Pygmy Blue Whale Diving Behaviour Reflects Song Structure
Abstract
:1. Introduction
1.1. Function of Blue Whale Sounds
1.2. Context of Sound Production
1.3. Eastern Indian Ocean Pygmy Blue Whale
1.4. Conservation Monitoring of EIOPB Whale
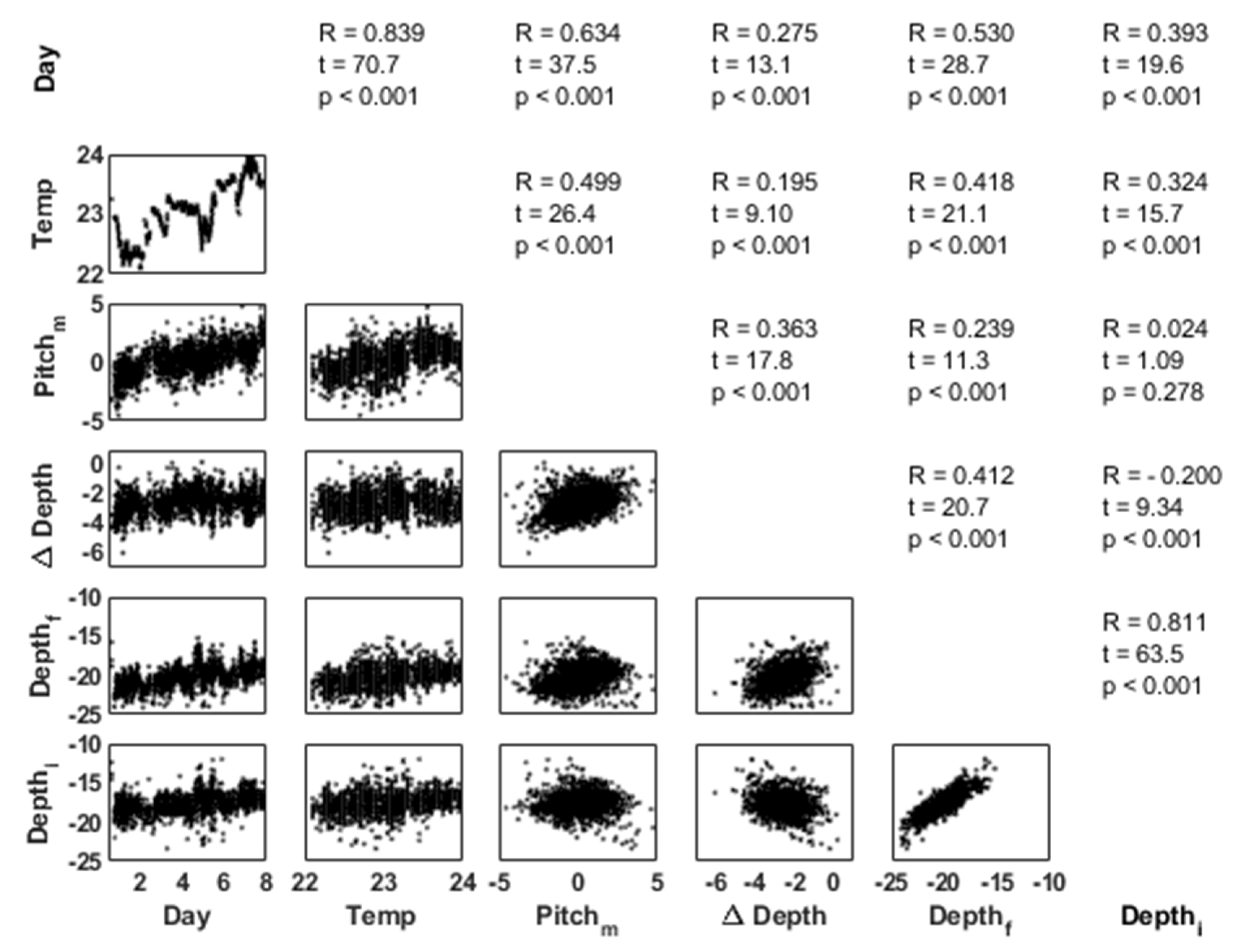
2. Materials and Methods
2.1. Tag Deployment and Sensors
2.2. Dive Definition
2.3. Dive Characteristic Generation
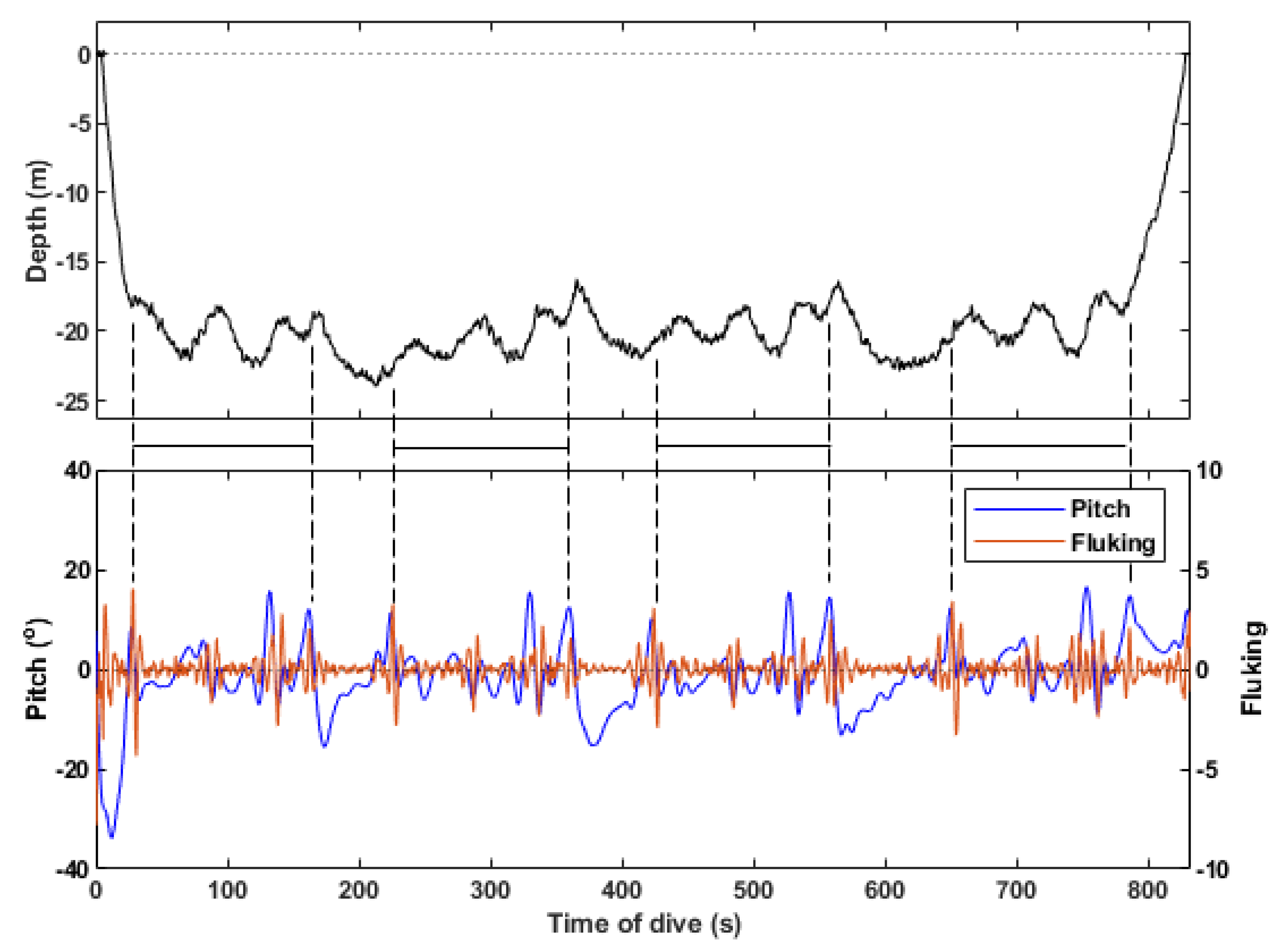
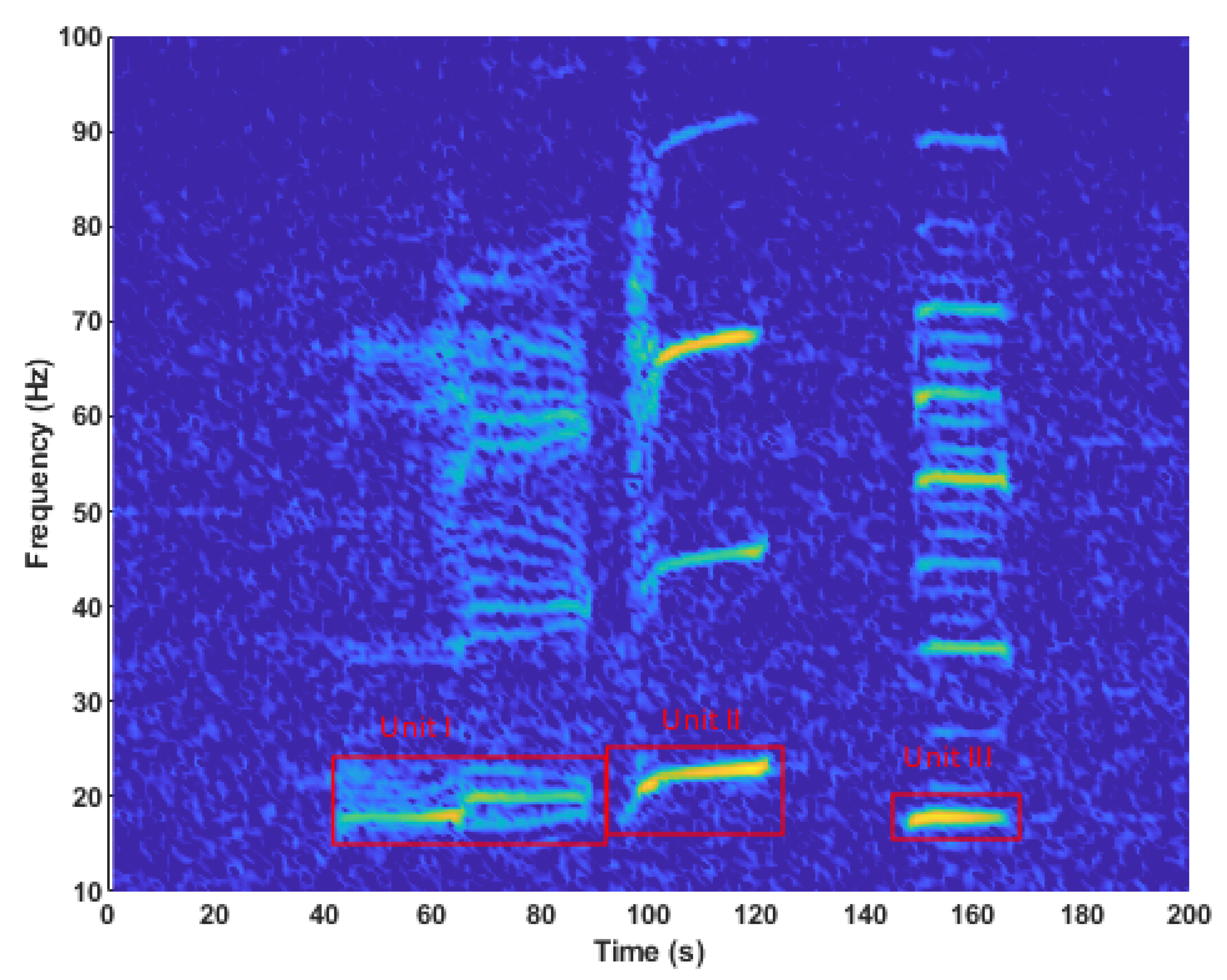
2.4. Song Identified in the Dive Profile
2.5. Verification of Song Identification
- (P2) Unit II, 20 s pause, then Unit III; and
2.6. Context of Sound Production
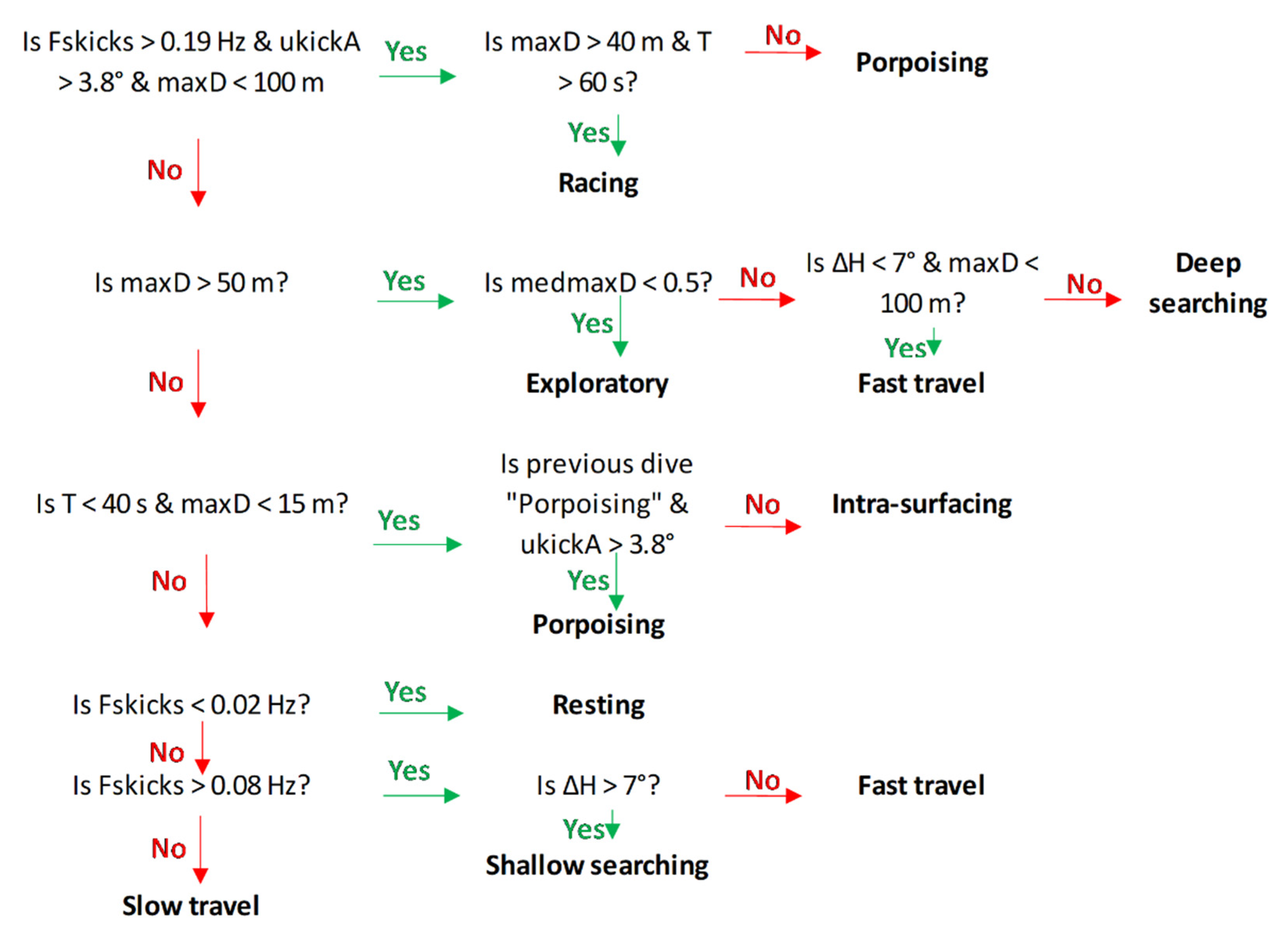
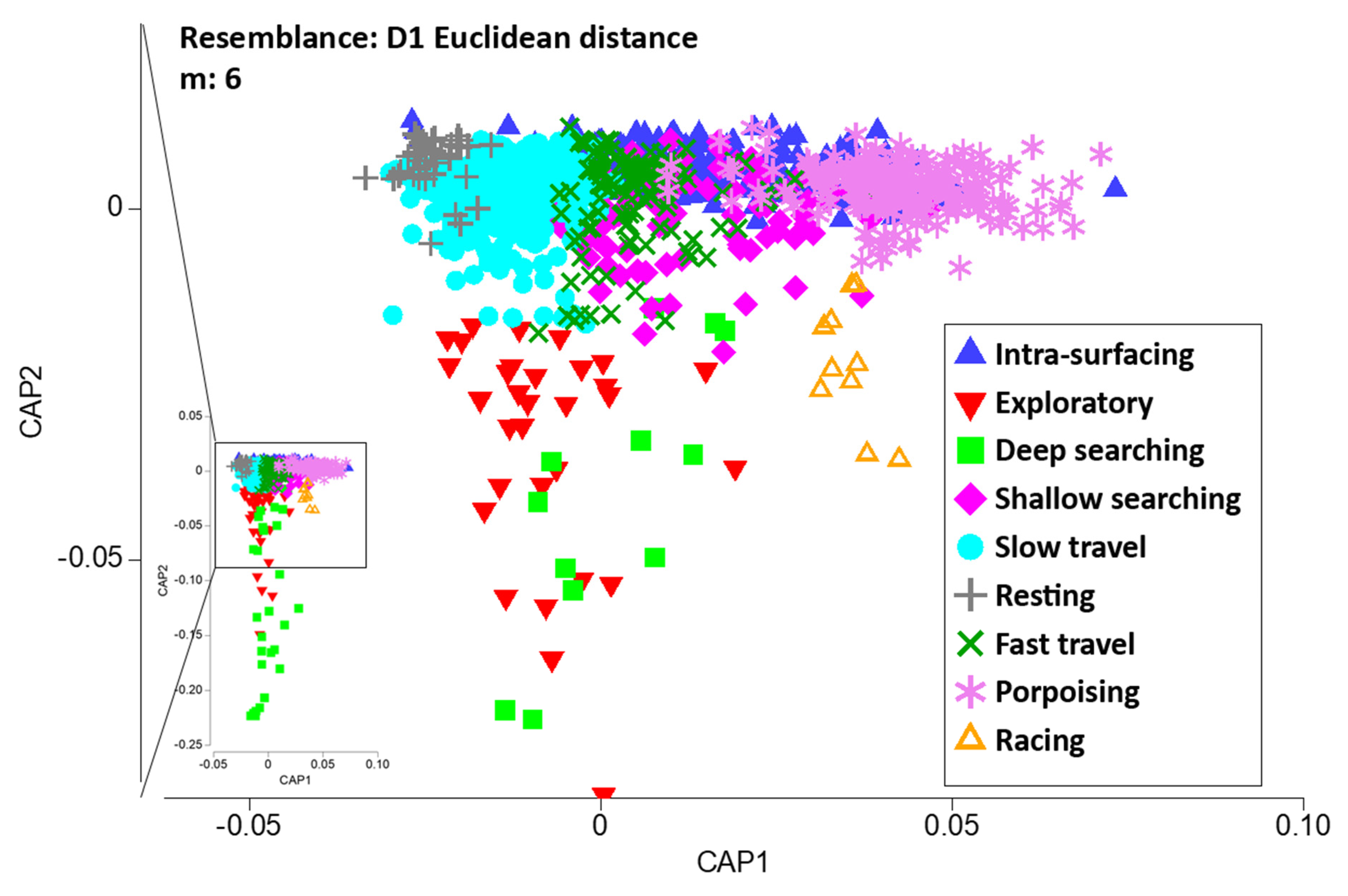
2.7. Dive Type Classification Schema
2.8. Behavioural Analysis
3. Results
3.1. Song Identified in the Dive Profile
3.2. Verification of Song Identification
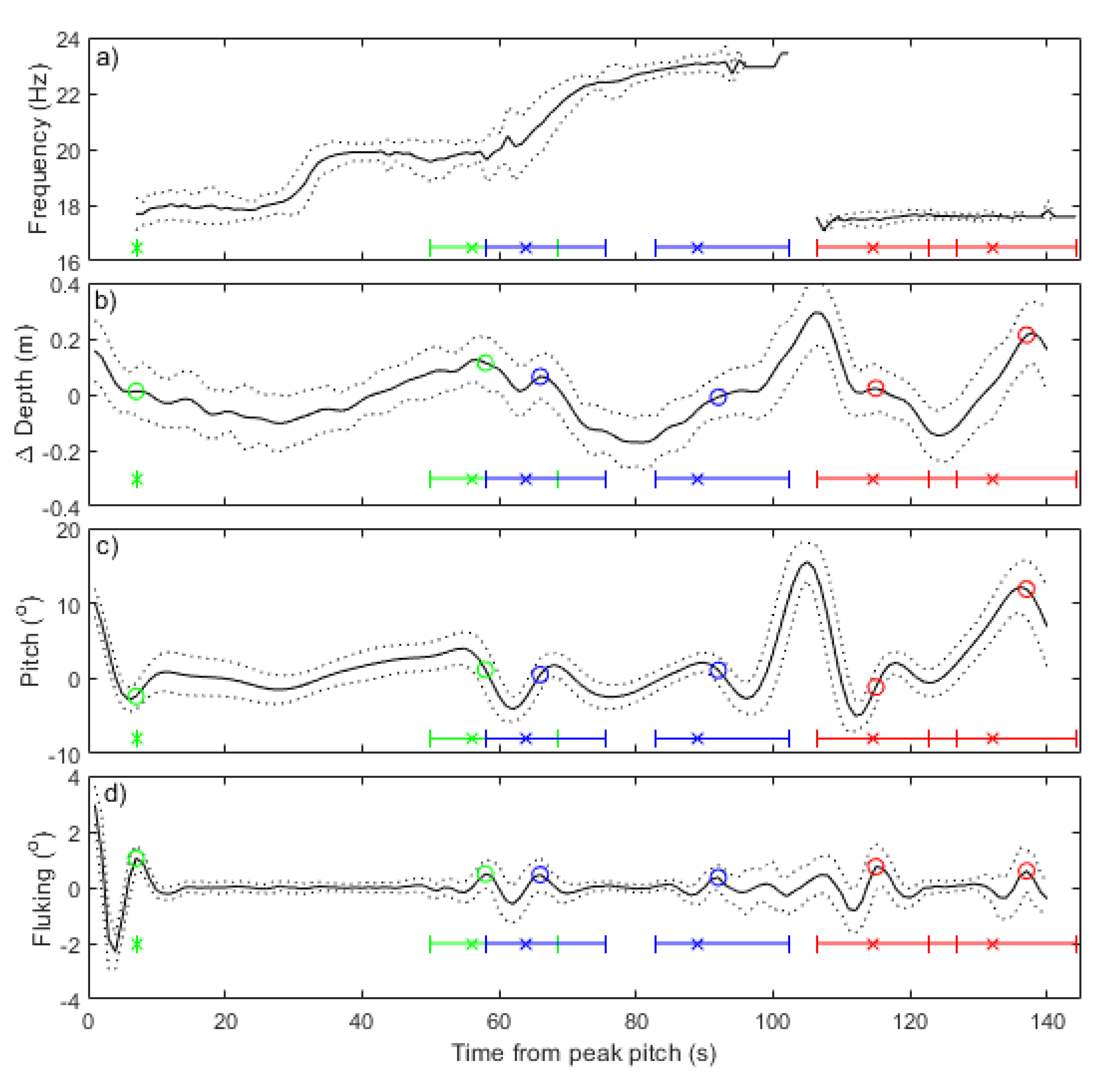
3.3. Context of Sound Production
3.4. Dive Type Classification Schema
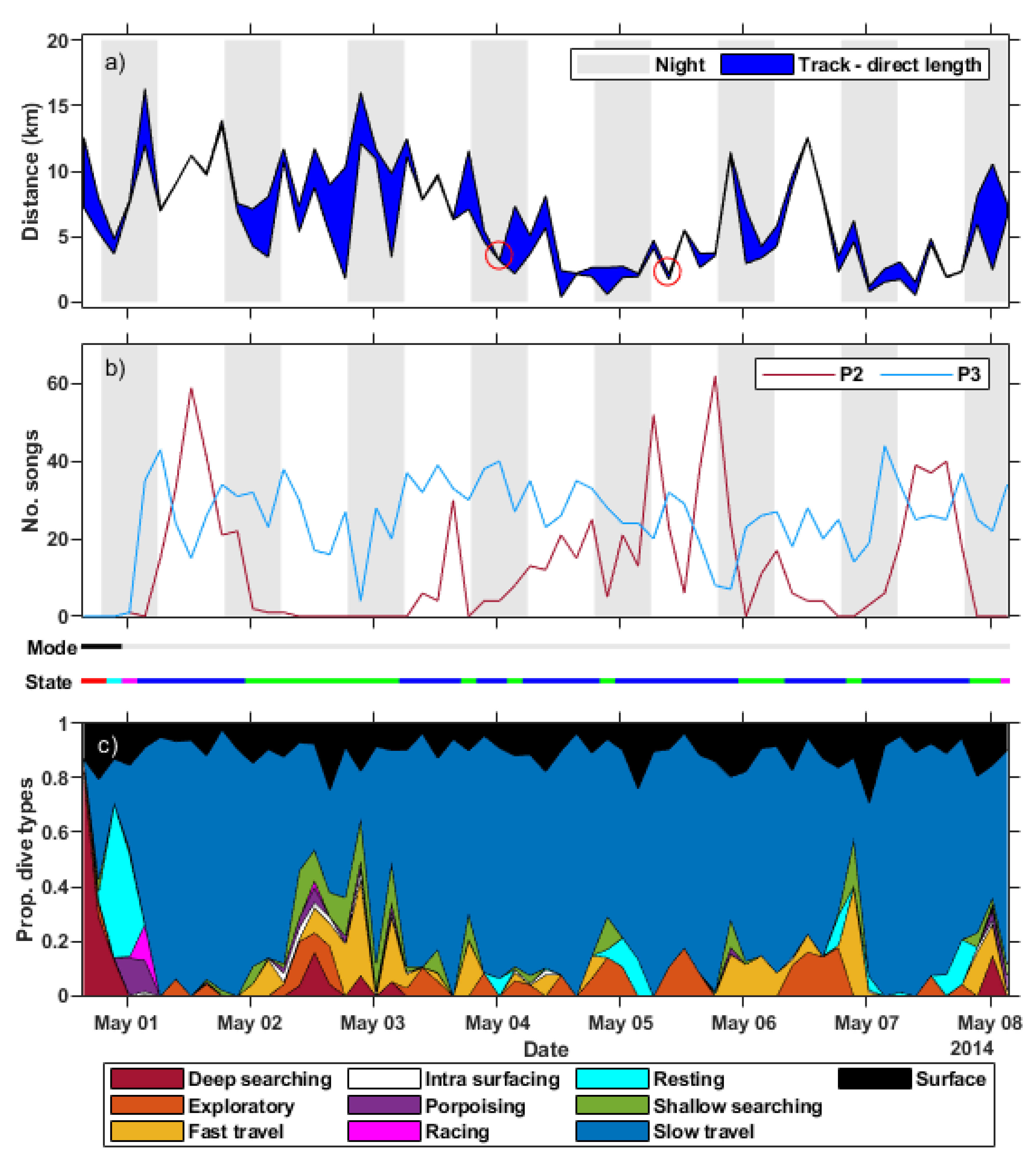
3.5. Behavioural Analysis
3.5.1. Behavioural States
3.5.2. Behavioural Modes
3.5.3. Surface Activity
4. Discussion
4.1. Limitations of Study
4.2. Physical Context of Song
4.3. Classification of Diving Behaviour
4.4. Singing Behavioural Context
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCauley, R.D.; Gavrilov, A.N.; Jolliffe, C.D.; Ward, R.; Gill, P.C. Pygmy Blue and Antarctic Blue Whale Presence, Distribution and Population Parameters in Southern Australia Based on Passive Acoustics. Deep Sea Res. Part II Top. Stud. Oceanogr. 2018, 157–158, 154–168. [Google Scholar] [CrossRef]
- Rice, A.; Širović, A.; Hildebrand, J.A.; Wood, M.; Carbaugh-Rutland, A.; Baumann-Pickering, S. Update on Frequency Decline of Northeast Pacific Blue Whale (Balaenoptera musculus) Calls. PLoS ONE 2022, 17, e0266469. [Google Scholar] [CrossRef] [PubMed]
- Dziak, R.P.; Haxel, J.H.; Lau, T.-K.; Heimlich, S.; Caplan-Auerbach, J.; Mellinger, D.K.; Matsumoto, H.; Mate, B. A Pulsed-Air Model of Blue Whale B Call Vocalizations. Sci. Rep. 2017, 7, 9122. [Google Scholar] [CrossRef] [PubMed]
- Oleson, E.M.; Calambokidis, J.; Burgess, W.C.; McDonald, M.A.; LeDuc, C.A.; Hildebrand, J.A. Behavioral Context of Call Production by Eastern North Pacific Blue Whales. Mar. Ecol. Prog. Ser. 2007, 330, 269–284. [Google Scholar] [CrossRef]
- Lewis, L.A.; Calambokidis, J.; Stimpert, A.K.; Fahlbusch, J.; Friedlaender, A.S.; McKenna, M.F.; Mesnick, S.L.; Oleson, E.M.; Southall, B.L.; Szesciorka, A.R.; et al. Context-Dependent Variability in Blue Whale Acoustic Behaviour. R. Soc. Open Sci. 2018, 5, 180241. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.A.; Calambokidis, J.; Teranishi, A.M.; Hildebrand, J.A. The Acoustic Calls of Blue Whales off California with Gender Data. J. Acoust. Soc. Am. 2001, 109, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Cazau, D.; Adam, O.; Aubin, T.; Laitman, J.T.; Reidenberg, J.S. A Study of Vocal Nonlinearities in Humpback Whale Songs: From Production Mechanisms to Acoustic Analysis. Sci. Rep. 2016, 6, 31660. [Google Scholar] [CrossRef]
- Adam, O.; Cazau, D.; Gandilhon, N.; Fabre, B.; Laitman, J.T.; Reidenberg, J.S. New Acoustic Model for Humpback Whale Sound Production. Appl. Acoust. 2013, 74, 1182–1190. [Google Scholar] [CrossRef]
- Schall, E.; Di Iorio, L.; Berchok, C.; Filún, D.; Bedriñana-Romano, L.; Buchan, S.J.; Van Opzeeland, I.; Sears, R.; Hucke-Gaete, R. Visual and Passive Acoustic Observations of Blue Whale Trios from Two Distinct Populations. Mar. Mamm. Sci. 2020, 36, 365–374. [Google Scholar] [CrossRef]
- Tyack, P.L. Studying How Cetaceans Use Sound to Explore Their Environment. In Communication; Owings, D.H., Beecher, M.D., Thompson, N.S., Eds.; Springer: Boston, MA, USA, 1997; pp. 251–297. ISBN 9781489917454. [Google Scholar]
- Payne, R.; Webb, D. Orientation by Means of Long Range Acoustic Signaling in Baleen Whales. Ann. N. Y. Acad. Sci. 1971, 188, 110–141. [Google Scholar] [CrossRef]
- Clark, C.W.; Ellison, W.T. Potential Use of Low-Frequency Sounds by Baleen Whales for Probing the Environment: Evidence from Models and Empirical Measurements. In Echolocation in Bats and Dolphins; The University of Chicago Press: Chicago, IL, USA, 2004. [Google Scholar]
- Oestreich, W.K.; Fahlbusch, J.A.; Cade, D.E.; Calambokidis, J.; Margolina, T.; Joseph, J.; Friedlaender, A.S.; McKenna, M.F.; Stimpert, A.K.; Southall, B.L.; et al. Animal-Borne Metrics Enable Acoustic Detection of Blue Whale Migration. Curr. Biol. 2020, 30, 4773–4779.e3. [Google Scholar] [CrossRef]
- Aroyan, J.L.; McDonald, M.A.; Webb, S.C.; Hildebrand, J.A.; Clark, D.; Laitman, J.T.; Reidenberg, J.S. Acoustic Models of Sound Production and Propagation. In Hearing by Whales and Dolphins; Au, W.W.L., Fay, R.R., Popper, A.N., Eds.; Springer: New York, NY, USA, 2000; pp. 409–469. ISBN 9781461211501. [Google Scholar]
- Stimpert, A.K.; DeRuiter, S.L.; Falcone, E.A.; Joseph, J.; Douglas, A.B.; Moretti, D.J.; Friedlaender, A.S.; Calambokidis, J.; Gailey, G.; Tyack, P.L.; et al. Sound Production and Associated Behavior of Tagged Fin Whales (Balaenoptera Physalus) in the Southern California Bight. Anim. Biotelemetry 2015, 3, 23. [Google Scholar] [CrossRef]
- Jones, A.D.; McCauley, R.D.; Cato, D.H. Observations and Explanation of Low Frequency Clicks in Blue Whale Calls. In Proceedings of the Annual Conference of the Australian Acoustical Society, Adelaide, Australia, 12–15 November 2002; pp. 45–50. [Google Scholar]
- Brekhovskikh, L.M.; Lysanov, Y.P.; Lysanov, J.P. Fundamentals of Ocean Acoustics; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003; ISBN 9780387954677. [Google Scholar]
- Hooker, S.K.; Fahlman, A.; Moore, M.J.; de Soto, N.A.; de Quirós, Y.B.; Brubakk, A.O.; Costa, D.P.; Costidis, A.M.; Dennison, S.; Falke, K.J.; et al. Deadly Diving? Physiological and Behavioural Management of Decompression Stress in Diving Mammals. Proc. Biol. Sci. 2012, 279, 1041–1050. [Google Scholar] [CrossRef]
- Bostrom, B.L.; Fahlman, A.; Jones, D.R. Tracheal Compression Delays Alveolar Collapse during Deep Diving in Marine Mammals. Respir. Physiol. Neurobiol. 2008, 161, 298–305. [Google Scholar] [CrossRef]
- Moore, M.J.; Hammar, T.; Arruda, J.; Cramer, S.; Dennison, S.; Montie, E.; Fahlman, A. Hyperbaric Computed Tomographic Measurement of Lung Compression in Seals and Dolphins. J. Exp. Biol. 2011, 214, 2390–2397. [Google Scholar] [CrossRef]
- Bouffaut, L.; Landrø, M.; Potter, J.R. Source Level and Vocalizing Depth Estimation of Two Blue Whale Subspecies in the Western Indian Ocean from Single Sensor Observations. J. Acoust. Soc. Am. 2021, 149, 4422–4436. [Google Scholar] [CrossRef]
- Branch, T.A.; Stafford, K.M.; Palacios, D.M.; Allison, C.; Bannister, J.L.; Burton, C.L.K.; Cabrera, E.; Carlson, C.A.; Galletti Vernazzani, B.; Gill, P.C.; et al. Past and Present Distribution, Densities and Movements of Blue Whales Balaenoptera Musculus in the Southern Hemisphere and Northern Indian Ocean. Mamm. Rev. 2007, 37, 116–175. [Google Scholar] [CrossRef]
- Thums, M.; Ferreira, L.; Jenner, C.; Jenner, M.; Harris, D.; Davenport, A.; Andrews-Goff, V.; Double, M.; Möller, L.; Attard, C.R.M.; et al. Pygmy Blue Whale Movement, Distribution and Important Areas in the Eastern Indian Ocean. Glob. Ecol. Conserv. 2022, 35, e02054. [Google Scholar] [CrossRef]
- McCauley, R.D.; Jenner, C. Migratory Patterns and Estimated Population Size of Pygmy Blue Whales (Balaenoptera Musculus Brevicauda) Traversing the Western Australian Coast Based on Passive Acoustics. In Paper SC/62/SH26 Presented to the IWC Scientific Committee, Agadir, Morocco, 21–25 June 2010; International Whaling Commission: Cambridge, UK.
- Samaran, F.; Stafford, K.M.; Branch, T.A.; Gedamke, J.; Royer, J.-Y.; Dziak, R.P.; Guinet, C. Seasonal and Geographic Variation of Southern Blue Whale Subspecies in the Indian Ocean. PLoS ONE 2013, 8, e71561. [Google Scholar] [CrossRef]
- Leroy, E.C.; Samaran, F.; Stafford, K.M.; Bonnel, J.; Royer, J.Y. Broad-Scale Study of the Seasonal and Geographic Occurrence of Blue and Fin Whales in the Southern Indian Ocean. Endanger. Species Res. 2018, 37, 289–300. [Google Scholar] [CrossRef] [Green Version]
- Möller, L.M.; Attard, C.R.M.; Bilgmann, K.; Andrews-Goff, V.; Jonsen, I.; Paton, D.; Double, M.C. Movements and Behaviour of Blue Whales Satellite Tagged in an Australian Upwelling System. Sci. Rep. 2020, 10, 21165. [Google Scholar] [CrossRef]
- Double, M.C.; Andrews-Goff, V.; Jenner, K.C.S.; Jenner, M.-N.; Laverick, S.M.; Branch, T.A.; Gales, N.J. Migratory Movements of Pygmy Blue Whales (Balaenoptera musculus Brevicauda) between Australia and Indonesia as Revealed by Satellite Telemetry. PLoS ONE 2014, 9, e93578. [Google Scholar] [CrossRef]
- Andrews-Goff, V.; Bestley, S.; Gales, N.J.; Laverick, S.M.; Paton, D.; Polanowski, A.M.; Schmitt, N.T.; Double, M.C. Humpback Whale Migrations to Antarctic Summer Foraging Grounds through the Southwest Pacific Ocean. Sci. Rep. 2018, 8, 12333. [Google Scholar] [CrossRef]
- Kahn, B. Blue Whales of the Savu Sea, Indonesia. In Proceedings of the 17th Biennial Conference on the Biology of Marine Mammals, Cape Town, South Africa, 28 November–3 December 2007; p. 89. [Google Scholar]
- Owen, K.; Jenner, C.S.; Jenner, M.-N.M.; Andrews, R.D. A Week in the Life of a Pygmy Blue Whale: Migratory Dive Depth Overlaps with Large Vessel Drafts. Anim. Biotelemetry 2016, 4, 17. [Google Scholar] [CrossRef]
- Conservation Management Plan for the Blue Whale: A Recovery Plan under the Environment Protection and Biodiversity Conservation Act 1999, Commonwealth of Australia 2015. Available online: https://www.dcceew.gov.au/sites/default/files/documents/blue-whale-conservation-management-plan.pdf (accessed on 15 October 2021).
- Branch, T.A.; Allison, C.; Mikhalev, Y.A.; Tormosov, D.; Brownell, R.L. Historical Catch Series for Antarctic and Pygmy Blue Whales. In Paper SC/60/SH9 Presented to the IWC Scientific Committee, Santiago, Chile, 23–27 June 2008; International Whaling Commission: Cambridge, UK.
- Jenner, C.; Jenner, M.; Burton, C.; Sturrock, V.; Kent, C.S.; Morrice, M.; Attard, C.; Möller, L.; Double, M.C. Mark Recapture Analysis of Pygmy Blue Whales from the Perth Canyon, Western Australia 2000–2005. In Paper SC/60/SH16 Presented to the IWC Scientific Committee, Santiago, Chile, 23–27 June 2008; International Whaling Commission: Cambridge, UK.
- Rennie, S.; Hanson, C.E.; McCauley, R.D.; Pattiaratchi, C.; Burton, C.; Bannister, J.; Jenner, C.; Jenner, M.-N. Physical Properties and Processes in the Perth Canyon, Western Australia: Links to Water Column Production and Seasonal Pygmy Blue Whale Abundance. J. Mar. Syst. 2009, 77, 21–44. [Google Scholar] [CrossRef]
- Jolliffe, C.D.; McCauley, R.D.; Gavrilov, A.N.; Jenner, K.C.S.; Jenner, M.-N.M.; Duncan, A.J. Song Variation of the South Eastern Indian Ocean Pygmy Blue Whale Population in the Perth Canyon, Western Australia. PLoS ONE 2019, 14, e0208619. [Google Scholar] [CrossRef]
- Jolliffe, C.D.; McCauley, R.D.; Gavrilov, A.N.; Jenner, C.; Jenner, M.N. Comparing the Acoustic Behaviour of the Eastern Indian Ocean Pygmy Blue Whale on Two Australian Feeding Grounds. Acoust. Aust. 2021, 49, 331–344. [Google Scholar] [CrossRef]
- Marques, T.A.; Thomas, L.; Martin, S.W.; Mellinger, D.K.; Ward, J.A.; Moretti, D.J.; Harris, D.; Tyack, P.L. Estimating Animal Population Density Using Passive Acoustics. Biol. Rev. Camb. Philos. Soc. 2013, 88, 287–309. [Google Scholar] [CrossRef] [PubMed]
- Cade, D.E.; Gough, W.T.; Czapanskiy, M.F.; Fahlbusch, J.A.; Kahane-Rapport, S.R.; Linsky, J.M.J.; Nichols, R.C.; Oestreich, W.K.; Wisniewska, D.M.; Friedlaender, A.S.; et al. Tools for Integrating Inertial Sensor Data with Video Bio-Loggers, Including Estimation of Animal Orientation, Motion, and Position. Anim. Biotelemetry 2021, 9, 34. [Google Scholar] [CrossRef]
- Simon, M.; Johnson, M.; Madsen, P.T. Keeping Momentum with a Mouthful of Water: Behavior and Kinematics of Humpback Whale Lunge Feeding. J. Exp. Biol. 2012, 215, 3786–3798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldbogen, J.A.; Calambokidis, J.; Shadwick, R.E.; Oleson, E.M.; McDonald, M.A.; Hildebrand, J.A. Kinematics of Foraging Dives and Lunge-Feeding in Fin Whales. J. Exp. Biol. 2006, 209, 1231–1244. [Google Scholar] [CrossRef] [PubMed]
- Owen, K.; Dunlop, R.A.; Monty, J.P.; Chung, D.; Noad, M.J.; Donnelly, D.; Goldizen, A.W.; Mackenzie, T. Detecting Surface-Feeding Behavior by Rorqual Whales in Accelerometer Data. Mar. Mamm. Sci. 2016, 32, 327–348. [Google Scholar] [CrossRef]
- Sweeney, D.A.; DeRuiter, S.L.; McNamara-Oh, Y.J.; Marques, T.A.; Arranz, P.; Calambokidis, J. Automated Peak Detection Method for Behavioral Event Identification: Detecting Balaenoptera musculus and Grampus griseus Feeding Attempts. Anim. Biotelemetry 2019, 7, 7. [Google Scholar] [CrossRef]
- Erbe, C.; Verma, A.; McCauley, R.; Gavrilov, A.; Parnum, I. The Marine Soundscape of the Perth Canyon. Prog. Oceanogr. 2015, 137, 38–51. [Google Scholar] [CrossRef]
- Gavrilov, A.N.; McCauley, R.D.; Salgado-Kent, C.; Tripovich, J.; Burton, C. Vocal Characteristics of Pygmy Blue Whales and Their Change over Time. J. Acoust. Soc. Am. 2011, 130, 3651–3660. [Google Scholar] [CrossRef]
- Anderson, M.J.; Willis, T.J. Canonical Analysis of Principal Coordinates: A Useful Method of Constrained Ordination for Ecology. Ecology 2003, 84, 511–525. [Google Scholar] [CrossRef]
- Anderson, M.J.; Robinson, J. Generalized Discriminant Analysis Based on Distances. Aust. N. Z. J. Stat. 2003, 45, 301–318. [Google Scholar] [CrossRef]
- Recalde-Salas, A.; Salgado Kent, C.P.; Parsons, M.J.G.; Marley, S.A.; McCauley, R.D. Non-Song Vocalizations of Pygmy Blue Whales in Geographe Bay, Western Australia. J. Acoust. Soc. Am. 2014, 135, EL213–EL218. [Google Scholar] [CrossRef]
- Stimpert, A.K.; Lammers, M.O.; Pack, A.A.; Au, W.W.L. Variations in Received Levels on a Sound and Movement Tag on a Singing Humpback Whale: Implications for Caller Identification. J. Acoust. Soc. Am. 2020, 147, 3684. [Google Scholar] [CrossRef]
- Goldbogen, J.A.; Stimpert, A.K.; DeRuiter, S.L.; Calambokidis, J.; Friedlaender, A.S.; Schorr, G.S.; Moretti, D.J.; Tyack, P.L.; Southall, B.L. Using Accelerometers to Determine the Calling Behavior of Tagged Baleen Whales. J. Exp. Biol. 2014, 217, 2449–2455. [Google Scholar] [CrossRef] [Green Version]
- Saddler, M.R.; Bocconcelli, A.; Hickmott, L.S.; Chiang, G.; Landea-Briones, R.; Bahamonde, P.A.; Howes, G.; Segre, P.S.; Sayigh, L.S. Characterizing Chilean Blue Whale Vocalizations with DTAGs: A Test of Using Tag Accelerometers for Caller Identification. J. Exp. Biol. 2017, 220, 4119–4129. [Google Scholar] [CrossRef]
- Miller, B.S.; Leaper, R.; Calderan, S.; Gedamke, J. Red Shift, Blue Shift: Investigating Doppler Shifts, Blubber Thickness, and Migration as Explanations of Seasonal Variation in the Tonality of Antarctic Blue Whale Song. PLoS ONE 2014, 9, e107740. [Google Scholar] [CrossRef]
- Gavrilov, A.N.; McCauley, R.D.; Gedamke, J. Steady Inter and Intra-Annual Decrease in the Vocalization Frequency of Antarctic Blue Whales. J. Acoust. Soc. Am. 2012, 131, 4476–4480. [Google Scholar] [CrossRef]
- Leroy, E.C.; Royer, J.-Y.; Bonnel, J.; Samaran, F. Long-Term and Seasonal Changes of Large Whale Call Frequency in the Southern Indian Ocean. J. Geophys. Res. C Oceans 2018, 123, 8568–8580. [Google Scholar] [CrossRef]
- McDonald, M.A.; Hildebrand, J.A.; Mesnick, S. Worldwide Decline in Tonal Frequencies of Blue Whale Songs. Endanger. Species Res. 2009, 9, 13–21. [Google Scholar] [CrossRef]
- Malige, F.; Patris, J.; Hauray, M.; Giraudet, P.; Glotin, H. Camille Noûs Mathematical Models of Long Term Evolution of Blue Whale Song Types’ Frequencies. J. Theor. Biol. 2022, 548, 111184. [Google Scholar] [CrossRef]
- Malige, F.; Patris, J.; Buchan, S.J.; Stafford, K.M.; Shabangu, F.; Findlay, K.; Hucke-Gaete, R.; Neira, S.; Clark, C.W.; Glotin, H. Inter-Annual Decrease in Pulse Rate and Peak Frequency of Southeast Pacific Blue Whale Song Types. Sci. Rep. 2020, 10, 8121. [Google Scholar] [CrossRef]
- Erbe, C.; Peel, D.; Smith, J.N.; Schoeman, R.P. Marine Acoustic Zones of Australia. J. Mar. Sci. Eng. 2021, 9, 340. [Google Scholar] [CrossRef]
- Barham, E.G. Whales’ Respiratory Volume as a Possible Resonant Receiver for 20 Hz Signals. Nature 1973, 245, 220–221. [Google Scholar] [CrossRef]
- Hertel, H. Structure-Form-Movement; Reinhold: New York, NY, USA, 1966; ISBN 9780442150570. [Google Scholar]
- Thums, M.; Bradshaw, C.J.A.; Hindell, M.A. Tracking Changes in Relative Body Composition of Southern Elephant Seals Using Swim Speed Data. Mar. Ecol. Prog. Ser. 2008, 370, 249–261. [Google Scholar] [CrossRef] [Green Version]
- Goldman, J.A. Effects of the Free Water Surface on Animals That Jump out of Water; Duke University: Ann Arbor, MI, USA, 2001. [Google Scholar]
- Palacios, D.M.; Irvine, L.M.; Lagerquist, B.A.; Fahlbusch, J.A.; Calambokidis, J.; Tomkiewicz, S.M.; Mate, B.R. A Satellite-Linked Tag for the Long-Term Monitoring of Diving Behavior in Large Whales. Anim. Biotelemetry 2021, 10, 26. [Google Scholar] [CrossRef]
- de Vos, A.; Brownell, R.L.; Tershy, B.; Croll, D. Anthropogenic Threats and Conservation Needs of Blue Whales, Balaenoptera Musculus Indica, around Sri Lanka. J. Mar. Biol. 2016, 2016, 8420846. [Google Scholar] [CrossRef]
- Jenner, K.C.S. (Centre for Whale Research (WA) Inc., Fremantle, WA, Australia); Jenner, M.M. (Centre for Whale Research (WA) Inc., Fremantle, WA, Australia). Personal communication. 2022. [Google Scholar]
- Gill, P. (Deakin University, Warrnambool, VIC, Australia). Personal communication, 2022.
- Donnelly, D.M.; McInnes, J.D.; Jenner, K.C.S.; Jenner, M.-N.M.; Morrice, M. The First Records of Antarctic Type B and C Killer Whales (Orcinus orca) in Australian Coastal Waters. Aquat. Mamm. 2021, 47, 292–302. [Google Scholar] [CrossRef]
- Totterdell, J.A.; Wellard, R.; Reeves, I.M.; Elsdon, B.; Markovic, P.; Yoshida, M.; Fairchild, A.; Sharp, G.; Pitman, R.L. The First Three Records of Killer Whales (Orcinus orca) Killing and Eating Blue Whales (Balaenoptera musculus). Mar. Mamm. Sci. 2022, 38, 1286–1301. [Google Scholar] [CrossRef]
- McCauley, R.D.; Jenner, C.; Bannister, J.L.; Cato, D.H.; Duncan, A. Blue Whale Calling in the Rottnest Trench, Western Australia, and Low Frequency Sea Noise. In Proceedings of the Annual Conference of the Australian Acoustical Society, Joondalup, Australia, 15–17 November 2001; pp. 245–250. [Google Scholar]
- Gill, P.C.; Morrice, M.G.; Page, B.; Pirzl, R.; Levings, A.H.; Coyne, M. Blue Whale Habitat Selection and Within-Season Distribution in a Regional Upwelling System off Southern Australia. Mar. Ecol. Prog. Ser. 2011, 421, 243–263. [Google Scholar] [CrossRef] [Green Version]

| Song | Manual Songs | XC Signals | XC Songs | Wrong Song | False Positives |
|---|---|---|---|---|---|
| P2 | 797 (91.3%) | 907 | 728 (80.2%) | 134 (14.8%) | 45 (6.2%) |
| P3 | 1528 (90.1%) | 1410 | 1376 (97.6%) | 20 (1.4%) | 15 (1.1%) |
| Total Duration (h) | Unit II h−1 | P2 Songs h−1 | P3 Songs h−1 | Time in Singing Dives (%) | |
|---|---|---|---|---|---|
| Migrating | 171 | 13.6 | 4.6 | 9.0 | 73.8% |
| Day | 63 | 15.2 | 6.6 | 8.5 | 76.8% |
| Night | 66 | 10.6 | 1.9 | 8.6 | 64.9% |
| Twilight | 42 | 15.9 | 5.8 | 10.2 | 83.3% |
| Milling | 57 | 8.8 | 0.8 | 8.0 | 56.9% |
| Day | 9 | 7.1 | 0.0 | 7.0 | 48.1% |
| Night | 36 | 8.3 | 0.8 | 7.6 | 53.6% |
| Twilight | 12 | 11.6 | 1.5 | 10.2 | 73.4% |
| Travelling | 111 | 16.1 | 6.7 | 9.4 | 82.4% |
| Day | 54 | 16.6 | 7.7 | 8.8 | 81.6% |
| Night | 27 | 13.4 | 3.6 | 9.7 | 78.7% |
| Twilight | 30 | 17.6 | 7.5 | 10.2 | 87.3% |
| Dive Type | N | Total Duration (h) | Surface Interval (s) | Blows | Dive Duration (min) | Mean Bottom Depth (m) |
|---|---|---|---|---|---|---|
| Deep searching | 21 | 3.36 | 56.9 ± 36.5 | 4.4 ± 2.5 | 9.5 ± 3.3 | 189 ± 163 |
| Deep searching (F) | 8 | 1.79 | 150.4 ± 40.1 | 11.3 ± 2.6 | 13.4 ± 2.7 | 354 ± 97 |
| Exploratory | 14 | 2.87 | 93.6 ± 52.8 | 5.6 ± 2.4 | 12.3 ± 6.8 | 30 ± 18 |
| Exploratory (S) | 21 | 4.51 | 68.9 ± 44.0 | 4.3 ± 2.0 | 12.8 ± 4.6 | 44 ± 56 |
| Fast travel | 109 | 8.23 | 71.8 ± 67.8 | 4.5 ± 3.8 | 4.5 ± 4.0 | 21 ± 13 |
| Fast travel (S) | 5 | 0.68 | 46.2 ± 13.8 | 3.2 ± 0.9 | 8.1 ± 1.4 | 21 ± 3 |
| Intra surfacing | 231 | 1.03 | 16.4 ± 31.5 | 1.8 ± 1.7 | 0.2 ± 0.1 | 4 ± 2 |
| Porpoising | 205 | 1.71 | 2.1 ± 4.8 | 1.0 ± 0.2 | 0.5 ± 0.4 | 12 ± 6 |
| Racing | 10 | 0.53 | 1.8 ± 0.6 | 1.0 ± 0.0 | 3.1 ± 1.3 | 53 ± 21 |
| Resting | 33 | 3.02 | 70.1 ± 31.3 | 4.3 ± 1.8 | 5.4 ± 3.4 | 7 ± 6 |
| Resting (S) | 8 | 2.25 | 203.0 ± 347.1 | 8.0 ± 9.9 | 16.8 ± 4.1 | 15 ± 2 |
| Shallow searching | 85 | 4.37 | 27.7 ± 30.3 | 2.3 ± 1.5 | 3.0 ± 3.0 | 18 ± 9 |
| Shallow searching (S) | 8 | 1.19 | 43.4 ± 22.5 | 3.0 ± 1.2 | 8.9 ± 4.4 | 20 ± 4 |
| Slow travel | 130 | 8.75 | 79.6 ± 81.5 | 4.7 ± 4.3 | 4.0 ± 3.6 | 12 ± 8 |
| Slow travel (S) | 671 | 117.98 | 57.5 ± 53.1 | 3.5 ± 2.1 | 10.5 ± 4 | 18 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davenport, A.M.; Erbe, C.; Jenner, M.-N.M.; Jenner, K.C.S.; Saunders, B.J.; McCauley, R.D. Pygmy Blue Whale Diving Behaviour Reflects Song Structure. J. Mar. Sci. Eng. 2022, 10, 1227. https://doi.org/10.3390/jmse10091227
Davenport AM, Erbe C, Jenner M-NM, Jenner KCS, Saunders BJ, McCauley RD. Pygmy Blue Whale Diving Behaviour Reflects Song Structure. Journal of Marine Science and Engineering. 2022; 10(9):1227. https://doi.org/10.3390/jmse10091227
Chicago/Turabian StyleDavenport, Andrew M., Christine Erbe, Micheline-Nicole M. Jenner, K. Curt S. Jenner, Benjamin J. Saunders, and Robert D. McCauley. 2022. "Pygmy Blue Whale Diving Behaviour Reflects Song Structure" Journal of Marine Science and Engineering 10, no. 9: 1227. https://doi.org/10.3390/jmse10091227






