariales): Hybridization, growth and genetic comparisons of six Irish populations. J. Appl. Phycol. 2000, 12, 577–583. [Google Scholar] [CrossRef]
- Buck, B.H.; Buchholz, C.M. The offshore-ring: A new system design for the open ocean aquaculture of macroalgae. J. Appl. Phycol. 2004, 16, 355–368. [Google Scholar] [CrossRef]
- Peteiro, C.; Freire, Ó. Effect of outplanting time on commercial cultivation of kelp Laminaria saccharina at the southern limit in the Atlantic coast, N.W. Spain. Chin. J. Oceanol. Limnol. 2009, 27, 54–60. [Google Scholar] [CrossRef]
- Campbell, I.; Macleod, A.; Sahlmann, C.; Neves, L.; Funderud, J.; Øverland, M.; Hughes, A.D.; Stanley, M. The environmental risks associated with the development of seaweed farming in Europe—Prioritizing key knowledge gaps. Front. Mar. Sci. 2019, 6, 107. [Google Scholar] [CrossRef]
- Gao, G.; Burgess, J.G.; Wu, M.; Wang, S.; Gao, K. Using macroalgae as biofuel: Current opportunities and challenges. Bot. Mar. 2020, 63, 355–370. [Google Scholar] [CrossRef]
- Barbot, Y.N.; Al-Ghaili, H.; Benz, R. A review on the valorization of macroalgal wastes for biomethane production. Mar. Drugs 2016, 14, 120. [Google Scholar] [CrossRef]
- Milledge, J.J.; Harvey, P.J. Potential process ‘hurdles’ in the use of macroalgae as feedstock for biofuel production in the British Isles. J. Chem. Technol. Biotechnol. 2016, 91, 2221–2234. [Google Scholar] [CrossRef]
- Pandolfi, J.M.; Bradbury, R.H.; Sala, E.; Hughes, T.P.; Bjorndal, K.A.; Cooke, R.G.; McArdle, D.; McClenachan, L.; Newman, M.J.H.; Paredes, G.; et al. Global trajectories of the long-term decline of coral reef ecosystems. Science 2003, 301, 955–958. [Google Scholar] [CrossRef]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.B.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L., Jr.; Hughes, A.R.; et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Canensi, S.; Dell’Anno, A.; Tangherlini, M.; Capua, I.D.; Varrella, S.; Willis, T.J.; Cerrano, C.; Danovaro, R. Multiple impacts of microplastics can threaten marine habitat-forming species. Commun. Biol. 2021, 4, 431. [Google Scholar] [CrossRef]
- Danielsen, F.; Sørensen, M.K.; Olwig, M.F.; Selvam, V.; Parish, F.; Burgess, N.D.; Hiraishi, T.; Karunagaran, V.M.; Rasmussen, M.S.; Hansen, L.B.; et al. The Asian tsunami: A protective role for coastal vegetation. Science 2005, 310, 643. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, J.H.; Brumbaugh, R.D.; Conrad, R.F.; Keeler, A.G.; Opaluch, J.J.; Peterson, C.H.; Piehler, M.F.; Powers, S.P.; Smyth, A.R. Economic valuation of ecosystem services provided by oyster reefs. BioScience 2012, 62, 900–909. [Google Scholar] [CrossRef]
- McGlathery, K.J.; Sundback, K.; Anderson, I.C. Eutrophication in shallow coastal bays and lagoons: The role of plants in the coastal filter. Mar. Ecol. Prog. Ser. 2007, 348, 1–18. [Google Scholar] [CrossRef]
- Moberg, F.; Folke, C. Ecological goods and services of coral reef ecosystems. Ecol. Econ. 1999, 29, 215–233. [Google Scholar] [CrossRef]
- Jackson, E.L.; Rees, S.E.; Wilding, C.; Attrill, M.J. Use of a seagrass residency index to apportion commercial fishery landing values and recreation fisheries expenditure to seagrass habitat service. Conserv. Biol. 2015, 29, 899–909. [Google Scholar] [CrossRef]
- Jayathilake, D.R.M.; Costello, M.J. A modelled global distribution of the kelp biome. Biol. Conserv. 2020, 252, 108815. [Google Scholar] [CrossRef]
- Morton, D.N. The Effects of Parasites on the Kelp-Forest Food Web. Ph.D. Thesis, UC Santa Barbara, Santa Barbara, CA, USA, 2020. [Google Scholar]
- Bennett, S.; Wernberg, T.; Connell, S.D.; Hobday, A.J.; Johnson, C.R.; Poloczanska, E.S. The ‘Great Southern Reef’: Social, ecological and economic value of Australia’s neglected kelp forest. Mar. Freshw. Res. 2015, 67, 47–56. [Google Scholar] [CrossRef]
- Hobday, A.J.; Alexander, L.V.; Perkins, S.E.; Smale, D.A.; Straub, S.C.; Oliver, E.C.J.; Benthuysen, J.A.; Burrows, M.T.; Donat, M.G.; Feng, M.; et al. A hierarchical approach to defining marine heatwaves. Prog. Oceanogr. 2016, 141, 227–238. [Google Scholar] [CrossRef]
- Oliver, E.C.J.; Benthuysen, J.A.; Darmaraki, S.; Donat, M.G.; Hobday, A.J.; Holbrook, N.J.; Schlegel, R.W.; Gupta, A.S. Marine heatwaves. Annu. Rev. Mar. Sci. 2021, 13, 313–342. [Google Scholar] [CrossRef]
- Oliver, E.C.J.; Donat, M.G.; Burrows, M.T.; Moore, P.J.; Smale, D.A.; Alexander, L.V.; Benthuysen, J.A.; Feng, M.; Gupta, A.S.; Hobday, A.J.; et al. Longer and more frequent marine heatwaves over the past century. Nat. Commun. 2018, 9, 1324. [Google Scholar] [CrossRef]
- Benthuysen, J.A.; Oliver, E.C.J.; Chen, K.; Wernberg, T. Advances in understanding marine heatwaves and their impacts. Front. Mar. Sci. 2020, 7, 147. [Google Scholar] [CrossRef]
- Holbrook, N.J.; Hernaman, V.; Koshiba, S.; Lako, J.; Kajtar, J.B.; Amosa, P.; Singh, A. Impacts of marine heatwaves on tropical western and central Pacific Island nations and their communities. Glob. Planet. Chang. 2022, 208, 103680. [Google Scholar] [CrossRef]
- Tegner, M.J.; Dayton, P.K.; Edwards, P.B.; Riser, K.L. Large-scale, low-frequency oceanographic effects on kelp forest succession: A tale of two cohorts. Mar. Ecol. Prog. Ser. 1997, 146, 117–134. [Google Scholar] [CrossRef]
- Gerard, V.A. The role of nitrogen nutrition in high-temperature tolerance of the kelp, Laminaria saccharina (Chromophyta). J. Phycol. 1997, 33, 800–810. [Google Scholar] [CrossRef]
- Wernberg, T.; Smale, D.A.; Tuya, F.; Thomsen, M.S.; Langlois, T.J.; de Bettignies, T.; Bennett, S.; Rousseaux, C.S. An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nat. Clim. Chang. 2013, 3, 78–82. [Google Scholar] [CrossRef]
- Straub, S.C.; Wernberg, T.; Thomsen, M.S.; Moore, P.J.; Burrows, M.T.; Harvey, B.P.; Smale, D.A. Resistance, extinction, and everything in between—The diverse responses of seaweeds to Marine Heatwaves. Front. Mar. Sci. 2019, 6, 763. [Google Scholar] [CrossRef]
- Arafeh-Dalmau, N.; Schoeman, D.S.; Montaño-Moctezuma, G.; Micheli, F.; Rogers-Bennett, L.; Olguin-Jacobson, C.; Possingham, H.P. Marine heat waves threaten kelp forests. Science 2020, 367, 635. [Google Scholar] [CrossRef]
- Gao, G.; Zhao, X.; Jiang, M.; Gao, L. Impacts of marine heatwaves on algal structure and carbon sequestration in conjunction with ocean warming and acidification. Front. Mar. Sci. 2021, 8, 758651. [Google Scholar] [CrossRef]
- Rogers-Bennett, L.; Catton, C.A. Marine heat wave and multiple stressors tip bull kelp forest to sea urchin barrens. Sci. Rep. 2019, 9, 15050. [Google Scholar] [CrossRef]
- McPherson, M.L.; Finger, D.J.I.; Houskeeper, H.F.; Bell, T.W.; Carr, M.H.; Rogers-Bennett, L.; Kudela, R.M. Large-scale shift in the structure of a kelp forest ecosystem co-occurs with an epizootic and marine heatwave. Commun. Biol. 2021, 4, 298. [Google Scholar] [CrossRef]
- Tanaka, K.; Ohno, M.; Largo, D.B. An update on the seaweed resources of Japan. Bot. Mar. 2020, 63, 105–117. [Google Scholar] [CrossRef]
- Moy, F.E.; Christie, H. Large-scale shift from sugar kelp (Saccharina latissima) to ephemeral algae along the south and west coast of Norway. Mar. Biol. Res. 2012, 8, 309–321. [Google Scholar] [CrossRef]
- Johnson, C.R.; Banks, S.C.; Barrett, N.S.; Cazassus, F.; Dunstan, P.K.; Edgar, G.J.; Frusher, S.D.; Gardner, C.; Haddon, M.; Helidoniotis, F.; et al. Climate change cascades: Shifts in oceanography, species’ ranges and subtidal marine community dynamics in eastern Tasmania. J. Exp. Mar. Biol. Ecol. 2011, 400, 17–32. [Google Scholar] [CrossRef]
- Falkenberg, L.J.; Russell, B.D.; Connell, S.D. Stability of strong species interactions resist to the synergistic effects of local and global pollution in kelp forests. PLoS ONE 2012, 7, e33841. [Google Scholar] [CrossRef]
- Filbee-Dexter, K.; Scheibling, R.E. Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Mar. Ecol. Prog. Ser. 2014, 495, 1–25. [Google Scholar] [CrossRef]
- Ling, S.D.; Scheibling, R.E.; Rassweiler, A.; Johnson, C.R.; Shears, N.; Connell, S.D.; Salomon, A.K.; Norderhaug, K.M.; Pérez-Matus, A.; Hernández, J.C.; et al. Global regime shift dynamics of catastrophic sea urchin overgrazing. Philos. Trans. R. Soc. B 2015, 370, 1659. [Google Scholar] [CrossRef]
- Connell, S.D.; Foster, M.S.; Airoldi, L. What are algal turfs? Towards a better description of turfs. Mar. Ecol. Prog. Ser. 2014, 495, 299–307. [Google Scholar] [CrossRef]
- Barber, R.T.; Chavez, F.P. Biological consequences of El Niño. Science 1983, 222, 1203–1210. [Google Scholar] [CrossRef]
- Dayton, P.K.; Tegner, M.J.; Parnell, P.E.; Edwards, P.B. Temporal and spatial patterns of disturbance and recovery in a kelp forest community. Ecol. Monogr. 1992, 62, 421–445. [Google Scholar] [CrossRef]
- Hollarsmith, J.A.; Buschmann, A.H.; Camus, C.; Grosholz, E.D. Varying reproductive success under ocean warming and acidification across giant kelp (Macrocystis pyrifera) populations. J. Exp. Mar. Biol. Ecol. 2020, 522, 151247. [Google Scholar] [CrossRef]
- Tegner, M.J.; Dayton, P.K. El Niño effects on southern California kelp forest communities. Adv. Ecol. Res. 1987, 17, 243–279. [Google Scholar] [CrossRef]
- Hernández-Carmona, G.; García, O.; Robledo, D.; Foster, M. Restoration techniques for Macrocystis pyrifera (Phaeophyceae) populations at the southern limit of their distribution in México. Bot. Mar. 2000, 43, 273–284. [Google Scholar] [CrossRef]
- Damatac II, A.M.; Santos, M.D. Possible effects of El Niño on some Philippine marine fisheries resources. Philipp. J. Sci. 2016, 145, 283–295. [Google Scholar]
- Trono, G.C., Jr.; Valdestamon, R.G. New aspects in the ecology and culture of Kappaphycus and Eucheuma. Algae 1994, 9, 205–216. [Google Scholar]
- Peters, A.F.; Breeman, A.M. Temperature tolerance and latitudinal range of brown algae from temperate Pacific South America. Mar. Biol. 1993, 115, 143–150. [Google Scholar] [CrossRef]
- Cole, R.G.; Babcock, R.C. Mass mortality of a dominant kelp (Laminariales) at Goat Island, North-eastern New Zealand. Mar. Freshw. Res. 1996, 47, 907–911. [Google Scholar] [CrossRef]
- Cole, R.G.; Syms, C. Using spatial pattern analysis to distinguish causes of mortality: An example from kelp in north-eastern New Zealand. J. Ecol. 1999, 87, 963–972. [Google Scholar] [CrossRef]
- Norderhaug, K.M.; Christie, H.C. Sea urchin grazing and kelp re-vegetation in the NE Atlantic. Mar. Biol. Res. 2009, 5, 515–528. [Google Scholar] [CrossRef]
- Perreault, M.C.; Borgeaud, I.A.; Gaymer, C.F. Impact of grazing by the sea urchin Tetrapygus niger on the kelp Lessonia trabeculata in Northern Chile. J. Exp. Mar. Biol. Ecol. 2014, 453, 22–27. [Google Scholar] [CrossRef]
- Barrientos, S.; Piñeiro-Corbeira, C.; Barreiro, R. Temperate kelp forest collapse by fish herbivory: A detailed demographic study. Front. Mar. Sci. 2022, 9, 817021. [Google Scholar] [CrossRef]
- Melis, R.; Ceccherelli, G.; Piazzi, L.; Rustici, M. Macroalgal forests and sea urchin barrens: Structural complexity loss, fisheries exploitation and catastrophic regime shifts. Ecol. Complex. 2019, 37, 32–37. [Google Scholar] [CrossRef]
- Himmelman, J.H.; Cardinal, A.; Bourget, E. Community development following removal of urchins, Strongylocentrotus droebachiensis, from the rocky subtidal zone of the St. Lawrence Estuary, Eastern Canada. Oecologia 1983, 59, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Pinnegar, J.K.; Polunin, N.V.C.; Francour, P.; Badalamenti, F.; Chemello, R.; Harmelin-Vivien, M.L.; Hereu, B.; Milazzo, M.; Zabala, M.; D’Anna, G.; et al. Trophic cascades in benthic marine ecosystems: Lessons for fisheries and protected-area management. Environ. Conserv. 2000, 27, 179–200. [Google Scholar] [CrossRef]
- Tuya, F.; Boyra, A.; Sanchez-Jerez, P.; Barbera, C.; Haroun, R.J. Relationships between rocky-reef fish assemblages, the sea urchin Diadema antillarum and macroalgae throughout the Canarian Archipelago. Mar. Ecol. Prog. Ser. 2004, 278, 157–169. [Google Scholar] [CrossRef]
- Guidetti, P.; Dulčić, J. Relationships among predatory fish, sea urchins and barrens in Mediterranean rocky reefs across a latitudinal gradient. Mar. Environ. Res. 2007, 63, 168–184. [Google Scholar] [CrossRef]
- Gianguzza, P.; Agnetta, D.; Bonaviri, C.; Trapani, F.D.; Visconti, G.; Gianguzza, F.; Riggio, S. The rise of thermophilic sea urchins and the expansion of barren grounds in the Mediterranean Sea. Chem. Ecol. 2011, 27, 129–134. [Google Scholar] [CrossRef]
- Lang, C.; Mann, K.H. Changes in sea urchin populations after the destruction of kelp beds. Mar. Biol. 1976, 36, 321–326. [Google Scholar] [CrossRef]
- Mann, K.H. Kelp, sea urchins and predators: A review of strong interactions in rocky subtidal systems of Eastern Canada, 1970–1980. Neth. J. Sea Res. 1982, 16, 414–423. [Google Scholar] [CrossRef]
- Miller, K.I.; Blain, C.O.; Shears, N.T. Sea urchin removal as a tool for macroalgal restoration: A review on removing “the spiny enemies”. Front. Mar. Sci. 2022, 9, 831001. [Google Scholar] [CrossRef]
- Leinaas, H.P.; Christie, H. Effects of removing sea urchins (Strongylocentrotus droebachiensis): Stability of the barren state and succession of kelp forest recovery in the east Atlantic. Oecologia 1996, 105, 524–536. [Google Scholar] [CrossRef]
- Gagnon, P.; Himmelman, J.H.; Johnson, L.E. Temporal variation in community interfaces: Kelp-bed boundary dynamics adjacent to persistent urchin barrens. Mar. Biol. 2004, 144, 1191–1203. [Google Scholar] [CrossRef]
- Estes, J.E.; Smith, N.S.; Palmisano, J.F. Sea otter predation and community organization in the western Aleutian Islands, Alaska. Ecology 1978, 59, 822–833. [Google Scholar] [CrossRef]
- Amsler, C.D. Induced defenses in macroalgae: The herbivore makes a difference. J. Phycol. 2001, 37, 353–356. [Google Scholar] [CrossRef]
- Elner, R.W.; Vadas Sr, R.L. Inference in ecology: The sea urchin phenomenon in the Northwestern Atlantic. Am. Nat. 1990, 136, 108–125. [Google Scholar] [CrossRef]
- Bredvik, J.J.; Boerger, C.; Allen, L.G. Age and growth of two herbivorous, kelp forest fishes, the opaleye (Girella nigricans) and halfmoon (Medialuna californiensis). Bull. South. Calif. Acad. Sci. 2011, 110, 25–34. [Google Scholar] [CrossRef]
- Dobkowski, K. The role of kelp crabs as consumers in bull kelp forests—Evidence from laboratory feeding trials and field enclosures. PeerJ 2017, 5, e3372. [Google Scholar] [CrossRef]
- Gutow, L.; Poore, A.G.B.; Díaz Poblete, M.A.; Villalobos, V.; Thiel, M. Small burrowing amphipods cause major damage in a large kelp. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200330. [Google Scholar] [CrossRef]
- Loureiro, R.; Gachon, C.M.M.; Rebours, C. Seaweed cultivation: Potential and challenges of crop domestication at an unprecedented pace. New Phytol. 2015, 206, 489–492. [Google Scholar] [CrossRef]
- Valero, M.; Guillemin, M.-L.; Destombe, C.; Jacquemin, B.; Gachon, C.M.M.; Badis, Y.; Buschmann, A.H.; Camus, C.; Faugeron, S. Perspectives on domestication research for sustainable seaweed aquaculture. Perspect. Phycol. 2017, 4, 33–46. [Google Scholar] [CrossRef]
- Badis, Y.; Klochkova, T.A.; Strittmatter, M.; Garvetto, A.; Murúa, P.; Sanderson, J.C.; Kim, G.H.; Gachon, C.M.M. Novel species of the oomycete Olpidiopsis potentially threaten European red algal cultivation. J. Appl. Phycol. 2019, 31, 1239–1250. [Google Scholar] [CrossRef]
- Ribera, M.A.; Boudouresque, C.F. Introduced marine plants, with special reference to macroalgae: Mechanisms and impact. In Progress in Phycological Research; Round, F.E., Chapman, D.J., Eds.; Biopress Limited: Bristol, UK, 1995; Volume 11, pp. 187–268. ISBN 978-0-948-73720-6. [Google Scholar]
- Ward, G.M.; Faisan, J.P., Jr.; Cottier-Cook, E.J.; Gachon, C.; Hurtado, A.Q.; Lim, P.E.; Matoju, I.; Msuya, F.E.; Bass, D.; Brodie, J. A review of reported seaweed diseases and pests in aquaculture in Asia. J. World Aquacult. Soc. 2020, 51, 815–828. [Google Scholar] [CrossRef]
- Bermejo, R.; Buschmann, A.; Capuzzo, E.; Cottier-Cook, E.; Fricke, A.; Hernández, I.; Hofmann, L.C.; Pereira, R.; van den Burg, S. State of Knowledge Regarding the Potential of Macroalgae Cultivation in Providing Climate-Related and Other Ecosystem Services; Eklipse Working Group: Leipzig, Germany, 2022; pp. 1–23. ISBN 978-3-9442-8028-8. [Google Scholar]
- Zhang, J.; Hansen, P.K.; Fang, J.; Wang, W.; Jiang, Z. Assessment of the local environmental impact of intensive marine shellfish and seaweed farming—Application of the MOM system in the Sungo Bay, China. Aquaculture 2009, 287, 304–310. [Google Scholar] [CrossRef]
- Zhou, J. Impacts of Mariculture practices on the temporal distribution of macrobenthos in Sandu Bay, South China. Chin. J. Oceanol. Limnol. 2012, 30, 388–396. [Google Scholar] [CrossRef]
- Mouritsen, O.G.; Rhatigan, P.; Pérez-Lloréns, J.L. The rise of seaweed gastronomy: Phycogastronomy. Bot. Mar. 2019, 62, 195–209. [Google Scholar] [CrossRef]
- Smit, A.J. Medicinal and pharmaceutical uses of seaweed natural products: A review. J. Appl. Phycol. 2004, 16, 245–262. [Google Scholar] [CrossRef]
- Bixler, H.J.; Porse, H. A decade of change in the seaweed hydrocolloids industry. J. Appl. Phycol. 2011, 23, 321–335. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N.; et al. Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Xiao, X.; Agusti, S.; Lin, F.; Li, K.; Pan, Y.; Yu, Y.; Zheng, Y.; Wu, J.; Duarte, C.M. Nutrient removal from Chinese coastal waters by large-scale seaweed aquaculture. Sci. Rep. 2017, 7, 46613. [Google Scholar] [CrossRef]
- Food and Agricultural Organization (FAO). Fisheries and Aquaculture Statistics 2015; FAO: Rome, Italy, 2017; pp. 1–107. ISBN 978-9-2500-9987-3. [Google Scholar]
- Food and Agriculture Organization (FAO). The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018; pp. 1–227. ISBN 978-9-2513-0562-1. [Google Scholar]
- Dayton, P.K. Ecology of kelp communities. Ann. Rev. Ecol. Syst. 1985, 16, 215–245. [Google Scholar] [CrossRef]
- Rogers, C.S. Responses of coral reefs and reef organisms to sedimentation. Mar. Ecol. Prog. Ser. 1990, 62, 185–202. [Google Scholar] [CrossRef]
- Duarte, C.M. Submerged aquatic vegetation in relation to different nutrient regimes. Ophelia 1995, 41, 87–112. [Google Scholar] [CrossRef]
- Terrados, J.; Duarte, C.M.; Fortes, M.D.; Borum, J.; Agawin, N.S.R.; Bach, S.; Thampanya, U.; Kamp-Nielsen, L.; Kenworthy, W.J.; Geertz-Hansen, O.; et al. Changes in community structure and biomass of seagrass communities along gradients of siltation in SE Asia. Estuar. Coast. Shelf Sci. 1998, 46, 757–768. [Google Scholar] [CrossRef]
- Saiz Salinas, J.I.; Isasi Urdangarin, I. Response of sublittoral hard substrate invertebrates to estuarine sedimentation in the outer harbour of Bilbao (N. Spain). Mar. Ecol. 1994, 15, 105–131. [Google Scholar] [CrossRef]
- Ellis, J.; Cummings, V.; Hewitt, J.; Thrush, S.; Norkko, A. Determining effects of suspended sediment on condition of a suspension feeding bivalve (Atrina zelandica): Results of a survey, a laboratory experiment and a field transplant experiment. J. Exp. Mar. Biol. Ecol. 2002, 267, 147–174. [Google Scholar] [CrossRef]
- Fabricius, K.E. Effects of terrestrial runoff on the ecology of corals and coral reefs: Review and synthesis. Mar. Pollut. Bull. 2005, 50, 125–146. [Google Scholar] [CrossRef]
- Kroon, F.J.; Kuhnert, P.M.; Henderson, B.L.; Wilkinson, S.N.; Kinsey-Henderson, A.; Abbott, B.; Brodie, J.E.; Turner, R.D.R. River loads of suspended solids, nitrogen, phosphorus and herbicides delivered to the Great Barrier Reef lagoon. Mar. Pollut. Bull. 2012, 65, 167–181. [Google Scholar] [CrossRef]
- Orpin, A.R.; Ridd, P.V. Exposure of inshore corals to suspended sediments due to wave resuspension and river plumes in the central Great Barrier Reef: A reappraisal. Cont. Shelf Res. 2012, 47, 55–67. [Google Scholar] [CrossRef]
- Jones, R.J.; Bessell-Browne, P.; Fisher, R.; Klonowski, W.; Slivkoff, M. Assessing the impacts of sediments from dredging on corals. Mar. Pollut. Bull. 2016, 102, 9–29. [Google Scholar] [CrossRef]
- Hughes, T.P.; Barnes, M.L.; Bellwood, D.R.; Cinner, J.E.; Cumming, G.S.; Jackson, J.B.C.; Kleypas, J.; van de Leemput, I.A.; Lough, J.M.; Morrison, T.H.; et al. Coral reefs in the Anthropocene. Nature 2017, 546, 82–90. [Google Scholar] [CrossRef]
- Smith, S.J.; Friedrichs, C.T. Size and settling velocities of cohesive flocs and suspended sediment aggregates in a trailing suction hopper dredge plume. Cont. Shelf Res. 2011, 31, S50–S63. [Google Scholar] [CrossRef]
- López-Jiménez, I.T.; Quan-Young, L.I.; Florez-Leiva, L. Effect of terrigenous sediments on macroalgae functional-form groups of coral reefs in Capurganá, Colombian Caribbean. Sci. Mar. 2021, 85, 125–135. [Google Scholar] [CrossRef]
- Clausing, R.J.; Bittick, S.J.; Fong, C.R.; Fong, P. Sediments influence accumulation of two macroalgal species through novel but differing interactions with nutrients and herbivory. Coral Reefs 2016, 35, 1297–1309. [Google Scholar] [CrossRef]
- Eriksson, B.K.; Johansson, G. Effects of sedimentation on macroalgae: Species-specific responses are related to reproductive traits. Oecologia 2005, 143, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, B.K.; Johansson, G.; Snoeijs, P. Long-term changes in the sublittoral zonation of brown algae in the southern Bothnian Sea. Eur. J. Phycol. 1998, 33, 241–249. [Google Scholar] [CrossRef]
- Pedersén, M.; Snoeijs, P. Patterns of macroalgal diversity, community composition and long-term changes along the Swedish west coast. Hydrobiologia 2001, 459, 83–102. [Google Scholar] [CrossRef]
- Watanabe, H.; Ito, M.; Matsumoto, A.; Arakawa, H. Effects of sediment influx on the settlement and survival of canopy-forming macrophytes. Sci. Rep. 2016, 6, 18677. [Google Scholar] [CrossRef]
- Matsumoto, A.; Sato, M.; Arakawa, H. Impacts of sub-micrometer sediment particles on early-stage growth and survival of the kelp Ecklonia bicyclis. Sci. Rep. 2020, 10, 20689. [Google Scholar] [CrossRef]
- Galarno, A.J. Coral vs. Macroalgae: Relative Susceptibility to Sedimentation and Ocean Warming. Master’s Thesis, Nova Southeastern University, Davie, FL, USA, 2017; pp. 1–36. Available online: https://nsuworks.nova.edu/occ_stuetd/450 (accessed on 5 April 2022).
- Airoldi, L.; Cinelli, F. Effects of sedimentation on subtidal macroalgal assemblages: An experimental study from a mediterranean rocky shore. J. Exp. Mar. Biol. Ecol. 1997, 215, 269–288. [Google Scholar] [CrossRef]
- Airoldi, L. The effects of sedimentation on rocky coast assemblages. Oceanogr. Mar. Biol. 2003, 41, 161–236. [Google Scholar] [CrossRef]
- Traiger, S.B.; Konar, B. Mature and developing kelp bed community composition in a glacial estuary. J. Exp. Mar. Biol. Ecol. 2018, 501, 26–35. [Google Scholar] [CrossRef]
- Traiger, S.B. Effects of elevated temperature and sedimentation on grazing rates of the green sea urchin: Implications for kelp forests exposed to increased sedimentation with climate change. Helgol. Mar. Res. 2019, 73, 5. [Google Scholar] [CrossRef]
- Layton, C.; Coleman, M.A.; Marzinelli, E.M.; Steinberg, P.D.; Swearer, S.E.; Vergés, A.; Wernberg, T.; Johnson, C.R. Kelp forest restoration in Australia. Front. Mar. Sci. 2020, 7, 74. [Google Scholar] [CrossRef]
- Hamilton, S.L.; Gleason, M.G.; Godoy, N.; Eddy, N.; Grorud-Colvert, K. Ecosystem-based management for kelp forest ecosystems. Mar. Pol. 2022, 136, 104919. [Google Scholar] [CrossRef]
- Tegner, M.J.; Dayton, P.K.; Edwards, P.B.; Riser, K.L.; Chadwick, D.B.; Dean, T.A.; Deysher, L. Effects of a large sewage spill on a kelp forest community: Catastrophe or disturbance? Mar. Environ. Res. 1995, 40, 181–224. [Google Scholar] [CrossRef]
- Coleman, M.A.; Kelaher, B.P.; Steinberg, P.D.; Millar, A.J.K. Absence of a large brown macroalga on urbanized rocky reefs around Sydney, Australia, and evidence for historical decline. J. Phycol. 2008, 44, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.K.; Edwards, M.S. Bioaccumulation of copper and zinc by the giant kelp Macrocystis pyrifera. Algae 2011, 26, 265–275. [Google Scholar] [CrossRef]
- Foster, M.S.; Schiel, D.R. Loss of predators and the collapse of southern California kelp forests (?): Alternatives, explanations and generalizations. J. Exp. Mar. Biol. Ecol. 2010, 393, 59–70. [Google Scholar] [CrossRef]
- Campbell, A.H.; Marzinelli, E.M.; Vergés, A.; Coleman, M.A.; Steinberg, P.D. Towards restoration of missing underwater forests. PLoS ONE 2014, 9, e84106. [Google Scholar] [CrossRef]
- Konik, M.; Darecki, M.; Pavlov, A.K.; Sagan, S.; Kowalczuk, P. Darkening of the Svalbard Fjords waters observed with satellite ocean color imagery in 1997–2019. Front. Mar. Sci. 2021, 8, 699318. [Google Scholar] [CrossRef]
- Blain, C.O.; Hansen, S.C.; Shears, N.T. Coastal darkening substantially limits the contribution kelp to coastal carbon cycles. Glob. Chang. Biol. 2021, 27, 5547–5563. [Google Scholar] [CrossRef]
- Mustaffa, N.I.H.; Kallajoki, L.; Biederbick, J.; Binder, F.I.; Schlenker, A.; Striebel, M. Coastal ocean darkening effects via terrigenous DOM addition on plankton: An indoor mesocosm experiment. Front. Mar. Sci. 2020, 7, 547829. [Google Scholar] [CrossRef]
- Opdal, A.F.; Lindemann, C.; Aksnes, D.L. Centennial decline in North Sea water clarity causes strong delay in phytoplankton bloom timing. Glob. Chang. Biol. 2019, 25, 3946–3953. [Google Scholar] [CrossRef] [PubMed]
- McGovern, M.; Evenset, A.; Borgå, K.; de Wit, H.A.; Braaten, H.F.V.; Hessen, D.O.; Schultze, S.; Ruus, A.; Poste, A. Implications of coastal darkening for contaminant transport, bioavailability, and trophic transfer in northern coastal waters. Environ. Sci. Technol. 2019, 53, 7180–7182. [Google Scholar] [CrossRef] [PubMed]
- Frontier, N.; Mulas, M.; Foggo, A.; Smale, D.A. The influence of light and temperature on detritus degradation rates for kelp species with contrasting thermal affinities. Mar. Environ. Res. 2022, 173, 105529. [Google Scholar] [CrossRef]
- Thushari, G.G.N.; Senevirathna, J.D.M. Plastic pollution in the marine environment. Heliyon 2020, 6, e04709. [Google Scholar] [CrossRef]
- Hongthong, S.; Leese, H.S.; Allen, M.J.; Chuck, C.J. Assessing the conversion of various nylon polymers in the hydrothermal liquefaction of macroalgae. Environments 2021, 8, 34. [Google Scholar] [CrossRef]
- Gutow, L.; Eckerlebe, A.; Giménez, L.; Saborowski, R. Experimental evaluation of seaweeds as a vector for microplastics into marine food webs. Environ. Sci. Technol. 2016, 50, 915–923. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, J.; Liu, D.; Sun, Z.; Tang, R.; Ma, X.; Feng, Z. Loading of Microplastics by two related macroalgae in a sea area where gold and green tides occur simultaneously. Sci. Total Environ. 2022, 814, 152809. [Google Scholar] [CrossRef]
- Li, W.C.; Tse, H.F.; Fok, L. Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total Environ. 2016, 566–567, 333–349. [Google Scholar] [CrossRef]
- Galgani, F.; Hanke, G.; Maes, T. Global distribution, composition and abundance of marine litter. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Cham, Switzerland, 2015; pp. 29–56. ISBN 978-3-319-16509-7. [Google Scholar]
- Li, Q.; Feng, Z.; Zhang, T.; Ma, C.; Shi, H. Microplastics in the commercial seaweed nori. J. Hazard. Mater. 2020, 388, 122060. [Google Scholar] [CrossRef]
- Reed, D.C.; Rassweiler, A.; Arkema, K.K. Biomass rather than growth rate determines variation in net primary production by giant kelp. Ecology 2008, 89, 2493–2505. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, K.C.; Siegel, D.A.; Reed, D.C.; Dennison, P.E. Environmental controls of giant-kelp biomass in the Santa Barbara Channel, California. Mar. Ecol. Prog. Ser. 2011, 429, 1–17. [Google Scholar] [CrossRef]
- Edwards, M.S.; Estes, J.A. Catastrophe, recovery and range limitation in NE Pacific kelp forests: A large-scale perspective. Mar. Ecol. Prog. Ser. 2006, 320, 79–87. [Google Scholar] [CrossRef]
- Glynn, P.W. El Niño-Southern Oscillation 1982–1983: Nearshore population, community, and ecosystem responses. Annu. Rev. Ecol. Syst. 1988, 19, 309–346. [Google Scholar] [CrossRef]
- Chavez, F.P. Forcing and biological impact of onset of the 1992 El Niño in central California. Geophys. Res. Lett. 1996, 23, 265–268. [Google Scholar] [CrossRef]
- Chelton, D.B.; Bernal, P.A.; McGowan, J.A. Large-scale interannual physical and biological interaction in the California Current. J. Mar. Res. 1982, 40, 1095–1125. [Google Scholar]
- Dayton, P.K.; Tegner, M.J. Catastrophic storms, El Niño, and patch stability in a southern California kelp community. Science 1984, 224, 283–285. [Google Scholar] [CrossRef]
- Edwards, M.S. Estimating scale-dependency in disturbance impacts: El Niños and giant kelp forests in the Northeast Pacific. Oecologia 2004, 138, 436–447. [Google Scholar] [CrossRef]
- Zimmerman, R.C.; Robertson, D.L. Effects of El Niño on local hydrography and growth of the giant kelp, Macrocystis pyrifera, at Santa Catalina Island, California. Limnol. Oceanogr. 1985, 30, 1298–1302. [Google Scholar] [CrossRef]
- Dayton, P.K.; Tegner, M.J.; Edwards, P.B.; Riser, K.L. Temporal and spatial scales of kelp demography: The role of oceanographic climate. Ecol. Monogr. 1999, 69, 219–250. [Google Scholar] [CrossRef]
- Ladah, L.B.; Zertuche-González, J.A.; Hernández-Carmona, G. Giant kelp (Macrocystis pyrifera, Phaeophyceae) recruitment near its southern limit in Baja California after mass disappearance during ENSO 1997–1998. J. Phycol. 1999, 35, 1106–1112. [Google Scholar] [CrossRef]
- Hernández-Carmona, G.; Robledo, D.; Serviere-Zaragoza, E. Effect of nutrient availability on Macrocystis pyrifera recruitment and survival near its southern limit off Baja California. Bot. Mar. 2001, 44, 221–229. [Google Scholar] [CrossRef]
- Edwards, M.S. Comparing the impacts of four ENSO events on giant kelp (Macrocystis pyrifera) in the northeast Pacific Ocean. Algae 2019, 34, 141–151. [Google Scholar] [CrossRef]
- Connell, S.D.; Russell, B.D.; Turner, D.J.; Shepherd, S.A.; Kildea, T.; Miller, D.; Airoldi, L.; Cheshire, A. Recovering a lost baseline: Missing kelp forests from a metropolitan coast. Mar. Ecol. Prog. Ser. 2008, 360, 63–72. [Google Scholar] [CrossRef]
- Ling, S.D.; Johnson, C.R.; Frusher, S.D.; Ridgway, K.R. Overfishing reduces resilience of kelp beds to climate-driven catastrophic phase shift. Proc. Natl. Acad. Sci. USA 2009, 106, 22341–22345. [Google Scholar] [CrossRef] [PubMed]
- Vergés, A.; Doropoulos, C.; Malcolm, H.A.; Skye, M.; Garcia-Pizá, M.; Marzinelli, E.M.; Campbell, A.H.; Ballesteros, E.; Hoey, A.S.; Vila-Concejo, A.; et al. Long-term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp. Proc. Natl. Acad. Sci. USA 2016, 113, 13791–13796. [Google Scholar] [CrossRef]
- Carnell, P.E.; Keough, M.J. Reconstructing historical marine populations reveals major decline of a kelp forest ecosystem in Australia. Estuaries Coasts 2019, 42, 765–778. [Google Scholar] [CrossRef]
- Berry, H.D.; Mumford, T.F.; Christiaen, B.; Dowty, P.; Calloway, M.; Ferrier, L.; Grossman, E.E.; VanArendonk, N.R. Long-term changes in kelp forests in an inner basin of the Salish Sea. PLoS ONE 2021, 16, e0229703. [Google Scholar] [CrossRef]
- Woo, J.; Kim, D.; Yoon, H.S.; Na, W.B. Efficient placement models of labyrinth-type artificial concrete reefs according to wake volume estimation to support natural submerged aquatic vegetation. Bull. Mar. Sci. 2018, 94, 1259–1272. [Google Scholar] [CrossRef]
- Choi, C.G.; Lee, H.W.; Hong, B.K. Marine algal flora and community structure in Dokdo, East Sea, Korea. Korean J. Fish. Aquat. Sci. 2009, 42, 503–508. [Google Scholar] [CrossRef]
- Kim, Y.D.; Hong, J.P.; Song, H.I.; Park, M.S.; Moon, T.S.; Yoo, H.I. Studies on technology for seaweed forest construction and transplanted Ecklonia cava growth for an artificial seaweed reef. J. Environ. Biol. 2012, 33, 969–975. [Google Scholar] [PubMed]
- Hwang, S.I.; Kim, D.K.; Sung, B.J.; Jun, S.K.; Bae, J.I.; Jeon, B.H. Effects of climate change on whitening event proliferation the Coast of Jeju. Korean J. Environ. Ecol. 2017, 31, 529–536. [Google Scholar] [CrossRef]
- Okuda, K. Coastal environment and seaweed-bed ecology in Japan. Kuroshino Sci. 2008, 2, 15–20. [Google Scholar]
- Pratt, J.R. Artificial habitats and ecosystem restoration: Managing for the future. Bull. Mar. Sci. 1994, 55, 268–275. [Google Scholar]
- Svane, I.; Petersen, J.K. On the problems of epibioses, fouling and artificial reefs, a review. Mar. Ecol. 2001, 22, 169–188. [Google Scholar] [CrossRef]
- Cebrian, E.; Tamburello, L.; Verdura, J.; Guarnieri, G.; Medrano, A.; Linares, C.; Hereu, B.; Garrabou, J.; Cerrano, C.; Galobart, C.; et al. A roadmap for the restoration of Mediterranean macroalgal forests. Front. Mar. Sci. 2021, 8, 709219. [Google Scholar] [CrossRef]
- Eger, A.M.; Marzinelli, E.M.; Christie, H.; Fagerli, C.W.; Fujita, D.; Gonzalez, A.P.; Hong, S.W.; Kim, J.H.; Lee, L.C.; McHugh, T.A.; et al. Global kelp forest restoration: Past lessons, present status, and future directions. Biol. Rev. 2022, 97, 1449–1475. [Google Scholar] [CrossRef]
- Halling, C.; Aroca, G.; Cifuentes, M.; Buschmann, A.H.; Troell, M. Comparison of spore inoculated and vegetative propagated cultivation methods of Gracilaria chilensis in an integrated seaweed and fish cage culture. Aquac. Int. 2005, 13, 409–422. [Google Scholar] [CrossRef]
- Yu, Y.Q.; Zhang, Q.S.; Tang, Y.Z.; Zhang, S.B.; Lu, Z.C.; Chu, S.H.; Tang, X.X. Establishment of intertidal seaweed beds of Sargassum thunbergii through habitat creation and germling seeding. Ecol. Eng. 2012, 44, 10–17. [Google Scholar] [CrossRef]
- Fredriksen, S.; Filbee-Dexter, K.; Norderhaug, K.M.; Steen, H.; Bodvin, T.; Coleman, M.A.; Moy, F.; Wernberg, T. Green gravel: A novel restoration tool to combat kelp forest decline. Sci. Rep. 2020, 10, 3983. [Google Scholar] [CrossRef]
- Largo, D.B.; Ohno, M. Constructing an artificial seaweed beds. In Seaweed Cultivation and Marine Ranching; Ohno, M., Critchley, A.T., Eds.; Kanagawa International Fisheries Training Center, Japan International Cooperative Agency: Yokosuka, Kanagawa, Japan, 1993; pp. 113–130. [Google Scholar]
- Choi, C.G.; Serisawa, Y.; Ohno, M.; Sohn, C.H. Construction of artificial seaweed beds; using the spore bag method. Algae 2000, 15, 179–182. [Google Scholar]
- Poza, A.M.; Fernández, C.; Latour, E.A.; Raffo, M.P.; Dellatorre, F.G.; Parodi, E.R.; Gauna, M.C. Optimization of the rope seeding method and biochemical characterization of the brown seaweed Asperococcus ensiformis. Algal Res. 2022, 64, 102668. [Google Scholar] [CrossRef]
- Schiel, D.R.; Foster, M.S. The Biology and Ecology of Giant Kelp Forests; University of California Press: Oakland, CA, USA, 2015; pp. 235–264. ISBN 978-0-5202-7886-8. [Google Scholar]
- North, W.J. Aquacultural techniques for creating and restoring beds of giant kelp, Macrocystis spp. J. Fish. Res. Bd. Can. 1976, 33, 1015–1023. [Google Scholar] [CrossRef]
- Wilson, K.C.; Haaker, P.L.; Hanan, D.A. Kelp restoration in southern California. In The Marine Plant Biomass of the Pacific Northwest Coast; Krauss, R.W., Ed.; Oregon State University Press: Corvallis, OR, USA, 1978; pp. 183–202. [Google Scholar]
- Peteiro, C. Alginate Production from Marine Macroalgae, with Emphasis on Kelp farming. In Alginates and Their Biomedical Applications; Rehm, B.H.A., Moradali, M.F., Eds.; Springer: Singapore, 2018; pp. 27–66. ISBN 978-9-8110-6909-3. [Google Scholar]
- Macchiavello, J.; Araya, E.; Bulboa, C. Production of Macrocystis pyrifera (Laminariales; Phaeophyceae) in northern Chile on spore-based culture. J. Appl. Phycol. 2010, 22, 691–697. [Google Scholar] [CrossRef]
- Camus, C.; Buschmann, A.H. Macrocystis pyrifera aquafarming: Production optimization of rope-seeded juvenile sporophytes. Aquaculture 2017, 468, 107–114. [Google Scholar] [CrossRef]
- Vásquez, X.; Gutiérrez, A.; Buschmann, A.H.; Flores, R.; Farías, D.; Leal, P. Evaluation of repopulation techniques for the giant kelp Macrocystis pyrifera (Laminariales). Bot. Mar. 2014, 57, 123–130. [Google Scholar] [CrossRef]
- Harger, B.W.W.; Neushul, M. Test-farming of the giant kelp, Macrocystis, as a marine biomass producer. J. World Aquacult. Soc. 1983, 14, 392–403. [Google Scholar] [CrossRef]
- Westermeier, R.; Murúa, P.; Patiño, D.J.; Muñoz, L.; Ruiz, A.; Atero, C.; Müller, D.G. Utilization of holdfast fragments for vegetative propagation of Macrocystis integrifolia in Atacama, Northern Chile. J. Appl. Phycol. 2013, 25, 639–642. [Google Scholar] [CrossRef]
- Terawaki, T.; Yoshikawa, K.; Yoshida, G.; Uchimura, M.; Iseki, K. Ecology and restoration techniques for Sargassum beds in the Seto Inland Sea, Japan. Mar. Pollut. Bull. 2003, 47, 198–201. [Google Scholar] [CrossRef]
- Reed, D.C.; Foster, M.S. The effect of canopy shadings on algal recruitment and growth in a giant kelp forest. Ecology 1984, 65, 937–948. [Google Scholar] [CrossRef]
- Santelices, B.; Ojeda, F.P. Effects of canopy removal on the understory algal community structure of coastal forest of Macrocystis pyrifera from Southern South America. Mar. Ecol. Prog. Ser. 1984, 14, 165–173. [Google Scholar] [CrossRef]
- Clark, R.P.; Edwards, M.S.; Foster, M.S. Effects of shade from multiple kelp canopies on an understory algal assemblage. Mar. Ecol. Prog. Ser. 2004, 267, 107–119. [Google Scholar] [CrossRef]
- Wood, G.; Marzinelli, E.M.; Coleman, M.A.; Campbell, A.H.; Santini, N.S.; Kajlich, L.; Verdura, J.; Woodak, J.; Steinberg, P.D.; Vergés, A. Restoring subtidal marine macrophytes in the Anthropocene: Trajectories and future-proofing. Mar. Freshw. Res. 2019, 70, 936–951. [Google Scholar] [CrossRef]
- Ohno, M.; Serisawa, Y. Recent reports on seaweed and seagrass establishment and restoration. Fish. Sci. 2002, 68, 1737–1742. [Google Scholar] [CrossRef]
- Ambrose, R.F. Mitigating the effects of a coastal power plant on a kelp forest community: Rationale and requirements for an artificial reef. Bull. Mar. Sci. 1994, 55, 694–708. [Google Scholar]
- Deysher, L.E.; Dean, T.A.; Grove, R.S.; Jahn, A. Design considerations for an artificial reef to grow giant kelp (Macrocystis pyrifera) in Southern California. ICES J. Mar. Sci. 2002, 59, S201–S207. [Google Scholar] [CrossRef]
- Carter, J.W.; Carpenter, A.L.; Foster, M.S.; Jessee, W.N. Benthic succession on an artificial reef designed to support a kelp reef community. Bull. Mar. Sci. 1985, 37, 86–113. [Google Scholar]
- Reed, D.C.; Schroeter, S.C.; Raimondi, P.T. Spore supply and habitat availability as sources of recruitment limitation in the giant kelp Macrocystis pyrifera (Phaeophyceae). J. Phycol. 2004, 40, 275–284. [Google Scholar] [CrossRef]
- Reed, D.C.; Schroeter, S.C.; Huang, D.; Anderson, T.W.; Ambrose, R.F. Quantitative assessment of different artificial reef designs in mitigating losses to kelp forest fishes. Bull. Mar. Sci. 2006, 78, 133–150. [Google Scholar]
- Carter, J.W.; Jessee, W.N.; Foster, M.S.; Carpenter, A.L. Management of artificial reefs designed to support natural communities. Bull. Mar. Sci. 1985, 37, 114–128. [Google Scholar]
- Ohno, M.; Arai, S.; Watanabe, M. Seaweed succession on artificial reefs on different bottom substrata. J. Appl. Phycol. 1990, 2, 327–332. [Google Scholar] [CrossRef]
- Ohno, M. Succession of seaweed communities on artificial reefs in Ashizuri, Tosa Bay, Japan. Algae 1993, 8, 191–198. [Google Scholar]
- Serisawa, Y.; Ohno, M. Succession of seaweed communities on artificial reefs in Tei, Tosa Bay, Japan. Nippon. Suisan Gakkaishi 1995, 61, 854–859. [Google Scholar] [CrossRef]
- Serisawa, Y.; Taino, S.; Ohno, M.; Aruga, Y. Succession of seaweeds on experimental plates immersed during different seasons in Tosa Bay, Japan. Bot. Marina 1998, 41, 321–328. [Google Scholar] [CrossRef]
- Choi, C.G.; Takayama, H.; Segawa, S.; Ohno, M.; Sohn, C.H. Early stage of algal succession on artificial reefs at Muronohana, Ikata, Japan. J. Fish. Sci. Tech. 2000, 3, 1–7. [Google Scholar]
- Choi, C.G.; Takeuchi, Y.; Terawaki, T.; Serisawa, Y.; Ohno, M.; Sohn, C.H. Ecology of seaweed beds on two types of artificial reef. J. Appl. Phycol. 2002, 14, 343–349. [Google Scholar] [CrossRef]
- Watanuki, A.; Yamamoto, H. Settlement of seaweeds on coastal structures. Hydrobiologia 1990, 204, 275–280. [Google Scholar] [CrossRef]
- Kato, T.; Kosugi, C.; Kiso, E.; Torii, K. Application of steelmaking slag to marine forest restoration. Nippon. Steel Sumitomo Met. Tech. Rep. 2015, 109, 79–84. [Google Scholar]
- Hwang, E.K.; Choi, H.G.; Kim, J.K. Seaweed resources of Korea. Bot. Mar. 2020, 63, 395–405. [Google Scholar] [CrossRef]
- Lee, M.O.; Kim, J.K.; Kim, B.K. A review-status of development and research of artificial reefs in the east Asian countries. J. Fish. Mar. Sci. Educ. 2016, 28, 630–644. [Google Scholar] [CrossRef]
- Korea Fisheries Resources Agency (FIRA). Marine Forest 5 Features. Available online: https://www.fira.or.kr/english/english_tap_010302.jsp (accessed on 30 May 2022).
- Korea Fisheries Resources Agency (FIRA). Artificial Reef Information Book; FIRA: Busan, Korea, 2021; pp. 1–99. [Google Scholar]
- Kim, D.; Woo, J.; Yoon, H.S.; Na, W.B. Wake lengths and structural responses of Korean general artificial reefs. Ocean. Eng. 2014, 92, 83–91. [Google Scholar] [CrossRef]
- Ministry of Oceans and Fisheries. Artificial Reef Facility Business Execution and Management Regulations; Ministry of Oceans and Fisheries Ordinance No. 572; Ministry of Oceans and Fisheries: Sejong, Korea, 2020; pp. 1–11. [Google Scholar]
- POSCO Uses Steel Slag to Create a Sea Forest and Save the Marine Ecosystem. Available online: https://newsroom.posco.com/en/posco-uses-steel-slag-to-create-a-sea-forest-and-save-the-marine-ecosystem (accessed on 6 May 2022).
- Lee, I.C.; Park, S.; Woo, H.E.; Jeong, I.; Choi, C.G.; Kim, K. A study on macroalgae establishment on concrete substratum covered by oyster shells. J. Korean Soc. Mar. Environ. Saf. 2021, 27, 639–646. [Google Scholar] [CrossRef]
- East Sea Fisheries Research Institute. A Study on Construction of Seaweed Forests in the East Sea; TR-2008-RE-021; National Fisheries Research and Development Institute: Busan, Korea, 2007; pp. 1–198. [Google Scholar]
- Korea Marine Environment and Ecology Institute. Improvement of Adhesion Substrate and Seed Bank Formation Technique Development for Marine Forest Restoration. Ministry of Oceans and Fisheries: Future Marine Industry Technology Development R&D Report; Ministry of Oceans and Fisheries: Sejong, Korea, 2018; pp. 1–132. [Google Scholar]
- Fishery Resources Management Act, No. 13385, 22 June 2015. Available online: https://elaw.klri.re.kr/eng_service/lawView.do?hseq=36363&lang=ENG (accessed on 6 May 2022).
- Cai, J.; Lovatelli, A.; Aguilar-Manjarrez, J.; Cornish, L.; Dabbadie, L.; Desrochers, A.; Diffey, S.; Garrido Gamarro, E.; Geehan, J.; Hurtado, A.; et al. Seaweeds and Microalgae: An Overview for Unlocking Their Potential in Global Aquaculture Development; No. 1229; FAO Fisheries and Aquaculture Circular: Rome, Italy, 2021; pp. 1–36. ISBN 978-9-2513-4710-2. [Google Scholar]
- Niwa, K.; Sano, F.; Sakamoto, T. Molecular evidence of allodiploidy in F1 gametophytic blades from a cross between Neopyropia yezoensis and a cryptic species of the Neopyropia yezoensis complex (Bangiales, Rhodophyta) by the use of microsatellite markers. Aquacult. Rep. 2020, 18, 100489. [Google Scholar] [CrossRef]
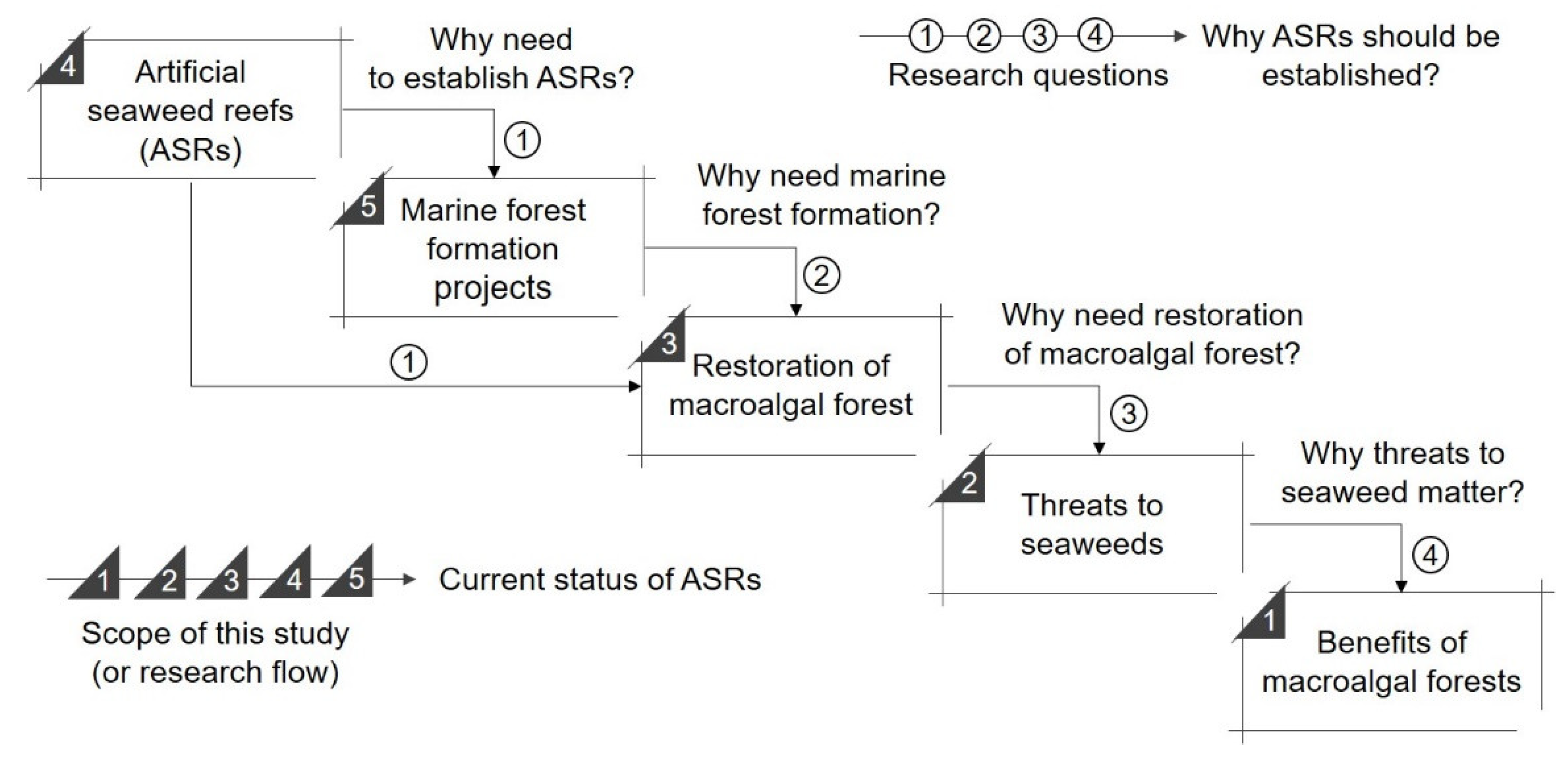
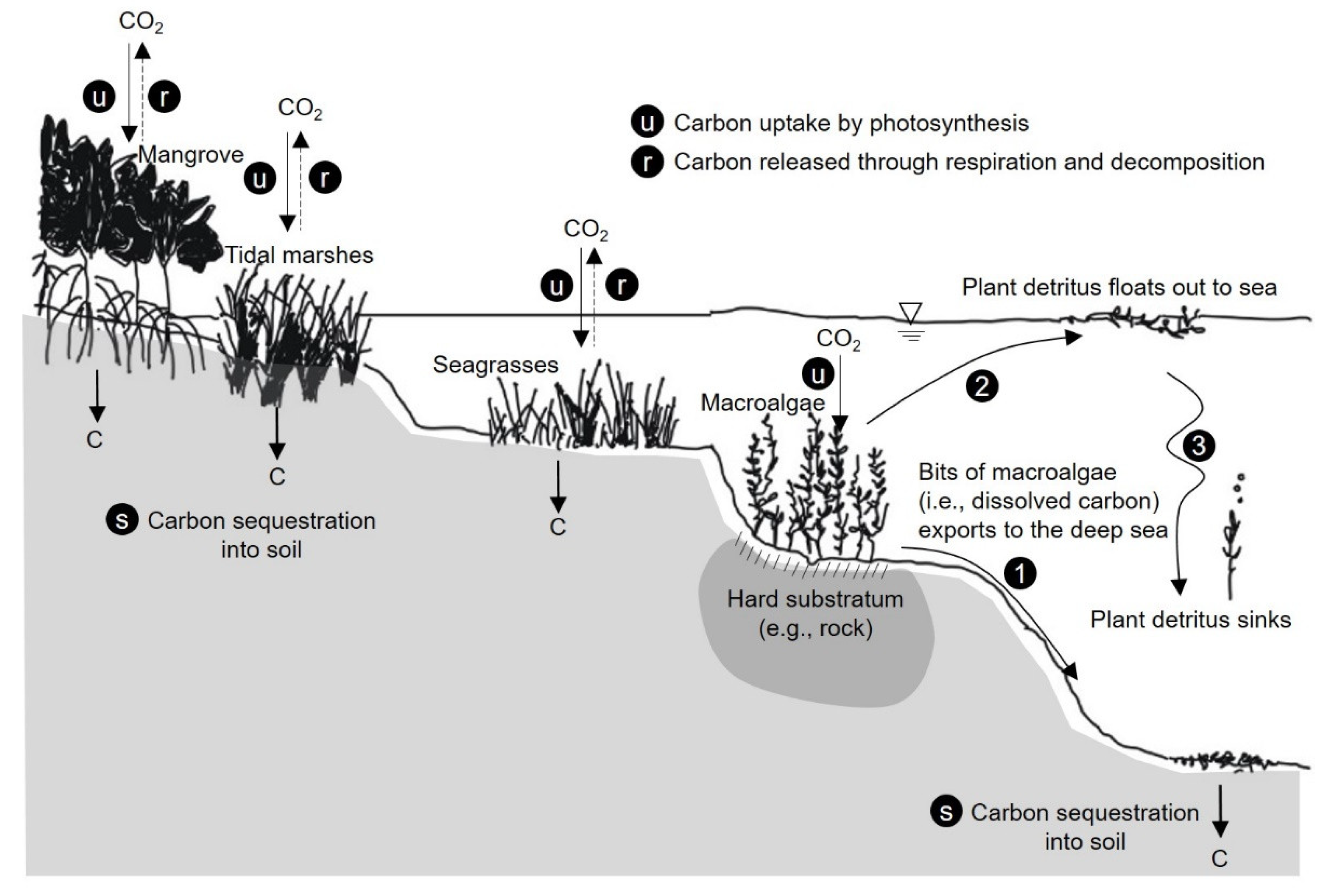
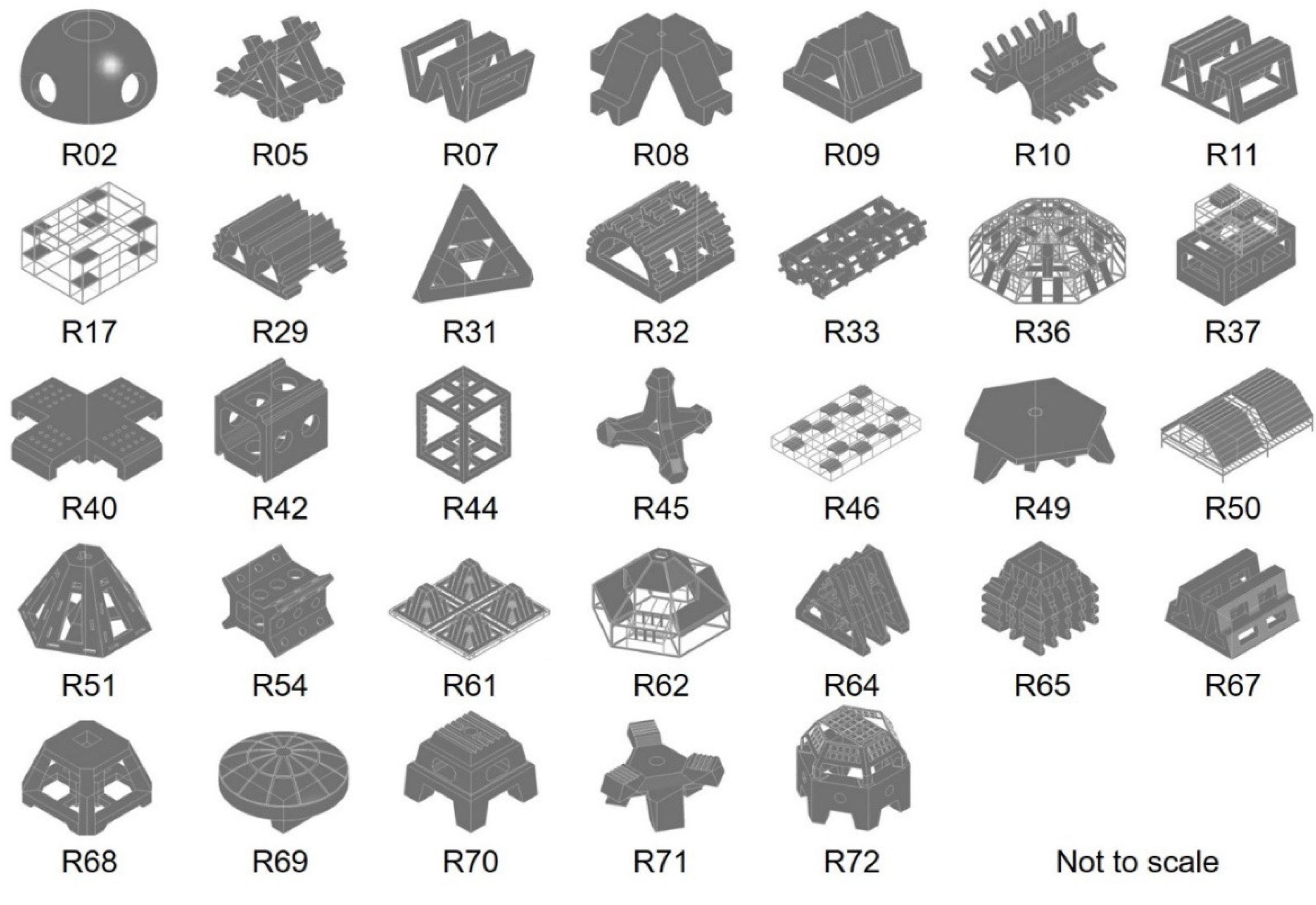
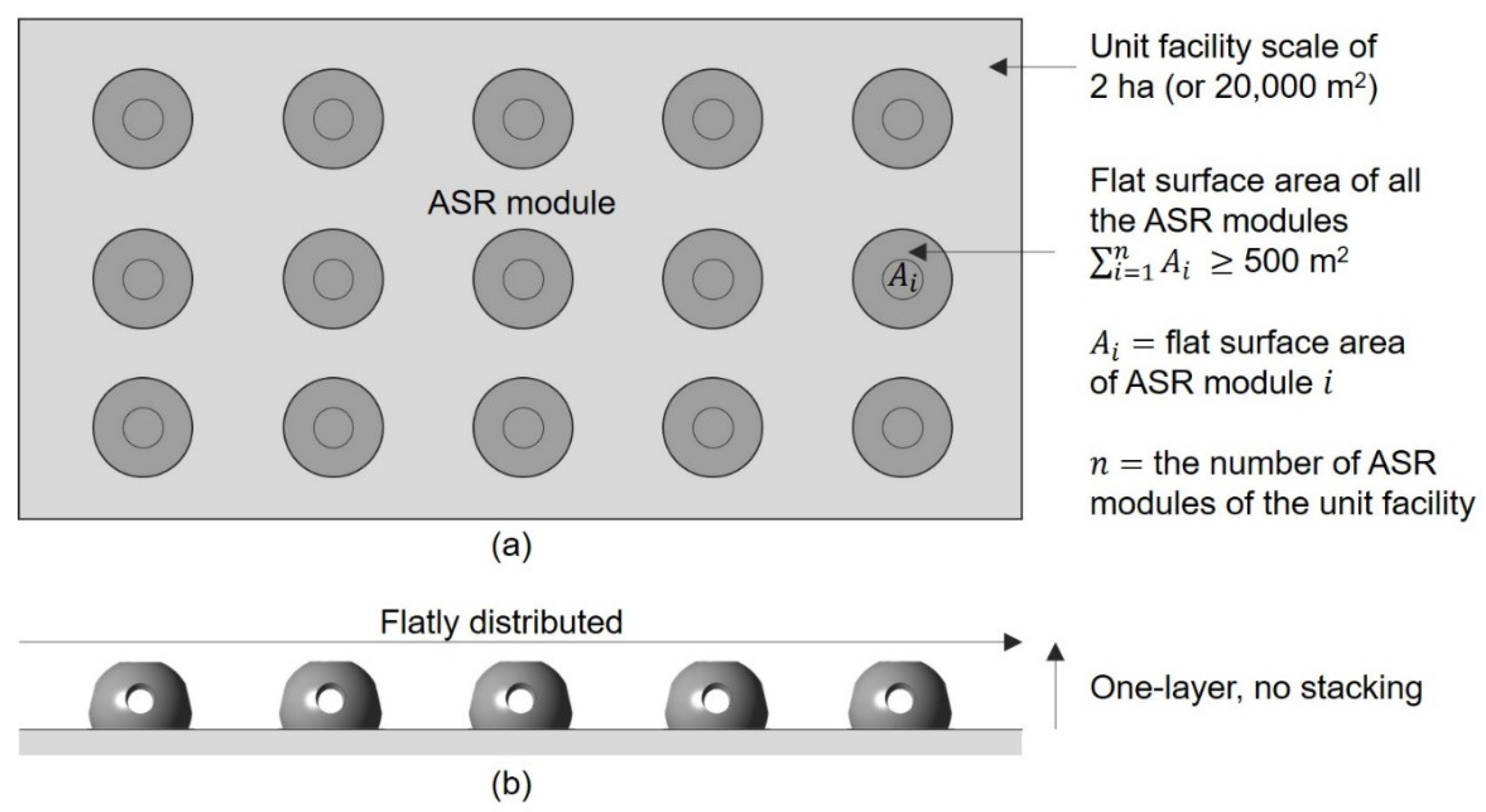
| Theme | Search String |
|---|---|
| Marine afforestation | (marine afforestation OR marine forest* OR macroalgal forest* OR formation* OR creation* OR aquatic vegetation bed* OR macroalgae OR seaweed OR kelp OR ecosystem* OR enhancement* OR function* OR restoration* OR benefit* OR threat* OR project*) |
| Benefits | (macroalgae OR seaweed OR kelp OR CO2 reduction OR marine habitats OR human well-being OR food* OR material* OR bioenergy) |
| Threats | (macroalgae OR seaweed OR kelp OR ocean warming OR marine heatwave* OR El Niño OR grazing OR harvesting OR sediment OR pollution OR storm* OR swell*) |
| Artificial seaweed reefs | (artificial OR man-made OR macroalgae OR seaweed OR kelp OR reef* OR marine forest OR bed*) |
| Criterion | Inclusion | Exclusion |
|---|---|---|
| Study type | Empirical and theoretical/conceptual studies. Peer-reviewed; technical books/conference articles/technical reports included if high quality; current practices and web data included if valuable | Current practices proposed, but no evidence in use |
| Language | English; Korean if necessary | Any other language |
| Date | 2000 to 2022; subsequently extended to the 1990s, 1980s, 1970s, and 1960s if necessary | Any study published before 1960 |
| Relevance | (i) Marine afforestation, marine forest; (ii) benefits/functions of macroalgae (or seaweed or kelp); (iii) threats to macroalgae (or seaweed or kelp); (iv) restoration/enhancement of macroalgae (or seaweed or kelp), relevant techniques and methods; (v) artificial seaweed reefs (Japan, Korea, and USA); (vi) Korean involvement in marine forest formation projects | (i) Not directly relevant to the research question; (ii) artificial reefs not oriented to marine forest formation; (iii) seaweed aquaculture oriented techniques/methods; (iv) level of analysis: not firm-level practices and processes |
| No. | Benefits (or Functions) |
|---|---|
| 1 | It reduces greenhouse gas by absorbing CO2 from the water and air. It has excellent efficiency in reducing CO2, compared to temperate forests and tropical rain forests. It purifies the marine environment by providing dissolved oxygen and eliminating water pollutants. |
| 2 | The forest provides a habitat for marine life. It acts as a spawning ground and growing area for reproduction of marine life, provides food for algae-eating marine creatures, and enhances the basic productive capacity of coastal areas as the primary producer in the ocean. |
| 3 | It is food that contributes to well-being, highlighted by high-protein levels while being a low-calorie diet food. It contains many useful elements for the human body including vitamins and minerals (e.g., iodine and magnesium). |
| 4 | It provides useful/functional materials for medicine, food, and industrial goods (e.g., fucoidan, seanol, alginic acid, and sun block). It absorbs and supplies rare industrial metals (e.g., uranium and lithium) from the sea. |
| 5 | It is a source of pure bio energy as bioethanol, superior to biomass from grains and wood (3rd-generation biomass). |
| Material | Total | Intended Purpose (Target Species) | ||||
|---|---|---|---|---|---|---|
| Fish | Fish–Shellfish | Shellfish– Seaweed | Marine Forest | Sea Cucumber | ||
| RC § | 41 | 10 | 5 | 18 | 7 | 1 |
| Concrete | 3 | – | – | 1 | 1 | 1 |
| Steel | 20 | 20 | – | – | – | – |
| Complex | 25 | 9 | 3 | 9 | 4 | – |
| Total | 89 | 39 | 8 | 28 | 12 | 2 |
| Ranks | Criteria | Causes |
|---|---|---|
| 1 | Coverage of crustose coralline algae: 40–60% (coverage of seaweed: 60–80%) | ① Seaweed feeding by herbivores: 30 g m−2 day−1; ② Number of herbivores: 5–10 m−2; ③ Seaweed state: decrease in large brown algae and perennial seaweeds, increase in small red algae |
| 2 | Coverage of crustose coralline algae: 60–80% (coverage of seaweed: 20–40%) | ① Seaweed feeding by herbivores: 40–60 g m−2 day−1; ② Number of herbivores: 10–20 m−2; ③ Seaweed state: signs of disappearance of large brown algae, colonies of small perennial red algae |
| 3 | Coverage of crustose coralline algae: ≥80% (coverage of seaweed: <20%) | ① Seaweed feeding by herbivores: 70 g m−2 day−1; ② Number of herbivores: ≥20 m−2; ③ Seaweed state: disappearance of large brown algae, colonies of small perennial red algae |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.; Chau, T.V.; Kim, M.; Na, W.-B. Artificial Seaweed Reefs That Support the Establishment of Submerged Aquatic Vegetation Beds and Facilitate Ocean Macroalgal Afforestation: A Review. J. Mar. Sci. Eng. 2022, 10, 1184. https://doi.org/10.3390/jmse10091184
Jung S, Chau TV, Kim M, Na W-B. Artificial Seaweed Reefs That Support the Establishment of Submerged Aquatic Vegetation Beds and Facilitate Ocean Macroalgal Afforestation: A Review. Journal of Marine Science and Engineering. 2022; 10(9):1184. https://doi.org/10.3390/jmse10091184
Chicago/Turabian StyleJung, Somi, Than Van Chau, Minju Kim, and Won-Bae Na. 2022. "Artificial Seaweed Reefs That Support the Establishment of Submerged Aquatic Vegetation Beds and Facilitate Ocean Macroalgal Afforestation: A Review" Journal of Marine Science and Engineering 10, no. 9: 1184. https://doi.org/10.3390/jmse10091184
APA StyleJung, S., Chau, T. V., Kim, M., & Na, W.-B. (2022). Artificial Seaweed Reefs That Support the Establishment of Submerged Aquatic Vegetation Beds and Facilitate Ocean Macroalgal Afforestation: A Review. Journal of Marine Science and Engineering, 10(9), 1184. https://doi.org/10.3390/jmse10091184







